Abstract
Enterobacteriaceae-associated blaCTX-M genes have become globally widespread within the past 30 years. Among isolates from Washington State cattle, Escherichia coli strains carrying blaCTX-M (CTX-M E. coli strains) were absent from a set of 2008 isolates but present in a set of isolates from 2011. On 30 Washington State dairy farms sampled in 2012, CTX-M E. coli prevalence was significantly higher on eastern than on northwestern Washington farms, on farms with more than 3,000 adult cows, and on farms that recently received new animals. The addition of fresh bedding to calf hutches at least weekly and use of residual fly sprays were associated with lower prevalence of CTX-M E. coli. In Washington State, the occurrence of human pathogens carrying blaCTX-M genes preceded the emergence of blaCTX-M-associated E. coli in cattle, indicating that these resistance determinants and/or their bacterial hosts may have emerged in human populations prior to their dissemination to cattle populations.
INTRODUCTION
Emergence and dissemination of antibiotic resistance traits in Gram-negative bacteria can occur rapidly and across wide geographic distances (1). Bacterial strains carrying the blaCTX-M family of extended-spectrum β-lactamase (ESBL) genes have spread globally in less than 30 years (2, 3). Unlike other families of ESBL genes which result from point mutations in preexisting, narrower-spectrum β-lactamase genes (blaTEM-1 and blaSHV) (4), blaCTX-M β-lactamase genes originated from chromosomal genes of Kluyvera species (2). The earliest Escherichia coli plasmid-associated blaCTX-M was detected from laboratory dogs used in pharmaceutical research in Japan in 1986, followed by isolations from human patients in Munich, Germany (1989), Argentina (1989), France (1989), and Poland (1996) (2). In North America, blaCTX-M-bearing Escherichia coli strains (CTX-M E. coli strains) were detected in humans in Canada in 2000 (5) and in the United States between 2001 and 2002 (6). By 2007 in the United States, 80% of 15 geographically dispersed medical centers reported E. coli or Klebsiella pneumoniae infections with associated blaCTX-M genes (7). In Washington State, the earliest reported clinical human E. coli strain carrying blaCTX-M was isolated in 2001 in Seattle (6). Currently, CTX-M enzymes are considered the most prevalent ESBLs in isolates of E. coli, K. pneumoniae, and Proteus mirabilis associated with human infections globally (8).
In the United States, dairy cattle-associated CTX-M E. coli strains were first reported by Wittum and others in Ohio in 2009 (9). This Ohio research group later reported CTX-M in Salmonella enterica isolates from equine, swine, and turkey sources from six different states (10) and from swine finishing barns in 5 states (11). In the current study, we first tested E. coli isolates from Washington State dairy cattle banked from several earlier research projects to determine the time window of CTX-M E. coli emergence in this region and host species. The results of this retrospective study indicated recent emergence of CTX-M E. coli in Washington State; in order to determine factors associated with CTX-M E. coli prevalence, we conducted a study of management and other factors influencing the prevalence of this novel bacterial resistance determinant among dairy farms.
(Preliminary results from this study were presented at the 113th General Meeting of the American Society for Microbiology, 18 to 21 May 2013, Denver, CO, USA.)
MATERIALS AND METHODS
Retrospective analysis.
Ceftiofur, a veterinary third-generation cephalosporin, is used commonly to treat infectious diseases in dairy cattle (12). Resistance to ceftiofur is correlated with clinically relevant drug resistance in human medicine (13) and has been used as a phenotypic marker for targeted research of third-generation cephalosporin resistance genes in bovine bacterial flora (14–16). The E. coli isolates used in the retrospective study were obtained during previous studies using various selection methods focused primarily on ceftiofur resistance.
Isolates from the years 2002 and 2003 were obtained from a study in which 3,673 fecal E. coli isolates were collected from dairy cattle and calves in Washington State (17) and then tested for resistance to a panel of 12 antibiotics (see Table S1 in the supplemental material) using breakpoint agar dilution (17). From the ceftiofur-resistant E. coli isolates (n = 478 from 162 individual animals) detected in that study, we tested one isolate per animal by PCR for the presence of blaCMY-2 and blaCTX-M. Five isolates were not available, leaving 157 isolates from different animals on 14 different farms for testing.
Isolates from 2008 originated from a study of the dynamics of antibiotic-resistant E. coli in dairy calves in which individual dairy calves were sampled repeatedly over time (W. M. Sischo, unpublished data). In that study, fecal samples were plated onto either unsupplemented MacConkey agar or MacConkey agar supplemented with ceftiofur (8 μg/ml) (Pfizer, New York, NY, USA). Lactose-positive (pink) colonies were transferred to bank tubes containing brain heart infusion (BHI) agar (Hardy Diagnostics, Santa Maria, CA, USA) and incubated overnight at 37°C and later confirmed as E. coli by PCR detection of uidA (18). E. coli isolates were tested for resistance to a panel of 12 antibiotics (see Table S2 in the supplemental material) by agar diffusion assay (19, 20). Isolates with reduced susceptibility to ceftiofur (inhibition zone size of ≤22 mm) were systematically chosen to represent different animals, farms, and collection dates, yielding 65 isolates that were tested by PCR for the presence of blaCMY-2 and blaCTX-M.
