Abstract
Burkholderia pseudomallei is a saprophytic bacterium that causes melioidosis and is often isolated from rice fields in Southeast Asia, where the infection incidence is high among rice field workers. The aim of this study was to investigate the relationship between this bacterium and rice through growth experiments where the effect of colonization of domestic rice (Oryza sativa L. cv Amaroo) roots by B. pseudomallei could be observed. When B. pseudomallei was exposed to surface-sterilized seeds, the growth of both the root and the aerosphere was retarded compared to that in controls. The organism was found to localize in the root hairs and endodermis of the plant. A biofilm formed around the root and root structures that were colonized. Growth experiments with a wild rice species (Oryza meridionalis) produced similar retardation of growth, while another domestic cultivar (O. sativa L. cv Koshihikari) did not show retarded growth. Here we report B. pseudomallei infection and inhibition of O. sativa L. cv Amaroo, which might provide insights into plant interactions with this important human pathogen.
INTRODUCTION
Melioidosis is caused by the aerobic Gram-negative bacillus Burkholderia pseudomallei, which is common in Northern Australia and Southeast Asia, where the mortality rate can be as high as 40% (1). B. pseudomallei is recognized as a soil- and waterborne pathogen and is commonly isolated from the soil of rice fields and other environmental sources where the organism is endemic (2–7). Exposure to the organism in rice paddies is a significant risk to rice farmers, particularly those who are also diabetic and are generally more predisposed to infection (8).
The rhizosphere is a rich habitat for bacteria. Microbial interaction with plants can result in a variety of effects, ranging from pathogenesis to growth promotion (9), and plants may facilitate the persistence of the microorganism (10). Bacteria in the Burkholderia genus are well known to be plant endophytes, some with plant growth promotion capacity and some as pathogens (11). As B. pseudomallei has a broad host range (12–17) and has been shown to infect other species of plants (18, 19), it may be possible that it will infect some cultivars of rice and a plant-microbe interaction may assist in the persistence of B. pseudomallei in the rice fields where it is commonly found. However, no studies have so far identified any growth promotion or pathogenic relationship between B. pseudomallei and rice. Lee et al. (19) found no effects on a Japanese rice cultivar (Oryza sativa cv. Nipponbare), while Kaestli et al. (18) found no effects on an Australian wild rice species (Oryza rufipogon). An examination of the interaction of B. pseudomallei with more cultivars and species of rice could provide more information about whether rice is, in general, resistant to B. pseudomallei or identify a potential host for an infection model. The development of a rice model of B. pseudomallei infection has the potential to assist in further plant-microbe interaction studies and the development of biocontrol measures.
The aims of this project were to investigate if B. pseudomallei can infect domestic rice (O. sativa L. cv Amaroo) and to demonstrate the effect of this infection on plant growth; this effect was also compared with those of other species of Burkholderia and other cultivars of rice.
MATERIALS AND METHODS
Bacteria.
All of the bacterial isolates used in this study were subcultured from the Biomedicine collection, James Cook University, Queensland, Australia. B. pseudomallei was removed from storage (−80°C), streaked onto Ashdown agar, and incubated at 37°C for 48 h in a biosafety level 3 (BSL3) laboratory. A single colony was subcultured into 10 ml of Luria-Bertani (LB) broth. The culture was incubated at 37°C with agitation (166 rpm) overnight. Other Burkholderia species were processed as described above, except that the incubation temperature of both Ashdown agar and LB broth was 30°C. Other non-Burkholderia species were streaked onto LB agar, incubated at 37°C for 24 h, and subcultured into LB broth at 37°C for 24 h.
Biosafety protocols.
All experimental work with B. pseudomallei was carried out in Australia at James Cook University. Australian/New Zealand standard 2243.3:2010 (safety in laboratories) was adhered to, with work on live organisms carried out in a BSL3 laboratory to a BSL3 standard. All protocols were approved by the Institutional Biosafety Committee, and biological samples were handled in a C2 cabinet with all waste autoclaved. Confirmation of sterilization was carried out with duplicate samples prior to the removal of any processed, killed organism from the laboratory to a BSL2 laboratory.
Preparation and cleaning of seeds.
Rice (O. sativa L. cv Amaroo) seeds were sown, grown, and harvested at the Biomedicine department, James Cook University, during 2012 and 2013. Wild rice (Oryza meridionalis) seeds were harvested at Woodstock, Townsville, in May 2012 and kept for 1 year until the seed dormancy period had passed (20, 21). A Japanese rice cultivar (O. sativa L. cv. Koshihikari) was provided by a local supplier. The harvested and provided seeds were cleaned by a modified form of the method of Oyebanji et al. (22) by soaking and agitation (200 rpm) in 3.5% bleach (sodium hypochlorite) at 30°C for 10 min. Disinfectant solutions were discharged, and seeds were washed by soaking and agitation in sterile distilled water for 3 min (washes were repeated four times). The last sterile distilled water was decanted and inoculated onto Ashdown's agar to confirm that no B. pseudomallei bacteria were present. Selective agar was used, as surface cleaning does not remove all of the bacteria from inside seeds and typically other bacteria will still replicate in nonselective medium (23, 24). Seeds were soaked at a depth of approximately 1 cm in sterile distilled water at 30°C for 2 to 6 days to encourage uniform imbibition and germination (25). This sterile distilled water was also tested for contamination with B. pseudomallei by incubation on Ashdown's agar for 48 h.
