Abstract
Leaf-cutter ants use plant matter to culture the obligate mutualistic basidiomycete Leucoagaricus gongylophorus. This fungus mediates ant nutrition on plant resources. Furthermore, other microbes living in the fungus garden might also contribute to plant digestion. The fungus garden comprises a young sector with recently incorporated leaf fragments and an old sector with partially digested plant matter. Here, we show that the young and old sectors of the grass-cutter Atta bisphaerica fungus garden operate as a biphasic solid-state mixed fermenting system. An initial plant digestion phase occurred in the young sector in the fungus garden periphery, with prevailing hemicellulose and starch degradation into arabinose, mannose, xylose, and glucose. These products support fast microbial growth but were mostly converted into four polyols. Three polyols, mannitol, arabitol, and inositol, were secreted by L. gongylophorus, and a fourth polyol, sorbitol, was likely secreted by another, unidentified, microbe. A second plant digestion phase occurred in the old sector, located in the fungus garden core, comprising stocks of microbial biomass growing slowly on monosaccharides and polyols. This biphasic operation was efficient in mediating symbiotic nutrition on plant matter: the microbes, accounting for 4% of the fungus garden biomass, converted plant matter biomass into monosaccharides and polyols, which were completely consumed by the resident ants and microbes. However, when consumption was inhibited through laboratory manipulation, most of the plant polysaccharides were degraded, products rapidly accumulated, and yields could be preferentially switched between polyols and monosaccharides. This feature might be useful in biotechnology.
INTRODUCTION
In the nests of leaf-cutter ants of the genera Atta and Acromyrmex, gardens of the obligate mutualistic basidiomycete fungus Leucoagaricus gongylophorus (1, 2) are cultivated on fresh vegetal materials (3–5). Although few leaf-cutter species prefer monocots, most leaf-cutter ants collect plant materials from both monocots and dicots (6). The ants collect and transport these plants to their nests and then fractionate and extensively clean the leaf fragments (7, 8). Subsequently, the ants deposit a drop of fecal fluid onto the leaf fragment surface and inoculate the leaf fragment with L. gongylophorus hyphae. The plant-digesting enzymes in the fecal fluid facilitate fungal development (9).
This fungus produces swollen hyphal tips, called gongylidia, that cluster together to form macroscopic structures known as staphylae, which are food sources for the ants (3–5). L. gongylophorus also secretes enzymes that act synergistically with ant enzymes to degrade plant matter, generating soluble compounds that are subsequently ingested by the ants (10–13). These enzymes attack plant polysaccharides, including starch, hemicellulose, pectin, and, to a lesser extent, cellulose (10, 14–23). In addition, other microbes living in the fungus garden might mediate ant nutrition on plant polysaccharides (24–27).
New substrates are incorporated in the top section of the fungus garden and are likely continuously degraded in the middle and the bottom sections (21–28). As waste material, the exhausted substrate is eventually discarded in a refuse dump (3-5, 27). The fungus garden and refuse dump differ with respect to enzyme activity, amount of mutualistic fungus, and microbial diversity (27–29).
In the present study, we observed that the monocot-cutter ant Atta bisphaerica adds fresh leaves and L. gongylophorus hyphal fragments to compose a green peripheral sector, almost completely circumventing the fungus garden, and that the fungus garden core contains decaying leaf fragments on which the fungi have been growing for a long time. We generated respiration data to estimate the microbial growth rate, active biomass, and kinetic parameters in ant-free fungus garden sectors. In addition, we determined the enzyme activity on polysaccharides and sucrose and used liquid chromatography (LC) coupled with mass spectrometry (MS) to analyze the substrate degradation products generated by microbes in the fungus garden.
The results indicate that the fungus garden is a biphasic solid-state bioreactor with peripheral and core sectors working synergistically to convert plant matter to monosaccharides and polyols, which are subsequently consumed by microbes and ants. Moreover, L. gongylophorus plays a central, but not exclusive, role in this mixed-culture fermentative process, which can be manipulated for biotechnological purposes.
MATERIALS AND METHODS
Collection and handling of fungus garden material.
A. bisphaerica nests were located in an open area dominated by Brachiaria and Paspalum species at the Universidade Estadual Paulista Júlio de Mesquita Filho, Campus Rio Claro. Eight different young nests (up to 6 months after they were founded) were excavated per month from January to May 2012, for a total of 40 sampled nests. The first underground nest chamber below the nest entrance, containing the fungus garden, was opened, and the fungus garden temperature was immediately determined. The entire chamber content was gently collected, packed into a plastic bottle (15 by 15 by 15 cm) with a plaster base, and maintained in a biochemical oxygen demand incubator in the dark at 25°C and 70% relative humidity for 24 h. This incubation was necessary for the ants to clean and recover the fungus garden structure. The samples were subsequently collected from the whole, peripheral, or core sector of the fungus garden, and the ants, larvae, pupae, and eggs were manually removed under a stereo microscope and discarded. The resulting material was used as ant-free fungus garden material.
Wet or dry mass and water content determination.
The masses of ant-free fungus garden material were determined before and after drying at 105 ± 2°C for 24 h.
pH determination.
Samples (1.00 ± 0.02 g, wet mass) of whole ant-free fungus garden material were suspended in 50 ml of deionized water in an Erlenmeyer flask, which was subsequently maintained in a reciprocating shaker at 150 rpm and 4°C for 10 min. The suspension obtained was vacuum filtered with a 0.45-μm Millipore HAWP04700 filter, and the pH of the filtrate was determined.
Respiration.
Respiration was determined with solid-state or suspended ant-free whole fungus garden material and a Bartha and Pramer respirometer (30) filled with CO2-free air. Solid-state material was sprayed with 400 μl of 7% (vol/vol) Vogel medium (31) to avoid dehydration. The evolved CO2 was trapped in 25 ml of 0.1 N NaOH, and the alkali excess was determined by potentiometric titration, followed by calculation of the amount of CO2 produced. The results were converted to O2 consumed by using a stoichiometric ratio of 44 mg of CO2 to 32 mg of O2. The stability of fungus garden respiration (see Fig. S2A and B in the supplemental material) was assessed as the respiration rate (Δy · Δt−1) expressed as milligrams of O2 consumed per gram (wet mass) of fungus garden material per hour. The effect of sodium azide on respiration (see Fig. S2C) was determined as the cumulative respiration (y), which corresponded to milligrams of O2 consumed per gram (wet mass) of fungus garden material. Five young nests were opened, and the total fungus garden content of the first chamber was collected. Each of the sampled ant-free whole fungus gardens was divided into two fractions: a solid-state garden and a fungus garden suspension. For the solid-state tests, 100 μl of water was added to the controls (213.33 ± 2.44 mg of wet mass) and 100 μl of 10 mM sodium azide (Sigma-Aldrich S2002) was added to the test samples (245.12 ± 1.98 mg of wet mass). Suspensions of 204.00 ± 1.87 mg of fungus garden wet mass in 25 ml of 10 mM sodium azide were also examined, and the results were compared with those obtained with control suspensions of 200 ± 0.56 mg of fungus garden wet mass in 25 ml of water. To determine the effect of sodium acetate on respiration (see Fig. S2D), a single nest chamber from 15 young nests was opened and whole ant-free fungus garden samples (200.00 ± 0.47 mg of wet mass) were treated with different amounts of sodium acetate. One hundred milligrams of chemical oxygen demand (COD) acetate refers to the fact that 98.8 mg of sodium acetate reacts with 100 mg of O2 (32). Amounts ranging from 100 to 1,000 mg of COD acetate/g (wet mass) of fungus garden material were used. The mixture was incubated for 15 h at 25°C, and cumulative respiration (y) was calculated.
Estimation of microbial kinetic and physiological parameters.
Microbial kinetic and physiological parameters were estimated by using peripheral (195.32 ± 0.36 mg of wet mass), core (201.43 ± 0.27 mg of wet mass), or whole (203.28 ± 0.78 mg of wet mass) ant-free fungus garden materials obtained from six young nests (one chamber per nest). A saturating amount of substrate corresponding to 500 mg of COD acetate (494 mg of sodium acetate)/g (wet mass) of ant-free fungus garden material was used. The kinetic parameters were assessed by the Van de Werf and Verstraete modeling method (32) adapted for CO2 release by using the O2-to-CO2 molar ratio of 1:1 observed in acetate oxidation. This method uses a model based on Monod kinetics [μ = (μmax · S−1) (KS + S)−1] and three differential equations: −dS · dt−1 = (1 · Ymax−1) dX · dt−1 + mX, dX · dt−1 = μX − mYmaxX, and dy · dt−1 = [(1 − fYmax) Ymax−1] μX + mX. The conversion factor f is 1.33 (32), KS is the semisaturation constant for substrate affinity (milligrams of COD per gram of wet fungus garden mass), m is the coefficient of cell maintenance (milligrams of COD consumed per milligram of dry biomass per hour), μ is the specific microbial growth rate (per hour), μmax is the maximum specific growth rate (per hour), S is the substrate concentration (milligrams of COD per gram of wet fungus garden mass), X is the active microbial dry mass (milligrams of dry mass per gram of wet fungus garden mass), y is the cumulative respiration (milligrams of O2 consumed per gram of wet fungus garden material mass), and Ymax is the maximum microbial yield (milligrams of dry biomass generated per milligram of COD consumed). The three differential equations were solved by using the LSODA algorithm (Runge-Kutta fourth order) from the deSolve package (33) implemented in the R 3.0.1 software package (34) with the nls function for modeling. The microbial assimilation rates were defined as q = μYmax−1 + m, and the CO2 assimilation rate (Q) was defined as Q = qX.