Isolates from 2011 originated from a project that sampled calves on four dairy farms visited five times at 2-week intervals. Approximately 50 individual calf fecal samples were obtained at each visit and were processed as described for isolates from 2008, except that selective MacConkey plates were supplemented with 2 μg/ml ceftiofur. To capture isolates with intermediate susceptibility, the ceftiofur concentration of 2 μl/ml was chosen, because it is the breakpoint between susceptibility and intermediate susceptibility (19). This resulted in a total of 882 samples which generated 863 E. coli isolates from unsupplemented MacConkey and 755 isolates from MacConkey supplemented with 2 μg/ml ceftiofur. From those isolates, a subset was chosen by random selection of up to three isolates per farm per date. This subset included 44 E. coli isolates from unsupplemented MacConkey medium and 45 isolates picked from ceftiofur-supplemented MacConkey medium. The resulting 89 isolates were tested by PCR for the presence of blaCMY-2 and blaCTX-M and were tested for resistance to ceftiofur using a disk agar diffusion assay as described above (19, 20). Forty-seven of these isolates had reduced susceptibility to ceftiofur (inhibition zone size of ≤22 mm), and their PCR results are reported in Table 2.
TABLE 2.
Results of blaCMY-2 and blaCTX-M PCR of E. coli isolates from cattle feces in the Pacific Northwest
| Time period | Isolate selection or ceftiofur concn in MacConkey medium | Total no. of isolates tested | No. of farms |
blaCMY-2 positive |
blaCTX-M positive |
||
|---|---|---|---|---|---|---|---|
| No. | % (95% CI)a | No. | % (95% CI)a | ||||
| 2002-2003 | Reduced susceptibility to ceftiofur per breakpoint agar test (17) | 157 | 14 | 137 | 87.3 (80.8–91.9) | 0 | 0 (0–3.0) |
| 2008 | Reduced susceptibility to ceftiofur per breakpoint agar test (17) | 41 | 4 | 40 | 97.6 (85.6–99.9) | 0 | 0 (0–10.7) |
| 2008 | 8 μg/ml ceftiofur | 24 | 4 | 23 | 95.8 (76.9–99.8) | 0 | 0 (0–17.2) |
| 2008 total | 65 | 4 | 63 | 96.9 (88.4–99.5) | 0 | 0 (0–7.0) | |
| 2011 | 0 μg/ml ceftiofur | 45 | 4 | 14 | 31.1 (18.6–46.8) | 2 | 4.4 (0.1–16.4) |
| 2011 | 2 μg/ml ceftiofur | 44 | 4 | 24 | 54.5 (39.0–69.3) | 5 | 11.4 (3.0–20.2) |
| 2011 total | 89 | 4 | 38 | 42.7 (32.4–53.6) | 7 | 7.9 (3.5–16.1) | |
| 2011 | Reduced susceptibility to ceftiofur according to disk diffusion results (inhibition zone size of ≤22 mm) (19, 20) | 47 | 4 | 37 | 78.7 (63.9–88.8) | 7 | 14.9 (6.7–28.9) |
CI, confidence interval (23).
PCR protocols.
We detected the presence of blaCTX-M and blaCMY-2 genes using previously published protocols (8, 21, 22). Boiled cell lysate was used (5 μl in a 25-μl PCR mixture) for the reaction template. Standard amplification reaction volumes were 25-μl final concentrations of 1× buffer, 100 μM deoxynucleoside triphosphate (dNTP), 1.5 μM MgCl2, 1 to 0.1 pmol/μl primer, and 0.5 to 1 U Taq. Cycling conditions for blaCMY-2 PCR included an initial denaturation of 95°C for 3 min and 30 cycles of 94°C for 30 s, 55°C for 30 s,72°C for 30 s, and 72°C for 10 min. Cycling conditions for the global blaCTX-M included initial denaturation at 94°C for 3 min, 30 cycles of 94°C for 15 s, annealing at 52°C for 30 s, extensions at 72°C for 30 s, and then a final extension at 72°C for 7 min. Primer sequences are as shown in Table 1.
TABLE 1.
PCR primer sequences used in the study
Prevalence risk factor study.
During the summer and fall of 2012, a convenience sample of 30 Washington State dairy farms was identified, and each farm was visited once. The 30 farms were distributed throughout the dairy-intensive agricultural areas of Washington State. For the analysis, we divided the state into two regions, northwestern Washington (region 1; 9 farms) and eastern Washington (region 2; 21 farms) (Fig. 1). Samples collected at each farm included individual animal fecal samples from up to 5 preweaned dairy calves per age group (0 to 6 days old, 7 to 13 days old, 14 to 20 days old, 21 to 27 days old, 28 to 34 days old, 35 to 41 days old, 42 to 48 days old, 49 to 55 days old, 56 to 62 days old, and >62 days old), 5 pooled fecal pat samples from each of the lactation pen, fresh cow pen, hospital pen, and maternity or closeup pens, a milk filter, and one wastewater lagoon sample. Approximately 5 g of fecal material and 5 ml lagoon water were collected for each sample. Individual calf fecal samples were obtained aseptically per rectum. These procedures were approved by the Washington State University Institutional Animal Care and Use Committee. Fecal samples were held on ice during transport to the laboratory.
FIG 1.
County boundary map of Washington State. Nine dairies were visited in the northwestern region (region 1), and 21 dairies were visited in the eastern region (region 2) for the blaCTX-M prevalence risk factor study. (Map adapted from the 2012 USDA Census of Agriculture.)
Microbiological assessments.
