Abstract
Bacteria such as Escherichia coli are frequently grown to high density to produce biomolecules for study in the laboratory. To achieve this, cells can be incubated in extremely rich media that increase overall cell yield. In these various media, bacteria may have different metabolic profiles, leading to changes in the amounts of toxic metabolites produced. We have previously shown that stresses experienced during short-term growth can affect the survival of cells during the long-term stationary phase (LTSP). Here, we incubated cells in LB, 2× yeast extract-tryptone (YT), Terrific Broth, or Super Broth medium and monitored survival during the LTSP, as well as other reporters of genetic and physiological change. We observe differential cell yield and survival in all media studied. We propose that differences in long-term survival are the result of changes in the metabolism of components of the media that may lead to increased levels of protein and/or DNA damage. We also show that culture pH and levels of protein glycation, a covalent modification that causes protein damage, affect long-term survival. Further, we measured mutation frequency after overnight incubation and observed a correlation between high mutation frequencies at the end of the log phase and loss of viability after 4 days of LTSP incubation, indicating that mutation frequency is potentially predictive of long-term survival. Since glycation and mutation can be caused by oxidative stress, we measured expression of the oxyR oxidative stress regulator during log-phase growth and found that higher levels of oxyR expression during the log phase are consistent with high mutation frequency and lower cell density during the LTSP. Since these complex rich media are often used when producing large quantities of biomolecules in the laboratory, the observed increase in damage resulting in glycation or mutation may lead to production of a heterogeneous population of plasmids or proteins, which could affect the quality of the end products yielded in some laboratory experiments.
INTRODUCTION
Escherichia coli is often used both as a model organism to understand fundamental biological process and as a tool to produce biomolecules, including plasmids and proteins. While many of these applications require relatively short periods of incubation, the E. coli life cycle in batch culture in the laboratory can last for extended periods and include five phases. During the first phase after inoculation into fresh medium, termed the “lag phase,” cells are adjusting their metabolism to the new, nutrient-rich environment, with little cell division. During the second phase, called the “exponential phase” or “logarithmic phase,” cells are replicating at a relatively rapid and constant rate, which can be as fast as 1 cell division every 20 min for many E. coli strains (1). This is followed by the “stationary phase,” where cell density ceases to increase, most likely due to some combination of a reduction in available nutrients, a buildup of metabolic wastes or toxins, and/or signals to cease growth. After the stationary phase, cells can enter the fourth phase, termed the “death phase,” where at least 99% of the cells in the culture lose viability, depending on the strain, growth medium, and other culture conditions (2–4). Cells that survive the death phase may then enter the “long-term stationary phase” (LTSP), where the total number of cells remains roughly constant, although the cells are in a dynamic equilibrium where significant shifts in subpopulation density can occur (3, 5). Survival of cells better suited to the culture conditions of the LTSP leads to selection of mutants with beneficial alleles and subsequent adaptive evolution (6–9), including expression of the growth advantage in stationary phase (GASP) phenotype (3, 5–9).
Although the mechanisms controlling the transition into the LTSP are not well understood, several physiological outputs often correspond with the viability of cells in batch culture, including changes in the extent of glycation, culture pH, and mutation frequency. Glycation, or nonenzymatic glycosylation, can lead to damage of proteins and nucleic acids in cells through covalent modification of terminal amines on proteins and N2 of guanine bases (10–13), leading to cross-linking of proteins and/or DNA and loss of function of those molecules. An increase in glycation is associated with increased oxidative stress levels; therefore, conditions that lead to high levels of oxidative stress also lead to higher levels of glycation. It has been demonstrated that glycation can lead to cell death in E. coli (10–12), and when glycation levels are reduced, for example, by the addition of antiglycation agents, such as carnosine or aminoguanidine, cell death also decreases (10). Another factor modulating the survival of cells in batch culture is pH (14–17). When E. coli is grown aerobically in Luria-Bertani broth (LB), cultures become more alkaline during the log phase due to metabolism of amino acids, with further increases in pH during the stationary phase. This increase in pH contributes to cell death and affects the timing of entry into the long-term stationary phase (17–19). We have also observed differences in spontaneous mutation frequency in LB batch culture dependent on culture volume (2). While it is clear that glycation, culture pH, and mutation frequency contribute to death phase and survival in the LTSP, the specific relationships between these phenotypes are not well understood. However, it is clear that the composition of the culture medium plays an important role.
A wide variety of different culture media are used to grow cells, depending on the requirements of a particular experiment or application. Although LB is used commonly for many laboratory experiments, other rich media may be used to increase protein, plasmid, and/or cell yield (20). The 2× yeast extract-tryptone (YT) medium was specifically developed for propagation of M13 bacteriophages (21, 22). The increased amounts of yeast extract and tryptone compared to LB provide additional nutrient and growth factors so that the bacteriophage can multiply rapidly without depleting host cell resources (21, 22). Terrific Broth (TB) was developed in 1987 to increase plasmid yield by extending the exponential phase of E. coli (23). Super Broth (SB), which replaces tryptone with soy hydrolysate, was developed to avoid the use of the cow's milk protein casein, due to concerns of contamination of biomolecules with the bovine spongiform encephalopathy agent (BD Bionutrients Technical Manual; http://www.bd.com/ds/technicalcenter/misc/br_3_2547.pdf). The differences in compositions of these media likely affect cellular physiology, including oxidative stress due to higher nutrient availability, which results in changes in long-term survival patterns, mutation frequency, and ultimately adaptation, relative to growth in standard LB. Here, we incubated cells in all four rich media and monitored long-term survival and other physiological outputs. We determined that medium composition affects the stresses that cells experience during the exponential and early stationary phases, which results in differences in survival during LTSP. Further, these differential survival responses are affected by the type of vessel in which cells are incubated. In fact, even seemingly small changes in medium composition can result in drastic changes to the physiology of cells, indicating that bacteria can be exquisitely sensitive to minor perturbations in otherwise very similar environments.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli K-12 lineage strain PFM2 (24), derived from MG1655, was used in this study. Unless otherwise stated, cultures were initiated by transferring cells directly from a frozen 20% glycerol stock into 5 ml of Luria-Bertani (Lennox) medium (LB) (Difco) in 18- by 150-mm borosilicate test tubes and were incubated overnight with aeration in a TC-7 rolling drum (New Brunswick Scientific, Edison, NJ) at 37°C. Cells were then inoculated into either LB, 2× yeast extract-tryptone (YT), Terrific Broth (TB), or Super Broth (SB) at 1:1,000 (vol/vol) to initiate experiments. All media were obtained from BD Biosciences, and the composition of each is shown in Table 1, as well as the calculated total carbohydrate and total amino acid concentrations. Ca2+ and Mg2+ cation concentrations were also calculated and ranged from 3.2 to 6.1 mg/liter and 5.7 to 39.0 mg/liter, respectively. Cultures were incubated in either 5 ml in test tubes, as described above, or in 12.5 ml (10% volume) in 125-ml long-neck Erlenmeyer flasks (Kimble Chase) on a shaking platform (200 rpm) at 37°C. For inoculation of cultures into multiple vessels, test tubes, and/or flasks, one large volume of medium was inoculated and then distributed into individual culture vessels at the appropriate final volumes.