Enzyme activity.
A single nest chamber of six nests was opened, and samples (200.00 ± 0.41 mg of wet mass) of whole, peripheral, or core ant-free fungus garden material were suspended in 50 ml of McIlvaine buffer (35), pH 6.3. The buffer contained 10 mM sodium azide to inhibit microbial activity and a proteolysis inhibition cocktail [23 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Sigma-Aldrich A8456), 2 mM aprotinin (Sigma A3428), 130 μM bestatin (Sigma B8385), 100 mM EDTA (Sigma E6758), 0.3 mM E-64 (Sigma E3132), 0.3 mM pepstatin A (Sigma P5318), and 10 mM phenylmethylsulfonyl fluoride (Sigma P7626)]. The suspension was maintained at 4°C at 150 rpm for 30 min in the dark for protein extraction, followed by vacuum filtration with a 0.45-μm Millipore filter (HAWP04700). To determine enzyme activities, 1.0 ml of filtrate and 3.0 ml of substrate at 2 g · 100 ml−1 in McIlvaine (35) buffer, pH 6.3, were used. The blanks were boiled for 10 min immediately after filtrate addition. The enzyme assays were conducted in a water bath at 40°C for 2 h, and the reducing sugars were determined by using 200-μl aliquots sampled at 30-min intervals (36). The substrate used to determine exocellulase activity in crystalline cellulose was either low-polymerization Avicel (Sigma [Fluka] PH-101PhEur 11363) containing approximately 200 glucose residues per molecule (37) or high-polymerization cotton fiber (Johnson & Johnson) containing 10,000 to 15,000 glucose residues per molecule (37). The substrates used to determine exocellulase on amorphous celluloses were Walseth cellulose, a cotton fiber treated with phosphoric acid (38) and ball-milling cellulose. Carboxymethyl (CM)-cellulose sodium salt (Sigma C5678) was also used to determine endocellulases; xylan “birchwood” (Sigma-Aldrich X4252) was used to determine hemicellulase activity; starch (Sigma S9765) was used for amylases; citrus pectin (Vetec 1219) was used for pectinases; sucrose (Mallinckrodt 8360) was used for invertase; and chitin (Sigma C9752), a major component of fungal cell walls (39, 40), was used to quantify chitinases. One enzyme unit (U) was defined as the amount of enzyme producing 1 μmol of reducing ends per minute. Enzyme activity is expressed in units per gram of fungus garden dry mass.
Monosaccharide concentration variation in ant-free fungus garden material suspensions.
Ant-free whole, peripheral, or core fungus garden samples were collected from four distinct nests (one chamber per nest). Each of the 12 samples was further divided into two fractions (212.00 ± 2.09 mg of wet mass). The first fraction was added to a 250-ml Erlenmeyer flask containing 50 ml of distilled water, and the second fraction was added to a similar flask containing 50 ml of 10 mM azide. The flasks were incubated in the dark at 25°C for 48 h at 150 rpm. Aliquots of 500 μl were collected at fixed incubation times and heated at 100°C for 10 min to inactivate the enzymes in the solution. Subsequently, each aliquot was centrifuged (15,000 × g for 20 min), and the monosaccharide and polyol concentrations in the supernatant were determined.
Analysis of L. gongylophorus staphyla exudates.
Staphylae were collected from 1.00 ± 0.34 g of whole fungus garden material (two young nests, one chamber per nest) with microtweezers and gently washed with 100 μl of 0.1 M ammonium acetate. After centrifugation at 3,500 × g for 20 min at 4°C, the supernatant was collected and the polyol concentration was determined. After extraction, staphyla integrity was confirmed through microscopy.
Monosaccharide and polyol variations in fungus garden material without ants.
Ant-free whole, peripheral, or core fungus garden samples were aseptically collected from nests 1, 2, and 3 (one chamber per nest). The ants were removed from each of the nine samples, and these garden samples were divided into two fractions; one was treated with 100 μl of 10 mM sodium azide; and the other was treated with 100 μl of distilled water. The 18 fractions produced had 214.03 ± 3.23 mg of wet mass. Nine of these fractions (see Table 2 for fraction sorting) were immediately subjected to monosaccharide and polyol extraction and analysis, representing time zero (t0). Each of the remaining nine fractions was incubated in a 50-ml Erlenmeyer flask at 25°C for 15 h (t15); this was followed by monosaccharide and polyol extraction and analysis.
TABLE 2.
Monosaccharide and polyol contents of fungus garden materiala
| Fungus garden type | Mean (SD) concn, mg · g−1 (dry mass) of fungus garden material |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Mannose | Xylose | Arabinose | Sorbitol | Mannitol | Inositol | Arabitol | Total | Δc | |
| Without ants | ||||||||||
| Whole | ||||||||||
| − | ||||||||||
| 0 h (1)d | 24.7b (3.7) | 3.1b (0.5) | 6.8b (1.0) | 2.3b (0.3) | 8.3b (1.2) | 103.0b (15.0) | 7.5b (1.1) | 54.6b (8.2) | 210.3b (31.0) | 671.3 (172.2)e |
| 15 h (2, 3) | 90.3b (13.5) | 10.2b (1.5) | 70.6b (10.6) | 23.5b (3.5) | 27.2b (4.1) | 289.3b (49.4) | 24.7b (3.7) | 345.8b (54.9) | 881.6b (141.2) | |
| + | ||||||||||
| 0 h (1, 3) | 26.8b (4.0) | 3.4b (0.5) | 7.4b (1.1) | 2.5b (0.4) | 9.0 (1.3) | 108.9 (16.3) | 8.2 (1.2) | 59.4 (8.9) | 225.6b (33.7) | 200.1 (98.2) |
| 15 h (2) | 112.8b (16.9) | 5.5b (0.2) | 88.2b (13.2) | 29.4b (4.4) | 9.2 (1.4) | 111.0 (16.0) | 8.3 (2.2) | 61.3 (10.2) | 425.7b (64.5) | |
| Peripheral | ||||||||||
| − | ||||||||||
| 0 h (2) | 27.6b (4.1) | 3.4b (0.5) | 7.6b (1.1) | 2.5b (0.4) | 9.6b (2.1) | 111.8b (16.8) | 8.4b (1.3) | 61.0b (9.1) | 231.5b (34.4) | 518.6 (151.1) |
| 15 h (1, 3) | 108.1b (16.2) | 8.4b (1.3) | 84.6b (12.7) | 28.2b (4.2) | 22.4b (3.4) | 271.1b (40.7) | 20.3b (3.1) | 207.0b (34.1) | 750.1b (115.7) | |
| + | ||||||||||
| 0 h (1, 3) | 30.2b (4.5) | 3.8b (0.6) | 8.3b (1.3) | 2.8b (0.4) | 10.1 (1.7) | 122.3 (18.4) | 9.2 (1.4) | 66.7 (10.0) | 253.4b (38.3) | 242.3 (115.7) |
| 15 h (2) | 135.5b (20.3) | 5.8b (1.3) | 106.0b (15.9) | 35.3b (5.3) | 10.3 (4.5) | 124.4 (18.7) | 9.3 (1.0) | 69.1 (10.4) | 494.7b (77.4) | |
| Core | ||||||||||
| − | ||||||||||
| 0 h (2) | 23.6 (3.5) | 3.0b (0.4) | 6.5b (1.0) | 2.2b (0.3) | 7.9b (1.2) | 95.8b (14.4) | 7.2b (1.1) | 52.3b (7.8) | 198.5b (29.7) | 85.3 (72.2) |
| 15 h (1, 3) | 25.8 (3.9) | 2.0b (0.3) | 20.2b (3.0) | 6.7b (1.0) | 5.3b (0.8) | 64.7b (9.7) | 4.9b (0.7) | 154.2b (23.1) | 283.8b (42.5) | |
| + | ||||||||||
| 0 h (2, 3) | 26.8 (4.0) | 3.4 (0.5) | 7.4b (1.1) | 2.5b (0.4) | 9.0 (3.3) | 108.9 (16.3) | 8.2 (1.2) | 59.4 (18.9) | 225.6 (45.7) | 69.2 (107.4) |
| 15 h (1) | 30.2 (7.5) | 3.5 (1.2) | 53.6b (8.0) | 17.9b (2.7) | 9.3 (4.2) | 111.4 (16.7) | 8.4 (2.3) | 60.5 (19.1) | 294.8 (61.7) | |
| With ants | ||||||||||
| Whole (−) | ||||||||||
| 0 h (4) | 25.8 (3.9) | 3.2b (0.5) | 7.1 (1.1) | 2.4 (0.4) | 8.6 (1.3) | 104.4 (15.7) | 7.8 (1.2) | 57.0b (8.5) | 220.3 (32.6) | 0.1 (72.4) |
| 15 h (5, 6) | 25.2 (3.8) | 3.3 (1.1) | 7.0 (1.0) | 2.4 (1.0) | 8.8 (4.3) | 102.1 (15.3) | 8.0 (3.5) | 48.6b (9.8) | 220.4 (39.8) | |
| Peripheral (−) | ||||||||||
| 0 h (5) | 28.9 (4.3) | 3.6 (0.5) | 8.0 (1.2) | 2.7 (0.4) | 9.7 (1.4) | 117.1 (17.6) | 8.8 (1.3) | 63.9 (9.6) | 247.7 (36.3) | 15.4 (81.3) |
| 15 h (4, 6) | 29.5 (4.4) | 3.7 (0.6) | 8.2 (2.6) | 2.8 (1.1) | 9.9 (3.5) | 119.7 (18.0) | 9.0 (2.0) | 65.3 (12.8) | 263.1 (45.0) | |
| Core (−) | ||||||||||
| 0 h (6) | 25.2 (3.8) | 3.2 (0.5) | 7.0 (1.0) | 2.3 (0.3) | 8.4 (1.3) | 102.3 (15.4) | 7.7b (1.2) | 55.8 (10.4) | 217.9 (33.9) | 5.9 (87.8) |
| 15 h (4, 5) | 26.0 (8.9) | 3.0 (1.4) | 7.2 (1.1) | 2.1 (1.0) | 7.0 (2.0) | 100.6 (25.1) | 5.3b (0.8) | 57.6 (13.6) | 223.8 (53.9) | |
Values are mean monosaccharide or polyol contents (mg · g−1 dry mass [standard deviation]) of fungus garden material. +, sodium azide present; −, sodium azide absent. Italic type highlights microbial production of mannose, sorbitol, mannitol, and inositol in the peripheral sector of the fungus garden, and boldface type highlights microbial consumption of these metabolites in the core sector. Six nests were sampled, and nest numbers are shown in parentheses.