To select for blaCTX-M- and blaCMY-2-associated phenotypes, samples from all farms were processed as previously described (22). Briefly, feces (5 g) were incubated in 45 ml nutrient broth (Hardy Diagnostics, Santa Maria, CA, USA) supplemented with cefotaxime (2 μg/ml) at 37°C for 18 to 24 h without shaking. Following incubation, 0.1 ml of broth was independently plated onto MacConkey agar supplemented with cefepime (4 μg/ml) or cefoxitin (4 μg/ml). Cefepime was used to screen for a blaCTX-M phenotype and cefoxitin for a blaCMY-2 phenotype (22). Bacterial isolates grown on cefepime- and cefoxitin-supplemented media were tested for the presence of blaCTX-M and blaCMY-2, respectively. Three to five isolated colonies with morphology consistent with E. coli were picked from each plate for banking and further characterization. Isolates were confirmed as E. coli by PCR detection of uidA (18). In addition to selective plating and in order to estimate the prevalence of blaCTX-M-and blaCMY-2-bearing E. coli in unselected populations of fecal E. coli, samples from 21 farms (9 in region 1 and 12 in region 2) were directly swabbed onto MacConkey agar without antibiotic supplement and incubated overnight at 37°C. Two isolates were picked from each of these plates and characterized by PCR for uidA, blaCTX-M, and blaCMY-2 as described above.
Questionnaires.
At each farm visit, a questionnaire was administered to the farm herd manager, calf manager, or owner. The questionnaire elicited information about the number of animals (adult cows, preweaned calves, heifers, and bull calves), the number of physical locations occupied by the farm, whether heifer calves were raised elsewhere and returned to the dairy, recent animal purchases from other premises, whether feed was raised on site or purchased elsewhere, calving pen practices, frequency and method of cleaning maternity pens and calf hutches, calf feeding practices, protocols for cleaning bottles and buckets used for feeding milk and dry feeds, calf housing practices, fly control, calf treatment crew hygiene, the number of employees and visitors and frequency of visits to the farm, and antibiotics used for calf and cow diseases (see the supplemental material).
Data analysis.
To calculate farm-level prevalence of blaCTX-M- and blaCMY-2-positive samples, a sample producing one or more E. coli isolates PCR positive for blaCTX-M and/or blaCMY-2 on any media was counted as positive for the relevant gene. Farm-level bacterial data were joined with the questionnaire data and analyzed using SAS v. 9.2 for Windows (SAS Institute Corp., Cary, NC, USA). To describe the distribution of prevalence by farm, all sample types were included in the analysis. For comparisons between proportions, a Mantel-Haenszel chi-square test was computed using Proc Freq in SAS, and 95% confidence intervals surrounding proportions were calculated according to Fleiss et al. (23) by using WinPepi (24). For risk factor assessment, farm prevalence of calves positive for blaCTX-M was the outcome variable, and risk factors from the questionnaire data were independent variables. Each potential risk factor was assessed for a univariable association with the outcome of interest using Wilcoxon's two-sample test calculated using Proc Univariate in SAS. A P value of ≤0.05 was considered significant.
RESULTS AND DISCUSSION
Retrospective analysis.
All E. coli isolates obtained from studies conducted prior to 2011 were PCR negative for blaCTX-M. In isolates obtained in the 2011 study, blaCTX-M was detected in 2/45 (4.4%) isolates derived from nonselective (unsupplemented MacConkey agar plates) and in 5/44 (11.4%) isolates derived from ceftiofur-supplemented plates (Table 2).
Risk factor study.
Each of 30 Washington State farms was visited once between June and October 2012. The herd size distribution in the northwestern part of Washington was significantly smaller than that in eastern Washington (Kruskal-Wallis P value = 0.003) (see Fig. S1 in the supplemental material). An average of 45 fecal samples were collected from each farm for a total of 1,351 samples. The majority of fecal samples were from preweaned calves (see Table S3 in the supplemental material). The numbers of samples collected were uniform across the 10 age interval categories (average number per age category, 4.2; standard deviation [SD], 0.73). Growth on cefepime-supplemented MacConkey plates was highly correlated with blaCTX-M: of 2,785 isolates obtained from cefepime-supplemented plates, blaCTX-M was detected in 2,508 (90.1%). Similarly, growth on cefoxitin-supplemented plates correlated strongly with the presence of blaCMY-2: of 3,914 isolates from cefoxitin-supplemented plates, blaCMY-2 was detected in 3,080 (78.7%). Carriage of both beta-lactamase genes in single isolates was also common: blaCMY-2 was detected in 339 of 2,169 (13.5%) CTX-M E. coli isolates isolated from cefepime-supplemented plates (see Tables S4 and S5 in the supplemental material).