TABLE 1.
Components of the culture media used in this study
| Medium | Amt of component |
|||||||
|---|---|---|---|---|---|---|---|---|
| Yeast extract (%) | Tryptone (%) | Soytone (%) | NaCl (%) | KH2PO4/K2HPO4 (mM) | Glycerol (%) | Total amino nitrogen (%) | Total carbohydrate (%) | |
| LB | 0.5 | 1.0 | 0.5 | 0.9 | 0.1 | |||
| YT | 1.0 | 1.6 | 0.5 | 1.4 | 0.2 | |||
| TB | 2.4 | 1.2 | 90 | 0.4 | 3.4 | 0.4 | ||
| SB | 2.4 | 1.2 | 90 | 0.5 | 1.9 | 0.8 | ||
Monitoring cell growth, survival, culture pH, and mutation frequency.
To monitor cell growth and survival, viable cell counts were determined by serial dilution of cells sampled periodically from the cultures, followed by plating on LB agar (2, 25). The limit of detection in all experiments was >1,000 CFU/ml (25). At each time point, 10 μl (equivalent to 0.02% culture volume) was removed for viable count determination. pH was monitored using pH paper with a range of 6.0 to 10.0 and increments of 0.3 to 0.5 pH unit or with a range of 2.0 to 9.0 and increments of 0.5 pH unit (EMD Chemicals, La Jolla, CA).
Mutation frequency, as monitored by the spontaneous appearance of colonies showing resistance to the antibiotic rifampin (Rifr), was determined by plating ∼109 cells on LB agar supplemented with 100 μg/ml rifampin (Sigma-Aldrich) (26). To determine mutation frequency, the number of Rifr CFU per milliliter observed after 1 day of incubation was divided by the total number of CFU per milliliter for each culture. Significant differences in the distribution of the mutation frequencies for each sample were determined using the nonparametric Kolmogorov-Smirnov (K-S) test (http://www.physics.csbsju.edu/stats/KS-test.html).
Isolation of E. coli protein for glycation analysis.
Whole-cell protein was isolated as previously described (2, 10). Briefly, 5-ml cultures of PFM2, grown in different media, were incubated for 1 to 3 days. At each time point, cultures were sacrificed, and 2 ml from each tube was processed for protein isolation. Cells were pelleted by centrifugation at 13,000 rpm for 1 min, followed by resuspension in 10 mM Tris-HCl (pH 7.5) and 0.15 M NaCl. Cells were then sonicated on ice, twice at the maximum setting at 1-min intervals (Bronson Scientific), followed by digestion with DNase I (50 μg/ml), RNase A (50 μg/ml), and lysozyme (20 μg/ml) (Sigma-Aldrich) at 37°C for 1 h. Protein levels in whole-cell lysates were quantified using the Quick Start Bradford dye reagent, as per the manufacturer's instructions (Bio-Rad).
ELISA to determine glycation levels.
Enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (2). Briefly, 50 μl of whole-cell lysate (protein concentration, 0.5 to 1.0 mg/ml) were loaded into standard polystyrene 96-well plates (Corning, Inc.) with 100 μl sodium carbonate binding buffer and incubated overnight at 4°C. After 16 to 20 h, the wells were washed twice with phosphate-buffered saline (PBS)–Tween 20 (0.05%), blocked with 200 μl of blocking solution (5% bovine serum albumin in PBS) for 1 h at 37°C, washed again three times in PBS–Tween 20, and incubated (1 h at 37°C) with 100 μl of horseradish peroxidase (HRP)-conjugated anti-carboxymethyl lysine (anti-CML) antibody (diluted 1:1,000 [vol/vol] in blocking solution) obtained from Cosmobio (Tokyo, Japan). After three additional washes (PBS–Tween 20) to remove excess antibody, 100 μl of color reagent (G Biosciences) was added, and the mixture was incubated for 10 min at room temperature. The color developed in the wells was quantified at 630 nm using a plate reader (BioTek ELx808), as specified by the manufacturer.
β-Galactosidase assay to measure oxyR expression.
Strain SF2562, which is PFM2/pAQ23 (plasmid pAQ23 expresses lacZ from the E. coli oxyR promoter [PoxyR]) (2, 27), was grown in medium supplemented with ampicillin, as appropriate, in 5-ml cultures in test tubes as described above. Cultures were sacrificed at 2, 4, or 24 h to measure β-galactosidase activity as described previously (2, 21). Modified Miller units were calculated based on the cell titer (CFU per milliliter) of each culture, rather than the optical density at 600 nm (OD600), since the OD600 does not fully reflect the viable cell counts of cells grown in the different media. Endogenous activity of LacZ in PFM2 was not detected (data not shown).
RESULTS
Long-term growth and survival dynamics vary in different rich growth media.
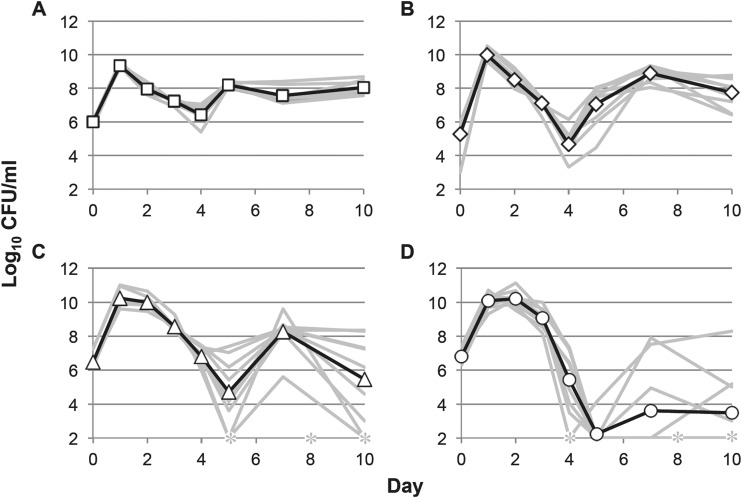
We have observed that E. coli K-12 strains can have very different survival profiles when grown in different vessels under standard laboratory conditions, including batch culture in test tubes and flasks (2). However, these long-term growth experiments were only performed in LB. Here we compare growth and long-term survival in four different rich media: LB, 2× yeast extract-tryptone (YT), Terrific Broth (TB), and Super Broth (SB) (see Table 1 for composition). In test tubes, the cell densities reached in overnight culture and the observed long-term survival dynamics vary greatly in the four different culture media (Fig. 1). Overnight cell densities are as much as 10-fold higher in YT, TB, and SB (the “richer” media) than in LB. These initial cell yield differences do not correspond with the timing of entry into the death phase or other long-term survival parameters, such as final cell yield or severity of the death phase; the viable counts of cultures at their peak and lowest culture densities are shown in Table 2. While cells incubated in LB and YT enter the death phase before the second day of incubation, cells in TB and SB remain in the stationary phase until the third day of incubation. Cells in LB reach their lowest cell density on the fourth day of incubation, at ∼106 to 107 CFU/ml, while the viable counts of cells in YT at this time are ∼100-fold lower at ∼104 CFU/ml. However, cells cultured in both LB and YT recover by the fifth day of incubation, with viable counts stabilized at ∼107 to 108 CFU/ml for the remainder of the experiment. Cells cultured in TB have an intermediate phenotype: after their delayed entry into the death phase, they reach their lowest cell density on the fifth day of incubation, at viable counts of ∼104 to 106 CFU/ml, and then, similar to LB- and YT-grown cells, recover to ∼107 to 108 CFU/ml by day 7, where they remain for the remainder of the experiment. Unlike cells cultured in the other media, upon entry into death phase on the third day of incubation, cell yields of SB cultures go below the limit of detection, and ∼50% of those cultures never recover, indicating a significant loss of viability in this medium. In those cultures that do recover, viable counts can approach those of other media.
