Abstract
The PilZ domain proteins have been demonstrated to be one of the major types of receptors mediating cyclic di-GMP (c-di-GMP) signaling pathways in several pathogenic bacteria. However, little is known about the function of PilZ domain proteins in c-di-GMP regulation of virulence in the bacterial blight pathogen of rice Xanthomonas oryzae pv. oryzae. Here, the roles of PilZ domain proteins PXO_00049 and PXO_02374 in c-di-GMP binding, regulation of virulence and motility, and subcellular localization were characterized in comparison with PXO_02715, identified previously as an interactor with the c-di-GMP receptor Filp to regulate virulence. The c-di-GMP binding motifs in the PilZ domains were conserved in PXO_00049 and PXO_02374 but were less well conserved in PXO_02715. PXO_00049 and PXO_02374 but not PXO_02715 proteins bound to c-di-GMP with high affinity in vitro, and the R141 and R10 residues in the PilZ domains of PXO_00049 and PXO_02374, respectively, were crucial for c-di-GMP binding. Gene deletion of PXO_00049 and PXO_02374 resulted in significant increases in virulence and hrp gene transcription, indicating their negative regulation of virulence via type III secretion system expression. All mutants showed significant changes in sliding motility but not exopolysaccharide production and biofilm formation. In trans expression of the full-length open reading frame (ORF) of each gene in the relevant mutants led to restoration of the phenotype to wild-type levels. Moreover, PXO_00049 and PXO_02374 displayed mainly multisite subcellular localizations, whereas PXO_02715 showed nonpolar distributions in the X. oryzae pv. oryzae cells. Therefore, this study demonstrated the different functions of the PilZ domain proteins in mediation of c-di-GMP regulation of virulence and motility in X. oryzae pv. oryzae.
INTRODUCTION
Cyclic dimeric GMP (c-di-GMP) was originally identified as a positive allosteric regulator of cellulose synthase in Gluconacetobacter xylinus (1). Now, it has been recognized as an important intracellular signaling molecule implicated in the control of various bacterial properties, including motility, biofilm formation, adherence, and production of virulence factors (2, 3). The intracellular level of c-di-GMP is controlled by diguanylate cyclases (DGCs), which contain the GGDEF domain, and phosphodiesterases (PDEs), which harbor EAL or HD-GYP domains (4). Also, the downstream cellular functions regulated by c-di-GMP often depend on signal transduction by its receptors or effectors. A wide variety of c-di-GMP receptors have been discovered, such as PilZ domains (5), the transcription factors (6–8), proteins containing degenerate GGDEF or EAL domains (9–11), and the RNA processing polynucleotide phosphorylase (PNPase) and riboswitches (12–14). Therefore, c-di-GMP can exert a regulatory function at various levels of gene transcription, translation, and posttranslation.
The PilZ domain is the first c-di-GMP binding module identified in G. xylinus and is named PilZ domain after being identified from Pseudomonas aeruginosa (5). Two highly conserved motifs, RxxxR and N/DxxxxG, in the PilZ domain are recognized to bind c-di-GMP by the crystal structure of the PilZ domain–c-di-GMP complex and biological analysis (15). PilZ domains are widely distributed in bacterial genomes, and bacterial genomes generally encode one or multiple PilZ domain proteins which contain a single PilZ domain alone or conjugated with other domains. Many PilZ domain proteins have been experimentally identified in different bacteria and implicated in the regulation of motility, polysaccharide synthesis, biofilm formation, and virulence. YcgR from Escherichia coli and Salmonella sp. was shown to regulate flagellar motility by directly interacting with flagellar motor components FliG and FliM in the presence of c-di-GMP (15–18). The PilZ domain proteins Alg44 and FlgZ in P. aeruginosa are required for alginate biosynthesis, swimming motility, and biofilm formation (19, 20). In Vibrio cholerae, five PilZ domain proteins function as c-di-GMP receptors, which are involved in the regulation of biofilm formation, motility, and virulence (21). Four PilZ domain proteins (XC_0965, XC_02249, XC_2317, and XC_3221) have been reported to be involved in the virulence regulation of Xanthomonas campestris pv. campestris (22). These observations suggest that downstream regulation mediated by PilZ domain proteins in the c-di-GMP signal pathway varies in different bacterial species.
X. oryzae pv. oryzae, the causal agent of bacterial blight of rice, has been one of the model systems to study the molecular mechanism of bacterial pathogenesis in plants (23). X. oryzae pv. oryzae produces many virulence factors, such as exopolysaccharide (EPS), extracellular enzymes, adhesins, and the type III secretion system (T3SS) and its effectors (24). For the type III secretion system of X. oryzae pv. oryzae, HrpG and HrpX are two master regulators which control the expression of hrp genes and type III effectors. In the genome of X. oryzae pv. oryzae PXO99A, 26 genes encoding GGDEF, EAL, or HD-GYP domain proteins have been identified through bioinformatics analysis (25). Some of these proteins have been identified as PDEs to regulate EPS production and virulence in X. oryzae pv. oryzae, such as PdeR and RpfG, which were response regulators in two-component systems (25, 26). However, little is known about the downstream regulation of the c-di-GMP signal pathway in X. oryzae pv. oryzae. The transcription factor Clp and degenerate GGDEF and EAL domain protein Filp have been identified as c-di-GMP receptors in X. oryzae pv. oryzae (11, 27). Interestingly, Filp specially interacts with PilZ domain protein PXO_02715 to regulate bacterial virulence (11). There are three PilZ-encoding sequences, PXO_00049, PXO_02374, and PXO_02715, in the X. oryzae pv. oryzae PXO99A genome (28), which showed protein similarities of 85.4%, 85.5%, and 92.1%, respectively, to the PilZ domain proteins XC_2317, XC_0965, and XC_3221 from X. campestris pv. campestris. Our previous studies have shown that PXO_02715 is required for the full virulence and T3SS-related hrp gene expression in X. oryzae pv. oryzae (11). However, whether other PilZ domain proteins besides PXO_02715 bind to c-di-GMP and participate in regulation of X. oryzae pv. oryzae virulence and motility remain unknown.
In this work, we identified these PilZ domain proteins and examined their c-di-GMP binding ability and function in regulating virulence and motility of X. oryzae pv. oryzae. Our evidence showed specific c-di-GMP binding in vitro of PXO_00049 and PXO_02374 but not PXO_02715. The PXO_00049 gene deletion resulted in increased virulence and decreased sliding motility, and the PXO_02374 mutation caused increased virulence and sliding motility of X. oryzae pv. oryzae. Meanwhile, the expression of type III secretion system (T3SS)-related genes (hrpX, hrpG, and hpa1) was regulated by PXO_00049 and PXO_02374. Moreover, subcellular localization analysis showed that the spatial distribution of three PilZ domain proteins varied in X. oryzae pv. oryzae cells. These results imply that PilZ domain proteins function differentially in c-di-GMP binding, regulation of bacterial virulence and motility, and subcellular localization in X. oryzae pv. oryzae.
MATERIALS AND METHODS
All biological experiments with the pathogens described in this publication were conducted at the Chinese Academy of Agricultural Sciences in Beijing, China.