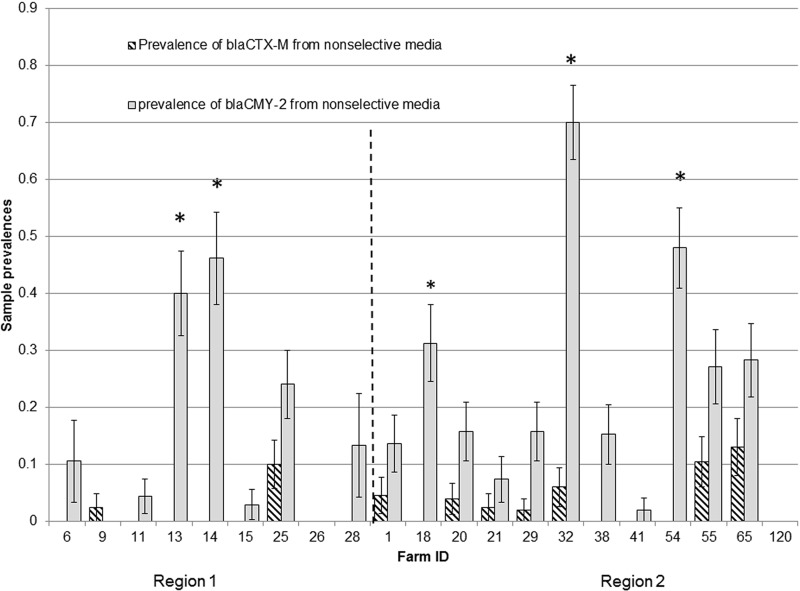
On the basis of selective media and PCR testing, 70.0% (21/30) of farms had a prevalence of E. coli isolates carrying blaCMY-2 of greater than 80%, and 33.3% (10/30) of farms had a CTX-M E. coli prevalence greater than 80% (Table 3). Samples from 21 farms plated onto unsupplemented MacConkey agar showed an overall prevalence of CTX-M E. coli of 4.4% (95% confidence interval [CI] of 3.0 to 6.5) and an overall prevalence of blaCMY-2-positive E. coli isolates of 32.1% (95% CI of 28.3 to 35.3). On 20/21 farms, the prevalence of blaCMY-2-positive E. coli isolates was higher than the prevalence of CTX-M isolates based on nonselective culture, and overall this difference was significant (Wilcoxon signed-rank test P value of <0.0001) (Fig. 2). E. coli isolates carrying blaCMY-2 had consistently higher prevalence than CTX-M E. coli isolates in preweaned calves across all calf age groups (Wilcoxon signed-rank test P value of 0.002) (see Fig. S2 in the supplemental material), and the prevalence of E. coli isolates carrying blaCMY-2 did not differ between regions (data not shown), suggesting that E. coli isolates carrying blaCMY-2 are well established at a population level across dairy farms in Washington State. CTX-M E. coli strains were present in 62/116 (53.5% [95% CI, 44.0 to 62.7%]) pooled adult cow fecal samples from 30 farms, 16 (57.1% [95% CI, 37.4 to 75.0]) lagoon samples from 28 farms, and 14 (50.0% [95% CI, 31.1 to 67.0]) milk filter samples from 28 farms.
TABLE 3.
Number of farms by prevalence of E. coli strains with blaCMY-2 and blaCTX-M genes
| Prevalence rangea | No. (%) of farms in prevalence category for E. coli with: |
|
|---|---|---|
| blaCMY-2 | blaCTX-M | |
| 0–0.2 | 0 (0) | 6 (20.0) |
| 0.2–0.4 | 0 (0) | 1 (3.3) |
| 0.4–0.6 | 4 (13.3) | 5 (16.7) |
| 0.6–0.8 | 5 (16.7) | 8 (26.7) |
| 0.8–1.0 | 21 (70.0) | 10 (33.3) |
Proportion of samples plated on selective media that yielded at least one E. coli isolate positive for blaCMY-2 or blaCTX-M.
FIG 2.
Prevalence of samples that yielded E. coli isolates positive for blaCTX-M and blaCMY-2 from nonselective media from 21 dairy farms. Error bars represent ±standard errors of the mean (SEM). Farms to the left of the vertical dotted line are located in region 1 (northwestern Washington), and farms to the right of the dotted line are located in region 2 (eastern Washington). Mean farm prevalence of blaCMY-2 was higher than the prevalence of blaCTX-M (Wilcoxon signed-rank test P value of <0.0001).
Fourteen antibiotics were reportedly used to treat calf and cow diseases across all farms. Ceftiofur was reported to be used for treating all surveyed cattle diseases. Other antibiotics reported to be commonly used on study farms included penicillin, ampicillin, enrofloxacin, and florfenicol. The most frequently used antibiotic for intramammary treatment of mastitis was ceftiofur, followed by cephapirin and pirlimycin. Nearly all the antibiotics that were available on study farms were reportedly used to treat calf diarrhea, with enrofloxacin and ceftiofur the most frequently used. Florfenicol and enrofloxacin were the antibiotics most frequently used to treat calf respiratory disease (Table 4).
TABLE 4.
Number of farms out of 30 reporting specific antibiotics used for cow and calf diseases
| Antibiotic | No. of farms reporting diseases |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calf diseases |
Cow diseases |
||||||||||||
| Diarrhea | Mycoplasma (ears) | Pneumonia | Sepsis | Swollen joint | Umbilical infection | Displaced abomasum | Dystocia | Foot rot | Intramammary treatment | Metritis | Pneumonia | Retained placenta | |
| Ampicillin | 3 | 7 | 1 | 2 | 8 | 6 | 6 | 5 | 11 | 0 | 9 | 13 | 6 |
| Ceftiofur | 11 | 4 | 3 | 8 | 4 | 5 | 14 | 15 | 8 | 21 | 22 | 14 | 20 |
| Cephapirin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 |
| Enrofloxacin | 15 | 0 | 13 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Florfenicol | 4 | 1 | 22 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gentamicin | 7 | 0 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oxytetracycline | 3 | 0 | 2 | 0 | 1 | 0 | 1 | 1 | 6 | 0 | 1 | 3 | 1 |
| Penicillin | 4 | 11 | 3 | 3 | 17 | 20 | 4 | 3 | 14 | 0 | 2 | 4 | 3 |
| Pirlimycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Sulfamethazine | 7 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Tilmicosin | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tilosin | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Sulfa-trimethoprim | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tulathromycin | 3 | 1 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Univariable nonparametric analysis results indicated that a northwestern Washington location, smaller herd size, adding fresh bedding to calf hutches weekly or more frequently, and use of residual fly sprays for insect control were associated with significantly lower prevalence of CTX-M E. coli in calves (exact Wilcoxon two-sample test P value of <0.05). Additionally, recent animal movements onto the farm were significantly associated with higher CTX-M E. coli prevalence in calves (exact Wilcoxon two-sample test P value of 0.03) (Table 5). Management factors for which no associations with CTX-M E. coli prevalence were detected included reported use of antibiotics (either any use or the number of diseases for which the antibiotic was used), addition of antibiotics to grain, milk, or milk replacers, frequency of cleaning feeding containers (milk and water containers) or esophageal feeders, type of calf hutch, method of calf hutch cleaning, frequency of hutch cleaning, and type of bedding used in calf hutches. Other variables for which no associations with CTX-M E. coli prevalence in calves were detected included calf treaters' hand hygiene, direction of feeding in the calf rows (youngest to oldest, oldest to youngest, or both directions), frequency of visits by feed delivery trucks, veterinarians, nutritionists, hoof trimmers, and breeders, and the number of people on the farm on any given day.
