Abstract
Background
A thorough understanding of the patient's genotype and their functional response to a medication is necessary for improving event free survival. Several outcome studies support this view particularly if the patient is to be started on clopidogrel due to the prevalence of clopidogrel resistance. Such guided therapy has reduced the incidence of Major Adverse Cardiac Events (MACE) after stent implantation.
Methods
Between August 2013 and August 2014, 200 patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) were prescribed any one of the anti-platelet medications such as clopidogrel, prasugrel or ticagrelor and offered testing to detect CYP2C19 gene mutations along with a platelet reactivity assay (PRA). Intended outcome was modification of anti-platelet therapy defined as either dose escalation of clopidogrel or replacement of clopidogrel with prasugrel or ticagrelor for the patients in clopidogrel arm, and replacement of ticagrelor or prasugrel with clopidogrel if those patients were non-carrier of mutant genes and also if they demonstrated bleeding tendencies in the ticagrelor and prasugrel arms.
Conclusion
Clopidogrel resistance was observed to be 16.5% in our study population. PRA was useful in monitoring the efficacy of thienopyridines. By having this test, one can be safely maintained on clopidogrel in non-carriers, or with increased dose of clopidogrel in intermediate metabolizers or with newer drugs such as ticagrelor or prasugrel in poor metabolizers. Patients on ticagrelor and prasugrel identified as non-carriers of gene mutations for clopidogrel metabolism could be offered clopidogrel resulting in economic benefits to the patients. Patients at high risk of bleeding were also identified by the PRA.
Keywords: PCI, CYP2C19 genotype, Platelet reactivity assay
1. Introduction
Over the last decade, dual anti-platelet therapy (APT) has been the mainstay of the management of acute coronary symptoms, and also Percutaneous Coronary Intervention (PCI). Aspirin, while effective, has been found to be relatively ineffective alone, in comparison to using it in combination with one of the three thienopyridines.1
However, despite advances in therapeutic options post PCI, many patients continue to suffer recurrent ischemia or bleeding events. High or Low on-treatment platelet reactivity is a major cause of not only increased morbidity but also mortality during post coronary interventions.2 This sub-optimal effect of APTs is mainly due to the choice of the medication and to the variability in response of a patient's platelets.
Of the thienopyridines, clopidogrel is most commonly prescribed due to lower cost and better efficacy compared to aspirin alone. A major disadvantage of using clopidogrel is the high variability in its bioavailability. Several variants of the CYP2C19 gene affect the conversion of clopidogrel pro-drug into its active form thus altering the platelet reactivity in an individual.3 In addition, several concomitant medications are also known to affect the efficacy of the active form of clopidogrel. These factors prompt the need for tests that determine the genotype of the patient and also measure the functional response of the patient's platelets in response to the APT administered. Previous studies have shown that such guided therapy not only reduces the Major Adverse Cardiac Events (MACE) post PCI, but also improves overall progression free survival.4–6
1.1. Clopidogrel resistance
It is the persistent activity of P2Y12 receptors on platelets even after treatment with clopidogrel. In laboratory terms, it depends on the different tests used to quantify residual platelet activity and also the cut off values. Three variants (*2, *3, and *17, with *1 being the wild-type) have been demonstrated to have a profound effect on the bio-availability of clopidogrel. Poor metabolizers of clopidogrel (*2/*2, *2/*3, and *3/*3) have a lack of bioavailability of active clopidogrel hence they are termed clopidogrel resistant, while the intermediate metabolizers (*1/*2, *1/*3, *2/*17, and *3/*17) have a reduced bioavailability but still have the ability to inhibit platelet activity but at a higher dosage. Rapid metabolizers (*1/*17) and ultra-rapid metabolizers (*17/*17) have a higher than normal bioavailability of clopidogrel and may benefit by a reduced dose. The ‘Poor Metabolizer’ variants *2 and *3 are loss-of-function mutations leading to a reduction in the availability of the active form of clopidogrel.5 Previous studies have shown that there is 55–76% increase in relative risk or cardiovascular death, myocardial infarction, or stroke as well as a 2.6–4.0 fold increase in the risk of stent thrombosis in patients with decreased response to clopiogrel.7
Conversely, the ‘Rapid Metabolizer’ variant *17, leads to increase in function and so patients have an increased risk of bleeding. In PCI patients with homozygous gain of function alleles, bleeding risk can be as high as 4-fold compared to similar patients with normal CYP2C19 genotype.8
In addition to genotype, several other factors affect the bio-availability of all thienopyridines. Changes in absorption of the medication, co-morbid conditions like diabetes mellitus, high BMI, low ejection fraction, and drug–drug interactions are some of these. Platelet reactivity monitoring has therefore, proven effective in reducing post-PCI complications in patients on prasugrel or ticagrelor.9–11
1.2. The platelet reactivity assay (PRA)
It is highly specific and sensitive compared to the commonly used Light Transmission Aggregometry (LTA), and correlates well with the plasma levels of anti-platelet medication. The value of this assay in preventing post-procedural MACE in patients who underwent PCI has been demonstrated in several studies world-wide.12–14
A Platelet Reactivity Index (PRI), determined as the VASP phosphorylation between the activated and inactivated states of P2Y12 receptor. It is used to express the functional status of the platelets with a cutoff value of >50% implies significant platelet activity and <16% implies significant bleeding risk.