Significant difference between values that resulted from incubation times of 0 and 15 h at P ≤ 0.05 according to the Mann-Whitney test.
Difference between 15- and 0-h metabolite concentrations.
Nest number.
Value corresponding to degradation of ∼67% of plant matter.
Monosaccharide and polyol variations in fungus garden material with ants.
Ant-free whole, peripheral, or core fungus garden samples were aseptically collected from nests 4, 5, and 6 (one chamber per nest). The nine fungus garden materials had 623.87 ± 45.10 mg of wet mass. Three of these materials (see Table 2 for material sorting) were immediately subjected to monosaccharide and polyol extraction and analysis (t0). The other six materials were reintegrated with the original ants (42 ± 10 gardeners per material) and incubated in a 50-ml Erlenmeyer flask at 25°C for 15 h; this was followed by ant removal and monosaccharide and polyol extraction and analysis (t15).
Polyol and monosaccharide extraction from the fungus garden.
The experimental design for the assessment of fungus garden monosaccharide and polyol variations resulted in either one or two replicates for each of the 18 conditions tested (for this information, see Table 2). Polyol and monosaccharide analyses were repeated three times for conditions with a single replicate and twice for conditions with two replicates. The total fungus garden material mass under each condition was transferred to a test tube and extracted for 10 min through vigorous stirring with 1.00 ml of 100 mM ammonium acetate at room temperature, followed by centrifugation for 20 min at 15,000 × g. The monosaccharide and polyol contents the supernatant were determined, and the results are expressed in milligrams of polyol or monosaccharide per gram (dry mass) of sample.
HPLC-ESI-MS analysis of monosaccharides and polyols.
A Shimadzu Prominence high-performance LC (HPLC) system with a Phenomenex Rezex RPM monosaccharide Pb2+ column connected to a Bruker micrOTOF-QII mass spectrometer was used for analysis. The chromatographic conditions were a column temperature of 85°C, an injection volume of 5 μl, and an isocratic mobile phase of 100% water at 0.4 ml · min−1. The LMS conditions were as follows: end plate offset, −500 V (electron spray ionization [ESI] in negative mode); capillary, 3.5 kV; nebulizer, 5.0 × 105 Pa; drying gas, N2 at 9.0 liters · min−1; drying temperature, 250°C; rate of spectrum acquisition, 2 Hz; range, 50 to 3,000 m/z; and MS analysis mode, full MS. Sodium trifluoroacetate was used for calibration. Calibration curves were generated by using the chromatographic standards in sugar alcohol kit 47266 from Supelco containing isolated flasks of d-(+)-arabitol, dulcitol (galactitol), iso-erythritol, glycerol, maltitol, and ribitol (adonitol); monosaccharide kit 47267 from Supelco containing individual standards of d-(−)arabinose, fructose, d-(+)galactose, and d-(−)ribose; and individual standards of d-(+)-cellobiose (Fluka 22150), d-(+)-maltose monohydrate (Sigma BioUltra 63418), glucose (Fluka G0350500) pure anomer, inositol (Fluka PHR1351), lactose (Fluka A1206000), l-arabitol (Sigma A3506), mannitol (Fluka M0200000) pure anomer, mannose (Sigma M2069), sorbitol (Fluka S1000000), sucrose (Sigma 84097), trehalose (Fluka PHR1344), xylitol (Fluka PHR1280), xylobiose (Sigma X1501), xylose (Supelco 47253), galacturonic acid (Fluka 48280), inositol (Fluka PHR1351), and l-arabitol (Sigma A3506). The chromatograms and extracted peaks of the carbohydrates (glucose, mannose, xylose, arabinose) and polyols (sorbitol, mannitol, inositol, arabitol) detected were determined through chromatography with Bruker Compass DataAnalysis and QuantAnalysis software. The values are expressed in milligrams per gram of dry fungus garden mass.
Variations in mass and fiber glucose after incubation of ant-free whole fungus garden material suspensions.
Ant-free whole fungus garden samples were collected from six distinct nests (one chamber per nest). Each sample was divided into two fractions, and each of the 12 fractions (588.65 ± 8.65 mg of wet mass) was individually suspended in 50 ml of distilled water in Erlenmeyer flasks. Six flasks were vigorously stirred for 5 min, and the other six were incubated at 150 rpm for 10 h at 25°C. The contents of each flask were vacuum filtered (0.22-μm Millipore HAWP04700 membrane), and the filtrates were dried at 65°C for 48 h, followed by mass determination. The residual material retained in the filters was washed with distilled water and dried at 100°C for 24 h. After mass determination, the residual material was ground with a mortar and pestle. The ground material sample (5.00 ± 0.02 mg of dry mass) was suspended in 25 ml of 0.5 M K2SO4, maintained in an orbital shaker at 25°C for 4 h, filtered (0.22-μm Millipore HAWP04700 membrane), washed with ultrapure water, and then dried at 102 ± 5°C for 24 h. The material retained in the filter was ground with a mortar and pestle and suspended in a Teflon pressure flask containing 3.00 ml of 72% sulfuric acid. The flasks were incubated in a water bath at 30 ± 1°C for 60 min and stirred every 5 min. After the first 60 min of hydrolysis, 84 ml of deionized water was added to the suspension and the reaction mixture was autoclaved for 1 h at 121 ± 3°C. The hydrolyzed material was cooled to room temperature and neutralized to pH 5 to 6 by the careful addition of NaOH with constant stirring. The pH was determined with pH paper. The reaction mixture was decanted, and the glucose concentration in the supernatant was determined according to the method of Bergmeyer and Bernt (41) with the Sigma GAGO-20 glucose oxidase kit. The results are expressed in milligrams of glucose per milligram of dry mass of ant-free fungus garden material.
Statistical analyses.
The normality of the results was assessed by the D'Agostino-Pearson test (42). The results were analyzed by analysis of variance at a significance level of P ≤ 0.05. Tukey's test was used to evaluate multiple contrasts between averages. The chi-square goodness-of-fit procedure (P ≤ 0.05) was used to test nonlinear regressions, which were applied to estimate microbial kinetics and physiological parameters. The results from endpoint incubations of solid-state fungus garden material did not present normality and were analyzed by Mann-Whitney test at P ≤ 0.05. Statistical analyses were implemented with the R 3.0.1 software (34), and the graphics were constructed with the OriginPro 8 software.
RESULTS
Nests and fungus gardens of A. bisphaerica.
The nests presented a single mound with openings leading to an oval chamber ranging from 10.5 by 9.0 by 8.0 cm to 15.0 by 10.6 by 9.0 cm in width, length, and height, respectively. The medial or basal points of the chambers were connected to one to three tunnels and almost entirely filled with the fungus garden, which was seated at the chamber base.