Maintenance of bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown in LB medium at 37°C (29). X. oryzae pv. oryzae strain PXO99A and derived mutant strains were cultured at 28°C on peptone sucrose agar (PSA) medium or M210 liquid medium with appropriate antibiotics (30). The antibiotics used were ampicillin (Ap), kanamycin (Km), and gentamicin (Gm) at concentrations of 100 μg ml−1, 50 μg ml−1, and 20 μg ml−1, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| DH5α | supE44 ΔlacU169(ϕ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 27 |
| BL21 | For protein expression | Novagen |
| Xanthomonas oryzae pv. oryzae | ||
| PXO99A | Wild-type strain, Philippine race 6 | Lab collection |
| ΔPXO_00049 mutant | PXO_00049 gene deletion mutant derived from PXO99A, Gmr | This study |
| ΔPXO_02374 mutant | PXO_02374 gene deletion mutant derived from PXO99A, Gmr | This study |
| ΔPXO_02715 mutant | PXO_02715 gene deletion mutant derived from PXO99A, Gmr | 11 |
| Plasmids | ||
| pColdSUMO | Expression vector to generate an N-terminal SUMO-His6 tag, Apr | Haigene |
| pK18mobsacB | Suicidal vector carrying sacB gene for mutagenesis, Kmr | 29 |
| pBBR1MCS-4 | Broad-host-range expression vector, Apr | 30 |
| pMD18-T | Cloning vector, Apr | TaKaRa |
| pBlueGFP1 | Plasmid containing the GFP coding sequence, Apr | 55 |
| pMDGFP | pMD18-T containing the GFP coding sequence, Apr | This study |
| pBBG | pBBR1MCS-4 containing the GFP coding sequence, Apr | This study |
| pC49 | pColdSUMO containing the PXO_00049 coding sequence, Apr | This study |
| pC2374 | pColdSUMO containing the PXO_02374 coding sequence, Apr | This study |
| pC49R141D | pColdSUMO containing the point mutant of R141D in PXO_00049 sequence, Apr | This study |
| pC2374R10D | pColdSUMO containing the point mutant of R10D in PXO_02374 sequence, Apr | This study |
| pK49G | pK18mobSacB with PXO_00049 inserted by Gm resistance gene, Kmr Gmr | This study |
| pK2374G | pK18mobSacB with PXO_02374 inserted by Gm resistance gene, Kmr Gmr | This study |
| pB49 | pBBR1MCS-4 carrying the PXO_00049 coding sequence, Apr | This study |
| pB2374 | pBBR1MCS-4 carrying the PXO_02374 coding sequence, Apr | This study |
| pB49 R141D | pBBR1MCS-4 carrying the PXO_00049 coding sequence, Apr | This study |
| pB2374 R10D | pBBR1MCS-4 carrying the PXO_02374 coding sequence, Apr | This study |
| pBBG49 | pBBG carrying the PXO_00049 coding sequence, Apr | This study |
| pBBG2374 | pBBG carrying the PXO_02374 coding sequence, Apr | This study |
| pBBG2715 | pBBG carrying the PXO_02715 coding sequence, Apr | This study |
Protein expression and purification.
The DNA fragments encoding PXO_00049 and PXO_02374 were amplified by primer pairs 49PF1/49PR1 and 2374PF1/2374PR1, respectively. For the mutation of R141 of PXO_00049, the upstream and downstream fragments were amplified using primers 49PF1/49PR2 and 49PF2/49PR1. Then, two fragments were used as the templates to amplify fragment 49R141D by primers 49PF1/49PR1. We also amplified 2374R10D using the same methods. The primers are listed in Table S1 in the supplemental material. The PCR fragments were gel purified and cloned into the pMD18-T vector (TaKaRa, Tokyo, Japan), following verification by sequencing. The fragments were digested with the appropriate restriction enzymes and cloned into pColdSUMO, resulting in plasmids pC49, pC2374, pC49R141D, and pC2374R10D. The plasmids were transformed into E. coli BL21 strains for expression of the protein. Also, the E. coli strain used for the expression of PXO_02715 was from our previous study (11). Expression of the target protein was induced by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.5 mM, and the bacterial cultures were collected after incubation at 16°C for 12 h. Subsequently purification of the protein was performed according to the methods described by Yang et al. (11, 25). SUMO-His6 tag expressed from the empty vector pColdSUMO was purified as a negative control.
Isothermal titration calorimetry (ITC) assay.
The binding analysis of the proteins was carried out on an ITC200 calorimeter (MicroCal, Northampton, MA), according to the manufacturer's directions. In brief, 2 μl c-di-GMP solution (200 μM, 300 μM, or 500 μM) was injected at 2-min intervals via a 60-μl syringe into the sample cell containing 30 μM SUMOHis6-PXO_00049, 50 μM SUMOHis6-PXO_02374m, 50 μM SUMOHis6-PXO_02715, or 20 μM SUMOHis6 tag protein with constant stirring, and the heat changes accompanying these additions were recorded. The titration experiment was repeated three times, and the data were calibrated with a buffer control and fitted with the single-site model to determine the dissociation constant (Kd) using the MicroCal ORIGIN version 7.0 software.
Construction of gene deletion mutant and complementation analysis.
For generating the mutants, homologous recombination as described previously was used in this study (31). The upstream and downstream fragments of PXO_00049 were amplified using chromosomal DNA of PXO99A as the template with 49MF1/49MR1 and 49MF2/49MR2 as primers. The DNA fragments corresponding to downstream and upstream regions of PXO_02374 were amplified using specific primers 2374MF1/2374MR1 and 2374MF2/2374MR2, respectively. The DNA fragment was digested with corresponding restriction enzymes and ligated to pK18mobsacB. A gentamicin resistance (Gmr) gene was amplified from pBBR1MCS-5 plasmid (32) using the primers GmF/GmR and digested with BamHI and HindIII, and then each gene fragment carried by pK18mobsacB was inserted, resulting in plasmids pK49G and pK2374G. pK49G and pK2374G were introduced into PXO99A by electroporation. Potential mutants were selected on PSA plates containing gentamicin and 10% sucrose. The single colonies that were resistant to gentamicin and high concentrations of sucrose but sensitive to kanamycin were further confirmed as mutants by sequencing the PCR products.
For complementation analysis, coding regions for the full length of PXO_00049 and PXO_02374 were amplified by primer pairs 49CF/49PR1 and 2374CF/2374PR1, respectively. PXO_00049R141D and PXO_02374R10D fragments were amplified using the primers 49CF/49PR1 and 2374CF/2374PR1 by pC49R141D and pC2374R10D as the template, respectively. The information on the primers is listed in Table S1 in the supplemental material. The PCR fragments were gel purified and cloned into the middle vector pMD18-T for sequencing, and each fragment was digested from pMD18-T-derived constructs with KpnI/HindIII and ligated into the vector pBBR1MCS-4 (32), resulting in constructs pB49, pB2374, pB49R141D, and pB2374R10D to express the proteins under the lac promoter. These constructs were electroporated into the ΔPXO_00049 or ΔPXO_02374 mutant for complementation studies. The PXO_02715 deletion mutant and complement strain were from our previous work (11).
Virulence test.
The wild-type and mutant strains of PXO99A were grown for 72 h at 28°C in M210 medium with appropriate antibiotics. The cells were collected by centrifugation and resuspended in sterile distilled water at a concentration with an optical density at 600 nm (OD600) of 0.8. The bacterial suspensions were clip inoculated on leaves of approximately 6-week-old rice plants of the susceptible rice variety (Oryza sativa subsp. japonica) under relevant conditions (33). For the biosafety of the genetically modified X. oryzae pv. oryzae strains, the pathogenicity tests were conducted in separate and dedicated greenhouse rooms, where the X. oryzae pv. oryzae-inoculated rice plants were strictly isolated and contained. After the completion of the experiments, all of the tested rice plants were collected and autoclaved. Pictures were taken using a digital camera (Canon EOS 550D), and disease lesion lengths were measured 14 days after inoculation.