The aim of this study was to determine the impact of assessment of CYP2C19 genetic variation and platelet reactivity assay in post PCI patients. The patients were given any of the three thienopyridines on cardiologist's choice without compromising patient treatment. Based on the results obtained, recommendations were made either as dose escalation of clopidogrel, or replacement with prasugrel or ticagrelor for patients with mutations in the CYP2C19 gene in the clopidogrel arm. Patients on prasugrel or ticagrelor with normal genotype but with increased risk of bleeding were recommended to switch to clopidogrel.
2. Materials and methods
2.1. Recruitment of patients
Between August 2013 and August 2014, 200 patients with coronary artery disease undergoing PCI were prescribed any one of the APTs such as clopidogrel, prasugrel or ticagrelor. These patients were offered a combination of genetic profiling for the presence of three variants of CYP2C19 and a platelet function test (PFT) for evaluating platelet reactivity. Two centres of Apollo hospitals at Hyderabad and at Bhubaneswar participated in the study.
All the patients were recruited for the data collection with due informed consent. Ethical norms were followed and the study was approved by the Apollo ethics committee.
2.2. Collection of blood sample
Peripheral blood was collected in a citrate tube for PRA. Care was taken to minimize agitation of the platelets during the process of blood collection and transport. Samples were processed within 72 h.
2.3. Genotyping assay
Genomic DNA was extracted from whole blood using the GenElute Blood Genomic DNA extraction kit (Sigma–Aldrich Company LLC, St Louis, MO, USA) according to the manufacturer's recommendations. The amount of DNA was quantitated using a UV spectrophotometer (Nanodrop Spectrophotometer, Thermo Scientific, Wilmington, DE).
The presence or absence of the variants were detected using the PCR-RFLP method.15,16 In the first step, 100–200 ng of genomic DNA was PCR amplified using a touchdown PCR method (Mastercycler Pro S, Eppendorf, Hamburg, Germany). The positions of the SNP and the primer sequences used are given in Table 1.
Table 1.
Sequence of primers used for PCR amplification of the region of interest of the CYP2C19 gene.
| Variant | Position on gene | Change | Primer name | Primer sequence | Length | Size of product |
|---|---|---|---|---|---|---|
| *2 | 681 | G to A | V2f | AATTACAACCAGAGCTTGGC | 20 | 169 bp |
| V2r | TATCACTTTCCATAAAAGCAAG | 22 | ||||
| *3 | 636 | G to A | V3f | TATTATTATCTGTTAACTAATATGA | 25 | 329 bp |
| V3r | ACTTCAGGGCTTGGTCAATA | 20 | ||||
| *17 | −806 | C to T | V17f | AAATTTGTGTCTTCTGTTCTCAATG | 25 | 143 bp |
| V17r | AGACCCTGGGAGAACAGGAC | 20 |
In the second step, the PCR product obtained was digested with specific restriction enzymes (New England Biolabs, Ipswich, MA) as given in Table 2.
Table 2.