The fungus garden presented a particulate and brittle aspect, containing aggregates of mycelia dispersed on the surface of grass fragments and ranging from 5 to 8 mm in length, connected through thin hyphal filaments. The fungus garden had low substrate compression and two clearly distinguished morphological sectors (see Fig. S1 in the supplemental material). In the peripheral sector, the fungus garden showed a brittle structure with fresh green grass fragments. The peripheral sector surface presented fungal mycelia distributed in aggregates containing short thick hyphal fragments and a high staphyla number (approximately 6/20 mm2 of leaf surface; see Fig. S1 in the supplemental material). In the core sector, the fungus gardens maintained a cohesive structure comprising pale yellow grass fragments. This sector also contained diffusely distributed mycelia with thin hyphal fragments, which invaded the vegetation structure, and a low number of staphylae (approximately 1/20 mm2 of leaf surface; see Fig. S1).
Physical parameters.
Whole fungus garden samples (n = 15) had an extracellular pH of 6.31 ± 0.26, which remained stable during the collection period extending from January to May 2012. The dry mass ranged between 37 and 42% of the wet mass. The solutes accounted for 64 to 75% of the dry mass. The temperature remained stable at 24.8 ± 0.7°C. The dry mass of the fungus garden per chamber was 98.42 ± 23.12 g.
Microbial respiration.
The respiration of ant-free whole garden material remained constant for 18 h, followed by exponential growth (see Fig. S2A and B in the supplemental material). This exponential growth reflects changes in the physiology of the microbial community likely resulting from the lack of management by the ants and involving the activation of the dormant microbial biomass and invader microorganisms. Thus, all respiration tests with ant-free fungus garden material were limited to a maximum of 15 h. Sodium azide inhibited respiration in both fungus garden material and the material suspension (see Fig. S2C).
Ant-free whole garden material showed maximum respiration values following the addition of 500 mg of COD acetate g−1, and respiration did not significantly increase with the addition of higher acetate concentrations, indicating saturation (see Fig. S2D).
Kinetic and physiological parameters.
The acetate-induced respiration results were modeled (32), indicating that microbial biomass (i.e., all respiring microbes living in the fungus garden) is rapidly produced and constantly removed from the fungus garden peripheral sector, as microbes grow slowly and are stocked in larger amounts in the core.
Table 1 shows that microbes in the peripheral fungus garden material had a high cell mass yield (Ymax) and low cell maintenance coefficient (m), indicating that the metabolism of these microbes primarily supports microbial growth. The assimilation per biomass unit (q) was high; however, because of the small biomass amounts (X), energy consumption (Q) was low. Compared with the peripheral sector, the fungus garden core had a low cell yield and a high cell maintenance coefficient, indicating that the metabolism of these microbes contributes to cell maintenance. The assimilation per biomass unit was reduced in these slow-growing microbes, although the high microbial mass amounts led to high energy consumption (Table 1).
TABLE 1.
Kinetic and physiological parameters of ant-free fungus garden materiala
| Garden sample | Ymax (mg · mg−1 COD) | m (mg COD · mg−1 · h−1) | q (mg COD · mg−1 · h−1) | X (mg · g−1) | Q (mg COD · mg−1 · h−1) | μ (h−1) |
|---|---|---|---|---|---|---|
| Peripheral | 0.741 (0.037)* | 0.043 (0.008)‡ | 0.637 (0.160)* | 32.11 (1.93)‡ | 20.448 (0.164)* | 0.42 (0.02)‡ |
| Core | 0.693 (0.073)† | 0.083 (0.008)† | 0.545 (0.057)† | 54.28 (3.53)† | 29.570 (0.058)† | 0.32 (0.02)† |
| Whole | 0.738 (0.049)* | 0.074 (0.001)* | 0.602 (0.099)* | 39.63 (2.38)* | 23.875 (0.104)* | 0.39 (0.02)* |
Data are means and standard deviations of six fungus garden material samples from different nests. Values followed by different symbols are statistically significantly different from the others in that column by Tukey test at P ≤ 0.05.
Intermediate parameter values were obtained for the whole fungus garden material, except for the maximum cell yield, which was similar to that observed in the peripheral sector. These results reflect the high intensity of vegetal biomass conversion to microbial biomass in the fungus garden periphery. The whole fungus garden material microbial biomass (X) was only 4% of the fungus garden biomass but presented a high specific growth rate (μ) (Table 1).
Monosaccharides and polyols in ant-free fungus garden material suspensions and polyol production by L. gongylophorus.
The results obtained with fungus garden material suspended in distilled water indicated that the peripheral sector accumulates predominantly glucose and xylose at high rates and the core sector accumulates xylose and arabinose at low rates. In the peripheral sector, these monosaccharides are converted to polyols through the mutualistic fungus L. gongylophorus and another, unidentified, microbe.
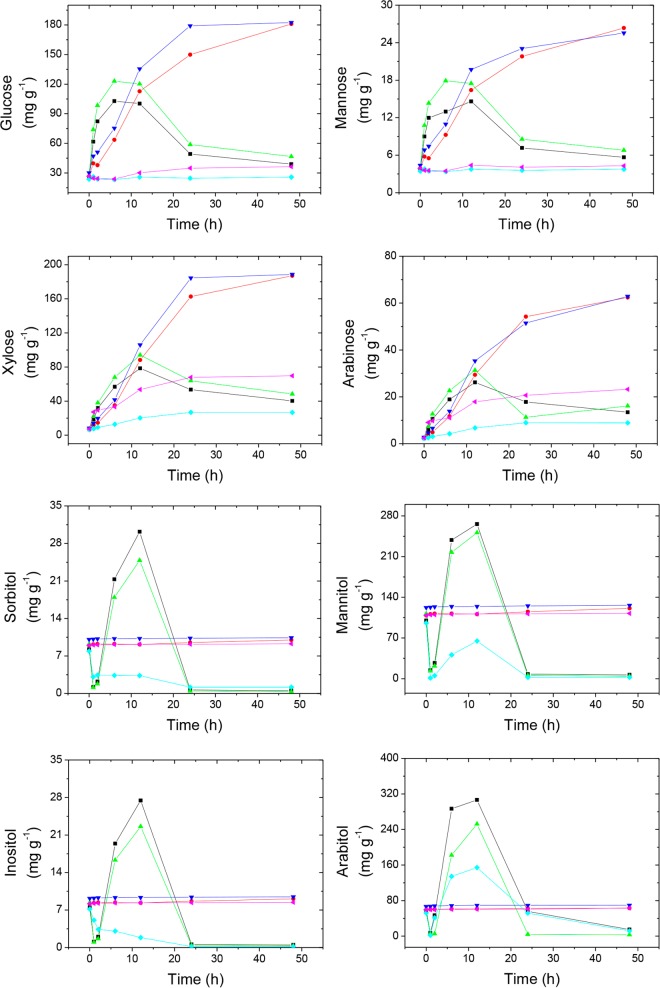
As shown in Fig. 1, the concentration increased in both suspensions of whole and peripheral materials, reaching a maximum at 5 h for C6 monosaccharides (glucose and mannose) and 12 h for C5 monosaccharides (xylose and arabinose), followed by a decrease thereafter because of microbial consumption. Glucose accumulated in larger amounts, followed by xylose, arabinose, and mannose. When microbial oxidative metabolism was inhibited with sodium azide, the monosaccharide concentrations continuously increased for up to 48 h, although at lower rates than those observed in the absence of azide, when polysaccharidases are likely continuously secreted by active microbes. However, in core sector material suspensions, monosaccharides accumulated slowly. Peak concentrations contained 42 to 54% glucose and 36 to 43% xylose in peripheral material suspensions and 31 to 75% xylose and 10 to 25% arabinose in core material suspensions. Galacturonic acid, a pectin degradation product; disaccharides; and oligosaccharides were not detected.
FIG 1.
Monosaccharide and polyol concentrations in suspensions of ant-free fungus garden material incubated for 48 h at 25°C. Values are expressed per gram of fungus garden material dry mass. n = 12. Symbols: ■, whole; ●, whole plus NaN3; ▲, peripheral; ▼, peripheral plus NaN3; ◆, core; ◀, core plus NaN3. Sample size: four nests, one chamber per nest. Each point in the graph represents the results of four determinations.
The polyols mannitol and arabitol were massively produced in fungus garden material in suspension, as minor amounts of sorbitol and inositol were also present. The polyol concentration peaked after 12 h and decreased thereafter, reflecting microbial consumption. The peripheral material rapidly produced polyols compared with the core material, and azide blocked this polyol production (Fig. 1). The L. gongylophorus staphyla exudates collected from the whole fungus garden material were rich in inositol (4.9%), arabitol (22.1%), and mannitol (73.0%), and sorbitol was not detected. Therefore, sorbitol might be produced by microbes other than L. gongylophorus.
A delay in the conversion of monosaccharides to polyols was observed, as peaks of C6 polyols (sorbitol, mannitol, inositol) occurred at 7 h after peaks of C6 monosaccharides (glucose and mannose). This delay was not observed with the C5 monosaccharide arabinose or the C5 polyol arabitol, and the concentrations of both compounds peaked after 12 h of incubation (Fig. 1).
Variations in monosaccharide and polyol concentrations in ant-free fungus garden material.