TABLE 5.
Mean and median farm prevalence of blaCTX-M-positive E. coli strains according to farm-level characteristics
| Characteristic | No. of farms | Farm prevalence of blaCTX-M-positive E. coli strains |
Wilcoxon 2-sample P value | |
|---|---|---|---|---|
| Mean (SEM) | Median (range) | |||
| Region | ||||
| Northwest Washington | 9 | 0.439 (0.121) | 0.487 (0.822) | |
| Eastern Washington | 21 | 0.718 (0.063) | 0.822 (0.930) | 0.03 |
| No. of adult cows in the herd | ||||
| <3,000 | 19 | 0.551 (0.081) | 0.714 (1.0) | |
| ≥3,000 | 11 | 0.779 (0.076) | 0.860 (0.822) | 0.04 |
| Any animal movements onto the farm | ||||
| Yes | 11 | 0.824 (0.046) | 0.86 (0.442) | |
| No | 19 | 0.525 (0.083) | 0.714 (1.0) | 0.03 |
| Frequency of adding fresh bedding to calf hutches | ||||
| ≥weekly | 5 | 0.295 (0.703) | 0.178 (0.714) | |
| <weekly | 25 | 0.703 (0.059) | 0.8 (1.0) | 0.02 |
| Use of residual fly sprays | ||||
| No | 17 | 0.774 (0.045) | 0.822 (0.692) | |
| Yes | 13 | 0.452 (0.111) | 0.511 (1.0) | 0.04 |
CTX-M E. coli strains were not detected among isolates obtained from Washington State dairy cattle prior to 2009 but were detected among isolates from 2011. By 2012, CTX-M E. coli strains were widespread among dairy farms throughout Washington State. Although the number of isolates in the retrospective set was limited, they were screened for resistance to ceftiofur, which would increase the probability of detecting E. coli strains carrying β-lactamase genes, including blaCTX-M (25). Our estimate of the timing of the appearance of CTX-M E. coli in Washington State dairy cattle is consistent with findings from dairy studies in Ohio (9, 10) and feedlot cattle in Texas (26), where CTX-M E. coli strains were detected in cattle in 2009.
Human infections with Enterobacteriaceae strains carrying blaCTX-M were reported from Europe, Asia, Africa, and South America during the late 1980s and 1990s, while the earliest reports of blaCTX-M Enterobacteriaceae infections in human patients from North America were in the early 2000s (2, 6, 27–29). Between 2000 and 2006, blaCTX-M became the predominant ESBL determinant among E. coli strains in a U.S. hospital (30). By 2010, CTX-M genes were the most frequent ESBL determinants among E. coli, Klebsiella sp., and Proteus mirabilis strains from 72 hospitals in the United States (31). E. coli and Klebsiella sp. strains carrying blaCTX-M in Seattle hospitals were reported between 2003 and 2007 (7, 32).
A frequently cited concern is that antimicrobial use in food-producing animals generates a population of resistant bacteria that will serve as a reservoir of resistance genes for human enteric pathogens (33–37). Our data show that CTX-M E. coli strains first emerged as frequently occurring members of the bovine enteric E. coli community only considerably after its emergence as a significant cause of human clinical infection (6, 31, 32) (Table 2). It is possible that these strains were present during earlier years, but if so at most they represented uncommon or rare members of the cattle commensal E. coli populations. This observed order of emergence is clearly inconsistent with the hypothesis that emergence in livestock commensal E. coli populations is a necessary precursor to emergence in human-pathogenic E. coli (35, 38–41).
The results of culture and isolation from fecal and environmental samples in 2012 confirmed that CTX-M E. coli strains have become widespread on dairies around the state; however, the distribution of CTX-M E. coli prevalence on farms was skewed, limiting analysis of risk factors to nonparametric tests (Table 3). Single-variable comparisons indicated associations between high prevalence of CTX-M E. coli and eastern Washington location, large herd size, and recent animal movements onto the farm (Table 5). These variables are correlated, as large herds were more likely to be in eastern Washington and to have recent animal movements onto the farm. Large herd size is a plausible contributor to CTX-M E. coli prevalence, because large farms are associated with a high frequency of visits by feed trucks and other services, such as veterinarians and hoof trimmers, high volumes of feed imported to the farm, and a high probability of animal movements onto the farm.
Two surprising findings in the current study were the association of frequency of adding fresh bedding to calf hutches and the use of residual fly sprays with reduced CTX-M E. coli prevalence (Table 5). Lack of fly control is an established risk factor for infections on dairy farms (42, 43), and residual sprays provide long-lasting insecticide activity. Relatively few farms (5 of 30 [16.7%]) reported adding fresh bedding to calf hutches weekly or more frequently, but the difference in prevalence was large enough to be statistically significant, even with this relatively small sample size. Although the frequency of adding bedding may be a proxy for general good calf management on a farm, other “good management” farm-level management factors, such as frequency of cleaning water and milk bottles and buckets, hand hygiene of treatment crews, and how hutches were cleaned between calves, were not associated with CTX-M E. coli prevalence. Fresh bedding may provide a barrier between calves and old bedding that is contaminated with feces and urine.