FIG 1.
Growth and survival in rich media in test tubes. Viable counts of cells incubated in test tubes are shown. (A) LB (open squares); (B) YT (open diamonds); (C) TB (open triangles); (D) SB (open circles). Average values are shown in black, and data from all 9 replicates are shown in gray. Asterisks indicate cell densities below the limit of detection (<1,000 CFU/ml).
TABLE 2.
Timing of average peak and trough viable counts for E. coli cultures grown in each culture medium and vessel
| Medium and vessel | Peak daya | CFU/ml on peak day | Trough dayb | CFU/ml on trough day | % of cultures with viable counts below LODc at trough day |
|---|---|---|---|---|---|
| LB | |||||
| Tube | 1 | 2.2 × 109 | 4 | 2.5 × 106 | 0 |
| Flask | 1 | 1.6 × 109 | 3.4 | 2.4 × 105 | 0 |
| YT | |||||
| Tube | 1 | 9.3 × 109 | 4 | 4.6 × 104 | 0 |
| Flask | 1 | 1.6 × 1010 | 4 | 6.2 × 101 | 67 |
| TB | |||||
| Tube | 1–2 | 1.7 × 1010 | 4.9 | 8.3 × 104 | 22 |
| Flask | 1 | 1.6 × 1010 | 7.1 | 8.3 × 105 | 11 |
| SB | |||||
| Tube | 1–2 | 1.6 × 1010 | 4.8 | 2.6 × 101 | 89 |
| Flask | 1 | 3.3 × 1010 | 4 | <LOD | 100 |
Day of highest average viable cell count.
Day of lowest average viable cell count.
LOD, limit of detection.
Differential effect of culture vessel on survival in various rich media.
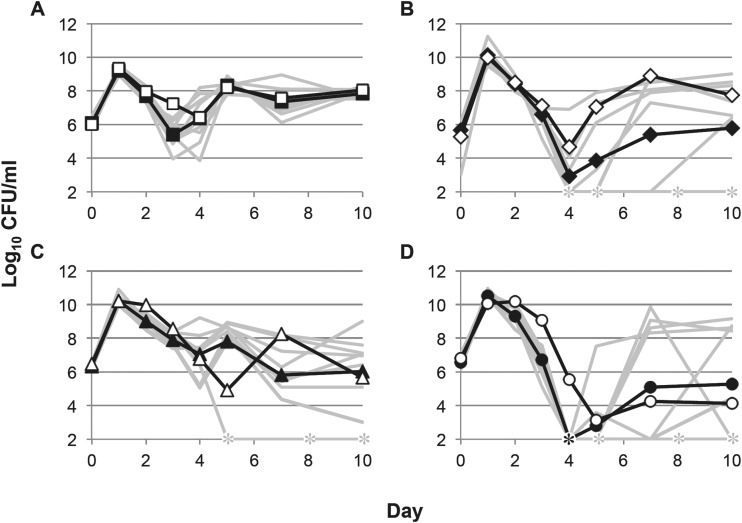
We have previously shown that the type of culture vessel—test tubes versus flasks—can affect long-term survival and that this effect is partly dependent on oxygen availability (2). Here, we determined how vessel type affects growth in the four rich media, including determination of the timing or severity of the “trough point,” the time the lowest cell density is reached in the culture. Cells cultured in flasks experience either a lower trough point (YT), an earlier trough point (SB), or both (LB) compared to cells grown in test tubes, except in TB (Fig. 2). The timing and viable counts of cultures at their peak and lowest densities are shown in Table 2. At their lowest, flask culture viable counts in LB and YT are more than 100-fold lower than those in test tubes on the third day of incubation in LB (Fig. 1A) and the fourth day of incubation in YT (Fig. 2B). Viable counts in YT cultures dip below the limit of detection until recovery on the fifth day of incubation; however, ∼10% of cultures never recover from this severe death phase.
FIG 2.
Growth and survival in rich media in flasks. Viable counts of cells incubated in flasks are shown. (A) LB (solid squares); (B) YT (solid diamonds); (C) TB (solid triangles); (D) SB (solid circles). All flask replicates are shown in gray, with average data from all 9 samples shown in black. For reference, the average values of cells incubated in test tubes from Fig. 1 are shown by the open symbols. Asterisks indicate cell densities below the limit of detection (<1,000 CFU/ml).
In both SB and TB cultures, the delay of entry into the death phase observed in test tubes does not occur when cells are incubated in flasks. In flask cultures, cells enter the death phase after day 1, with cell yields ∼10-fold and ∼100-fold lower than those in test tube cultures, respectively (Fig. 2C and D). Though cells in TB cultures enter the death phase sooner in flasks, it is less severe than when cells are grown in test tubes, with only an ∼50-fold reduction in viable counts compared to flasks by the fourth day of incubation. However, in about three-quarters of the flasks, there is a second death phase after the fifth day of incubation (Fig. 2C). In SB cultures, the viable counts in all of the flasks decrease below the limit of detection by the fourth day of incubation compared to day 5 in test tubes (Fig. 2D). Similar to test tube growth, about 50% of flask cultures do not recover after the death phase.
Differential effect of culture vessel on pH in various rich media.