Motility assay.
Fresh colonies from PSA plates were stabbed into plates composed of 0.03% (wt/vol) Bacto peptone, 0.03% yeast extract, and 0.3% agar to detect swimming motility (34) and an SB medium plate containing 0.5% (wt/vol) Bacto peptone, 0.5% yeast extract, 0.5% sugar, 0.1% l-glutamic acid, and 0.6% agar to detect sliding motility (35). The inoculated cells were cultured for 4 days at 28°C and examined for bacteria swimming and sliding away from the inoculated site. The diameter of each swimming or sliding zone was measured, and its dimensions were calculated. The numbers represented the average from at least three independent repeats.
EPS production assay.
For analyzing EPS production, bacterial strains were cultured in M210 medium for 48 h. Supernatants were collected by centrifugation at 12,000 × g for 10 min at room temperature. Two volumes of absolute ethanol was added to the supernatants. The mixtures were held at −20°C for half an hour and centrifuged to collect the precipitated EPS molecules. After drying at 55°C overnight, EPS molecules were weighed. The experiments were repeated three times (36).
Biofilm formation assay.
Bacterial strains were grown to log phase in M210 medium, and then the bacteria were diluted to an OD600 of 0.002 using L medium containing 1% tryptone, 0.5% yeast extract, 0.5% NaCl, and 0.001% glucose, and 5 ml was incubated at 28°C for 72 h in the tubes. Bacterial pellicles were stained by gently pouring off the medium, washing twice with water, and staining with 0.1% crystal violet. Tubes were washed and rinsed with water until all unbound dye was removed. The adherent dye was later redissolved with 90% ethanol and quantified by spectrophotometry at A490 (37).
RNA isolation and qRT-PCR analysis.
The expression of T3SS-related genes in X. oryzae pv. oryzae strains was induced by culture in the medium XOM2 (38). A bacterial RNAout (Tiandz) kit was used for total RNA isolation according to the manufacturer's instructions. Total RNA was treated with DNase I (Tiandz) to remove any DNA contamination. Five micrograms of RNA was used for cDNA synthesis with the random hexanucleotide primers. Quantitative reverse transcription-PCR (qRT-PCR) analysis was carried out using iQTMSYBRR Green Supermix (Bio-Rad) and the gene-specific primers (see Table S1 in the supplemental material) with cDNAs as the template. The signals during amplification were detected by the iCycleriQ real-time detection system (Bio-Rad). The relative levels of gene expression were determined by using the threshold cycle (2−ΔΔCT) method (39) with the DNA gyrase subunit B gene (gyrB) as the internal control (40). Three technical replicates were used each time.
Localization of PilZ-GFP fusion proteins.
The green fluorescent protein (GFP) was amplified using the primers GFPF and GFPR from the vector pBlueGFP1, and the PCR fragments were cloned into the vector pMD18-T for sequencing. We constructed pBBG by digesting an XbaI/SacI GFP fragment from pMDGFP into the vector pBBR1MCS-4. This allows the GFP fusions to be constitutively expressed by the lac promoter from pBBR1MCS-4. PCR primers 49CF/49LR, 2374CF/2374LR, and 2715LF/2715LR were used to amplify the fragments PXO_00049, PXO_02374, and PXO_02715, respectively. All the coding sequences were without the termination codon. The PCR products were cloned into pMD18-T, and the fragments digested with KpnI/HindIII were subcloned into pBBG, resulting in pBBG49, pBBG2374, and pBBG2715. The plasmids were electroporated into PXO99A or mutant strains. The locations of different proteins in X. oryzae pv. oryzae cells were detected by fluorescence microscopy (Olympus BX61), and the CellSens Dimension software was used for analysis of the average brightness value of the selected area in bacterial cells.
RESULTS
Analysis of PilZ domain proteins in the genome of X. oryzae pv. oryzae.
Bioinformatics analysis using BLAST indicates that three genes, PXO_00049, PXO_02374, and PXO_02715, encoding typical PilZ domains, are found in the genome of X. oryzae pv. oryzae strain PXO99A. The domain architectures of the proteins were analyzed by the simplified modular architecture research tool (SMART) online service. PXO_00049 is a YcgR-like protein containing an N-terminal YcgR domain, which is a predicted domain involved in regulating flagellar rotation (15, 18), and a C-terminal PilZ domain, which is a c-di-GMP-binding domain. PXO_02374 and PXO_02715 contain a single PilZ domain (Fig. 1A). Alignment of the PilZ domain protein sequences revealed that the RxxxR and N/DxxxxG motifs were highly conserved in PXO_00049 and PXO_02374, while those in PXO_02715 were less well conserved due to the changes to QxxxS and RxxxxD (Fig. 1B).
FIG 1.
Domain organization and sequence alignment of PilZ domain proteins in Xanthomonas oryzae pv. oryzae wild-type strain PXO99A. (A) Schematic representation of the domain organization of three PilZ domain proteins from Xanthomonas oryzae pv. oryzae strain PXO99A. The numbers represent amino acid residues where the predicted domains start and end, based on NCBI's conserved domain database and the SMART database. The PilZ domain and YcgR domain are shown. (B) Sequence alignment of the PilZ domain of PXO_00049, PXO_02374, and PXO_02715 with PilZ (PA4608) from P. aeruginosa, PlzD (VCA0042) from V. cholerae, and YcgR from E. coli, which represent the conserved PilZ domain. Black and gray highlighting show amino acid residues with ≥75% and ≥50% homology, respectively. An asterisk highlights the residue confirmed by isothermal titration calorimetry assays to be involved in c-di-GMP binding.
c-di-GMP binding efficiency of PilZ domain proteins.