Infection of seeds for plant growth experiments.
A dose trial for infection was carried out on the basis of the lower dose used by Kaestli et al. (18) and the higher dose used by Mattos et al. (26). Cleaned, primed seeds of O. sativa L. cv Amaroo were infected by using a modified form of the protocol of Kaestli et al. (18) by placing 10 ml of 104 or 108 CFU/ml B. pseudomallei TSV189 in LB for an hour prior to removal from bacterial broth. Control seeds were treated by soaking in LB for the same amount of time. Forty-five seeds infected with each dose and 45 control seeds were used for this study. For comparison of bacterial strains and species, 45 control seeds and 45 seeds infected by soaking with each bacterial strain and species at 108 CFU/ml were used. The other bacteria included two strains of B. pseudomallei (TSV192, environmental, Australia; K96243, clinical, Thailand), as well as bacteria in the same genus (Burkholderia cenocepacia (17sp), B. vietnamiensis (38sp), and B. ubonensis (A21). The seeds were then transferred to experimental chambers. The seeds were transferred for propagation on the basis of the method of Hoagland and Arnon (27), modified by Watt et al. (28) to 1% (wt/vol) (0.25×) Hoagland agar. Survival kinetics in 0.25× Hoagland broth were also determined, and bacteria were not inhibited in this medium (see the supplemental material). The seedlings were grown in Hoagland agar in sealed glass bottles and incubated under cycles of 12 h of fluorescent light and 12 h of darkness for 7 days at 30°C. B. pseudomallei TSV189 was also used at the higher dose as described above to infect Australian wild rice (O. meridionalis, 30 seeds per group) and Japanese rice (O. sativa L. cv. Koshihikari, 45 seeds per group). All experiments included an uninfected rice group to control for error between experiments.
Plant measurement and statistical analysis of plant growth determination.
Rice seedlings were removed from agar and photographed alongside a scale. Images were analyzed by using the area measurement commands in Adobe Photoshop CS6 to calculate area as described by Villar et al. (29). When an analysis consisted of only a control and an experimental group, independent t tests (P = 0.05) were performed by using IBM SPSS version 20, and where three or more groups are compared, a one-way analysis of variance (ANOVA) was performed (P = 0.05) by using a Gabriel post hoc test. When results were compared across different experiments, actual areas were converted to percentages of the average within-experiment uninfected control area. This was done to control for variation between experiments.
Reactivity of the antibody specific for B. mallei with B. pseudomallei and other bacteria.
No commercial anti-B. pseudomallei antibodies could be obtained at the beginning of our experiments. However, antibodies specific for B. mallei are available and considering that some lipopolysaccharides (LPSs) are common to B. pseudomallei and B. mallei, a monoclonal antibody (MAb) specific for B. mallei LPS (MCA2823; AbD Serotec/Bio-Rad) was tested.
Various species and strains of bacteria (Table 1) were cultured and centrifuged at 3,000 × g for 15 min, and the supernatants were discharged. Pellets were washed three times with 0.85% NaCl, and 15-μl volumes of bacterial suspensions were placed on slides (Menzel GmbH & Co. KG, Braunschweig, Germany) in duplicate and allowed to dry for 1 h at room temperature (RT).
TABLE 1.