Table 2 shows that polysaccharide metabolites accumulated in whole or peripheral fungus garden material without ants. However, the core sector accumulated only minor amounts of glucose, xylose, arabinose, and arabitol and consumed mannose, sorbitol, mannitol, and inositol. In the presence of ants, the metabolite concentration did not vary significantly, suggesting that the ants consumed the metabolites produced by the microbes. Therefore, these results suggest that peripheral microbes degrade plant polysaccharides to generate monosaccharides and polyols, which are subsequently consumed by core microbes and ants. In the presence of azide, ant-free fungus garden material showed only sugar accumulation; i.e., polyol production was inhibited.
Degradation of solids and fiber glucose in the fungus garden.
We estimated the percentage of solids degraded as the total amount of monosaccharides and polyols generated per gram of fungus garden material. This amount, 641.76 ± 32.50 mg · g−1 after 12 h of incubation in the absence of azide, was observed in ant-free whole fungus garden material suspensions (Fig. 1), corresponding to 64.2% degraded solids; 67.0% degradation was observed in ant-free whole fungus garden material incubated without azide for 15 h (Table 2); and 82.0% (18% polyol plus 64% monosaccharide) degradation was observed in the peripheral sector material suspension incubated without azide for 6 to 10 h (Fig. 1).
These estimates are approximations obtained after hydrolysis adds water molecules to the monosaccharides and polyols produced and these metabolites are partially consumed by microbes. The amount of degradation could be more accurately calculated by determining the fungus garden material mass variation. As shown in Table S1 in the supplemental material, after 10 h of incubation, ant-free whole fungus garden material suspensions lost almost 71 mg (87%) of their solid material, reflecting microbial degradation, resulting in the production of 58 mg of solutes. The 13-mg difference might be explained, in part, by microbial consumption. The fibers in the solid residue resistant to degradation after 10 h contained 91% glucose residue, which is 3.8 times as high as the 24% glucose residue observed in the starting material, indicating a high level of preservation of the glucose residue in fibers.
Enzyme activity in the fungus garden.
The finding that distinct sectors within the fungus garden contained different amounts of glucose, xylose, and arabinose suggests differences in substrate degradation, likely reflecting differences in enzyme activity. To examine this hypothesis, enzyme activities in the fungus garden material were determined. Whole fungus garden material showed low cellulase activity, high pectinase and xylanase (hemicellulase) activities, and intermediate chitinase, invertase, and amylase activities (Fig. 2). The peripheral sector of the fungus garden exhibited chitinase, invertase, and amylase activities, whereas xylanases were more active in the core sector. The two sectors had equivalent pectinase activities (Fig. 2). Chitinase activity and the growth rate of the peripheral sector were 34 and 31%, respectively, higher than those in the core sector of the fungus garden. The production of the remaining enzymes was not associated with the cell growth rate.
FIG 2.
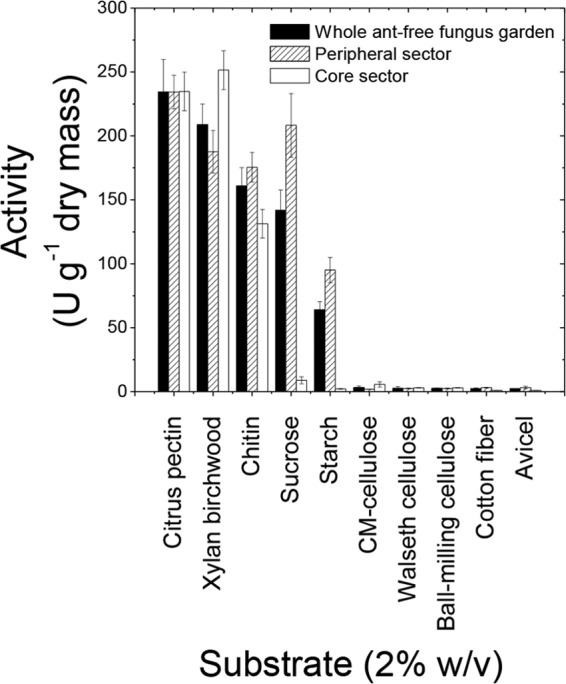
Enzyme activities of ant-free fungus garden material from different sectors on different substrates.
DISCUSSION
A. bisphaerica fungus garden sectors.
The 40 field nests of A. bisphaerica sampled showed clear morphological differences between the peripheral and core sectors (see Fig. S1 in the supplemental material). The peripheral sector contained a new fungus garden region almost completely circumventing the core fungus garden material, which contained pale yellow leaf fragments, corresponding to an older fungus garden region (see Fig. S1). This pattern differs from that reported for field nests of Atta colombica (21) or laboratory nests of Acromyrmex echinatior (28), in which top, middle, and bottom sections have been described. A. bisphaerica harvests monocots, generating a fungus garden markedly distinct from those observed in field nest gardens of attines that cut dicots. For instance, the monocot fragments in A. bisphaerica fungus gardens were larger than the dicot fragments observed in Atta sexdens fungus gardens. However, in A. sexdens field nests fed with dicots, we also observed the incorporation of new plant fragments, thereby circumventing the fungus garden (M. Bacci, personal observation). Therefore, plant processing dynamics in fungal gardens are highly complex, presenting plant matter processing from peripheral to core sectors, in addition to previously described plant matter flow from top to bottom sections.
Microbial community methods are useful to study the fungus garden microbiota.
The mutualistic fungus was originally considered a unique microbe in ant nests (43). However, it has been shown that the fungus garden contains a complex microbial community mediating ant nutrition on plant matter (29), infecting the ants (44) or L. gongylophorus (45, 46) and producing antibiotics (47, 48), further shaping microbial community diversity, which also includes many commensals (49–51). Therefore, the fungus garden is a mixture rather than a single microbial culture. The experimental approach applied in the present study aimed to preserve the characteristics of this mixed culture by using fungus garden material freshly collected from field nests to reduce potential contamination due to long-term laboratory manipulation. To assess the mixed-fungus garden microbial community physiology and preserve the potential interactions between microbes, we separated ants from the fungus garden material and did not isolate microbes in cultures.
The fungus garden microbial community showed remarkable physiological stability, consuming O2 at constant rates for up to 20 h. Subsequently, the O2 consumption was much faster (see Fig. S2A in the supplemental material), likely occurring through otherwise dormant microbial contaminants (52–54), which outcompeted the typically active microbes. Thus, the experiments on fungus garden microbial kinetics and physiology were limited to 15-h incubations.
Fungus garden peripheral and core sector functions.
The results indicated that the peripheral and core sectors of the fungus garden operate differently from and complementary to each other (Fig. 3). Staphyla production was less abundant in the core than in the peripheral sector (see Fig. S1 in the supplemental material), consistent with a previous report showing lower density of gongylidia in older regions than in newer fungus garden regions (55). The sectors were also distinct with respect to the enzymes produced (Fig. 2).
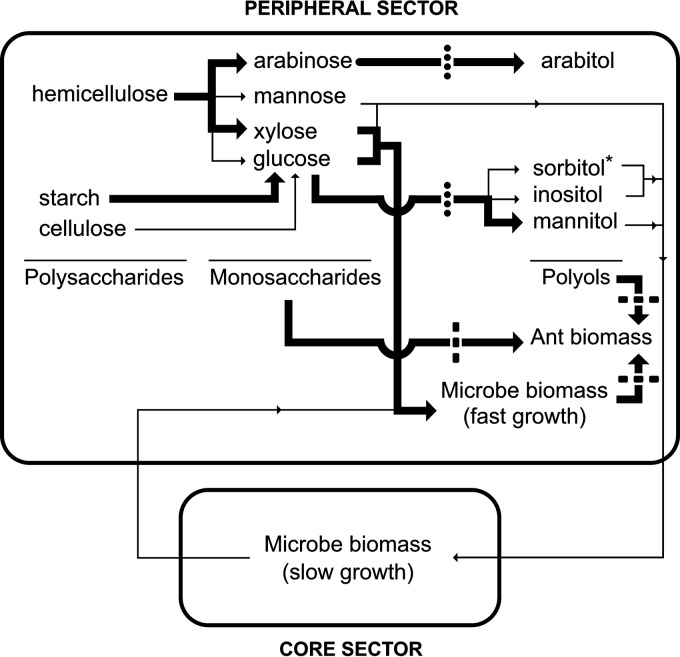
FIG 3.
Carbon flow from plant polysaccharides to microbe and ant biomass in the fungus garden and carbon flow manipulation in the laboratory. In the peripheral sector, plant polysaccharides are degraded into monosaccharides and some monosaccharides are reduced to polyols. L. gongylophorus produces most of the polyols, while sorbitol (*) is produced by another, unidentified, microbe. Thick arrows indicate the quantitatively prevalent steps. Some polyols and mannose support the slow growth of the microbial biomass in the core sector. Samples of L. gongylophorus stocked in the core sector might be transferred through ants for rapid growth on newly incorporated plant matter in the peripheral sector. Ants ultimately feed on fungal biomass, monosaccharides, and polyols. The artificial removal of ants from the fungus garden blocks carbon flow to the ant biomass, represented as dashed lines crossing thick black lines, thereby inducing the accumulation of monosaccharides, polyols, and microbe biomass. The inhibition of microbe respiration with sodium azide blocks polyol production, indicated as dotted crossing lines, further inducing the accumulation of monosaccharides. The manipulation of fungus garden carbon flow might be useful for biotechnological processing to generate monosaccharides, polyols, or biofuels from plant biomass.