We monitored pH changes in cultures grown in different media and vessels. While alkalization occurs before the second day of incubation in LB and YT test tube cultures, alkalization in TB and SB tube cultures is delayed until the third day of incubation (Fig. 3). This is due to at least two factors: the presence of glycerol as a carbon source in TB and SB, whose metabolism releases acid into the environment, and the presence of potassium phosphate, which naturally buffers these media. Since the alkalization of bacterial cultures is frequently associated with entry into the death phase (17, 18, 28), the delay of alkalization of TB and SB test tube cultures likely delays entry into the death phase. However, in TB and SB flask cultures, there is less of a delay in alkalization, which leads to an earlier entry into death phase than in test tube cultures of either medium.
FIG 3.
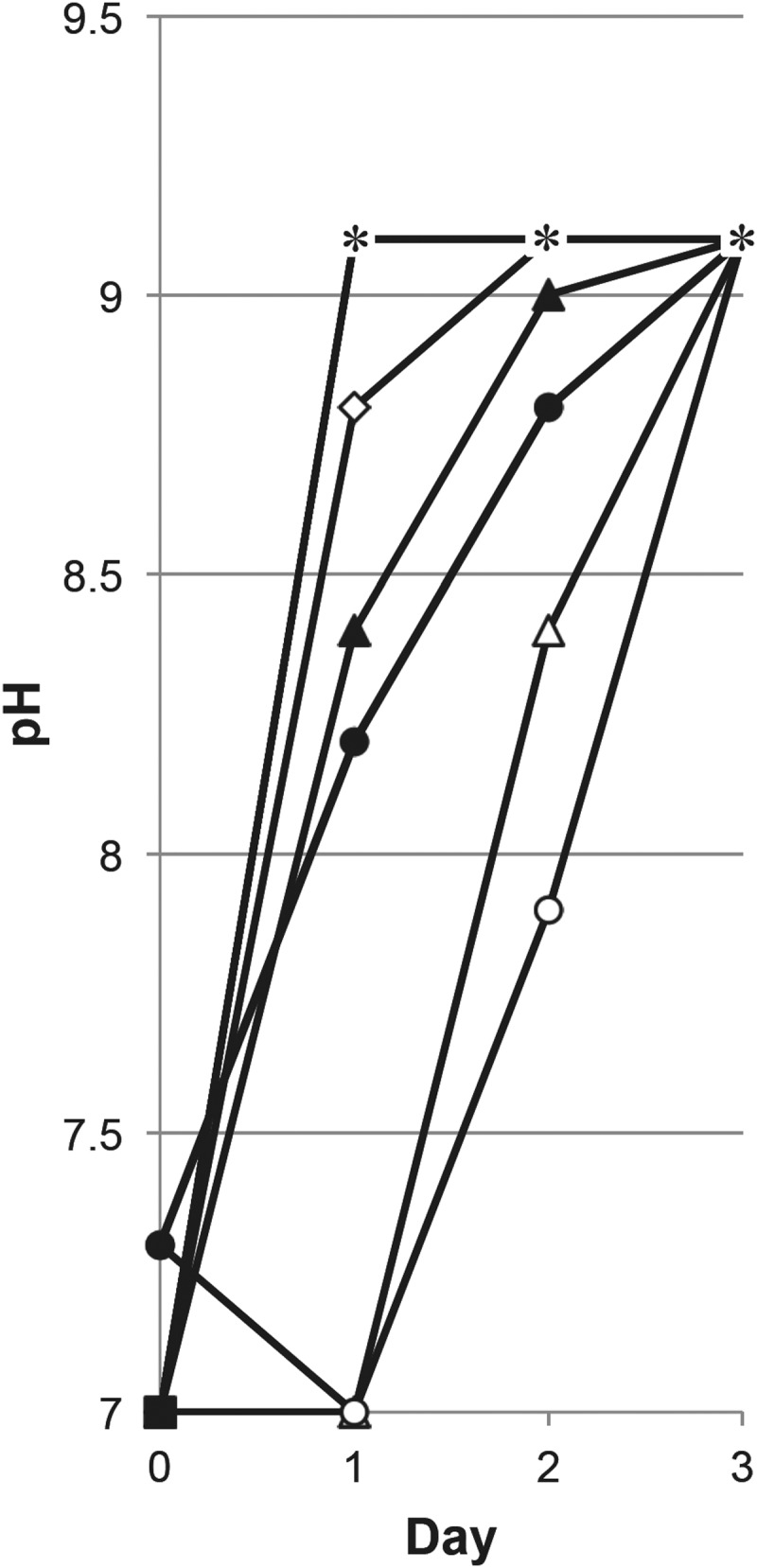
pH of cultures in rich media in different vessels. pH values of cells incubated in test tubes (open symbols) or flasks (closed symbols) in LB (squares), YT (diamonds), TB (triangles), or SB (circles) are shown. The asterisks correspond to multiple cultures that have reached pH 9.1. For example, on day 1, LB flasks, LB tubes, and YT flasks have all reached pH 9.1.
Presence of glycerol affects long-term survival.
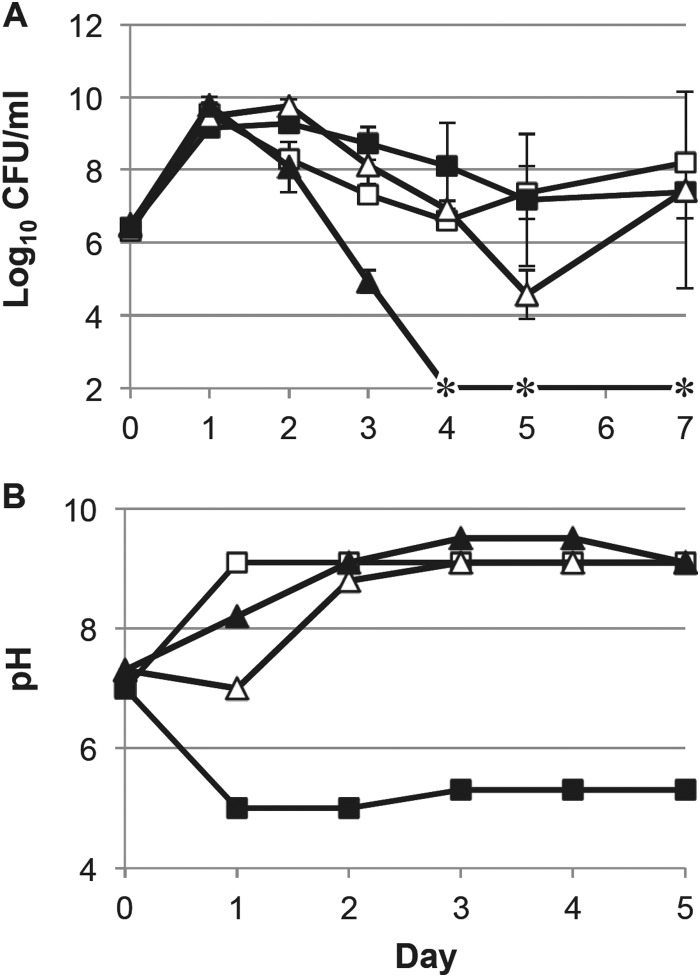
There are several significant differences in the components of the rich media studied here. TB and SB contain glycerol, which can be used by the cells as a carbohydrate source, while LB and YT do not have additional carbohydrate, relying almost entirely on amino acids as the source of carbon and energy. To determine whether the presence of glycerol affects long-term survival, we either added glycerol to standard LB [LB(+glycerol)] or prepared TB without glycerol [TB(−glycerol)] and monitored survival in test tube cultures compared to that with the standard composition.