Reactivity of MAb MCA2823 with B. pseudomallei, neighbor Burkholderia species, and other bacteria
| Isolate | Species | Source | Location | IFA result |
|---|---|---|---|---|
| 4 | B. pseudomalleia | Human clinical sample | Mornington Island, Australia | + |
| 8 | B. pseudomalleib | Human clinical sample | Cloncurry, Australia | + |
| C1 | B. pseudomalleic | Human clinical sample | Adiba, Sanabase, Papua New Guinea | + |
| C2 | B. pseudomalleic | Human clinical sample | Kimama, Teleme, Papua New Guinea | + |
| TSV189 | B. pseudomalleid | Alpaca necropsy | Townsville, Australia | + |
| 14-289 | B. pseudomalleid | Parrot necropsy | Townsville, Australia | + |
| 14-327 | B. pseudomalleid | Koala necropsy | Townsville, Australia | + |
| TSV192 | B. pseudomalleid | Soil | Townsville, Australia | + |
| TS5 | B. pseudomalleid | Soil | Townsville, Australia | + |
| K96243 | B. pseudomalleie | Human clinical sample | Thailand | + |
| TSV4 | B. arborisf | Water seep | Townsville, Australia | − |
| TSV19 | B. gladiolif | Bulk water | Townsville, Australia | − |
| TSV21 | B. cepaciaf | Water seep | Townsville, Australia | − |
| TSV87 | B. pyrrociniaf | Bulk water | Townsville, Australia | − |
| TSV88 | B. pseudomultivoransf | Bulk water | Townsville, Australia | − |
| E1 | B. thailandensisc | Clay | Biula, Papua New Guinea | − |
| A21 | B. ubonensisf | Soil | Adiba, Sanabase, Papua New Guinea | − |
| 17sp | B. cenocepaciaf | Rhizosphere soil | Townsville, Australia | − |
| 31sp | B. latensf | Rhizosphere soil | Townsville, Australia | − |
| 38sp | B. vietnamiensisf | Rhizosphere soil | Townsville, Australia | − |
| A03a | Bordetella speciesf | Rhizosphere soil | Adiba, Sanabase, Papua New Guinea | − |
| 13sp | Achromobacter xylosoxidansf | Rhizosphere soil | Townsville, Australia | − |
| ATCC 27895 | Pseudomonas aeruginosa | Control | − | |
| ATCC 25921 | Escherichia coli | Control | − | |
| ATCC 10876 | Bacillus cereus | Control | − | |
| ATCC 13076 | Salmonella enterica serovar Enteritidis | Control | − |
The bacterium was identified to the species level by API 20 NE.
The bacterium was identified to the species level by indirect hemagglutination assay and IgG/M enzyme immunoassay.
The bacterium was identified to the species level by multilocus sequence typing.
The bacterium was identified to the species level by PCR assay of the type III secretion system gene.
The bacterium was identified to the species level by determination of its full sequence.
The bacterium was identified to the species level by determination of its recA gene sequence.
Fixation, antibody concentration, and incubations were optimized by using B. pseudomallei TSV189 and B. ubonensis A21. After this, all bacteria were fixed in acetone for 4 h, blocked with goat serum, and incubated with MAb MCA2823 diluted 1/100 overnight at 4°C in a humidified chamber. After being washed in phosphate-buffered saline (PBS), the bacteria were incubated with goat anti-mouse IgG1 conjugated with Alexa Fluor 595 diluted 1/300 for 45 min at RT, washed in PBS, mounted with fluorescent mounting medium (KPL), and observed under an epifluorescence microscope (AxioImager.Z1; Zeiss). Pictures were taken with a digital camera (AxioCam MRm; Zeiss). As a negative control, a mouse MAb of the same isotype (IgG1) (MM1A, anti-CD3 receptor; Washington State University) was used at the same concentration as MAb MCA2823. The reactivity of all species and strains of bacteria with MAb MCA2823 was checked in duplicate.
Infection of seeds with B. pseudomallei for immunofluorescence assay (IFA).
Cleaned, primed seeds were incubated in petri dishes for 2 days for primary root germination. After that, the roots of three seedlings were inoculated with about 107 CFU of B. pseudomallei (isolate TSV189; 100 μl of a 108-CFU/ml concentration) as described by Kaestli et al. (18), while another three were not infected. The six seedlings were then transferred for propagation and incubated as described for the plant growth experiment.
Preparation of plant samples for IFA.
Plantlets were gently lifted from the agar surface, and their roots were washed in 0.85% NaCl three times to remove any loose bacteria or agar. Root pieces were cut to 0.5 to 1 cm long and fixed in acetone at –20°C for 3 days, until the sterility of the samples was confirmed. Further, the samples were embedded in optimum cutting temperature compound (Tissue-Tek, Sakura, Japan) and stored at −80°C until sections were cut. Five-micrometer-thick cryosections were cut from roots with a cryostat (Leica CM1850) at −20°C and transferred to slides (Menzel GmbH & Co. KG, Braunschweig, Germany). Sections were dried overnight at room temperature under a fan.
IFA of root sections.
Sections were immersed in PBS and blocked for 30 min at RT in 10% (vol/vol) goat serum diluted in 1% bovine serum albumin (BSA) in PBS. After a brief wash in PBS, the sections were incubated at RT for 40 min and then overnight at 4°C with MAb MCA2823 diluted 1:100 in 1% BSA in PBS (final concentration, 10 μg/ml). Negative controls using MAb MM1A as the primary antibody were processed similarly. The slides were washed gently with PBS, and the sections were incubated with goat anti-mouse IgG1 conjugated with Alexa Fluor 595 (Invitrogen) diluted 1/300 in 1% BSA in PBS for 45 min at room temperature. After three gentle washes with PBS, slides were mounted with fluorescent mounting medium (KPL) and observed under an epifluorescence microscope (AxioImager.Z1; Zeiss) as described above.
RESULTS
Preparation of seeds.
None of the seeds used showed any evidence of prior contamination with B. pseudomallei, so all of the B. pseudomallei bacteria found were deemed to be present because of infection. No uninfected plantlets showed any evidence of the presence of B. pseudomallei, further supporting this conclusion.