List of restriction enzymes used and the DNA band sizes obtained in the presence or absence of specific genetic variations in the CYP2C19 gene.
| Variant | Restriction enzyme | Fragment sizes |
|
|---|---|---|---|
| Presence of variant | Absence of variant | ||
| Variant 2 | SmaI | 120 bp 49 bp |
169 bp |
| Variant 3 | BamHI | 233 bp 96 bp |
329 bp |
| Variant 17 | NsiI | 116 bp 27 bp |
143 bp |
2.4. Functional assay
The response of platelets to the current APT was determined using the PLT VASP/P2Y12 flow cytometry kit (Biocytex, Marseille, France), according to the manufacturer's recommendations. Changes in the phosphorylation status of VASP protein were measured by flow cytometry (FC-500, Beckman–Coulter, Brea, CA), following which a Platelet Reactivity Index was calculated as per manufacturer recommendations to measure the reactivity of the platelets in the presence of APT.17
3. Results
Of the 200 patients, 50 each were on clopidogrel and prasugrel while the ticagrelor arm had 100 patients. Patient characteristics have been elucidated in Table 3. The patients were found to be compliant with the cardiologist prescribed regimen and were on the medication for enough duration for it to have achieved plasma steady state.
Table 3.
General characteristics of patients based on the medication prescribed.
| Total | Clopidogrel | Prasugrel | Ticagrelor | |
|---|---|---|---|---|
| No. of patients | 200 | 50 | 50 | 100 |
| Male | 123 | 27 | 28 | 68 |
| Female | 77 | 23 | 22 | 32 |
| Age (mean) | 62 | 60 | 61 | 63 |
| Co-morbidities | ||||
| Diabetes mellitus | 94 | 23 | 27 | 44 |
| Hypertension | 71 | 19 | 21 | 31 |
| Dyslipidemia | 112 | 33 | 36 | 43 |
| CAD lesions | ||||
| RCA | 54 | 16 | 17 | 21 |
| LAD | 110 | 23 | 20 | 67 |
| LCX | 36 | 12 | 9 | 15 |
3.1. High frequency of reduced or poor metabolizer mutations in Indian population
A functional wild-type CYP2C19 gene (*1/*1) is needed for the conversion of clopidogrel into its active form. Mutations in the gene can increase or decrease its metabolism causing the individual to either be an ultra-rapid metabolizer (*17/*17), or, Rapid (*1/*17), Intermediate (*1/*2, *1/*3, *2/*17, *3/*17) or Poor metabolizer (*2/*2, *3/*3, *2/*3).
Results obtained with the genotype assay from 200 patients suggest that only 19.5% (n = 39) of patients had normal function of the CYP2C19 gene (also called as Extensive Metabolizers), while the remaining patients were carriers of one of the three variants studied [Fig. 1]. The intermediate metabolizers formed the highest percentage (47%, n = 94) with regards to clopidogrel metabolism. Ultra-rapid metabolizers (2.5%, n = 5), rapid metabolizers (14.5%, n = 29), and poor metabolizers (16.5%, n = 33) made up the rest of the population.
Fig. 1.
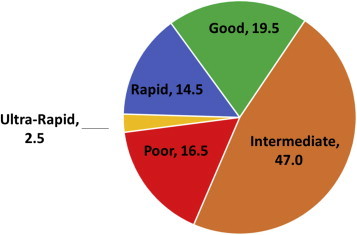
Percentage of the various genotypes in 200 post PCI patients.
Our results suggest that up to 80% the patients could benefit from clopidogrel potentially using the PRA to guide optimal dosage.
3.2. VASP assay showed good correlation of platelet reactivity with the genotype in patients on clopidogrel
Patients responding well to any of the three APTs are expected to have a PRI below 50%. In the clopidogrel cohort (n = 50), our results from the PRA demonstrate positive correlation with the genotype status of the patients [Fig. 2], in agreement with published data from Nakata T et al, Siller-Matula et al, and other studies.18,19
Fig. 2.
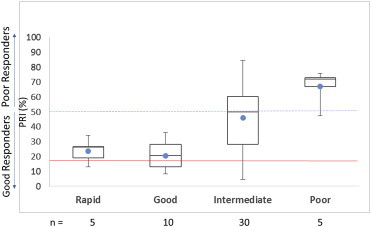
PRI in patients on clopidogrel as determined by the PRA categorized by their genotype. The solid red line denotes a PRI value of 16% which denotes the upper limit of an increased bleeding risk. The hashed blue line denotes PRI value of 50% representing the upper limit for good response in the PRA. Solid blue dot denotes the mean PRI value for that group.