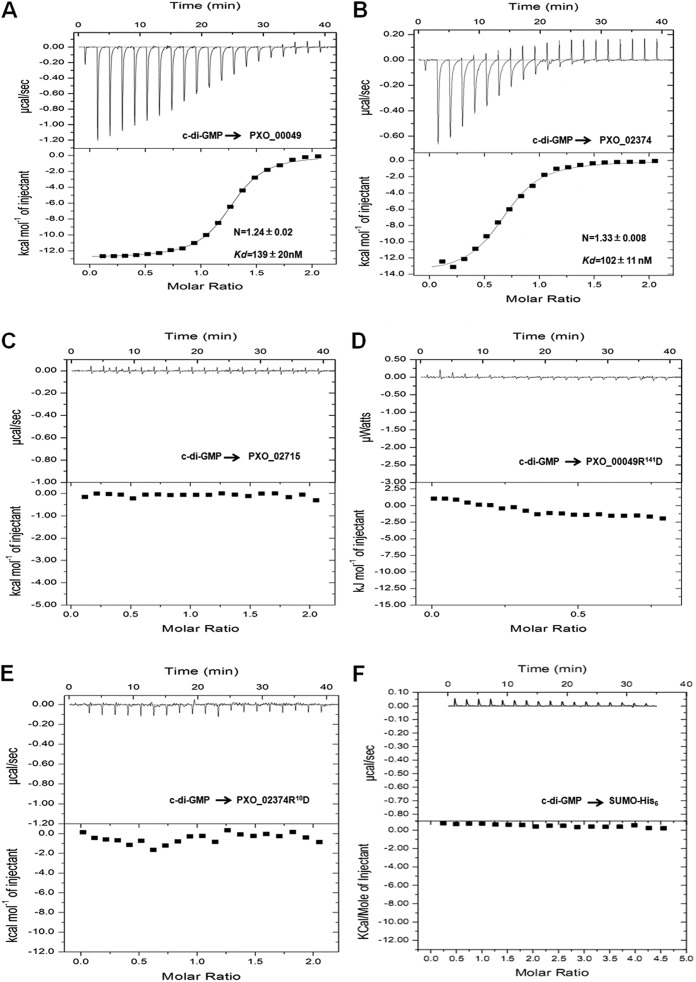
To determine whether three X. oryzae pv. oryzae proteins with PilZ domains function as receptors for c-di-GMP, we performed isothermal titration calorimetry (ITC) to detect the c-di-GMP binding affinity of these proteins. SUMOHis6-PXO_00049, SUMOHis6-PXO_02374, and SUMOHis6-PXO_02715 were titrated with an injected solution of c-di-GMP at 20°C, and the heat released upon binding was measured by the integration of power values over a range of ligand concentrations. The results showed that PXO_00049 and PXO_02374 bound to c-di-GMP in a 1:1 stoichiometry, with estimated dissociation constants (Kd) of 139 ± 20 nM and 102 ± 11 nM, respectively (Fig. 2A and B). In contrast, PXO_02715 did not bind to c-di-GMP (Fig. 2C), and no physical interaction was observed between the control protein SUMO-His6 and c-di-GMP molecule (Fig. 2F). The R residue in the RxxxR motif has been identified as the crucial site for the PilZ domain protein to bind c-di-GMP (15). To further characterize the importance of the PilZ domain for PXO_00049 and PXO_02374, we chose R141 of PXO_00049 and R10 of PXO_02374 to introduce point mutations within the proteins and test their ability to bind c-di-GMP. Results from ITC assays showed that PXO_00049R141D and PXO_02374R10D lost their ability to bind c-di-GMP (Fig. 2D and E). These observations indicate that both PXO_00049 and PXO_02374 bind c-di-GMP with high affinity through the PilZ domain.
FIG 2.
Analysis of c-di-GMP binding of PilZ domain proteins using isothermal titration calorimetry assays. The top panel in each panel shows the titration calorimetry of 20 μM SUMO-His6, 30 μM SUMO-His6-PXO_00049, and 50 μM SUMO-His6-PXO_02374 or SUMO-His6-PXO_02715 protein with 2-μl aliquots of 200 μM, 30 μM, or 500 μM c-di-GMP at 20°C. The derived values for Kd and the N value for the binding stoichiometry of c-di-GMP to PilZ domain proteins are shown in the bottom panel. c-di-GMP binding to the recombinant proteins SUMO-His6-PXO_00049 (A), SUMO-His6-PXO_02374 (B), and SUMO-His6-PXO_02715 (C); to the point mutant for R141 of recombinant protein SUMO-His6-PXO_00049 (D) and the point mutant for R10 of recombinant protein SUMO-His6-PXO_02374 (E); and to the control protein SUMO-His6 (F).
PilZ domain proteins regulate X. oryzae pv. oryzae virulence in rice.
PilZ domain proteins have been identified as c-di-GMP receptors to perform biological functions, including virulence in diverse bacteria (4, 41). In X. oryzae pv. oryzae, deletion of the PXO_02715 gene resulted in attenuated virulence on rice, implying that PXO_02715 is required for the full virulence of X. oryzae pv. oryzae (11). To further characterize whether PXO_00049 and PXO_02374 affect the virulence, we constructed deletion mutants of the X. oryzae pv. oryzae wild-type strain PXO99A. The bacterial cells of PXO99A and derived mutants were inoculated onto the tips of the leaves as described in Materials and Methods. The disease symptoms were recorded by photography, and the lesion lengths were measured 14 days postinoculation. As shown in Fig. 3, ΔPXO_00049 and ΔPXO_02374 mutants caused more severe disease symptoms and longer lesions than did the wild-type strain, suggesting that their ability to infect the host rice plant was significantly increased. When a plasmid to express PXO_00049 and PXO_02374 in trans was transformed into the relevant mutants, the disease phenotypes were restored to near-wild-type levels. In contrast, expression of PXO_00049R141D in the ΔPXO_00049 mutant and PXO_02374R10D in the ΔPXO_02374 mutant failed to complement its virulence, indicating that the PilZ domains of PXO_00049 and PXO_02374 were necessary for bacterial virulence. These findings demonstrate that PilZ domain proteins PXO_00049 and PXO_02374 regulate virulence of X. oryzae pv. oryzae.
FIG 3.
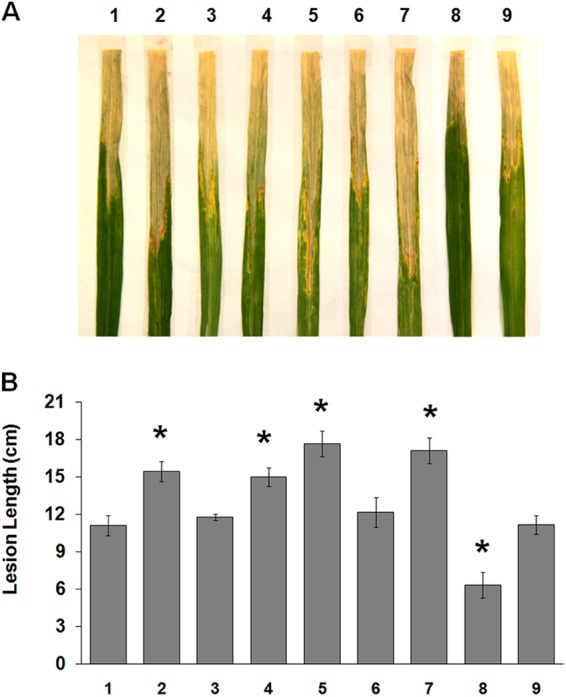
The PilZ domain proteins affected the virulence of X. oryzae pv. oryzae in rice. (A) X. oryzae pv. oryzae strains were inoculated by using the rice leaf clipping method. The disease symptoms were observed at 14 days after inoculation. (B) The lesion lengths were recorded. The error bars represent standard deviations of the lesion lengths from 10 leaves. Three independent experiments were performed with similar results. Lanes for both panels: 1, wild-type PXO99A; 2, ΔPXO_00049 mutant; 3, ΔPXO_00049 (pB49) mutant; 4, ΔPXO_00049 (pB49R141D) mutant; 5, ΔPXO_02374 mutant; 6, ΔPXO_02374 (pB2374) mutant; 7, ΔPXO_02374 (pB2374R10D) mutant; 8, ΔPXO_02715 mutant; 9, ΔPXO_02715 (pB2715) mutant. Asterisks indicate P values of <0.05 by t test.
PilZ domain proteins affect T3SS-related gene transcription.
Previous studies have shown that the PilZ domain protein PXO_02715 is required for the full virulence of X. oryzae pv. oryzae and that the expression of T3SS-related genes is regulated by PXO_02715 (11). Since the PXO_00049 and PXO_02374 mutants displayed increased virulence on rice, the T3SS expression might be speculated to be affected as well. Therefore, we examined the transcriptional levels of T3SS regulator genes hrpG and hrpX and harpin gene hpa1 in the ΔPXO_00049 and ΔPXO_02374 mutants by qRT-PCR experiments. The total RNA was extracted from the wild-type PXO99A and relevant mutants after the expression of the hrp genes was induced in the minimal medium XOM2 (38). As shown in Fig. 4, the mRNA levels of hrpX, hrpG, and hpa1 in ΔPXO_00049 and ΔPXO_02374 mutants increased about 1- to 6-fold compared with those in wild-type PXO99A, respectively. Complementation of ΔPXO_00049 and ΔPXO_02374 mutants by expression in trans of PXO_02374 or PXO_00049 restored the gene expression to near-wild-type levels. This is consistent with the virulence phenotypes on rice of the ΔPXO_00049 and ΔPXO_02374 mutants described above. These observations suggest that both PXO_00049 and PXO_02374 have a negative effect on the T3SS gene expression of X. oryzae pv. oryzae.
