Abstract
Pharmacological induction of the fetal γ globin gene and the consequent formation of HbF (α2/γ2) in adult erythroid cells are one feasible therapeutic strategy for sickle cell disease (SCD) and severe β-thalassemias. Hydroxyurea (HU) is the current drug of choice for SCD, but serious side effects limit its clinical use. Moreover, 30 to 50% of patients are irresponsive to HU treatment. We have used high-throughput screening to identify benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one and its derivatives (compounds I to VI) as potent γ globin inducers. Of the compounds, I to V exert superior γ globin induction and have better therapeutic potential than HU, likely because of their activation of the p38 mitogen-activated protein kinase (MAPK) signaling pathway and modulation of expression levels and/or chromosome binding of γ globin gene regulators, including BCL11A, and chromatin structure over the γ globin promoter. Unlike sodium butyrate (NaB), the global levels of acetylated histones H3 and H4 are not changed by compound II treatment. Remarkably, compound II induces the γ globin gene in HU-resistant primary human adult erythroid cells, the p38 signaling pathway of which appears to be irresponsive to HU and NaB as well as compound II. This study provides a new framework for the development of new and superior compounds for treating SCD and severe β-thalassemias.
INTRODUCTION
The expression of α-like globin chains (ζ [embryonic] and α [fetal/adult]) and β-like globin chains (ε [embryonic], γ [fetal], and β [adult]) is under temporal control during human development. Genetic defects such as deletions or mutations inside the globin gene loci result in abnormal expression of the hemoglobins and consequent hemoglobinopathies (1). β-Thalassemia and sickle cell disease (SCD) are the two most common hemoglobinopathies (2). Patients with severe β-thalassemia are defective in generation of the adult β globin chain and become profoundly anemic when the hemoglobin switch is completed from HbF (α2/γ2) to HbA (α2/β2). With the generation of functional HbA impaired, patients with β-thalassemia major require regular blood transfusion to replenish their HbA for survival. On the other hand, patients with SCD produce mutated sickle hemoglobin HbS (α2/βs2), which polymerizes under low-oxygen conditions and distorts the red blood cells into the characteristic sickle shape (3).
Pharmacologic induction of HbF expression has been suggested to be a useful strategy for modulation of anemia and related symptoms in severely hemoglobinopathic patients (4–6). The elevation of fetal γ globin chain synthesis balances the excess of α globin chain by formation of HbF, thus modulating severe anemia in β-thalassemia major patients and directly inhibiting the polymerization of HbS in SCD patients (7, 8). So far, several chemotherapeutic agents, such as the histone deacetylase inhibitors (HDACi), e.g., trichostatin A (TSA), apicidin, sodium butyrate (NaB), etc.; short-chain fatty acid (SCFA) derivatives, e.g., 2,2-dimethylbutyrate (HQK-1001), NaB, etc.; DNA methyltransferase inhibitors, e.g., 5′-azacytidine; and ribonucleotide reductase inhibitors, e.g., hydroxyurea (HU), have been shown to be able to stimulate HbF production (9–15). Among them, HU is the only U.S. FDA-approved therapeutic drug for the treatment of SCD despite its side effects, including leucopenia, thrombocytopenia, myelosuppression, and potential reproductive toxicity (16, 17). However, a notable proportion of hemoglobinopathic patients have poor outcomes or no response to HU treatment (18, 19). A search for new γ globin-inducing agents for treating the hemoglobinopathies, including β-thalassemia major and SCD, is thus warranted.
In this study, we used a simple dual-fluorescence reporter to screen for potential HbF-inducing compounds and investigated the possible mechanisms underlying the reactivation of the γ globin gene. Benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one as well as several of its derivatives was identified to potently increase the fetal γ globin gene expression in the adult erythroid cells. These heterocyclic compounds modulate the expression of several γ globin regulators as well as the activation of p38 mitogen-activated protein kinase (MAPK) signaling, both of which are known to contribute to γ globin gene activation in adult erythroid cells (20–24). Significantly, this series of compounds, as exemplified by compound II, could induce γ globin gene expression in HU-resistant as well as HU-responsive human adult erythroid cells. Therefore, these compounds can be considered highly promising for development into a new generation of therapeutic drugs for SCD and β-thalassemia major.
MATERIALS AND METHODS
Ethics statement.
This study was conducted with the approval of the Human Subject Research Ethics Committee/IRB, Academia Sinica (AS-IRB01-10039). Written informed consent was obtained from all blood donors.
Chemical compounds.
A chemical library used for the screening in this study was from the National Health Research Institutes (NHRI) at Taiwan and contains 10,000 heterocyclic compounds with drug-like structures. Compound II was purchased from ChemDiv.
Reporter constructs.