Patients with an increased rate of clopidogrel metabolism (ultra-rapid and rapid metabolizers combined) and normal metabolizer status responded well to typically prescribed dose of clopidogrel with a mean PRI value of 20.6% for patients with normal genotype and 23.8% for patients with increased metabolism genotype. As expected patients with poor metabolizer status demonstrated inadequate response in the function test with the mean PRI value of 67%. Interestingly, patients with an intermediate metabolizer genotype that formed the largest group, and were on clopidogrel (n = 30) varied greatly in their response to clopidogrel, ranging from 4.5% to 84.6% on the PRI, PRA was valuable in determining which of these patients could be maintained on clopidogrel versus those with PRI >50 needing a change in drug or dosage.
3.3. Higher incidence of bleeding risk with prasugrel and ticagrelor
For patients on prasugrel or ticagrelor, there was no correlation between the observed PRI and metabolizer status as expected [Fig. 3]. The average mean value across the different metabolizer groups for ticagrelor (PRI 15.6%) and for prasugrel (13.9%) was well below the average mean value seen for the rapid and normal metabolizer groups for clopidogrel (22.2%). Previous studies by several groups have shown that PRI values lower than 16 have been associated with increased risk of bleeding in patients undergoing PCI-stent procedure. The overall potential for risk of bleeding was higher in patients on prasugrel and ticagrelor across all the different metabolizer status.
Fig. 3.
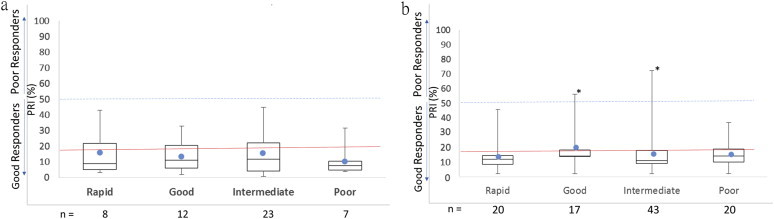
Platelet reactivity in patients on prasugrel (a) and on ticagrelor (b) as seen by the PRA categorized based on their genotype. The solid red line denotes a PRI value of 16% which denotes the upper limit of an increased bleeding risk. The hashed blue line denotes a PRI value of 50% recommended as the upper limit for a good response in the PR assay. Solid blue dot denotes the mean PRI value for that group.
3.4. Impact of genotype and PFT on choice of patient medication
The main impact of using a combination of the genotype and functional assay for platelet reactivity is in determining the optimal drug and dosage of thienopyridines. Our results suggest that only 20–25% of the patients were on optimal drug and dose at the time of the assay [Table 4]. More importantly about 60% of the patients were recommended a change in their current drug or dose to improve efficacy or safety. More importantly about 60% of the patients were recommended a change in their current drug or dose to improve efficacy or safety. In the clopidorgel arm, about 72% of the patients did not need to change the medication. In the Ticagrelor arm, 34% of the patients could be changed to clopidogrel because of economic reasons but about 40% were recommended a change due to bleeding risk. Similarly, in the prasugrel arm, about 37% of the patients were recommended a change in drug due to risk of bleeding while 52% of patients were recommended a change to get economic benefit.
Table 4.
Snapshot of changes recommended in the study population based on the results obtained.
| Observation | Percentage |
|---|---|
| Patients that were not on optimal drug or dose | 79 |
| A change in drug or dose was recommended for either efficacy or safety | 62 |
| Increased monitoring due to increased risk of bleeding as patients could not be switched to clopidogrel | 15 |
| Change to clopidogrel from a costlier medication based on genotype | 8–12 |
As seen in Fig. 4, though the risk of thrombosis was low based on the observed PRI in prasugrel and ticagrelor patients, the risk of bleeding is higher compared to clopidogrel. For prasugrel, 66% of the patients and 67% of patients in ticagrelor group had PRI value less than 16% while in clopidogrel cohort, only 20% patients fall in that category. However, 35% of the patients on clopidogrel do not respond to the medication at the prescribed dose and so have a higher risk of thrombosis.
Fig. 4.
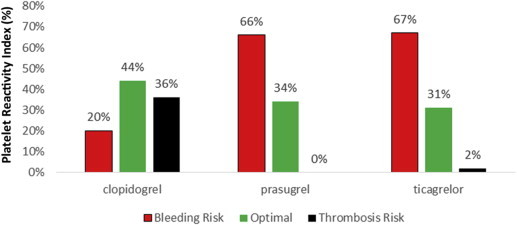
Observed platelet reactivity to the prescribed medication.