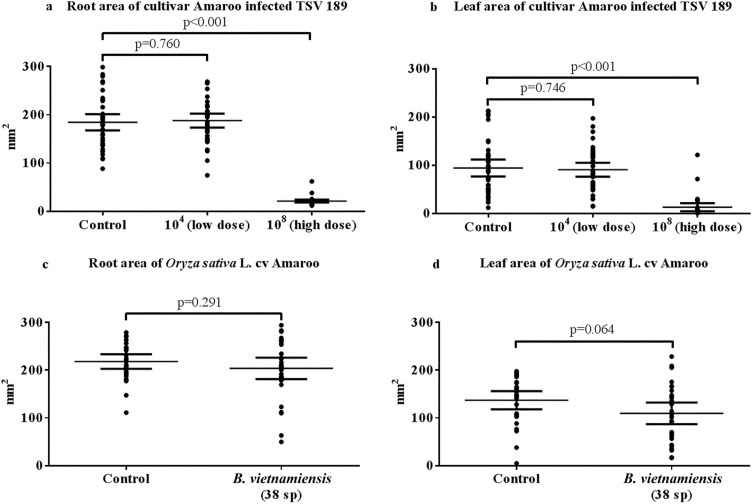
The pilot dose study determined that growth was inhibited at 108 CFU/ml but not at 104 CFU/ml (Fig. 1a and b). A concentration of 108 CFU/ml was selected for plant infection trials. Rice infected with B. vietnamiensis displayed growth that was not significantly different from that of uninfected control rice, indicating that the 108-CFU/ml dose of Gram-negative bacteria selected was not, in and of itself, able to stunt growth (Fig. 1c and d).
FIG 1.
Infection of O. sativa L. cv Amaroo with low (104 CFU/ml) and high (108 CFU/ml) concentrations of B. pseudomallei TSV189 (a, root; b, leaf) and a high concentration of B. vietnamiensis 38sp (108 CFU/ml) (c, root; d, leaf), as well as control uninfected groups for each infection. Area measurements include mean values with 95% confidence intervals. The low concentration of B. pseudomallei does not significantly stunt the root or leaf (root, P = 0.760; leaf, P = 0.746), while the high concentration of B. pseudomallei significantly stunts both roots and leaves (P < 0.001). The high concentration of B. vietnamiensis does not significantly stunt root or leaf growth (root, P = 0.291; leaf, P = 0.064; independent t tests, P = 0.05).
Trials of O. sativa L. cv Amaroo infection with Burkholderia species.
When germination of seeds infected with all strains of B. pseudomallei did occur, the majority of the plantlets did not show fully expanded leaves and had shorter roots than the controls. An example is shown in Fig. 2a and b. Infection with all of the Burkholderia species and strains except B. vietnamiensis showed statistically significant inhibition of growth relative to that of the control (Table 2). Of the species tested, B. pseudomallei and B. cenocepacia caused the most inhibition, followed by B. ubonensis (Fig. 3a and b; Table 3).
FIG 2.
Effect of B. pseudomallei TSV189 on rice seedlings. The growth of seedlings after 7 days without (a) and with (b) B. pseudomallei is shown. Seedlings exposed to B. pseudomallei have a shorter leaf and fewer and shorter roots than the unexposed control (a). Bars, 1 cm.
TABLE 2.
Summary statistics of infection of O. sativa L. cv Amaroo with various Burkholderia isolatesa
| Strain and plant part | t | df | P value | Sized effect (r) |
|---|---|---|---|---|
| B. vietnamiensis 38sp | ||||
| Root | 1.066 | 57 | 0.291 | 0.140 |
| Leaf | 1.888 | 57 | 0.064 | 0.243 |
| B. ubonensis A21 | ||||
| Root | 11.565 | 60.958 | <0.001 | 0.829 |
| Leaf | 6.396 | 52.259 | <0.001 | 0.663 |
| B. cenocepacia 17sp | ||||
| Root | 20.336 | 57 | <0.001 | 0.937 |
| Leaf | 10.651 | 46.409 | <0.001 | 0.842 |
| B. pseudomallei K96243 | ||||
| Root | 14.846 | 28.128 | <0.001 | 0.942 |
| Leaf | 7.477 | 28.693 | <0.001 | 0.813 |
| B. pseudomallei TSV192 | ||||
| Root | 14.968 | 32.779 | <0.001 | 0.934 |
| Leaf | 5.695 | 28.925 | <0.001 | 0.727 |
| B. pseudomallei TSV189 | ||||
| Root | 15.373 | 31.618 | <0.001 | 0.939 |
| Leaf | 5.918 | 28.134 | <0.001 | 0.745 |
Root and leaf areas of infected samples are compared to uninfected root and leaf areas in each case. Area measurements were compared by independent t test (P = 0.05).
FIG 3.