When cells are incubated in LB(+glycerol), entry into the death phase is significantly delayed (Fig. 4A). On the second day of incubation, viable cell counts are ∼10-fold higher than in standard LB, ∼100-fold higher on the third day, and ∼500-fold higher on the fourth day. In contrast, when cells are incubated in TB(−glycerol), viable counts decrease by at least 100-fold on the second day of incubation compared to cells in standard TB and continue to plummet through the fourth day of incubation, when viable counts drop below the limit of detection and never recover (Fig. 4A).
FIG 4.
Effect of glycerol on survival in LB and TB. (A) Viable counts of cells incubated in test tubes in LB (open squares), LB(+glycerol) (solid squares), TB (open triangles), and TB(−glycerol) (solid triangles). Averages from 6 replicates are shown. Error bars represent standard deviations. (B) pH of the same cultures shown in panel A. Asterisks indicate cell densities below the limit of detection (<1,000 CFU/ml).
When we examine the pH in these cultures, the effect of the presence or absence of glycerol is dramatic (Fig. 4B). In LB(+glycerol), instead of alkalizing to pH 9.1 as in standard LB, the pH decreases to 5.0 and remains at 5.0 to 5.3 for the remainder of the experiment. This is likely due to the metabolism of glycerol, during which E. coli produces acid, primarily as acetate (29). TB(−glycerol) cultures alkalize to pH 8.3 overnight, which is higher than in standard TB cultures, which remain at pH 7.0 during overnight incubation. The presence of glycerol appears to significantly delay alkalization in standard TB cultures. However, TB(−glycerol) cultures still have a delay in alkalization compared to cells grown in LB; LB cultures reach pH 9.1 during overnight growth, while TB(−glycerol) cultures do not reach pH 9.1 until the second day of incubation. This is most likely due to the presence of the potassium phosphate acting as a pH buffer. We have previously shown that pH likely affects timing of entry into the death phase and not necessarily survival in the LTSP (2), so it is unlikely that the earlier increase in pH in TB(−glycerol) cultures is the cause of severe death.
Glycation levels vary in each medium but increase during long-term incubation.
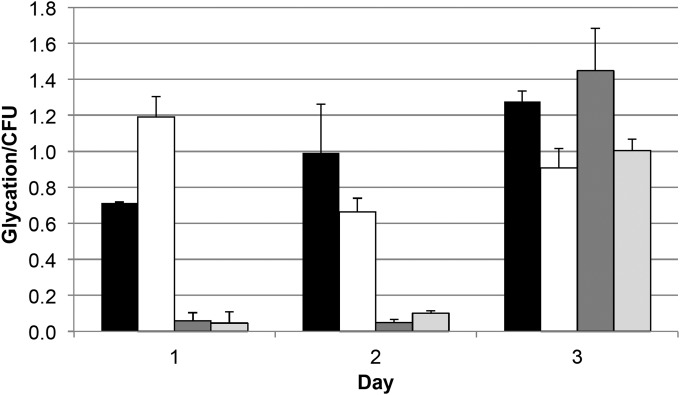
It has previously been shown that advanced glycation end products (AGEs) accumulate during the LTSP (2, 10–13). In the presence of increasing concentrations of sugar (i.e., glucose), both the accumulation of AGEs and the severity of the death phase increase (10). To determine how glycation is affected by growth in different rich media, we quantified the amount of carboxymethyl lysine (CML), a common glycation end product, in cells cultured in LB, YT, TB, or SB, using an ELISA with an anti-CML antibody.
After overnight incubation, cells cultured in YT have the highest glycation level (Fig. 5), ∼2-fold higher than that in cells incubated in LB. This corresponds with the more severe death phase seen in YT cultures (Fig. 2). Glycation levels in TB and SB are relatively low after overnight growth through the second day of incubation, but by day 3, glycation levels increase ∼10-fold (Fig. 5). Because pH does not increase as quickly in TB and SB cultures as it does for cells incubated in LB and YT, we determined if changes in pH are associated with changes in glycation levels. We observe that when the culture pH is below 8.5, glycation levels are low. However, when the pH rises above 8.5, glycation levels increase significantly.
FIG 5.
Glycation levels in rich media. Shown is glycation per milligram of protein per CFU of total isolated protein from cells cultured in LB (black bars), YT (white bars), TB (dark gray bars), and SB (light gray bars). Data are the averages from two biological replicates; error bars represent standard deviations.
Growth in different rich media affects mutation frequency.
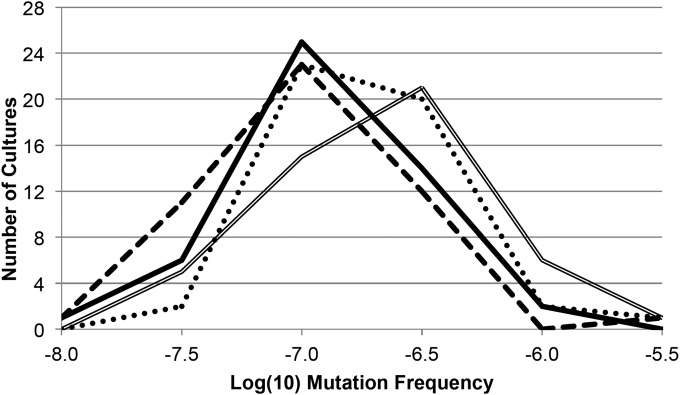
The observation that culture medium affects survival and glycation, a response to reactive oxidative species, led us to determine if mutation frequency was also affected by rich medium composition. Using spontaneous rifampin resistance as a reporter, we observed medium-specific differences in mutation frequency distributions (Fig. 6). While cells incubated in LB and TB have similar mutation frequency median values (6.55 × 10−8 and 6.83 × 10−8 mutants/CFU), the distribution of mutation frequencies for LB-cultured cells overall skews lower than in TB culture. Cells incubated in YT have a median mutation frequency (9.24 × 10−8 mutants/CFU) that is higher than that of cells incubated in LB and TB, and cells in SB have the highest median mutation frequency at 1.27 × 10−7 mutants/CFU. When the mutation frequency distributions are compared using the K-S test (see Materials and Methods), the YT and SB frequency distributions show a significant difference compared to those observed for LB and TB cultures (P = 0.026 and 0.007, respectively); the distribution of mutation frequencies between cells cultured in TB and SB are also significantly different (P = 0.003), even though the compositions of those culture media are quite similar.