When translated to the clinical setting, the impact of the test is in providing recommendation to the physician on the APT that would benefit the patient best both in terms of safety and efficacy, as well as economically.
4. Discussion
The safety and improved long term outcome in post-PCI patients are dependent upon the right medication and its dosage during the maintenance phase. Clopidogrel, which is the most commonly prescribed anti-platelet medication, needs to be metabolized to an active form with the activity of a cytochrome P450. Variants of this enzyme due to mutations in the gene CYP2C19, can lead to changes in the metabolism of clopidogrel and its bio-availability. Taking these into consideration, the USFDA and ACC/AHA (American College of Cardiology/American Heart Association) recommended a B2 status for personalized clopidogrel APT.
A thorough understanding of the genotype and functional response to the given medication is therefore needed to provide an optimal benefit to the patient. In case of prasugrel and ticagrelor, this is even more important as both the medications are more expensive compared to clopidogrel.
The goal of this study was to assess the impact of genotype and platelet functional assays on optimizing therapy options in patients who underwent PCI and were prescribed one of the thienopyridines as maintenance therapy.
Our results showed good correlation with previous studies on the distribution of the different variants in Indian population. Studies by Jose et al (2004),20 Adithan et al (2003)15 and Anichavezhi et al (2012)16 demonstrated that variant 2 had higher presence in healthy Indian volunteer population compared to Caucasians (Table 5).21,22
Table 5.
Indian data was compiled from Adithan et al and Anichhavezhi et al. Caucasian data shown here was adapted from Mega et al and shows a combination of both heterozygous and homozygous mutants (Mega et al, 2009).25
| Metabolizer status | CYP2C19 genotype | Observed frequency of CYP2C19 gene mutations (%) |
||
|---|---|---|---|---|
| Caucasians | Indian data | Present study | ||
| Extensive | *1/*1 | 63–69 | 19.5–39.4 | 19.5 |
| Intermediate | *1/*2 | 21.6–41.9 | 36.5 | |
| *1/*3 | 15 | 1 | ||
| *2/*17 | 12–30 | 9.5 | ||
| *3/*17 | NA | 0% | ||
| Poor | *2/*2 | 13–19 | 10.9–28.1 | 16.5 |
| *3/*3 | <1 | 0 | 0 | |
| *2/*3 | 1 | 0 | ||
| Rapid | *1/*17 | 12.8–30.7 | 14.5 | |
| Ultra-rapid | *17/*17 | 18* | 0.03–6.2 | 2.5 |
Based on a study with 112 subjects of Tamilian ethnicity, Adithan et al (2003)15 reported an allele frequency of 0.37 for the variant 2 compared to 0.4 in our study. Patients enrolled in our study are cosmopolitan yet compare similarly with the reported frequencies of the variant 2. In contrast, compiled data from European Americans, Turks and Germans show an allele frequency of 0.19 for the variant 2.
Clopidogrel is the only thienopyridine that is influenced by variations in the CYP2C19 gene and individuals having a null variant are at great risk when using clopidogrel. In addition to genetic variations several other factors including co-medications also influence the bio-availability of clopidogrel. Depending only on the genotype therefore may not provide all the relevant information to make a rationale choice of the drug and its dosage. This is especially true for prasugrel and ticagrelor which do not depend on the CYP2C19 gene on their bio-availability.
Even with the available new generation medications, the risk of MACE is still elevated. It was found that in patients undergoing PCI in ACS and who receive prasugrel, 25.2% of patients have a higher incidence of MACE even after a 60 mg loading dose. Moreover, the risk of increased bleeding after prasugrel loading dose is also higher in a subset of patients.23 PLATO study observed that in patients with ACS with or without ST-segment elevation, treatment with ticagrelor significantly reduced the rate of death from vascular causes, MI or stroke without increasing the overall rate of major bleeding. Nevertheless, there was an increase in the rate of non-procedure related bleeding.24
In our study with PCI patients, we observed a much lower on-treatment platelet reactivity in patients who were on maintenance dose of prasugrel or ticagrelor. The PRI value of all 50 patients in the prasugrel cohort, tested for platelet reactivity corresponded to good responder category with PRI ranging from 0.4 to 44.7%. However, the mean value of PRI across all different genotypes was 13.9% suggesting a higher potential for bleeding risk in patients on prasugrel. 64% of the population was under 16% PRI value which is the higher end for increased bleeding risk is of significant concern.