Ants stock high microbial cell mass densities in the core sector (Table 1), where microbes grow slowly on the monosaccharide mannose and the polyols sorbitol, mannitol, and inositol (Table 2) generated predominantly in the periphery (Fig. 1), suggesting that peripheral microbes mediate the transfer of carbons from plant material to core microbes (Fig. 3). This transfer might provide the large amount of energy (Q) necessary for cell maintenance and regeneration in the core sector (Table 1). The higher drainage of energy in the core resulted from the high density of microbial mass (X) and cell maintenance coefficients (m) in this sector (Table 1). Thus, ants might transfer microbial biomass from the core stock to the recently incorporated plant matter in the peripheral sector (Fig. 3). In this sector, the cells grew fast on hemicellulose and starch, presenting higher yields (Ymax) but sacrificing cell maintenance (m) (Table 1). In the periphery, low microbial energy drainage (Q) with a high assimilation rate (q) might reflect the reduced microbial mass (X) due to the removal or even consumption of microbial mass by the ants. This result is consistent with the constant pruning of fungal hyphae by ants and the use of these hyphae and staphylae to feed brood and reproductive ants (3–5, 54, 56). The peripheral sector energy draining was associated with a high specific growth rate (μ) (Table 1) and high polysaccharidase production (Fig. 2), resulting in the massive accumulation of glucose, mannose, xylose, arabinose, sorbitol, mannitol, inositol, and arabitol (Fig. 1). These products can be assimilated by leaf-cutter ants (11) (Table 2); thus, the peripheral and core regions complementarily function to ultimately degrade plant matter, generating monosaccharides and polyols for ant nutrition (Fig. 3).
Origins and fates of monosaccharides and polyols in the fungus garden.
Polyol secretion is a new and previously undiscovered function of L. gongylophorus staphylae described in the present study. Mannitol and arabitol accounted for 95.1% of the polyols secreted by staphylae as the major polyols produced in the peripheral fungus garden (Fig. 1; Table 2). Mannitol and arabitol are synthesized from glucose and arabinose, respectively (57). Glucose might result from plant starch degradation, but not significantly from cellulose, as suggested by the small amount of cellulase observed compared with high amylase activity (Fig. 2), as arabinose might result from plant hemicellulose degradation through the high hemicellulase (xylanase) activity observed in the fungus garden (Fig. 2). Therefore, major processes generating polyols in the fungus garden involve plant starch conversion to mannitol and plant hemicellulose conversion to arabitol through L. gongylophorus. Polyols that accumulate in minor amounts, i.e., sorbitol and inositol, can also be synthesized from glucose (57). Inositol was detected in staphyla secretions, but not sorbitol, which might be produced by microbial sources other than L. gongylophorus (Fig. 3). Xylitol can be generated from xylose in fungi (57), but this polyol was not detected in fungus gardens, indicating that the xylose generated in the fungus garden is preferentially used as a carbon source for microbial growth and maintenance. However, the rapid production of arabitol and temporal coincidence of arabitol and arabinose concentration peaks (Fig. 2) suggest that arabinose is not massively assimilated for microbial growth and maintenance but rather preferentially reduced to arabitol. In contrast to the fate of C5 arabinose originating arabitol, the conversion of C6 monosaccharide to C6 polyol occurred relatively slowly, evidenced as a 7-h delay in the concentration peaks of these compounds (Fig. 1). This result suggests that the C6 monosaccharides glucose and mannose are partially utilized for microbial cell maintenance and partially reduced to C6 polyols.
The finding that ants prevent monosaccharide and polyol accumulation in the fungus garden (Table 2) suggests that ants consume these compounds. The consumption of polysaccharide degradation products generated in the fungus garden has been previously shown in A. sexdens (11) and attributed to the licking of the fungus garden material by ants. Therefore, the results obtained in the present study suggest that, in addition to monosaccharides, polyols are also consumed when ants lick the fungus garden material.
Polyol accumulation as energy food stocks has many advantages over simple sugar accumulation. Polyols are more stable than monosaccharides and can be present at higher concentrations without damaging extracellular enzymes or disturbing the microbial osmotic balance (58–60).
Fungus garden enzyme regulation.
The positive correlation between chitinase activity and cell growth can be explained by the facts that the fungal cell walls comprise chitin (39, 40) and chitinases are involved in the remodeling of cell walls during hyphal growth (39). The remaining enzymatic activities might be induced through external carbon sources, as demonstrated for L. gongylophorus in laboratory cultures (18, 19, 61) or in fungus garden materials (15, 16, 25, 28). In addition, the detection of polysaccharidase activity in a high concentration of soluble sugars, notably, glucose and xylose, reinforces the finding (12) that these enzymes are not subject to severe catabolic repression or inhibition.
Grell and coworkers (23) have reported that L. gongylophorus expresses genes encoding cellulases, hemicellulases, amylases, and pectinases and that the expression levels of these enzymes are higher in the bottom (old) section than in the top (newer) section of the fungus garden of the leaf-cutter ant A. echinatior. This finding is consistent with the results of the present study showing higher xylanase activity in the core (old) sector but disagrees with the finding of much higher amylase activity in new fungus garden sections. This incongruence between L. gongylophorus amylase gene expression and amylase activity indicates that other microbes participate in starch metabolism in the fungus garden. However, this suggestion must be taken with caution, as these studies differ from each other in many aspects, such as leaf-cutter species and laboratory versus field nest locations.
Fungus gardens of leaf-cutter ants are solid-state bioreactors with biotechnological applications.
The name “fungus garden” was used in analogy with human agriculture at a time when fungi were regarded as close relatives of plants. The same reasoning led to the name “gardening ants.” However, the leaf-cutter fungus garden operation we describe here more accurately fits the description of a solid-state bioreactor because (i) the fungus garden not only produces biomass as feedstock, consistent with agriculture, but also produces microbial secretions, enzymes, and hydrolysis products; (ii) ants perform plant substrate preparation and feeding of the system; (iii) the microbial cell mass is continuously reinoculated into the newly incorporated plant substrate as in a continuous fermentation system; (iv) the waste material is eliminated as a postprocessed material; and (v) the peripheral sector functions as a log phase and the core sector functions as a stationary phase during the fermentation process. Therefore, the leaf-cutter fungus garden resembles, in all aspects, a solid-state bioreactor that works as a continuous fermentative system converting plant polysaccharides into polyols with high efficiency. Indeed, this operation is similar to the well-known and widely used solid-state fermentation process, which takes advantage of the low water activity of solid-state bioreactors to stress fungal cultures, which activate carbohydrate-reducing enzymes to produce polyols efficiently (62–64).
The results of the present study also indicate that the fungus garden of leaf-cutter ants is a promising source of tools for biofuel and polyol production from plant biomass. The hydrolysis of plant polysaccharides is a major issue in converting vegetal matter into biofuel (65). Chemical hydrolysis remains comparatively expensive, as hydrolysis by microbial cultures might generate low yields because microbes consume considerable fractions of the hydrolysis products (65, 66). However, in suspensions of peripheral ant-free fungus garden material, the high production of monosaccharides and polyols profusely surpassed microbial consumption (Fig. 1), resulting in a high product yield. In addition, the fungus garden material was very amenable to laboratory manipulation, preferentially producing either monosaccharides or polyols (Fig. 1). Moreover, the major polyols produced from the fungus garden, mannitol and arabitol, are valuable products in the market (67, 68).
The importance of slow cellulose degradation.
Cellulose was apparently poorly degraded in the fungus garden, consistent with the observed low cellulase activity (Fig. 2) and high glucose residue preservation in vegetal fibers (see Table S1 in the supplemental material). Low cellulase activity was observed on five different substrates (Fig. 2), including Walseth cellulose, which is cellulose treated with phosphoric acid to expose cleavage sites and facilitate degradation. Conversely, amylase activity was 50 to 100 times as high as cellulase activity (Fig. 2). Thus, it is reasonable to assume that glucose originating in the fungus garden materials (Fig. 1; Table 2) reflects primarily starch degradation. In addition, enzyme activities on other plant polysaccharides, such as pectin or hemicellulose (xylan) (Fig. 1), were more than 200 times higher than cellulase activity. Low cellulose degradation is consistent with the preservation of cellulosic structures in the fungus garden (28) and low cellulase activity in laboratory cultures of L. gongylophorus (10). However, histological analysis showed that cellulose degradation particularly occurs in old sectors of the leaf-cutter ants' fungus garden (69), and many studies have shown that L. gongylophorus harbors a full set of cellulosic enzymes (18, 21, 23, 25, 28). Therefore, the results obtained so far suggest that cellulose degradation is much slower than the degradation of other plant polysaccharides, suggesting that slow degradation preserves plant cellulose and contributes to the maintenance of the fungus garden three-dimensional structure, which might be important for ventilation, temperature control, and ant access to the different sectors of the fungus garden.