FIG 6.
Distribution of spontaneous rifampin resistance mutation frequencies in rich media. Histogram of spontaneous mutation frequencies measured for 48 independent cultures grown in each of the four different media. Mutation frequency values are binned in increments of 0.5 log10 mutation frequency unit. LB, solid line; YT, dotted line; TB, dashed line; and SB, double line.
The median values of mutation frequency (Fig. 6) for cells cultured in each medium are inversely related to the viable cell counts observed on the fourth day of incubation (Fig. 1), with cells in LB and TB having the highest viable counts and lowest mutation frequencies, while cells cultured in YT have intermediate values of both, and SB cultures show the lowest viable counts and highest mutation frequencies. In other words, the mutation frequency observed on day 1 is predictive of the number of surviving cells in culture on day 4.
Growth in different rich media affects oxyR expression.
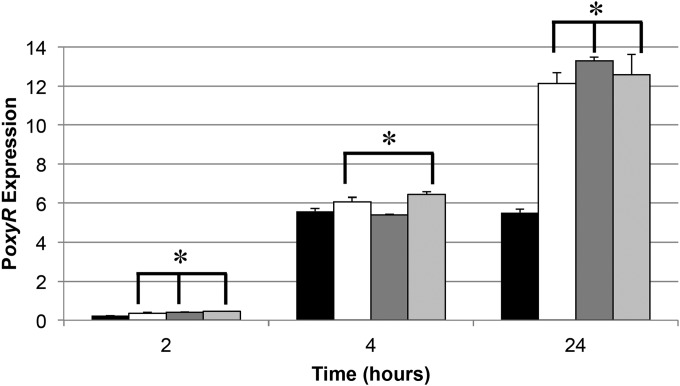
Because both glycation and mutation frequency can be affected by oxidative stress, we directly monitored oxyR expression using a transcriptional fusion lacZ reporter plasmid (pAQ23) (2, 27). OxyR is a transcriptional activator that is expressed upon detection of oxidative stress (27). β-Galactosidase levels were measured for cultures of strain SF2562 incubated in different media during the early log, mid-log, and stationary-phase incubations (Fig. 7). After 2 h of incubation, PoxyR expression is lowest for cultures incubated in LB, ∼50% higher in cells incubated in YT, ∼80% higher in cells incubated in TB, and more than 100% greater in cells incubated in SB. These data indicate that even very early on, cells in different media are experiencing various levels of oxidative stress.
FIG 7.
Expression of PoxyR in different media. β-Galactosidase assays were performed on PFM2/pAQ23 (SF2562) during incubation in LB (black bars), YT (white bars), TB (dark gray bars), or SB (light gray bars). Bars represent average modified Miller units (see Materials and Methods) of triplicate samples. Error bars represent standard deviations, and asterisks represent significant (P < 0.05) difference from LB cultures at the same time point.
After 4 h of incubation, during the mid-log phase, expression from PoxyR increases 13- to 24-fold in all four media. At this time, compared to LB and TB cultures, cultures in YT and SB show ∼10% and ∼15% greater expression, respectively. Similar to mutation frequency, increases in oxyR expression (Fig. 7) during the mid-log phase are associated with decreasing survival during the LTSP (Fig. 1).
After 24 h of incubation in LB, expression from PoxyR has not increased beyond the level observed at 4 h; however, expression in cells grown in YT, TB, and SB more than doubled, indicating that cells in these media are experiencing higher levels of oxidative stress, which may be related to their more severe death phase during the LTSP.
DISCUSSION
In the laboratory, cells are often cultured in various rich media. We examined long-term survival and physiological responses to incubation in frequently used rich media and found that long-term survival, glycation, mutation frequency, and oxidative stress level were affected by incubation in these different media. The media used can be grouped based on three sets of criteria: (i) overall nutrient level since YT, TB, and SB all have significantly higher levels of protein than LB; (ii) salt content and composition since LB and YT contain NaCl, while TB and SB contain no NaCl and instead have potassium phosphate salts, which act as a mild buffer; and (iii) additional carbon sources since LB and YT contain no additional carbohydrate, providing protein as the primary carbon source, while TB and SB also contain glycerol, which cells use as a carbon source.
Comparisons between the more similar media may help to identify specific factors that lead to the physiological responses of E. coli. The death phase in YT is much more severe than in LB, possibly reflecting an effect of the increased abundance of protein, which is the main difference in the compositions of these two media (Table 1). When comparing survival rates of cells cultured in TB and SB, the death phase is most severe for cells incubated in SB, where the viable counts in >80% of both test tube and flask cultures drop below the limit of detection, frequently not recovering. In contrast, viable cell counts in TB culture only drop below the limit of detection in about ∼10% of test tube and flask cultures. This is surprising because the components of TB and SB are very similar (Table 1). Both have high levels of protein sources and contain glycerol and potassium phosphate salts. The main difference between TB and SB is the protein source: TB contains tryptone, while SB contains soytone. Tryptone is a hydrolysate of casein (from cow's milk), while soytone is a hydrolysate of soy protein. According to the chemical analyses of these two protein sources (BD Bionutrients Technical Manual; http://www.bd.com/ds/technicalcenter/misc/br_3_2547.pdf), there is also a significant difference in the amount of total carbohydrate in each hydrolysate (Table 1). Tryptone has 4.3 mg/g carbohydrate, while soytone contains ∼80 times more at 336.2 mg/g. As shown here with respect to the presence or absence of glycerol, the amount of carbohydrate in the medium can affect survival, and the large amount of carbohydrate in soytone versus tryptone may contribute to both the initial high cell yield during the first 2 days of incubation and the sharp decrease in viable counts that occurs after the fourth day of incubation in SB.
We show that culture pH varies in different media during exponential-phase growth, and this likely contributes to the different survival dynamics observed in the LTSP. Bacterial cells normally catabolize the amino acids in rich media as a source of carbon and energy, which leads to excess nitrogen compound release and alkalization of the culture. Therefore, in LB and YT cultures, the medium pH increases rapidly. However, when cells use glycerol as a carbon source, as they can in TB or SB, the cells produce acid that is released into the medium, resulting in a delay in alkalization. The potassium phosphate buffering in these media also likely contributes to the delay in alkalization, which corresponds to a delay in entry into the death phase. This supports previous observations (2, 17) showing that the pH of the culture affects the timing of entry into the death phase.