The occurrence of bleeding risk was no different in the ticagrelor cohort, with the PRI ranging from 1.07 to 47.5% in good responders with a mean of 15.6% which is on the cusp of the cut-off value of 16% for the bleeding risk. By PRA, 65% of the population had an increased bleeding risk with the recommended dosage of ticagrelor.
As expected, patients on clopidogrel had a varied response with the PR assay. The observed on-treatment reactivity of platelets to clopidogrel correlated well with the genotype information for the patients. In the group consisting of rapid and ultra-rapid metabolizer genotype, the range of PRI values was 12.9%–34.2% with an average of 23.8%. In the extensive (normal) metabolizers, the PRI values ranged from 8.2% to 36.1% with an average of 20.6% outside the cut-off value for the bleeding risk and well within the good responder group according to the PR assay.
The intermediate metabolizers formed the major subset in the clopidogrel group with 60% based on the genotype. Within the intermediate metabolizer group, the PRI values ranged from 4.5% to 84.6% with a mean value of 46.1%. In our assessment, the intermediate metabolizers would benefit the most with a dose adjustment when clopidogrel was prescribed during the maintenance regimen. Bonello et al (2008)8 demonstrated benefit after clopidogrel dose adjustment in patients with heterozygous loss of function variant.
5. Study limitations
There are limitations to our study. Our study included a relatively small number of patients. However, it gives insights into clinical practice patterns. Though the genotype analysis is valuable, at present there are no clear cut recommendations to modify the treatment regimes based on the results obtained from genotype and phenotypic study. Those patients who were not willing to undergo the test mainly due to financial reasons were excluded from the study.
6. Conclusion
Genetic testing for polymorphism of clopidogrel metabolism and platelet reactivity assay demonstrates the value of having individualized anti-platelet therapy. Clopidogrel resistance is prevalent (16.5%) in our study population. By having this test, one can be safely maintained on clopidogrel in non-carriers, or with increased dose of clopidogrel in intermediate metabolizers or with newer drugs such as ticagrelor or prasugrel in poor metabolizers. In the era of newer potent anti-platelet drugs such as prasugrel and ticagrelor, this test will help in individualizing the anti-platelet therapy and also helps to predict the bleeding complications. Patient groups on ticagrelor and prasugrel when found non-carriers of mutation genes for clopidogrel metabolism can be switched over to clopidogrel, which proves the economical benefit and safety to the patients. Randomized control trials to study about the validity, cost effectiveness of this test and the clinical outcomes are needed.
Conflicts of interest
All authors have none to declare.
Contributions
Dr. P.C. Rath: Co ordinator and study design.
Dr. Sundar Chidambaram: Manuscript preparation, Follow up.
Dr. Pallavi Rath M.B.B.S: Clinical data collection.
Dr. Byomakesh Dikshit: Patient data maintenance.
Dr. Sudhir Naik: Manuscript correction and statistics.
Dr. Prashant K Sahoo: Patient follow up, Bhubaneshwar.
Dr. Brajraj Das: Data collection Bhubaneswar.
Dr. Jugnu Jain: Data collating and assistance in manuscript preparation.
Dr. Lakshmipathi Khandrika Ph.D.: Genotype assay design and validation.
Mr. Mohanshankar Mahalingam M.Sc.: Platelet reactivity assay data collection.
Acknowledgments
We wish to thank Dr. Sasidhar Manda Ph.D Apollo Hospital Education and Research Foundation. Dr. Vidya L Parsam Ph.D Sapiens Biosciences Pvt Ltd. Mr. Babul Reddy Tatireddy M.Sc Apollo Hospital education and research Foundation, Dr. Vanajakshi, Department of Haematology, Apollo, Jubilee Hills for access to flow cytometry analysis. Dr. Aparna Yerramilli, Mr. Dileep RR Vollala and Mr. Venkat S Shanker, Shri Venkateshwara College of pharmacy for help in data collection.