We did not detect any association between farm-level antibiotic use (including ceftiofur) and farm-level CTX-M E. coli prevalence, a finding that is consistent with previous observations (44, 45). A study of 65 dairy farms in the United Kingdom found a significant association between the use of a third- or fourth-generation cephalosporin in the previous 12 months and the presence of CTX-M E. coli on the farm (46). Therefore, an expanded Washington State study in which cephalosporin use is specifically targeted will be required to address this question locally. The United Kingdom study also found risk factors similar to ours, specifically an association between CTX-M E. coli and recent animal purchases or being an open herd. They also found disinfecting calf feeding equipment and rodent control to be protective against the presence of CTX-M E. coli, so low prevalence of CTX-M E. coli associated with reduced animal movements between farms, improving calf hygiene, and maintaining pest control were consistent findings of these two studies.
Between 2000 and 2010, third-generation cephalosporin resistance in E. coli and Salmonella enterica from food-producing animals in the United States was predominantly associated with plasmid-borne blaCMY-2 (44, 47, 48). In the current study, blaCMY-2 continued to be highly prevalent on most dairies (Table 2, Fig. 2). Although we identified management factors that were associated with lower CTX-M E. coli prevalence, we could not identify management changes to which we could attribute the emergence and dissemination of CTX-M E. coli strains between 2002 and 2011. A survey of antibiotic use in Washington dairies that was carried out in 2003 found that in 18% of herds, ceftiofur was the most commonly used antibiotic to treat calf respiratory disease (12). In the current study, in 3 of 30 herds (10.0% [95% CI, 2.6 to 27.7%]), ceftiofur was used for calf respiratory disease, suggesting that this use of ceftiofur was similar or even reduced since 2003. These data did not capture possible changes in dosing regimens or frequency of use, however, and the results of our antibiotic use survey indicate that ceftiofur was used for every indication listed. The use of multiple classes of antibiotics in calves may select for CTX-M E. coli strains carrying resistance determinants relevant to those other antibiotics.
Although differences in sampling and isolate selection between studies for the retrospective analyses represent a limitation of this study and we cannot rule out the presence of CTX-M E. coli in dairy cattle feces prior to 2008, we documented a significant increase in the prevalence of CTX-M E. coli between 2008 and 2011. Rapid dissemination of blaCTX-M genes may be in part attributable to their association with mobile elements (49, 50) and the use of specific antibiotics that select for CTX-M E. coli. Future characterization of blaCTX-M-associated mobile genetic elements will allow a high-resolution epidemiological investigation into patterns of dissemination.
We observed the recent emergence of CTX-M E. coli in dairies. This represents a rare opportunity to explore the determinants of population-level change in antibiotic resistance and to inform research efforts regarding the factors that affect resistance emergence and transmission. The temporal relationship between the emergence of Gram-negative human pathogens carrying blaCTX-M genes and the emergence of CTX-M E. coli in Washington dairies does not support the conventional hypothesis that cattle E. coli strains donate resistance genes to human bacterial populations in a unidirectional manner (37).
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by Agriculture and Food Research Initiative competitive grant no. 2010-51110-21131 from the USDA National Institute of Food and Agriculture.
We acknowledge Joshua B. Daniels, Thomas E. Wittum, and Dixie F. Mollenkopf, Ohio State University, for their generous intellectual contributions to this study. We also acknowledge Rian Calugcugan, Janie Clement, Neeraj Suthar, and Mariah Woodbury for help and support in the laboratory.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00463-15.
REFERENCES
- 1.Hawkey PM, Jones AM. 2009. The changing epidemiology of resistance. J Antimicrob Chemother 64(Suppl 1):i3–i10. doi: 10.1093/jac/dkp256. [DOI] [PubMed] [Google Scholar]
- 2.Canton R, Gonzalez-Alba JM, Galan JC. 2012. CTX-M enzymes: origin and diffusion. Front Microbiol 3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type beta-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Bush K. 2013. Proliferation and significance of clinically relevant beta-lactamases. Ann N Y Acad Sci 1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 5.Mulvey MR, Bryce E, Boyd D, Ofner-Agostini M, Christianson S, Simor AE, Paton S. 2004. Ambler class A extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. in Canadian hospitals. Antimicrob Agents Chemother 48:1204–1214. doi: 10.1128/AAC.48.4.1204-1214.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moland ES, Black JA, Hossain A, Hanson ND, Thomson KS, Pottumarthy S. 2003. Discovery of CTX-M-like extended-spectrum beta-lactamases in Escherichia coli isolates from five U.S. states. Antimicrob Agents Chemother 47:2382–2383. doi: 10.1128/AAC.47.7.2382-2383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castanheira M, Mendes RE, Rhomberg PR, Jones RN. 2008. Rapid emergence of blaCTX-M among Enterobacteriaceae in U.S. medical centers: molecular evaluation from the MYSTIC Program (2007). Microb Drug Resist 14:211–216. doi: 10.1089/mdr.2008.0827. [DOI] [PubMed] [Google Scholar]