FIG 4.
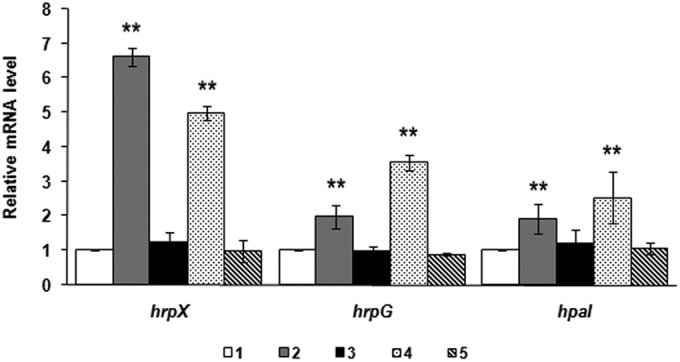
Expression of hrp genes was upregulated in ΔPXO_00049 and ΔPXO_02374 mutants as revealed by using qRT-PCR analysis. The expression level of each gene (hrpG, hrpX, and hpa1) in the wild-type strain PXO99A was mathematically designated 1 for each reaction. The housekeeping gene gyrB was used as a reference gene. Error bars represent standard deviations from three biological repeats. Bars: 1, wild-type PXO99A; 2, ΔPXO_00049 mutant; 3, ΔPXO_00049 (pB49) mutant; 4, ΔPXO_02374 mutant; 5, ΔPXO_02374 (pB2374) mutant. **, P values of <0.01 by t test.
PilZ domain proteins modulate X. oryzae pv. oryzae sliding motility.
Recent studies have uncovered that c-di-GMP receptors regulate the twitching motility in E. coli and P. aeruginosa (15, 42, 43). To characterize the effect of three PilZ domain proteins on motility in X. oryzae pv. oryzae, the wild-type strain and relevant mutants were cultured on semisolid plates containing 0.3% agar for detection of swimming motility (34). The ΔPXO_00049, ΔPXO_02374, and ΔPXO_02715 mutants showed the same swimming diameter as that of the wild-type strain PXO99A (see Fig. S1 in the supplemental material). It has been reported previously that some PilZ proteins regulate the sliding or gliding motility in X. campestris pv. campestris and Xanthomonas axonopodis pv. citri (22, 35). Therefore, we tested the sliding motility of X. oryzae pv. oryzae strains on SB medium containing 0.6% agar, which has been previously used to analyze the sliding motility of other Xanthomonas strains (11, 33). As shown in Fig. 5, the ΔPXO_00049 mutant showed a smaller sliding zone than that of the wild-type strain PXO99A, and the diameter decreased to approximately 2 mm, whereas the sliding zones of the ΔPXO_02374 and ΔPXO_02715 mutants were bigger than that of PXO99A. Expression in trans of the full-length open reading frames (ORFs) PXO_00049, PXO_02374, and PXO_02715 in the mutants restored sliding motility to near-wild-type levels, Moreover, expression in trans of PXO_00049R141D in the ΔPXO_00049 mutant and of PXO_02374R10D in the ΔPXO_02374 mutant failed to complement its sliding motility. These results indicate that three PilZ domain proteins differentially regulate sliding motility rather than swimming motility in X. oryzae pv. oryzae.
FIG 5.
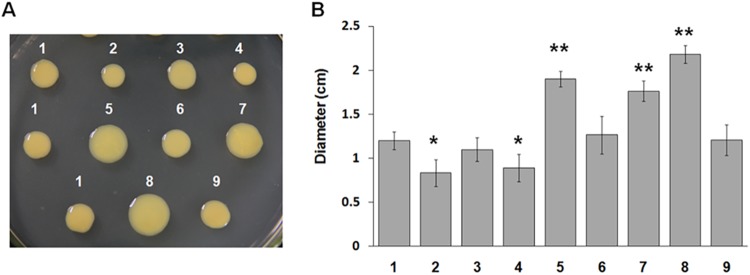
PXO_00049, PXO_02374, and PXO_02715 regulate sliding motility in PXO99A. X. oryzae pv. oryzae strains were inoculated on SB plates containing 0.6% agar at 28°C for 4 days. (A) Results of a representative motility assay. (B) Bar graph represents the mean sliding diameter and standard deviation for three independent replicates. Plates (A) and bars (B): 1, PXO99A; 2, ΔPXO_00049 mutant; 3, ΔPXO_00049 (pB49) mutant; 4, ΔPXO_00049 (pB49R141D) mutant; 5, ΔPXO_02374 mutant; 6, ΔPXO_02374 (pB2374) mutant; 7, ΔPXO_02374 (pB2374R10D) mutant; 8, ΔPXO_02715 mutant; 9, ΔPXO_02715 (pB2715) mutant. * and **, P values of <0.05 and 0.01, respectively, by t test.
PilZ domain proteins do not control EPS production and biofilm formation.
Based on the observation that PilZ domain proteins are required for bacterial virulence of X. oryzae pv. oryzae, we further investigated whether PXO_00049, PXO_02374, and PXO_02715 affect the production of virulence factors of X. oryzae pv. oryzae. EPS production and biofilm formation in the wild-type strain PXO99A and derived mutants were examined. No difference was detected between PXO99A and the three mutants in terms of EPS production by quantification (see Fig. S1A in the supplemental material) and colony surface checking (see Fig. S1B), indicating that PXO_00049, PXO_02374, and PXO_02715 did not regulate the EPS production of X. oryzae pv. oryzae. Similarly, results from the biofilm formation assays showed that there are no changes between the wild-type and relevant mutants as well (see Fig. S1E). These observations suggest that PilZ domain proteins are not involved in the regulation of both EPS production and biofilm formation in X. oryzae pv. oryzae.
Subcellular localizations of PilZ domain proteins in X. oryzae pv. oryzae.
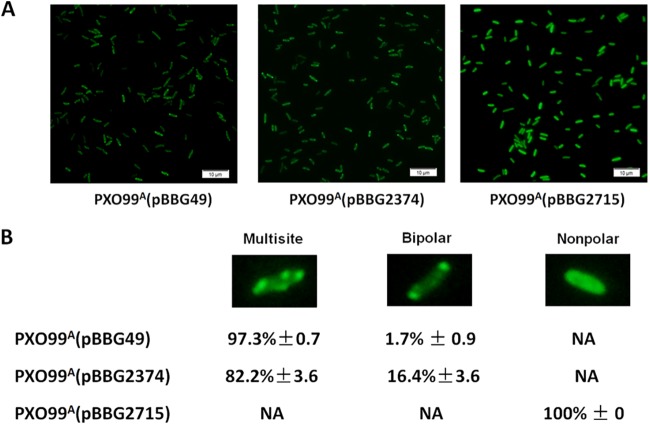
Since specific subcellular localization of a given protein might be required for its proper biological function in bacteria (44–46), we analyzed the subcellular localization of the X. oryzae pv. oryzae PilZ proteins by constructing C-terminal translational fusions of GFP to PXO_00049, PXO_02374, and PXO_02715. The restoration of sliding motility in the relevant mutants with transformed fusion proteins to the wild-type level was observed (data not shown), demonstrating that the fusion proteins retained their function. The wild-type strain PXO99A, which contained the plasmid pBBG (empty vector), showed very low fluorescence, with average brightness values below 60. Subcellular localization of the PilZ domain proteins was defined based on the average brightness value in different sites within the cytoplasm of the bacterium (see Fig. S2 in the supplemental material). As shown in Fig. 6, PXO_00049-GFP and PXO_02374-GFP proteins displayed multisite distributions of 97.3% and 82.2%, respectively, of all bacterial cells tested, while PXO_02715-GFP showed nonpolar localization with high-level fluorescence in 100% of cells. These results demonstrate that both PXO_00049 and PXO_02374 proteins mainly show similar multisite localization, whereas PXO_02715 protein displays nonpolar distribution in the X. oryzae pv. oryzae cells.