It has previously been shown that oxygen availability plays a role in long-term survival in LB (2). Since the shape and volume of the culture vessel can affect oxygenation through mixing, we examined vessel-dependent survival in all four rich media. Incubation in test tube versus flask cultures resulted in variations in long-term survival in each of the four media. For both TB and SB, flask cultures alkalized more rapidly than test tube cultures, indicating oxygen availability may affect the metabolic profiles of these cultures, leading to an increase in pH in flask cultures. It has previously been shown that the oxygen level in batch culture affects the cell's preference for which substrates to metabolize (30). Blommel et al. show that a shift from glucose utilization to lactose utilization occurs at a lower cell density in cultures that are O2 limited, indicating that cells may not always prefer the highest-energy carbon source (30). In the laboratory, TB and SB are often used in production of biomolecules in larger-volume flask batch cultures, rather than in test tubes. Based on data presented here, these culture methods will likely affect carbon metabolism, pH, mutation, frequency, and overall stress of the cell.
It has also been shown that glycerol utilization can be affected by culture volume (31). Losen et al. report that glycerol is used at a higher rate when the volume of the culture is lower, which means there is a higher oxygen transfer rate (31). We have previously shown, using an oxyR reporter construct, that cultures in flasks are likely experiencing higher levels of oxygen transfer than cultures in test tubes (2). Our data also show that during growth in TB(−glycerol) in flasks, the pH increases more rapidly and cells enter the death phase sooner than in standard TB. All of these data suggest that when cultured in flasks, the cells are in fact metabolizing glycerol more quickly and then shifting to amino acids earlier, leading to faster alkalization of the culture. We have also shown that the presence or absence of glycerol is a key factor in long-term survival in TB, regardless of vessel, as cells incubated without glycerol do not survive past a few days of incubation. This might partly be from the loss of acidification that metabolism of glycerol provides. However, alkalization of TB(−glycerol) test tube cultures is still slower than alkalization in LB tube cultures, indicating that the pH increase is not the sole cause of the severe death observed in TB(−glycerol) cultures. Interestingly, while both TB and SB contain glycerol, cultures incubated in SB often die out. So, while glycerol may be important for short-term survival in SB and TB, due in part to its ability to keep the culture from alkalizing quickly, it seems the addition of glycerol is not sufficient to guarantee long-term survival.
As shown previously (2), the ability to survive after the death phase can be attributed to stresses experienced during exponential- and early-stationary-phase growth. We hypothesized that the differences in long-term survival observed in cells cultured in different rich media may be due to altered levels of oxidative stress. Because the components of each medium may be metabolized at different rates or by different pathways, reactive oxidative species may be produced at different levels or rates. This could lead to damage of proteins, DNA, or other biomolecules in the cell. We tested this hypothesis by measuring both glycation levels and mutation frequencies in overnight cultures, as well as glycation during the death phase and the LTSP, which can both be affected by oxidative stress. We then measured oxyR levels during the log phase and in overnight cultures to demonstrate that cells in different media are experiencing different levels of oxidative stress.
Glycation is a nonenzymatic process in which proteins and nucleic acids are covalently modified by reactive electrophiles generated during metabolism (11–13). We monitored glycation levels in cells incubated in the different rich media over time as an assay for cell stress and found that mean glycation levels correspond with the severity and timing of the death phase in YT, TB, and SB. On the first day of incubation, glycation levels in YT cultures are higher than those observed in LB cultures, correlating with the more severe death phase in YT medium (shown in Fig. 5 and 1, respectively). Further, the delay in glycation in TB and SB cultures is coincident with both delay of the death phase and delay in alkalization. This is expected since glycation levels have been shown to increase with medium pH (10, 13, 28). Our data show that higher glycation levels early during incubation correspond to a more severe death phase, and low glycation levels are coincident with a delay in the death phase. This provides further support that glycation may contribute to loss of viability upon entry into the death phase or may be caused by the same stresses that ultimately cause cell death.
We hypothesize that the same stresses that lead to a more severe death phase in some types of media may also lead to an increase in mutation frequency in those media. In fact, median mutation frequency after overnight incubation in each medium corresponds with the severity of the death phase in those cultures. For example, cells incubated in YT and SB generate 2- to 5-fold more mutants than those cultured in LB, respectively, and those cells also have the lowest cell yield on the fourth day of incubation. However, high mutation frequency, in and of itself, does not necessarily lead to a more severe death phase since mutS, mutL, or dam strains, with mutation frequencies up to 100 times that of wild-type cells, can survive long-term stationary-phase incubation when grown in batch culture (S. Finkel, R. Kolter, and S J. Gould, unpublished observation; L. Westphal and S. Finkel, unpublished data). Both the glycation and mutation frequency data strongly imply that conditions present on the first day of incubation affect survival several days later.
To confirm the hypothesis that oxidative stress levels during the log phase may lead to higher mutation frequencies and glycation levels and ultimately lower survival in the death phase, we monitored oxyR expression in each medium during the early and mid-log phases and after overnight incubation. As early as 2 h after inoculation, oxyR levels are higher in YT, TB, and SB than in LB culture. Further, after 4 h of incubation, when DNA replication, transcription, and translation are likely at their peak, oxyR levels are highest in SB, slightly lower in YT, and lower still in LB. Increases in oxyR expression levels are coincident with both mutation frequency in overnight cultures and a more severe death phase. SB cultures show the highest level of oxidative stress after 4 h of incubation, the highest mutation frequency after overnight incubation, and the lowest cell density after 4 days of incubation. Further, in YT, TB, and SB cultures, oxyR expression continues to increase after the mid-log phase. The continuous oxidative stress the cells are experiencing in combination with increasing glycation levels and DNA damage together may determine the severity of the death phase for each culture and whether or not cells will survive incubation into the LTSP.
We have shown that there are significant changes in physiological and mutational responses due to the composition of the rich culture media frequently used in laboratory experiments. We propose that cells in very-rich-medium environments create more oxidative stress through metabolism of these nutrients, leading to higher glycation levels and mutation frequency and, ultimately, death. It does not escape our attention that overnutrition in humans can lead to high oxidative stress, which can affect the morbidity and mortality associated with diabetes, heart disease, and aging (32–34). In a similar way, E. coli in extremely rich media may be “overeating,” leading to larger amounts of oxidative stress and to more severe consequences in the death phase. It may be important to consider these factors when planning experiments or analyzing data, especially because highly rich media are often used to overproduce biomolecules that may be sensitive to the effects of increased oxidative stress, and the differences in oxidative stress are apparent even in the early log phase.
ACKNOWLEDGMENTS
We thank Lacey Westphal, Christina Ferraro, Nicole Ratib, Namita Shroff, Christopher Corzett, and Xuelin Wu for helpful suggestions and comments on the manuscript. We also thank Micheline Strand and Robert Kokoska for helpful suggestions regarding this project and Patricia Foster and Ellen Popodi for providing strains.