- 8.Zhao WH, Hu ZQ. 2013. Epidemiology and genetics of CTX-M extended-spectrum beta-lactamases in Gram-negative bacteria. Crit Rev Microbiol 39:79–101. doi: 10.3109/1040841X.2012.691460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wittum TE, Mollenkopf DF, Daniels JB, Parkinson AE, Mathews JL, Fry PR, Abley MJ, Gebreyes WA. 2010. CTX-M-type extended-spectrum beta-lactamases present in Escherichia coli from the feces of cattle in Ohio, United States. Foodborne Pathog Dis 7:1575–1579. doi: 10.1089/fpd.2010.0615. [DOI] [PubMed] [Google Scholar]
- 10.Wittum TE, Mollenkopf DF, Erdman MM. 2012. Detection of Salmonella enterica isolates producing CTX-M cephalosporinase in U.S. livestock populations. Appl Environ Microbiol 78:7487–7491. doi: 10.1128/AEM.01682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mollenkopf DF, Mirecki JM, Daniels JB, Funk JA, Henry SC, Hansen GE, Davies PR, Donovan TS, Wittum TE. 2013. Escherichia coli and Klebsiella pneumoniae producing CTX-M cephalosporinase from swine finishing barns and their association with antimicrobial use. Appl Environ Microbiol 79:1052–1054. doi: 10.1128/AEM.03169-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raymond MJ, Wohrle RD, Call DR. 2006. Assessment and promotion of judicious antibiotic use on dairy farms in Washington State. J Dairy Sci 89:3228–3240. doi: 10.3168/jds.S0022-0302(06)72598-X. [DOI] [PubMed] [Google Scholar]
- 13.CDC. 2014. National antimicrobial resistance monitoring system for enteric bacteria (NARMS): human isolates final report, 2012. Department of Health and Human Services, CDC, Atlanta, GA. [Google Scholar]
- 14.Hiki M, Usui M, Kojima A, Ozawa M, Ishii Y, Asai T. 2013. Diversity of plasmid replicons encoding the blaCMY-2 gene in broad-spectrum cephalosporin-resistant Escherichia coli from livestock animals in Japan. Foodborne Pathog Dis 10:243–249. doi: 10.1089/fpd.2012.1306. [DOI] [PubMed] [Google Scholar]
- 15.Zhao S, White DG, McDermott PF, Friedman S, English L, Ayers S, Meng J, Maurer JJ, Holland R, Walker RD. 2001. Identification and expression of cephamycinase blaCMY genes in Escherichia coli and Salmonella isolates from food animals and ground meat. Antimicrob Agents Chemother 45:3647–3650. doi: 10.1128/AAC.45.12.3647-3650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Call DR, Matthews L, Subbiah M, Liu J. 2013. Do antibiotic residues in soils play a role in amplification and transmission of antibiotic resistant bacteria in cattle populations? Front Microbiol 4:193. doi: 10.3389/fmicb.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berge AC, Hancock DD, Sischo WM, Besser TE. 2010. Geographic, farm, and animal factors associated with multiple antimicrobial resistance in fecal Escherichia coli isolates from cattle in the western United States. J Am Vet Med Assoc 236:1338–1344. doi: 10.2460/javma.236.12.1338. [DOI] [PubMed] [Google Scholar]
- 18.Bej AK, DiCesare JL, Haff L, Atlas RM. 1991. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl Environ Microbiol 57:1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (CLSI). 2013. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standard—fourth edition. CLSI document VET01-A4 CLSI, Wayne, PA. [Google Scholar]
- 20.Bauer AW, Kirby WM, Sherris JC, Turck M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496. [PubMed] [Google Scholar]
- 21.Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L. 2003. Prevalence and molecular epidemiology of CTX-M extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob Agents Chemother 47:3724–3732. doi: 10.1128/AAC.47.12.3724-3732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollenkopf DF, Weeman MF, Daniels JB, Abley MJ, Mathews JL, Gebreyes WA, Wittum TE. 2012. Variable within- and between-herd diversity of CTX-M cephalosporinase-bearing Escherichia coli isolates from dairy cattle. Appl Environ Microbiol 78:4552–4560. doi: 10.1128/AEM.00373-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleiss JL, Levin B, Paik MC. 2003. Statistical methods for rates and proportions, 3rd ed John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- 24.Abramson JH. 2011. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov 8:1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Okubo T, Usui M, Yokota S, Izumiyama S, Tamura Y. 2014. Association of veterinary third-generation cephalosporin use with the risk of emergence of extended-spectrum-cephalosporin resistance in Escherichia coli from dairy cattle in Japan. PLoS One 9:e96101. doi: 10.1371/journal.pone.0096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottell JL, Kanwar N, Castillo-Courtade L, Chalmers G, Scott HM, Norby B, Loneragan GH, Boerlin P. 2013. blaCTX-M-32 on an IncN plasmid in Escherichia coli from beef cattle in the United States. Antimicrob Agents Chemother 57:1096–1097. doi: 10.1128/AAC.01750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kariuki S, Corkill JE, Revathi G, Musoke R, Hart CA. 2001. Molecular characterization of a novel plasmid-encoded cefotaximase (CTX-M-12) found in clinical Klebsiella pneumoniae isolates from Kenya. Antimicrob Agents Chemother 45:2141–2143. doi: 10.1128/AAC.45.7.2141-2143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnet R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother 48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kariuki S, Revathi G, Corkill J, Kiiru J, Mwituria J, Mirza N, Hart CA. 2007. Escherichia coli from community-acquired urinary tract infections resistant to fluoroquinolones and extended-spectrum beta-lactams. J Infect Dev Ctries 1:257–262. [PubMed] [Google Scholar]