FIG 6.
Subcellular localization of PilZ domain proteins in PXO99A. X. oryzae pv. oryzae strains were cultured in M210 at 28°C until reaching an OD600 of 0.6. (A) Photograph of the GFP fusion protein in X. oryzae pv. oryzae strains detected using a fluorescence microscope (Olympus BX61). (B) Multisite, bipolar, and nonpolar locations were defined as three types for subcellular localization of the proteins. The different subcellular locations of PilZ domain proteins in PXO99A(pBBG49), PXO99A(pBBG2374), and PXO99A(pBBG2715) strains were counted. ± represents standard deviations from three biological repeats. NA, not available.
DISCUSSION
PilZ domain proteins are widely distributed in bacterial genomes (5). Several PilZ domain proteins have been reported to bind c-di-GMP signal molecules and function as the receptors in mediating c-di-GMP signaling and regulation of downstream phenotypes in pathogenic bacteria (20, 21, 47, 48). In this work, we comparatively characterized three PilZ domain proteins (PXO_00049, PXO_02374, and PXO_02715) encoded in the genome of X. oryzae pv. oryzae wild-type strain PXO99A. The observation that c-di-GMP can be effectively bound to PXO_00049 and PXO_02374, but not PXO_02715 (Table 2), might be due to the fact that c-di-GMP binding motifs in PilZ domains are conserved in PXO_00049 and PXO_02374 but are degenerate in PXO_02715 (Fig. 1). For PXO_00049 and PXO_02374, point mutations in the R141 and R10 residues within the PilZ domains completely abolish the c-di-GMP binding ability, confirming the requirement of the PilZ domain for c-di-GMP binding. For PXO_02715 showing no direct c-di-GMP binding activity, specific interaction with the EAL domain of the c-di-GMP receptor Filp was observed (unpublished data), implying that c-di-GMP sensing by Filp is required for its proper function in X. oryzae pv. oryzae.
TABLE 2.
Functional characterization of three PilZ domain proteins in Xanthomonas oryzae pv. oryzaea
| Strain | c-di-GMP binding/dissociation constant (Kd) | Virulence | Sliding motility | Subcellular location |
|---|---|---|---|---|
| PXO_00049 | Yes/139 ± 20 nM | ↑ | ↓ | Multisite |
| PXO_02374 | Yes/102 ± 11 nM | ↑ | ↑ | Multisite/bipolar |
| PXO_02715 | No/NA | ↓ | ↑ | Nonpolar |
↑ and ↓, increased and decreased virulence or sliding motility of the relevant mutant compared with the wild type, respectively. NA, not available.
X. oryzae pv. oryzae has been one of the most important bacterial pathogens of rice, leading to 20 to 50% yield losses, and has become an ideal model to study the molecular interaction between plant and pathogenic bacterium (23). It is of both scientific and economic significance to decipher the regulatory mechanisms in virulence of X. oryzae pv. oryzae. c-di-GMP receptors have been identified to play important roles in the regulation of virulence (49), while the regulatory functions of PilZ domain proteins in virulence expression were examined in a few pathogenic bacteria. For example, gene deletion of the PilZ domain protein from V. cholerae resulted in reduced colonization of the infant mouse small intestine (21). Mutation of X. campestris pv. campestris PilZ domain genes XC_0965 and XC_3221, but not XC_2317, led to significantly reduced virulence in Chinese radish, suggesting their role in virulence (22). The X. oryzae pv. oryzae PilZ protein PXO_02715, which is a homologue of XC3221, was previously shown to be an interactor with the c-di-GMP receptor FilP and to be required for the full virulence of X. oryzae pv. oryzae and regulated the expression of T3SS-related genes (11). These results mentioned above demonstrated that mutations of the PilZ domain proteins led generally to bacterial virulence reduction. However, the PilZ domain proteins PXO_00049 and PXO_02374 were identified in this study as the novel negative regulators of virulence and T3SS-related gene expression in X. oryzae pv. oryzae (Table 2). A few negative bacterial regulators in the c-di-GMP regulation of virulence expression have been reported, such as CgsB (a c-di-GMP synthase) in a mouse model of Brucella melitensis infection (50). To our knowledge, this is the first report that deletion of the PilZ-type protein gene produces a hypervirulent strain of pathogenic bacteria. Nevertheless, it remains to be determined what is the exact molecular mechanism by which the PilZ-type receptors affect bacterial virulence gene expression. In addition, based on the observation that the PilZ domain proteins with high similarities to X. oryzae pv. oryzae and X. campestris pv. campestris demonstrated either common or distinctive regulatory roles in virulence, we assume that their mechanistic role in virulence varies in the two species. Moreover, changes in the R141 and R10 residues within the PilZ domain of PXO_00049 and PXO_02374 proteins, respectively, resulted not only in a loss of c-di-GMP binding ability but also in complementation of the relevant phenotypes for virulence restoration in the mutant strains. This indicates that c-di-GMP binding via the PilZ domain is necessary for PXO_00049 and PXO_02374 to function properly in vivo. Moreover, expression of T3SS-related hrp genes was regulated by all PilZ domain proteins in the same ways as they exerted a virulence phenotype in rice (11) (Fig. 3). However, three PilZ proteins were all not involved in both EPS production and biofilm formation in X. oryzae pv. oryzae, which was not the case reported for X. campestris pv. campestris (22). Taken together, our data provide novel insights into the mechanisms underlying the PilZ protein-mediated c-di-GMP regulation of virulence via T3SS expression in X. oryzae pv. oryzae.
It has been reported elsewhere that PilZ domain proteins are involved in the regulation of swimming motility in E. coli, Salmonella, and Pseudomonas fluorescens (15–18, 20) and sliding or gliding but not swimming motility in Xanthomonas spp. (11, 22, 35). These observations implicate differential regulation by PilZ proteins of bacterial motility in different species. Sliding motility is the action of sliding or gliding on a semisolid surface and, in X. campestris pv. campestris and X. axonopodis pv. citri strains, is recognized to be dependent on the bacterial pilus (22, 35). In addition, type IV pili (T4P) have also been shown to be involved in virulence of pathogenic bacteria, including P. aeruginosa, Neisseria meningitidis, and Ralstonia solanacearum (51–53). In this study, the PilZ proteins were found to regulate sliding motility, among which PXO_00049 acts as a positive regulator, whereas both PXO_02374 and PXO_02715 function as the negative ones. However, where and how PilZ domain proteins affect pilus biogenesis, and what the relationship is between the T4P and virulence in X. oryzae pv. oryzae, remain to be further elucidated.