References
- 1.Yusuf S., Zhao F., Mehta S.R. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 2.Steinhubl S.R., Berger P.B., Mann J.T., III Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 3.Holmes D.R., Jr., Dehmer G.J., Kaul S., Leifer D., O'Gara P.T., Stein C.M. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56:321–341. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Scott S.A., Sangkuhl K., Stein C.M., Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonello L., Camoin-Jau L., Arques S. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol. 2008;51:1404–1411. doi: 10.1016/j.jacc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Tantry U.S., Bonello L., Aradi D. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;6:2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 7.Mega J.L., Simon T., Collet J.P. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibbing D., Koch W., Gebhard D. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–518. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos D., Xanthopoulou I., Storey R.F. Platelet reactivity during ticagrelor maintenance therapy: a patient-level data meta-analysis. Am Heart J. 2014;168:530–536. doi: 10.1016/j.ahj.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Cuisset T., Frere C., Poyet R. Clopidogrel response: head-to-head comparison of different platelet assays to identify clopidogrel non responder patients after coronary stenting. Arch Cardiovasc Dis. 2010;103:39–45. doi: 10.1016/j.acvd.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Angiolillo D.J., Badimon J.J., Saucedo J.F. A pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: results of the Optimizing anti-Platelet Therapy in diabetes MellitUS (OPTIMUS)-3 Trial. Eur Heart J. 2011;32:838–846. doi: 10.1093/eurheartj/ehq494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayla G., Macia J.C., Rabesandratana H. Flow cytometric assessment of vasodilator-stimulated phosphoprotein: prognostic value of recurrent cardiovascular events after acute coronary syndromes. Arch Cardiovasc Dis. 2008;101:743–751. doi: 10.1016/j.acvd.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Frere C., Cuisset T., Quilici J. ADP-induced platelet aggregation and platelet reactivity index VASP are good predictive markers for clinical outcomes in non-ST elevation acute coronary syndrome. Thromb Haemost. 2007;98:838–843. [PubMed] [Google Scholar]
- 14.Morel O., Faure A., Ohlmann P. Impaired platelet responsiveness to clopidogrel identified by flow cytometric vasodilator-stimulated phosphoprotein (VASP) phosphorylation in patients with subacute stent thrombosis. Thromb Haemost. 2007;98:896–899. [PubMed] [Google Scholar]
- 15.Adithan C., Gerard N., Vasu S., Rosemary J., Shashindran C.H., Krishnamoorthy R. Allele and genotype frequency of CYP2C19 in a Tamilian population. Br J Clin Pharmacol. 2003;56:331–333. doi: 10.1046/j.1365-2125.2003.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anichavezhi D., Chakradhara Rao U.S., Shewade D.G., Krishnamoorthy R., Adithan C. Distribution of CYP2C19*17 allele and genotypes in an Indian population. J Clin Pharm Ther. 2012 Jun;37:313–318. doi: 10.1111/j.1365-2710.2011.01294.x. [DOI] [PubMed] [Google Scholar]
- 17.Barragan P., Bouvier J.L., Roquebert P.O. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter Cardiovasc Interv. 2003;59:295–302. doi: 10.1002/ccd.10497. [DOI] [PubMed] [Google Scholar]
- 18.Siller-Matula J.M., Delle-Karth G., Lang I.M. Phenotyping vs. genotyping for prediction of clopidogrel efficacy and safety: the PEGASUS-PCI study. J Thromb Haemost. 2012;10:529–542. doi: 10.1111/j.1538-7836.2012.04639.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakata T., Miyahara M., Nakatani K. Relationship between CYP2C19 loss-of-function polymorphism and platelet reactivities with clopidogrel treatment in Japanese patients undergoing coronary stent implantation. Circ J. 2013;77:1436–1444. doi: 10.1253/circj.cj-12-1095. [DOI] [PubMed] [Google Scholar]
- 20.Jose R., Chandrasekaran A., Sam S.S. CYP2C9 and CYP2C19 genetic polymorphisms: frequencies in the south Indian population. Fundam Clin Pharmacol. 2005;19:101–105. doi: 10.1111/j.1472-8206.2004.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Scott S.A., Sangkuhl K., Gardner E.E. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90:328–332. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuisset T., Loosveld M., Morange P.E. CYP2C19*2 and *17 alleles have a significant impact on platelet response and bleeding risk in patients treated with prasugrel after acute coronary syndrome. JACC Cardiovasc Interv. 2012;5:1280–1287. doi: 10.1016/j.jcin.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Bonello L., Pansieri M., Mancini J. High on-treatment platelet reactivity after prasugrel loading dose and cardiovascular events after percutaneous coronary intervention in acute coronary syndromes. J Am Coll Cardiol. 2011;58:467–473. doi: 10.1016/j.jacc.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Wallentin L., Becker R.C., Budaj A. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 25.Mega J.L., Close S.L., Wiviott S.D. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]