Supplementary Material
ACKNOWLEDGMENTS
We thank Guilherme Miola Titato for support with LC-MS analyses. We also thank Christian Rabeling of the University of Rochester for valuable comments on the manuscript.
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for providing A.F.S. with a doctoral fellowship. This work was financially supported through grants from the Fundação de Apoio à Pesquisa do Estado de São Paulo (2011/50226-0) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (311562/2012-4 and 487639/2012-0).
Footnotes
This study represents the 48th contribution from the research group of Maurício Bacci, Jr., to the characterization of ant-fungus mutualism.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00046-15.
REFERENCES
- 1.Silva-Pinhati ACO, Bacci M, Hinkle G, Sogin ML, Pagnocca FC, Martins VG, Bueno OC, Hebling MJA. 2004. Low variation in ribosomal DNA and internal transcribed spacers of symbiotic fungi of leaf-cutting ants (Attini: Formicidae). Braz J Med Biol Res 37:1463–1472. [DOI] [PubMed] [Google Scholar]
- 2.Pagnocca FC, Bacci M Jr, Fungaro MH, Hebling MJ, Bueno OC, Sant'anna A, Capelari M. 2001. RAPD analysis of the sexual state and sterile mycelium of the fungus cultivated by the leaf-cutting ant Acromyrmex hispidus fallax. Mycol Res 105:173–176. doi: 10.1017/S0953756200003191. [DOI] [Google Scholar]
- 3.Hölldobler B, Wilson EO. 2008. The superorganism. The beauty, elegance, and strangeness of insect societies. W. W. Norton and Co., New York, NY. [Google Scholar]
- 4.Weber NA. 1966. Fungus-growing ants. Science 153:587–604. doi: 10.1126/science.153.3736.587. [DOI] [PubMed] [Google Scholar]
- 5.Weber NA. 1972. Gardening ants, the attines. American Philosophical Society, Philadelphia, PA. [Google Scholar]
- 6.Nagamoto NS, Carlos AA, Moreira SM, Verza SS, Hirose GL, Forti LC. 2009. Differentiation in selection of dicots and grasses by the leaf-cutter ants Atta capiguara, Atta laevigata and Atta sexdens rubropilosa. Sociobiology 54:127–138. [Google Scholar]
- 7.Autuori M. 1941. Contribuição para o conhecimento da saúva (Atta sp). (I) Evolução do sauveiro. Arch Inst Biol São Paulo 12:197–228. [Google Scholar]
- 8.Quinlan RJ, Cherret JM. 1977. The role of substrate preparation in the symbiosis between the leaf-cutting ant Acromyrmex octospinosus (Reich) and its food fungus. Ecol Entomol 2:161–170. [Google Scholar]
- 9.Boyd ND, Martin MM. 1975. Faecal proteinases of the fungus-growing ant, Atta texana: their fungal origin and ecological significance. J Insect Physiol 21:1815–1820. [Google Scholar]
- 10.Gomes de Siqueira C, Bacci M Jr, Pagnocca FC, Bueno OA, Hebling MJA. 1998. Metabolism of plant polysaccharides by Leucoagaricus gongylophorus, the symbiotic fungus of the ant Atta sexdens L. Appl Environ Microbiol 64:4820–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva A, Bacci M, Bueno OC, Pagnocca FC, Hebling MJA. 2003. Survival of Atta sexdens workers on different food sources. J Insect Physiol 49:307–313. doi: 10.1016/S0022-1910(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 12.Bacci M, Bueno OC, Rodrigues A, Pagnocca FC, Somera AF, Silva A. 2013. A metabolic pathway assembled by enzyme selection may support herbivory of leaf-cutter ants on plant starch. J Insect Physiol 59:525–531. doi: 10.1016/j.jinsphys.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Huang EL, Aylward FO, Kim YM, Webb-Robertson BJ, Nicora CD, Hu Z, Metz TO, Lipton MS, Smith RD, Currie CR, Burnum-Johnson KE. 2014. The fungus gardens of leaf-cutter ants undergo a distinct physiological transition during biomass degradation. Environ Microbiol Rep 6:389–395. doi: 10.1111/1758-2229.12163. [DOI] [PubMed] [Google Scholar]
- 14.Richard FJ, Mora P, Errard C, Rouland C. 2005. Digestive capacities of leaf-cutting ants and the contribution of their fungal cultivar to the degradation of plant material. J Comp Physiol B 175:297–303. doi: 10.1007/s00360-005-0485-1. [DOI] [PubMed] [Google Scholar]
- 15.De Fine Licht HH, Schiøtt M, Mueller U, Boomsma JJ. 2010. Evolutionary transitions in enzyme activity of ant fungus gardens. Evolution 64:2055–2069. doi: 10.1111/j.1558-5646.2010.00948.x. [DOI] [PubMed] [Google Scholar]
- 16.Erthal M Jr, Silva CP, Cooper RM, Samuels RI. 2009. Hydrolytic enzymes of leaf-cutting ant fungi. Comp Biochem Physiol Part B 152:54–59. doi: 10.1016/j.cbpb.2008.09.086. [DOI] [PubMed] [Google Scholar]
- 17.Semenova TA, Hughes DP, Boomsma JJ, Schiøtt M. 2011. Evolutionary patterns of proteinase activity in attine ant fungus gardens. BMC Microbiol 11:15. doi: 10.1186/1471-2180-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacci M Jr, Anversa Pagnocca MMFC. 1995. Cellulose degradation by Leucocoprinus gongylophorus, the fungus cultured by the leaf-cutting ant Atta sexdens rubropilosa. Antonie Van Leeuwenhoek 67:385–386. [DOI] [PubMed] [Google Scholar]
- 19.Silva A, Bacci M, Pagnocca FC, Bueno OC, Hebling MJA. 2006. Starch metabolism in Leucoagaricus gongylophorus, the symbiotic fungus of leaf-cutting ants. Microbiol Res 161:299–303. doi: 10.1016/j.micres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Abril AB, Bucher EH. 2002. Evidence that the fungus cultured by leaf-cutting ants does not metabolize cellulose. Ecol Lett 5:325–328. doi: 10.1046/j.1461-0248.2002.00327.x. [DOI] [Google Scholar]
- 21.Aylward FO, Burnum-Johnson KE, Tringe SG, Teiling C, Tremmel DM, Moeller JA, Scott JJ, Barry KW, Piehowski PD, Nicora CD, Malfatti SA, Monroe ME, Purvine SO, Goodwin LA, Smith RD, Weinstock GM, Gerardo NM, Suen G, Lipton MS, Currie CR. 2013. Leucoagaricus gongylophorus produces diverse enzymes for the degradation of recalcitrant plant polymers in leaf-cutter ant fungus gardens. Appl Environ Microbiol 79:3770–3778. doi: 10.1128/AEM.03833-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suen G, Scott JJ, Aylward FO, Adams SM, Tringe SG, Pinto-Tomás AA, Foster CE, Pauly M, Weimer PJ, Barry KW, Goodwin LA, Bouffard P, Li L, Osterberger J, Harkins TT, Slater SC, Donohue TJ, Currie CR. 2010. An insect herbivore microbiome with high plant biomass-degrading capacity. PLoS Genet 6:e1001129. doi: 10.1371/journal.pgen.1001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grell MN, Linde T, Nygaard S, Nielsen KL, Boomsma JJ, Lange L. 2013. The fungal symbiont of Acromyrmex leaf-cutting ants expresses the full spectrum of genes to degrade cellulose and other plant cell wall polysaccharides. BMC Genomics 14:928–938. doi: 10.1186/1471-2164-14-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto-Tomás AA, Anderson MA, Suen G, Stevenson DM, Chu FST, Cleland WW, Currie CR. 2009. Symbiotic nitrogen fixation in the fungus gardens of leaf-cutter ants. Science 326:1120–1123. doi: 10.1126/science.1173036. [DOI] [PubMed] [Google Scholar]
- 25.Aylward FO, Burnum KE, Scott JJ, Suen G, Tringe SG, Adams SM, Barry KW, Nicora CD, Piehowski PD, Purvine SO, Starrett GJ, Goodwin LA, Smith RD, Lipton MS, Currie CR. 2012. Metagenomic and metaproteomic insights into bacterial communities in leaf-cutter ant fungus gardens. ISME J 6:1688–1701. doi: 10.1038/ismej.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacci M, Anversa MM, Pagnocca FC. 1995. Cellulose degradation by Leucocoprinus gongylophorus, the fungus cultured by the leaf-cutting ant Atta sexdens rubropilosa. Braz J Med Biol Res 28:79–82. [DOI] [PubMed] [Google Scholar]
- 27.Scott Budsberg JJ, KJ, Suen G, Wixon DL, Balser TC, Currie CR. 2010. Microbial community structure of leaf-cutter ant fungus gardens and refuse dumps. PLoS One 5:e9922. doi: 10.1371/journal.pone.0009922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moller IE, De Fine Licht HH, Harholt J, Willats WGT, Boomsma JJ. 2011. The dynamics of plant cell-wall polysaccharide decomposition in leaf-cutting ant fungus gardens. PLoS One 6:e17506. doi: 10.1371/journal.pone.0017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carreiro SC, Pagnocca FC, Bueno OC, Bacci M, Hebling MJA, Silva OA. 1997. Yeasts associated with nests of the leaf-cutting ant Atta sexdens rubropilosa Forel, 1908. Antonie Van Leeuwenhoek 71:243–248. [DOI] [PubMed] [Google Scholar]