Protein subcellular localization is generally thought to be related to specific spatial functions in bacteria (44–46). In this study, the characteristics of PilZ domain protein localizations showed multisite localization of both PXO_00049 and PXO_02374 and nonpolar distribution of PXO_02715 in the X. oryzae pv. oryzae cells (Fig. 6). These results suggest that there might be a possible link among c-di-GMP binding, virulence, gene expression, and subcellular localization for the X. oryzae pv. oryzae PilZ domain proteins. Therefore, this provides informative clues for further exploration of the mechanistic regulation by the PilZ domain proteins of bacterial virulence and pathogenesis in rice.
For PilZ domain proteins, the interaction with downstream proteins has been identified as a major way to perform biological functions, including bacterial virulence and motility (17, 18, 54). For example, YcgR decreases bacterial swimming speed by directly binding to the flagellar motor component at high c-di-GMP levels, which is a signal for switching into a biofilm-associated lifestyle (15–18). The X. axonopodis pv. citri PilZ domain protein PilZXAC1133 and a PilB ATPase formed a complex to promote T4P formation and inhibit sliding motility (35, 53). The X. oryzae pv. oryzae PilZ domain protein PXO_02715 showed protein sequence similarity of 94.4% to PilZXAC1133, suggesting that its regulation of virulence and motility might be occurring by interacting with proteins involved with T4P formation. In addition, two novel PXO_02374-interacting proteins have been identified through yeast two-hybrid and glutathione S-transferase (GST) pulldown assays (unpublished data). We speculate that the interactions between the PilZ domain proteins and downstream proteins might occur and that the intracellular concentrations of c-di-GMP affect such protein-protein interactions and their mediation of downstream signaling mechanism, thereby regulating T3SS and virulence expression of X. oryzae pv. oryzae. Further identification and characterization of PilZ domain protein interaction, and their regulation of virulence or motility, are required to elucidate mechanisms by which PilZ domain proteins exert their biological functions in X. oryzae pv. oryzae.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the grants from the National Basic Research Program (2011CB100700) and National Science Foundation of China (31370160) to C. He and the National Science Foundation of China (31400117) and China Postdoctoral Science Foundation (2014T70149) to F. Yang.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04044-14.
REFERENCES
- 1.Romling U, Gomelsky M, Galperin MY. 2005. C-di-GMP: the dawning of a novel bacterial signalling system. Mol Microbiol 57:629–639. doi: 10.1111/j.1365-2958.2005.04697.x. [DOI] [PubMed] [Google Scholar]
- 2.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 3.Romling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schirmer T, Jenal U. 2009. Structural and mechanistic determinants of c-di-GMP signalling. Nat Rev Microbiol 7:724–735. doi: 10.1038/nrmicro2203. [DOI] [PubMed] [Google Scholar]
- 5.Amikam D, Galperin MY. 2006. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22:3–6. doi: 10.1093/bioinformatics/bti739. [DOI] [PubMed] [Google Scholar]
- 6.Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol 69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leduc JL, Roberts GP. 2009. Cyclic di-GMP allosterically inhibits the CRP-like protein (Clp) of Xanthomonas axonopodis pv. citri. J Bacteriol 191:7121–7122. doi: 10.1128/JB.00845-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao F, He YW, Wu DH, Swarup S, Zhang LH. 2010. The cyclic nucleotide monophosphate domain of Xanthomonas campestris global regulator Clp defines a new class of cyclic di-GMP effectors. J Bacteriol 192:1020–1029. doi: 10.1128/JB.01253-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarro MV, De N, Bae N, Wang Q, Sondermann H. 2009. Structural analysis of the GGDEF-EAL domain-containing c-di-GMP receptor FimX. Structure 17:1104–1116. doi: 10.1016/j.str.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duerig A, Abel S, Folcher M, Nicollier M, Schwede T, Amiot N, Giese B, Jenal U. 2009. Second messenger-mediated spatiotemporal control of protein degradation regulates bacterial cell cycle progression. Genes Dev 23:93–104. doi: 10.1101/gad.502409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang FH, Tian F, Li XT, Fan SS, Chen HM, Wu MS, Yang CH, He CY. 2014. The degenerate EAL-GGDEF domain protein Filp functions as a cyclic di-GMP receptor and specifically interacts with the PilZ-domain protein PXO_02715 to regulate virulence in Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 6:578–589. doi: 10.1094/MPMI-12-13-0371-R. [DOI] [PubMed] [Google Scholar]
- 12.Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR. 2010. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science 329:845–848. doi: 10.1126/science.1190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA. 2011. Cyclic di-GMP activation of polynucleotide phosphorylase signal-dependent RNA processing. J Mol Biol 407:633–639. doi: 10.1016/j.jmb.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Wang YC, Chin KH, Chuah ML, Liang ZX, Chou SH. 2012. Crystallization and preliminary X-ray diffraction studies of Xanthomonas campestris PNPase in the presence of c-di-GMP. Acta Crystallogr Sect F Struct Biol Cryst Commun 68:1247–1250. doi: 10.1107/S1744309112036202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryjenkov DA, Simm R, Romling U, Gomelsky M. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem 281:30310–30314. doi: 10.1074/jbc.C600179200. [DOI] [PubMed] [Google Scholar]
- 16.Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U. 2010. Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. 2010. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell 38:128–139. doi: 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pultz IS, Christen M, Kulasekara HD, Kennard A, Kulasekara B, Miller SI. 2012. The response threshold of Salmonella PilZ domain proteins is determined by their binding affinities for c-di-GMP. Mol Microbiol 86:1424–1440. doi: 10.1111/mmi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merighi M, Lee VT, Hyodo M, Hayakawa Y, Lory S. 2007. The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol Microbiol 65:876–895. doi: 10.1111/j.1365-2958.2007.05817.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Granero F, Navazo A, Barahona E, Redondo-Nieto M, Gonzalez de Heredia E, Baena I, Martin-Martin I, Rivilla R, Martin M. 2014. Identification of flgZ as a flagellar gene encoding a PilZ domain protein that regulates swimming motility and biofilm formation in Pseudomonas. PLoS One 9:e87608. doi: 10.1371/journal.pone.0087608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt JT, Tamayo R, Tischler AD, Camilli A. 2007. PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae. J Biol Chem 282:12860–12870. doi: 10.1074/jbc.M611593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy Y, Ryan RP, O'Donovan K, He YQ, Jiang BL, Feng JX, Tang JL, Dow JM. 2008. The role of PilZ domain proteins in the virulence of Xanthomonas campestris pv. campestris. Mol Plant Pathol 9:819–824. doi: 10.1111/j.1364-3703.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nino-Liu DO, Ronald PC, Bogdanove AJ. 2006. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7:303–324. doi: 10.1111/j.1364-3703.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 24.Das A, Rangaraj N, Sonti RV. 2009. Multiple adhesin-like functions of Xanthomonas oryzae pv. oryzae are involved in promoting leaf attachment, entry, and virulence on rice. Mol Plant Microbe Interact 22:73–85. doi: 10.1094/MPMI-22-1-0073. [DOI] [PubMed] [Google Scholar]