- 30.Lewis JS II, Herrera M, Wickes B, Patterson JE, Jorgensen JH. 2007. First report of the emergence of CTX-M-type extended-spectrum beta-lactamases (ESBLs) as the predominant ESBL isolated in a U.S. health care system. Antimicrob Agents Chemother 51:4015–4021. doi: 10.1128/AAC.00576-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castanheira M, Farrell SE, Deshpande LM, Mendes RE, Jones RN. 2013. Prevalence of beta-lactamase-encoding genes among Enterobacteriaceae bacteremia isolates collected in 26 U.S. hospitals: report from the SENTRY Antimicrobial Surveillance Program (2010). Antimicrob Agents Chemother 57:3012–3020. doi: 10.1128/AAC.02252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissman SJ, Adler A, Qin X, Zerr DM. 2013. Emergence of extended-spectrum beta-lactam resistance among Escherichia coli at a U.S. academic children's hospital is clonal at the sequence type level for CTX-M-15, but not for CMY-2. Int J Antimicrob Agents 41:414–420. doi: 10.1016/j.ijantimicag.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson AD, Nelson JM, Rossiter S, Angulo FJ. 2003. Public health consequences of use of antimicrobial agents in food animals in the United States. Microb Drug Resist 9:373–379. doi: 10.1089/107662903322762815. [DOI] [PubMed] [Google Scholar]
- 34.Angulo FJ, Nunnery JA, Bair HD. 2004. Antimicrobial resistance in zoonotic enteric pathogens. Rev Sci Tech 23:485–496. [DOI] [PubMed] [Google Scholar]
- 35.Hammerum AM, Heuer OE. 2009. Human health hazards from antimicrobial-resistant Escherichia coli of animal origin. Clin Infect Dis 48:916–921. doi: 10.1086/597292. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor AM, Poppe C, McEwen SA. 2002. Changes in the prevalence of resistant Escherichia coli in cattle receiving subcutaneously injectable oxytetracycline in addition to in-feed chlortetracycline compared with cattle receiving only in-feed chlortetracycline. Can J Vet Res 66:145–150. [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall BM, Levy SB. 2011. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aslam M, Diarra MS, Service C, Rempel H. 2009. Antimicrobial resistance genes in Escherichia coli isolates recovered from a commercial beef processing plant. J Food Prot 72:1089–1093. [DOI] [PubMed] [Google Scholar]
- 39.Karczmarczyk M, Walsh C, Slowey R, Leonard N, Fanning S. 2011. Molecular characterization of multidrug-resistant Escherichia coli isolates from Irish cattle farms. Appl Environ Microbiol 77:7121–7127. doi: 10.1128/AEM.00601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan RS, Sit TH, Wong SS, Wong RC, Chow KH, Mak GC, Yam WC, Ng LT, Yuen KY, Ho PL. 2006. Escherichia coli producing CTX-M beta-lactamases in food animals in Hong Kong. Microb Drug Resist 12:145–148. doi: 10.1089/mdr.2006.12.145. [DOI] [PubMed] [Google Scholar]
- 41.Wichmann F, Udikovic-Kolic N, Andrew S, Handelsman J. 2014. Diverse antibiotic resistance genes in dairy cow manure. mBio 5(2):e01017. doi: 10.1128/mBio.01017-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erskine RJ, Bartlett PC, Byrem TM, Render CL, Febvay C, Houseman JT. 2012. Herd-level determinants of bovine leukaemia virus prevalence in dairy farms. J Dairy Res 79:445–450. doi: 10.1017/S0022029912000520. [DOI] [PubMed] [Google Scholar]
- 43.Piepers S, Peeters K, Opsomer G, Barkema HW, Frankena K, De Vliegher S. 2011. Pathogen group specific risk factors at herd, heifer and quarter levels for intramammary infections in early lactating dairy heifers. Prev Vet Med 99:91–101. doi: 10.1016/j.prevetmed.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Daniels JB, Call DR, Hancock D, Sischo WM, Baker K, Besser TE. 2009. Role of ceftiofur in selection and dissemination of blaCMY-2-mediated cephalosporin resistance in Salmonella enterica and commensal Escherichia coli isolates from cattle. Appl Environ Microbiol 75:3648–3655. doi: 10.1128/AEM.02435-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heider LC, Funk JA, Hoet AE, Meiring RW, Gebreyes WA, Wittum TE. 2009. Identification of Escherichia coli and Salmonella enterica organisms with reduced susceptibility to ceftriaxone from fecal samples of cows in dairy herds. Am J Vet Res 70:389–393. doi: 10.2460/ajvr.70.3.389. [DOI] [PubMed] [Google Scholar]
- 46.Snow LC, Warner RG, Cheney T, Wearing H, Stokes M, Harris K, Teale CJ, Coldham NG. 2012. Risk factors associated with extended spectrum beta-lactamase Escherichia coli (CTX-M) on dairy farms in North West England and North Wales. Prev Vet Med 106:225–234. doi: 10.1016/j.prevetmed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Daniels JB, Call DR, Besser TE. 2007. Molecular epidemiology of blaCMY-2 plasmids carried by Salmonella enterica and Escherichia coli isolates from cattle in the Pacific Northwest. Appl Environ Microbiol 73:8005–8011. doi: 10.1128/AEM.01325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cobbold RN, Rice DH, Davis MA, Besser TE, Hancock DD. 2006. Long-term persistence of multi-drug-resistant Salmonella enterica serovar Newport in two dairy herds. J Am Vet Med Assoc 228:585–591. doi: 10.2460/javma.228.4.585. [DOI] [PubMed] [Google Scholar]
- 49.Poirel L, Lartigue MF, Decousser JW, Nordmann P. 2005. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob Agents Chemother 49:447–450. doi: 10.1128/AAC.49.1.447-450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng H, Zeng Z, Chen S, Liu Y, Yao Q, Deng Y, Chen X, Lv L, Zhuo C, Chen Z, Liu JH. 2012. Prevalence and characterisation of CTX-M beta-lactamases amongst Escherichia coli isolates from healthy food animals in China. Int J Antimicrob Agents 39:305–310. doi: 10.1016/j.ijantimicag.2011.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.