Comparison of the inhibition of O. sativa L. cv Amaroo growth by a range of Burkholderia species relative to that of uninfected-control growth. Percentages of root (a) and leaf (b) areas ± 95% confidence intervals are displayed, as are growth inhibition significance values. B. vietnamiensis does not significantly inhibit growth (leaf, P = 0.064; root, P = 0.291), while all of the other species do (P values also presented in Table 2) (independent t tests, P = 0.05). Inhibition by B. ubonensis is also significantly different from that by all of the other species tested (Table 3). B. cenocepacia and the B. pseudomallei strains are not significantly different from each other in inhibition (Table 3) (ANOVA and Gabriel post hoc test, P = 0.05).
TABLE 3.
Gabriel post hoc test results of one-way ANOVA comparing the relative growth (root and leaf) of O. sativa L. cv Amaroo upon inoculation with different Burkholderia species and strainsa
| Strain whose effect on leaf area growth was measured |
P value for comparison of effect of following strain on root area growth: |
|||||
|---|---|---|---|---|---|---|
| B. vietnamiensis 39sp | B. ubonensis A21 | B. cenocepacia 17sp | B. pseudomallei K96243 | B. pseudomallei TSV192 | B. pseudomallei TSV189 | |
| B. vietnamiensis 39sp | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| B. ubonensis A21 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| B. cenocepacia 17sp | <0.001 | 0.003 | 0.914 | 0.999 | 0.988 | |
| B. pseudomallei K96243 | <0.001 | <0.001 | 0.995 | 1.000 | 1.000 | |
| B. pseudomallei TSV192 | <0.001 | 0.002 | 1.000 | 0.988 | 1.000 | |
| B. pseudomallei TSV189 | <0.001 | 0.001 | 1.000 | 0.999 | 1.000 | |
P values for comparisons of leaf area growth percentages are shown in the bottom left half, and P values for comparisons of root area growth percentages are shown in the top right half.
Bacterial inhibition of other strains and species of rice.
While wild Australian rice (O. meridionalis) was inhibited by B. pseudomallei TSV189, Japanese rice (O. sativa L. cv. Koshihikari) was not inhibited in either root or leaf growth (Fig. 4).
FIG 4.
Inhibition of the growth of three rice species/cultivars by B. pseudomallei TSV189 relative to the control growth of each rice species/cultivar. Percentages of root (a) and leaf (b) areas ± 95% confidence intervals are displayed, as are the growth inhibition significance values. Cultivar Koshihikari growth is not significantly reduced relative to that of its control (root, P = 0.571; leaf, P = 0.599), while that of both other rice species/cultivars, O. sativa L. cv Amaroo (root, P < 0.001; leaf, P < 0.001) and O. meridionalis (root, P < 0.001; leaf, P < 0.001), is (independent t tests, P = 0.05).
B. pseudomallei IFA.
The reactivity of MAb MCA2823 was tested with a variety of isolates of B. pseudomallei, as well as other species of bacteria that are closely related to B. pseudomallei or can be found in the soil. B. mallei is not found in Australia, where this work was done, and was not available for testing because of restrictions on the importation of potentially pathogenic microorganisms. The IFA using this antibody was positive for B. pseudomallei and negative for B. pseudomallei near-neighbor species and other organisms (Table 1). In addition, no bacteria fluoresced in the roots of uninfected plants when the IFA was used; thus, it was possible to use MAb MCA2823 as a tool to detect B. pseudomallei in our plants.
Examination of infected plants by IFA showed the presence of B. pseudomallei as a multilayered structure around the epidermis and root hairs (Fig. 5a to c). Individual bacteria were also found inside the root hairs (Fig. 5d to f) and exodermis (Fig. 5g to i), indicating infection of the plant rather than just surface colonization.
FIG 5.
MAb MCA2823 immunofluorescence results. Panels a, d, and g show B. pseudomallei labeled by IFA; panels b, e, and h are pictures of the same field taken by Nomarski interference contrast; and panels c, f, and i are combined images. A biofilm of B. pseudomallei can be seen on the epidermis and root hairs in panels a, b, and c. B. pseudomallei can also be seen inside the plant root hair in panels d, e, and f, and inside the exodermis in panels g, h, and i. Bars, 10 μm.
DISCUSSION
The plant-microbe interaction between rice and B. pseudomallei is of interest, as B. pseudomallei is often found in rice fields and workers are exposed (2–4). Understanding more about the ecology of any interaction could improve our understanding of the persistence of the bacterium in this environment. Our study found that B. pseudomallei TSV189 retards the growth of Australian domestic rice (O. sativa L. cv Amaroo) and native Australian wild rice (O. meridionalis) but not that of Japanese rice (O. sativa L. cv. Koshihikari). The resistance of the Japanese rice cultivar O. sativa L. cv Nipponbare has previously been reported by Lee et al. (19); this cultivar is similar to Koshihikari (30). The wild rice (O. rufipogon) model of Kaestli et al. (18) was also not affected by B. pseudomallei inoculation; however, this wild rice species is different from the one we tested. Both of these other studies also utilized other models that were affected by B. pseudomallei, indicating that the B. pseudomallei isolates used were able to inhibit at least some host plant species. It is clear that there is a species and cultivar variation within the Oryza genus that determines the ability of B. pseudomallei to inhibit growth.