- 30.Bartha R, Pramer D. 1956. Features of a flask and method for measuring the persistence and biological effects of pesticides. Soil Sci 100:68–70. [Google Scholar]
- 31.Vogel HJA. 1956. A convenient growth medium for Neurospora (medium N). Microbiol Genet Bull 37:387–394. [Google Scholar]
- 32.Van de Werf H, Verstraete W. 1987. Estimation of active soil microbial biomass by mathematical analysis of respiration curves: development and verification of the model. Soil Biol Biochem 19:253–260. [Google Scholar]
- 33.Setzer W. 2008. Solvers for ordinary differential equations. The Comprehensive R Archive Network, Vienna, Austria. [Google Scholar]
- 34.R Development Core Team. 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 35.McIlvaine TC. 1921. A buffer solution for colorimetric comparison. J Biol Chem 49:183–186. [Google Scholar]
- 36.Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. [Google Scholar]
- 37.Henrissat B, Drigvez H, Viet C, Schülein M. 1985. Synergism of cellulases from Trichoderma reesei in the degradation of cellulose. Nat Biotechnology 3:722–726. [Google Scholar]
- 38.Walseth CS. 1952. Occurrence of cellulases in enzyme preparations from microorganisms. Tappi J 35:228–233. [Google Scholar]
- 39.Bartnicki-Garcia S. 1968. Cell wall chemistry, morphogenesis and taxonomy of fungi. Annu Rev Microbiol 22:87–108. [DOI] [PubMed] [Google Scholar]
- 40.Wessels JGH, Sietsma JH. 1981. Fungal cell walls: a survey, p 352–415. In Tanner W, Loewus FA (ed), Encyclopedia of plant physiology, vol. 13B Plant carbohydrates II Springer-Verlag, Berlin, Germany. [Google Scholar]
- 41.Bergmeyer HU, Bernt E. 1974. Determination of glucose with oxidase and peroxidase, p 1205–1215. In Bergmeyer HU. (ed), Methods of enzymatic analysis. Verlag Chemie/Academic Press, New York, NY. [Google Scholar]
- 42.D'Agostino R, Pearson E. 1973. Tests for departures from normality. Empirical results for the distribution of √b1 and b2. Biometrika 60:613–622. [Google Scholar]
- 43.Weber NA. 1955. Pure cultures of fungi produced by ants. Science 121:109. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro MMR, Amaral KD, Seide VE, Souza BMR, Della Lucia TMC, Kasuya MCM, de Souza DJ. 2012. Diversity of fungi associated with Atta bisphaerica (Hymenoptera: Formicidae): the activity of Aspergillus ochraceus and Beauveria bassiana. Psyche 2012:1–6. doi: 10.1155/2012/389806. [DOI] [Google Scholar]
- 45.Currie CR, Mueller UG, Malloch D. 1999. The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci U S A 96:7998–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds H, Currie C. 2004. Pathogenicity of Escovopsis weberi: the parasite of the attine ant-microbe symbiosis directly consumes the ant-cultivated fungus. Mycologia 96:955–959. doi: 10.2307/3762079. [DOI] [PubMed] [Google Scholar]
- 47.Currie CR, Scott JA, Summerbell RC, Malloch D. 1999. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398:701–704. [Google Scholar]
- 48.Santos AV, Dillon RJ, Dillon VM, Reynolds SE, Samuels RI. 2004. Occurrence of the antibiotic producing bacterium Burkholderia sp. in colonies of the leaf-cutting ant Atta sexdens rubropilosa. FEMS Microbiol Lett 239:319–323. doi: 10.1016/j.femsle.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues A, Bacci M, Mueller UG, Oritz A, Pagnocca FC. 2008. Microfungal “weeds” in the leafcutter ant symbiosis. Microb Ecol 56:604–614. doi: 10.1007/s00248-008-9380-0. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues A, Cable RN, Mueller UG, Bacci M, Pagnocca FC. 2009. Antagonistic interactions between garden yeasts and microfungal garden pathogens of leaf-cutting ants. Antonie Van Leeuwenhoek 96:331–341. doi: 10.1007/s10482-009-9350-7. [DOI] [PubMed] [Google Scholar]
- 51.Pagnocca FC, Legaspe MF, Rodrigues A, Ruivo CC, Nagamoto NS, Bacci M, Forti LC. 2010. Yeasts isolated from a fungus-growing ant nest, including the description of Trichosporon chiarellii sp. nov., an anamorphic basidiomycetous yeast. Int J Syst Evol Microbiol 60:1454–1459. doi: 10.1099/ijs.0.015727-0. [DOI] [PubMed] [Google Scholar]
- 52.Griffiths HM, Hughes WHO. 2010. Hitchhiking and the removal of microbial contaminants by the leaf-cutting ant Atta colombica. Ecol Entomol 35:529–537. doi: 10.1111/j.1365-2311.2010.01212.x. [DOI] [Google Scholar]
- 53.Poulsen M, Bot ANM, Nielsen MG, Boomsma JJ. 2002. Experimental evidence for the costs and hygienic significance of the antibiotic metapleural gland secretion in leaf-cutting ants. Behav Ecol Sociobiol 52:151–157. doi: 10.1007/s00265-002-0489-8. [DOI] [Google Scholar]
- 54.Currie CR, Stuart AE. 2001. Weeding and grooming of pathogens in agriculture by ants. Proc Biol Sci 268:1033–1039. doi: 10.1098/rspb.2001.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiøtt M, Licht HDE, Lange L, Boomsma JJ. 2008. Towards a molecular understanding of symbiont function: identification of a fungal gene for the degradation of xylan in the fungus gardens of leaf-cutting ants. BMC Microbiol 8:40. doi: 10.1186/1471-2180-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hölldobler B, Wilson EO. 1990. The ants. Belknap Press, Cambridge, MA. [Google Scholar]
- 57.Laere AV. 1995. Intermediary metabolism, p 211–238. In Gow NAR, Gadd GM (ed), The growing fungus. Chapman & Hall, London, United Kingdom. [Google Scholar]
- 58.Galinski EA, Trüper HG. 1994. Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol Rev 15:95–108. [Google Scholar]
- 59.Ruijter GJG, Bax M, Patel H, Flitter SJ, van de Vondervoort PJI, de Vries RP, van Kuyk PA, Visser J. 2003. Mannitol is required for stress tolerance in Aspergillus niger conidiospores. Eukaryot Cell 2:690–698. doi: 10.1128/EC.2.4.690-698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williamson JD, Jennings DB, Guo WW, Pharr M, Ehrenshaft M. 2002. Sugar alcohols, salt stress, and fungal resistance: polyols—multifunctional plant protection? J Am Soc Hortic Sci 127:467–473. [Google Scholar]
- 61.Silva A, Bacci Pagnocca M FC, Bueno OC, Hebling MJA. 2006. Production of polysaccharidases in different carbon sources by Leucoagaricus gongylophorus Möller (Singer), the symbiotic fungus of the leaf-cutting ant Atta sexdens Linnaeus. Curr Microbiol 53:68–71. doi: 10.1007/s00284-005-0431-1. [DOI] [PubMed] [Google Scholar]
- 62.Brown AD. 1990. Microbial water stress physiology: principles and perspectives. John Wiley and Sons, Chichester, United Kingdom. [Google Scholar]
- 63.Pandey A, Soccol CR, Mitchell D. 2000. New developments in solid-state fermentation: I. bioprocesses and products. Process Biochem 35:1153–1169. doi: 10.1016/S0032-9592(00)00152-7. [DOI] [Google Scholar]
- 64.Pandey A, Ashakumary L, Selvakumar P, Vijayalakshmi KS. 1994. Influence of water activity on growth and activity of Aspergillus niger for glycoamylase production in solid-state fermentation. World J Microbiol Biotechnol 10:485–486. [DOI] [PubMed] [Google Scholar]
- 65.Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schnepf R. 2010. Cellulosic ethanol: feedstocks, conversion technologies, economics, and policy options. Congressional Research Service, Washington, DC. [Google Scholar]
- 67.Bhatt SM, Mohan A, Srivastava SK. 2013. Challenges in enzymatic route of mannitol production. ISRN Biotechnol 2013:1–13. doi: 10.5402/2013/914187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Almeida JRM, Fávaro LCL, Quirino BF. 2012. Biodiesel biorefinery: opportunities and challenges for microbial production of fuels and chemicals from glycerol waste. Biotechnol Biofuels 5:48. doi: 10.1186/1754-6834-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagamoto NS, Garcia MG, Forti LC, Verza SS, Noronha NC, Rodella RA. 2011. Microscopic evidence supports the hypothesis of high cellulose degradation capacity by the symbiotic fungus of leaf-cutting ants. J Biol Res 16:308–312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.