This work was supported by U.S. Army Research Office grants W911NF1010444, W911NF1210321, and W911NF1410318.
REFERENCES
- 1.McFall E, Newman EB. 1996. Amino acids as carbon sources, p 358–379. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 2.Kram KE, Finkel SE. 2014. Culture volume and vessel affect long-term survival, mutation frequency, and oxidative stress in E. coli. Appl Environ Microbiol 80:1732–1738. doi: 10.1128/AEM.03150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel SE. 2006. Long-term survival during stationary phase: evolution and the GASP phenotype. Nat Rev Microbiol 4:113–120. doi: 10.1038/nrmicro1340. [DOI] [PubMed] [Google Scholar]
- 4.Gay FP. 1935. Bacteria growth and reproduction, p 1–38. In Gay FP, Bachman GW, Benham RH, Buchbinder L (ed), Agents of disease and host resistance. Charles C. Thomas, Springfield, IL. [Google Scholar]
- 5.Finkel SE, Kolter R. 1999. Evolution of microbial diversity during prolonged starvation. Proc Natl Acad Sci U S A 96:4023–4027. doi: 10.1073/pnas.96.7.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zambrano MM, Siegele DA, Almirón M, Tormo A, Kolter R. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]
- 7.Zinser ER, Kolter R. 2004. Escherichia coli evolution during stationary phase. Res Microbiol 155:328–336. doi: 10.1016/j.resmic.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Zinser ER, Schneider D, Blot M, Kolter R. 2003. Bacterial evolution through the selective loss of beneficial genes. Trade-offs in expression involving two loci. Genetics 164:1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinser ER, Kolter R. 2000. Prolonged stationary-phase incubation selects for lrp mutations in Escherichia coli K-12. J Bacteriol 182:4361–4365. doi: 10.1128/JB.182.15.4361-4365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepper ED, Farrell MJ, Nord G, Finkel SE. 2010. Antiglycation effects of carnosine and other compounds on the long-term survival of Escherichia coli. Appl Environ Microbiol 76:7925–7930. doi: 10.1128/AEM.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mironova R, Niwa T, Hayashi H, Dimitrova R, Ivanov I. 2001. Evidence for non-enzymatic glycosylation in Escherichia coli. Mol Microbiol 39:1061–1068. doi: 10.1046/j.1365-2958.2001.02304.x. [DOI] [PubMed] [Google Scholar]
- 12.Mironova R, Niwa T, Handzhiyski Y, Sredovska A, Ivanov I. 2005. Evidence for non-enzymatic glycosylation of Escherichia coli chromosomal DNA. Mol Microbiol 55:1801–1811. doi: 10.1111/j.1365-2958.2005.04504.x. [DOI] [PubMed] [Google Scholar]
- 13.Dimitrova R, Mironova R, Ivanov I. 2004. Glycation of proteins in Escherichia coli: effect of nutrient broth ingredients on glycation. Biotechnol Biotechnol Equip 18:99–103. doi: 10.1080/13102818.2004.10817094. [DOI] [Google Scholar]
- 14.Zilberstein D, Agmon V, Schuldiner S, Padan E. 1984. Escherichia coli intracellular pH, membrane potential, and cell growth. J Bacteriol 158:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booth IR. 1985. Regulation of cytoplasmic pH in bacteria. Microbiol Mol Biol Rev 49:359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner L, Model P. 1994. Role of an Escherichia coli stress-response operon in stationary-phase survival. Proc Natl Acad Sci U S A 91:2191–2195. doi: 10.1073/pnas.91.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell MJ, Finkel SE. 2003. The growth advantage in stationary-phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment. J Bacteriol 185:7044–7052. doi: 10.1128/JB.185.24.7044-7052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slonczewski JL, Rosen BP, Alger JR, Macnab RM. 1981. pH homeostasis in Escherichia coli: measurement by 31P nuclear magnetic resonance of methylphosphonate and phosphate. Proc Natl Acad Sci U S A 78:6271–6275. doi: 10.1073/pnas.78.10.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurer LM, Yohannes E, Bondurant SS, Radmacher M, Slonczewski JL. 2005. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J Bacteriol 187:304–319. doi: 10.1128/JB.187.1.304-319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lessard JC. 2013. Growth media for E. coli. Methods Enzymol 533:181–189. doi: 10.1016/B978-0-12-420067-8.00011-8. [DOI] [PubMed] [Google Scholar]
- 21.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 22.Sambrook JJ, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 23.Tartoff KD, Hobbs CA. 1987. Improved media for growing plasmid and cosmid clones. Bethesda Res Lab Focus 9:12. [Google Scholar]
- 24.Lee H, Popodi E, Tang H, Foster PL. 2012. Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing. Proc Natl Acad Sci U S A 109:2774–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraigsley AM, Finkel SE. 2009. Adaptive evolution in single species bacterial biofilms. FEMS Microbiol Lett 293:135–140. doi: 10.1111/j.1574-6968.2009.01526.x. [DOI] [PubMed] [Google Scholar]
- 26.Corzett CH, Goodman MF, Finkel SE. 2013. Competitive fitness during feast and famine: how SOS DNA polymerases influence physiology and evolution in Escherichia coli. Genetics 194:409–420. doi: 10.1534/genetics.113.151837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christman MF, Storz G, Ames BN. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci U S A 86:3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyström T. 2004. Stationary-phase physiology. Annu Rev Microbiol 58:161–181. doi: 10.1146/annurev.micro.58.030603.123818. [DOI] [PubMed] [Google Scholar]
- 29.Booth IR. 2005. Glycerol and methylglyoxal metabolism. EcoSal Plus doi: 10.1128/ecosalplus.3.4.3. [DOI] [PubMed] [Google Scholar]
- 30.Blommel PG, Becker KJ, Duvnjak P, Fox BG. 2007. Enhanced bacterial protein expression during auto-induction obtained by alteration of lac repressor dosage and medium composition. Biotechnol Prog 23:585–598. doi: 10.1021/bp070011x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Losen M, Frölich B, Pohl M, Büchs J. 2004. Effect of oxygen limitation and medium composition on Escherichia coli fermentation in shake-flask cultures. Biotechnol Prog 20:1062–1068. doi: 10.1021/bp034282t. [DOI] [PubMed] [Google Scholar]
- 32.Montezano AC, Touyz RM. 2012. Molecular mechanisms of hypertension—reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol 28:288–295. doi: 10.1016/j.cjca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Vazzana N, Santilli F, Sestili S, Cuccurullo C, Davi G. 2011. Determinants of increased cardiovascular disease in obesity and metabolic syndrome. Curr Med Chem 18:5267–5280. doi: 10.2174/092986711798184299. [DOI] [PubMed] [Google Scholar]
- 34.Wang C-H, Wu S-B, Wu Y-T, Wei Y-H. 2013. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med 238:450–460. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]