We also determined that both clinical and environmental Australian B. pseudomallei isolates and a Thai clinical isolate equally inhibited O. sativa L. cv Amaroo, indicating that B. pseudomallei may be generally inhibitory to some rice cultivars. This leads to the question of whether different rice cultivars will succeed in areas where B. pseudomallei is endemic.
As an example, the iRiceZoning map has three major rice group production areas in Thailand (http://carsr.agri.cmu.ac.th/projects/iRPZ/MAPRiceVarGroup.aspx). Aromatic rice and glutinous rice are produced in northeastern Thailand (an area where B. pseudomallei is highly endemic) (2, 31), while nonaromatic rice grows in the central part of Thailand, where B. pseudomallei is less commonly found (31). While there are differences in rainfall, irrigation patterns, and soil types in these regions (32), it is interesting to postulate that B. pseudomallei may also play a role in the success of different cultivars. Rice currently grown in northeastern Thailand may be more resistant to the effects of B. pseudomallei, and an infection trial of the different rice types and varieties would be useful in answering this question.
Low doses of B. pseudomallei did not inhibit the growth of O. sativa L. cv Amaroo, which may be due to competition with endophytic bacteria already present in seeds. Unfortunately, it was not possible to remove all of the potentially competing bacteria without killing the seeds. Previous experiments in which cleaning of seeds was studied have also identified this limitation (24). The high dose selected was designed to skew any potential interaction in favor of B. pseudomallei and was similar to the dose used in other plant-B pseudomallei experiments (18, 19, 26). B. pseudomallei has been found in soil at levels of up to 105 CFU/g (6), so growth retardation in natural soils may not be as obvious or may allow persistent interactions between the plant and the microbe. The brevity of this experiment (7 days) and growth in Hoagland agar, although it is also commonly used (26, 33, 34), are not adequate for full rice growth and may not mimic growth in soil. It would be interesting to study B. pseudomallei-O. sativa L. cv Amaroo interactions at this bacterial load over the life of rice in a soil environment; however, this was not possible with our facilities.
Rhizospheres usually produce nutrient sources for soil bacteria such as root exudates (sugar, amino acids, oxygen, etc.), border cells (root cap cells), and root debris (cell contents, lysates, etc.) (35). This can result in biofilm formation around the epidermis and root hairs. Root hairs develop during plant growth and autolyze, resulting in the proliferation of bacteria, which can then invade the epidermis and cortex (36). Using IFA, we found that in root sections, B. pseudomallei formed multiple layers around the epidermis. These were not removed by washing in 0.85% NaCl, which is indicative of a biofilm, although the presence of exopolysaccharide was not tested to confirm this. B. pseudomallei bacteria were also found inside root hairs and in the endodermis; however, images of the cortex were insufficient to clearly confirm the presence of B. pseudomallei in the cortex (data not shown). This is similar to results obtained by Kaestli et al. (18), who reported the presence of B. pseudomallei in the root hairs and cortex of grasses in epidemic areas.
This study successfully experimentally colonized the roots of a domestic rice cultivar with B. pseudomallei and identified differential inhibition of the growth of different species and cultivars of rice. However, the incubation period was short and the plants were grown in hydroponic agar. Growth in soil typical of rice paddies for longer periods may produce different results. In addition, this experiment used a high dose of bacteria, though other studies have also used relatively high inoculum concentrations and/or wounding of tissue to encourage inoculation or invasion (18, 19, 26, 34, 37). Natural environmental conditions may result in lower exposure levels. While B. pseudomallei can infect the roots of O. sativa L. cv Amaroo via root hairs and retard its growth, it does not retard the growth of Oryza sativa L. cv. Koshihikari. This cultivar difference could be a factor in the successful or unsuccessful growth of particular cultivars of rice in regions where B. pseudomallei is endemic. The relative susceptibility of plants may also affect the persistence, and thus the biogeographical boundaries, of B. pseudomallei. A susceptible rice cultivar also means that biocontrol experiments can be carried out with rice, and work is under way in this area.
Supplementary Material
ACKNOWLEDGMENTS
This research was aided by a grant from Royal Thai Government Scholarships, Ministry of Science and Technology.
We are grateful to Steve Ockerby for donating rice seed for this work.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00317-15.