- 25.Yang FH, Tian F, Sun L, Chen HM, Wu MS, Yang CH, He CY. 2012. A novel two-component system PdeK/PdeR regulates c-di-GMP turnover and virulence of Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 25:1361–1369. doi: 10.1094/MPMI-01-12-0014-R. [DOI] [PubMed] [Google Scholar]
- 26.He YW, Wu J, Cha JS, Zhang LH. 2010. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol 10:187. doi: 10.1186/1471-2180-10-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Tian F, Chen HM, Wu MS, He CY. 2013. Binding of transcription regulator ClpXoo to promoter of endoglucanase gene engAXoo was inhibited by c-di-GMP in Xanthomonas oryzae pv. oryzae. Acta Microbiol Sin 53:1166–1171. [PubMed] [Google Scholar]
- 28.Salzberg SL, Sommer DD, Schatz MC, Phillippy AM, Rabinowicz PD, Tsuge S, Furutani A, Ochiai H, Delcher AL, Kelley D, Madupu R, Puiu D, Radune D, Shumway M, Trapnell C, Aparna G, Jha G, Pandey A, Patil PB, Ishihara H, Meyer DF, Szurek B, Verdier V, Koebnik R, Dow JM, Ryan RP, Hirata H, Tsuyumu S, Won Lee S, Seo YS, Sriariyanum M, Ronald PC, Sonti RV, Van Sluys MA, Leach JE, White FF, Bogdanove AJ. 2008. Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics 9:204. doi: 10.1186/1471-2164-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins CM, White FF, Choi SH, Guo A, Leach JE. 1992. Identification of a family of avirulence genes from Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 5:451–459. doi: 10.1094/MPMI-5-451. [DOI] [PubMed] [Google Scholar]
- 31.Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 32.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 33.Ray SK, Rajeshwari R, Sonti RV. 2000. Mutants of Xanthomonas oryzae pv. oryzae deficient in general secretory pathway are virulence deficient and unable to secrete xylanase. Mol Plant Microbe Interact 13:394–401. doi: 10.1094/MPMI.2000.13.4.394. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y, Chern M, Silva FG, Ronald P. 2001. Isolation of a Xanthomonas oryzae pv. oryzae flagellar operon region and molecular characterization of flhF. Mol Plant Microbe Interact 14:204–213. doi: 10.1094/MPMI.2001.14.2.204. [DOI] [PubMed] [Google Scholar]
- 35.Guzzo CR, Salinas RK, Andrade MO, Farah CS. 2009. PILZ protein structure and interactions with PILB and the FIMX EAL domain: implications for control of type IV pilus biogenesis. J Mol Biol 393:848–866. doi: 10.1016/j.jmb.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 36.Tang JL, Feng JX, Li QQ, Wen HX, Zhou DL, Wilson TJ, Dow JM, Ma QS, Daniels MJ. 1996. Cloning and characterization of the rpfC gene of Xanthomonas oryzae pv. oryzae: involvement in exopolysaccharide production and virulence to rice. Mol Plant Microbe Interact 9:664–666. doi: 10.1094/MPMI-9-0664. [DOI] [PubMed] [Google Scholar]
- 37.An S, Wu J, Zhang LH. 2010. Modulation of Pseudomonas aeruginosa biofilm dispersal by a cyclic-di-GMP phosphodiesterase with a putative hypoxia-sensing domain. Appl Environ Microbiol 76:8160–8173. doi: 10.1128/AEM.01233-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furutanin A, Tsuge S, Oku T, Tsuno K, Inoue Y, Ochiai H, Kaku H, Kubo Y. 2003. HpaI secretion via type III secretion system in Xanthomonas oryzae pv. oryzae. J Gen Plant Pathol 69:271–275. doi: 10.1007/s10327-003-0042-2. [DOI] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Tsuge S, Nakayama T, Terashima S, Ochiai H, Furutani A, Oku T, Tsuno K, Kubo Y, Kaku H. 2006. Gene involved in transcriptional activation of the hrp regulatory gene hrpG in Xanthomonas oryzae pv. oryzae. J Bacteriol 188:4158–4162. doi: 10.1128/JB.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sondermann H, Shikuma NJ, Yildiz FH. 2012. You've come a long way: c-di-GMP signaling. Curr Opin Microbiol 15:140–146. doi: 10.1016/j.mib.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christen M, Christen B, Allan MG, Folcher M, Jeno P, Grzesiek S, Jenal U. 2007. DgrA is a member of a new family of cyclic diguanosine monophosphate receptors and controls flagellar motor function in Caulobacter crescentus. Proc Natl Acad Sci U S A 104:4112–4117. doi: 10.1073/pnas.0607738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi Y, Xu L, Dong X, Yau YH, Ho CL, Koh SL, Shochat SG, Chou SH, Tang K, Liang ZX. 2012. Functional divergence of FimX in PilZ binding and type IV pilus regulation. J Bacteriol 194:5922–5931. doi: 10.1128/JB.00767-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazmierczak BI, Lebron MB, Murray TS. 2006. Analysis of FimX, a phosphodiesterase that governs twitching motility in Pseudomonas aeruginosa. Mol Microbiol 60:1026–1043. doi: 10.1111/j.1365-2958.2006.05156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viollier PH, Sternheim N, Shapiro L. 2002. A dynamically localized histidine kinase controls the asymmetric distribution of polar pili proteins. EMBO J 21:4420–4428. doi: 10.1093/emboj/cdf454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao B, Lara-Tejero M, Lefebre M, Goodman AL, Galán JE. 2014. Novel components of the flagellar system in Epsilonproteobacteria. mBio 5(3):e01349-14. doi: 10.1128/mBio.01349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitzer JE, Sultan SZ, Hayakawa Y, Hobbs G, Miller MR, Motaleb MA. 2011. Analysis of the Borrelia burgdorferi cyclic-di-GMP-binding protein PlzA reveals a role in motility and virulence. Infect Immun 79:1815–1825. doi: 10.1128/IAI.00075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bian J, Liu X, Cheng YQ, Li C. 2013. Inactivation of cyclic di-GMP binding protein TDE0214 affects the motility, biofilm formation, and virulence of Treponema denticola. J Bacteriol 195:3897–3905. doi: 10.1128/JB.00610-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan RP. 2013. Cyclic di-GMP signalling and the regulation of bacterial virulence. Microbiology 159:1286–1297. doi: 10.1099/mic.0.068189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen E, Chaudhuri P, Gourley C, Harms J, Splitter G. 2011. Brucella melitensis cyclic di-GMP phosphodiesterase BpdA controls expression of flagellar genes. J Bacteriol 193:5683–5691. doi: 10.1128/JB.00428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Comolli JC, Hauser AR, Waite L, Whitchurch CB, Mattick JS, Engel JN. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect Immun 67:3625–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wairuri CK, van der Waals JE, van Schalkwyk A, Theron J. 2012. Ralstonia solanacearum needs Flp pili for virulence on potato. Mol Plant Microbe Interact 25:546–556. doi: 10.1094/MPMI-06-11-0166. [DOI] [PubMed] [Google Scholar]
- 53.Mahdavi J, Royer PJ, Sjolinder HS, Azimi S, Self T, Stoof J, Wheldon LM, Brannstrom K, Wilson R, Moreton J, Moir JW, Sihlbom C, Boren T, Jonsson AB, Soultanas P, Ala'Aldeen DA. 2013. Pro-inflammatory cytokines can act as intracellular modulators of commensal bacterial virulence. Open Biol 3(10):130048. doi: 10.1098/rsob.130048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guzzo CR, Dunger G, Salinas RK, Farah CS. 2013. Structure of the PilZ–FimXEAL–c-di-GMP complex responsible for the regulation of bacterial type IV pilus biogenesis. J Mol Biol 425:2174–2197. doi: 10.1016/j.jmb.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Burns C, Gregory KE, Kirby M, Cheung MK, Riquelme M, Elliott TJ, Challen MP, Bailey A, Foster GD. 2005. Efficient GFP expression in the mushroom Agaricus bisporus and Coprinus cinereus requires introns. Fungal Genet Biol 42:191–199. doi: 10.1016/j.fgb.2004.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.