REFERENCES
- 1.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol 4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 2.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NPJ, Peacock SJ. 2010. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg 82:1113–1117. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wuthiekanun V, Limmathurotsakul D, Chantratita N, Feil EJ, Day NPJ, Peacock SJ. 2009. Burkholderia pseudomallei is genetically diverse in agricultural land in northeast Thailand. PLoS Negl Trop Dis 3:e496. doi: 10.1371/journal.pntd.0000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rattanavong S, Wuthiekanun V, Langla S, Amornchai P, Sirisouk J, Phetsouvanh R, Moore CE, Peacock SJ, Buisson Y, Newton PN. 2011. Randomized soil survey of the distribution of Burkholderia pseudomallei in rice fields in Laos. Appl Environ Microbiol 77:532–536. doi: 10.1128/AEM.01822-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker A, Tahani D, Gardiner C, Bristow KL, Greenhill AR, Warner J. 2011. Groundwater seeps facilitate exposure to Burkholderia pseudomallei. Appl Environ Microbiol 77:7243–7246. doi: 10.1128/AEM.05048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas AD, Forbesfaulkner JC. 1981. Persistence of Pseudomonas pseudomallei in soil. Aust Vet J 57:535–536. doi: 10.1111/j.1751-0813.1981.tb05804.x. [DOI] [PubMed] [Google Scholar]
- 7.Inglis TJ, O'Reilly L, Merritt AJ, Levy A, Heath CH. 2011. The aftermath of the Western Australian melioidosis outbreak. Am J Trop Med Hyg 84:851–857. doi: 10.4269/ajtmh.2011.10-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suputtamongkol Y, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Ruchutrakool T, Budhsarawong D, Mootsikapun P, Wuthiekanun V, Teerawatasook N, Lulitanond A. 1999. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis 29:408–413. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- 9.Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C. 2013. Microbial interactions in the rhizosphere, p 29–44. In de Bruijn FJ. (ed), Molecular microbial ecology of the rhizosphere. Wiley-Blackwell, Hoboken, NJ. [Google Scholar]
- 10.Johnston-Monje D, Raizada MN. 2013. Surveying diverse Zea seed for populations of bacterial endophytes, p 445–455. In de Bruijn FJ. (ed), Molecular microbial ecology of the rhizosphere. Wiley-Blackwell, Hoboken, NJ. [Google Scholar]
- 11.Stoyanova M, Pavlina I, Moncheva P, Bogatzevska N. 2007. Biodiversity and incidence of Burkholderia species. Biotechnol Biotechnol Equip 21:306–310. doi: 10.1080/13102818.2007.10817465. [DOI] [Google Scholar]
- 12.White NJ. 2003. Melioidosis. Lancet 361:1715–1722. doi: 10.1016/S0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 13.Fisher NA, Ribot WJ, Applefeld W, DeShazer D. 2012. The Madagascar hissing cockroach as a novel surrogate host for Burkholderia pseudomallei, B. mallei and B. thailandensis. BMC Microbiol 12:117. doi: 10.1186/1471-2180-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elschner MC, Hnizdo J, Stamm I, El-Adawy H, Mertens K, Melzer F. 2014. Isolation of the highly pathogenic and zoonotic agent Burkholderia pseudomallei from a pet green Iguana in Prague, Czech Republic. BMC Vet Res 10:283. doi: 10.1186/s12917-014-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi SK, Lim TY, Lee SH, Nathan S. 2012. Burkholderia pseudomallei kills Caenorhabditis elegans through virulence mechanisms distinct from intestinal lumen colonization. Virulence 3:485–496. doi: 10.4161/viru.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampton V, Kaestli M, Mayo M, Choy JL, Harrington G, Richardson L, Benedict S, Noske R, Garnett ST, Godoy D, Spratt BG, Currie BJ. 2011. Melioidosis in birds and Burkholderia pseudomallei dispersal, Australia. Emerg Infect Dis 17:1310–1312. doi: 10.3201/eid1707.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Schaik E, Tom M, DeVinney R, Woods DE. 2008. Development of novel animal infection models for the study of acute and chronic Burkholderia pseudomallei pulmonary infections. Microbes Infect 10:1291–1299. doi: 10.1016/j.micinf.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Kaestli M, Schmid M, Mayo M, Rothballer M, Harrington G, Richardson L, Hill A, Hill J, Tuanyok A, Keim P, Hartmann A, Currie BJ. 2012. Out of the ground: aerial and exotic habitats of the melioidosis bacterium Burkholderia pseudomallei in grasses in Australia. Environ Microbiol 14:2058–2070. doi: 10.1111/j.1462-2920.2011.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YH, Chen YH, Ouyang XZ, Gan YH. 2010. Identification of tomato plant as a novel host model for Burkholderia pseudomallei. BMC Microbiol 10:28. doi: 10.1186/1471-2180-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veasey EA, Karasawa MG, Santos PP, Rosa MS, Manani RE, Oliveira GCX. 2004. Variation in the loss of seed dormancy during after-ripening of wild and cultivated rice species. Ann Bot 94:875–882. doi: 10.1093/aob/mch215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis RH, Hong TD, Roberts EH. 1983. Procedures for the safe removal of dormancy from rice seed. Seed Sci Technol 11:77–112. [Google Scholar]
- 22.Oyebanji OB, Nweke O, Odebunmi O, Galadima NB, Idris MS, Nnodi UN, Afolabi AS, Ogbadu GH. 2009. Simple, effective and economical explant-surface sterilization protocol for cowpea, rice and sorghum seeds. Afr J Biotechnol 8:5395–5399. [Google Scholar]
- 23.Liu Y, Zuo S, Zou YY, Wang JH, Song W. 2013. Investigation on diversity and population succession dynamics of endophytic bacteria from seeds of maize (Zea mays L., Nongda108) at different growth stages. Ann Microbiol 63:71–79. doi: 10.1007/s13213-012-0446-3. [DOI] [Google Scholar]
- 24.Kaga H, Mano H, Tanaka F, Watanabe A, Kaneko S, Morisaki H. 2009. Rice seeds as sources of endophytic bacteria. Microbes Environ 24:154–162. doi: 10.1264/jsme2.ME09113. [DOI] [PubMed] [Google Scholar]
- 25.Handelsman J, Raffel S, Mester EH, Wunderlich L, Grau CR. 1990. Biological control of damping-off of alfalfa seedlings with Bacillus cereus Uw85. Appl Environ Microbiol 56:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattos KA, Padua VLM, Romeiro A, Hallack LF, Neves BC, Ulisses TMU, Barros CF, Todeschini AR, Previato JO, Mendonca-Previato L. 2008. Endophytic colonization of rice (Oryza sativa L.) by the diazotrophic bacterium Burkholderia kururiensis and its ability to enhance plant growth. An Acad Bras Cienc 80:477–493. doi: 10.1590/S0001-37652008000300009. [DOI] [PubMed] [Google Scholar]
- 27.Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. College of Agriculture, University of California, Berkeley, CA. [Google Scholar]
- 28.Watt M, Hugenholtz P, White R, Vinall K. 2006. Numbers and locations of native bacteria on field-grown wheat roots quantified by fluorescence in situ hybridization (FISH). Environ Microbiol 8:871–884. doi: 10.1111/j.1462-2920.2005.00973.x. [DOI] [PubMed] [Google Scholar]
- 29.Villar R, Ruiz-Robleto J, Ubera JL, Poorter H. 2013. Exploring variation in leaf mass per area (LMA) from leaf to cell: an anatomical analysis of 26 woody species. Am J Bot 100:1969–1980. doi: 10.3732/ajb.1200562. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Nagasaki H, Yonemaru J, Ebana K, Nakajima M, Shibaya T, Yano M. 2010. Fine definition of the pedigree haplotypes of closely related rice cultivars by means of genome-wide discovery of single-nucleotide polymorphisms. BMC Genomics 11:267. doi: 10.1186/1471-2164-11-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuddhakul V, Tharavichitkul P, Na-Ngam N, Jitsurong S, Kunthawa B, Noimay P, Noimay P, Binla A, Thamlikitkul V. 1999. Epidemiology of Burkholderia pseudomallei in Thailand. Am J Trop Med Hyg 60:458–461. [DOI] [PubMed] [Google Scholar]
- 32.Boonsompopphan B, Vearasilp T, Yost RS, Attanandana T. 2008. Field identification of soil series: indexing and retrieving soil information while sharing experience and knowledge. Soil Sci 173:736–744. doi: 10.1097/SS.0b013e31818939cf. [DOI] [Google Scholar]
- 33.Rouws LFM, Meneses CHSG, Guedes HV, Vidal MS, Baldani JI, Schwab S. 2010. Monitoring the colonization of sugarcane and rice plants by the endophytic diazotrophic bacterium Gluconacetobacter diazotrophicus marked with gfp and gusA reporter genes. Lett Appl Microbiol 51:325–330. doi: 10.1111/j.1472-765X.2010.02899.x. [DOI] [PubMed] [Google Scholar]
- 34.Bernier SP, Silo-Suh L, Woods DE, Ohman DE, Sokol PA. 2003. Comparative analysis of plant and animal models for characterization of Burkholderia cepacia virulence. Infect Immun 71:5306–5313. doi: 10.1128/IAI.71.9.5306-5313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uren NC. 2007. Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants, p 1–22. In Pinton R, Varanini Z, Nannipieri P (ed), The rhizosphere: biochemistry and organic substances at the soil-plant interface, 2nd ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 36.Bell-Perkins LJ, Lynch JM. 2002. Rhizosphere microbiology, p 2713–2728. In Bitton G. (ed), Encyclopedia of environmental microbiology. Wiley, New York, NY. [Google Scholar]
- 37.Fujishige NA, Kapadia NN, Hirsch AM. 2006. A feeling for the micro-organism: structure on a small scale. Biofilms on plant roots. Bot J Linn Soc 150:79–88. doi: 10.1111/j.1095-8339.2006.00492.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.