The reporter plasmid pmLAR-Gp-DsRed-Bp-d2EGFP was constructed by multiple steps of a subcloning process. In brief, an 8,003-bp mini-LAR (mLAR) excised from pLAR-β (25) and a 1,622-bp β globin promoter (Bp) generated by PCR amplification were cloned into pd2EGFP-1 to generate pmLAR-Bp-d2EGFP. A 1,377-bp γ globin promoter (Gp) was subcloned into pDsRed-Monomer-C1 to generate pGp-DsRed-C1. The Gp-DsRed fragment was then excised from the pGp-DsRed-C1 plasmid and reinserted between the mLAR and Bp-d2EGFP of pmLAR-Bp-d2EGFP. A 759-bp enhancer-containing fragment 3′ to the Aγ globin gene (26) was generated by PCR and inserted downstream of the DsRed gene.
Cell culture.
Mouse erythroleukemic MEL cells (27) were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 20% fetal bovine serum (FBS), 1% penicillin, and 1% streptomycin. Human erythroleukemic K562 cells (28) were maintained in RPMI 1640 medium (Gibco) supplemented with 10% FBS, 1% penicillin, and 1% streptomycin. All cells were incubated in a 37°C chamber under a 5% CO2 humidified atmosphere. For establishment of the stable cell lines, MEL cells or K562 cells were transfected with the reporter pmLAR-Gp-DsRed-Bp-d2EGFP and selected with 700 μg/ml of neomycin. Preparation of the primary human adult erythroid cells followed the standard procedure of the two-phase liquid culture method (20). The peripheral blood from healthy blood donors was provided by the Taipei Blood Center. In brief, the culture was initiated by maintaining the purified peripheral blood mononuclear cells in the first-phase culture medium, the serum-free expansion medium (SFEM) with 1× cc100 cytokine mix (100 ng/ml Flt-3 ligand, 100 ng/ml stem cell factor [SCF], 20 ng/ml interleukin-3 [IL-3], 20 ng/ml IL-6) (StemSpan), for 7 days. In the phase II culture, cells were kept in the differentiation medium (SFEM with 20 ng/ml SCF, 1 U/ml erythropoietin [EPO], 5 ng/ml IL-3, 2 μM dexamethasone) for another 7 days at a density of 0.1 × 106 to 1 × 106 cells/ml. The differentiated primary erythroid cells were then treated with the indicated chemical compound(s) in the differentiation medium for 3 days and harvested for further analysis. The morphology of the compound-treated primary erythroid cells was analyzed by the Liu staining assay, a rapid and simple histologic stain modified from the Romanowsky stain (29). The culture medium and compound(s) were refreshed every 2 or 3 days. To pretest the responsiveness of a primary erythroid culture to HU treatment, one-half of each peripheral blood sample was differentiated into primary erythroid cells and then treated with HU (147 μM) for 3 days. Total mRNA was then harvested for analysis of the γ globin gene induction by reverse transcription-quantitative PCR (RT-qPCR). For high-performance liquid chromatography (HPLC) analysis, the cells were treated with 10 μM compound II at the beginning of phase II culture and continuously cultured for 10 days. The hemolysates were prepared from cells using osmotic lysis in water with three freeze-thaw cycles, and debris was removed by centrifugation. HPLC was carried out in the clinical laboratories of National Taiwan University Hospital using clinically calibrated standards for the human hemoglobins.
Robotic screening of chemical compounds.
MEL cells carrying the dual-fluorescence reporter were treated with 10 μM individual compounds for 3 days and primarily scanned for DsRed intensity using a Wallac Victor3 1420 multilabel counter (excitation, 550/9; emission, 620). The coefficient of variation (% CV) value of 8% and a z-factor value of 0.6 confirmed the quality of the screening. NaB and 0.1% dimethyl sulfoxide (DMSO) were used as positive and negative controls, respectively. The secondary screening was performed using a digital image detector, the Cellomics Arrayscan 3.0 system. The expression levels of the endogenous globin genes in the positive hits from the high-throughput screening were subsequently analyzed by RT-qPCR analysis. HU and NaB were used as reference compounds.
RT-qPCR analysis.
Total RNAs were isolated from the cells by using the RNAspin minikit (GE Healthcare). cDNA synthesis was carried out using SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was then performed using SYBR green PCR master mix (Applied Biosystems) and the ABI 7500 real-time system. All data were analyzed after normalization to the expression level of the human β-actin gene. All primer sequences used for RT-qPCR are as follows: ε globin forward primer, 5′-GCAAGAAGGTGCTGACTTC-3′; ε globin reverse primer, 5′-CCTTGCCAAAGTGAGTAGC-3′; γ globin forward primer, 5′-CTTCCTTGGGAGATGCCAT-3′; γ globin reverse primer, 5′-GAATTCTTTGCCGAAATGGAT-3′; δ globin forward primer, 5′-CTTTAGTGATGGCCTGGCT-3′; δ globin reverse primer, 5′-GATAGGCAGCCTGCATTTG-3′; β globin forward primer, 5′-CTTTAGTGATGGCCTGGCT-3′; β globin reverse primer, 5′-CACTGGTGGGGTGAATTCT-3′; BCL11A forward primer, 5′-TGGTATCCCTTCAGGACTAGGT-3′; BCL11A reverse primer, 5′-TCCAAGTGATGTCTCGGTGGT-3′; c-Myb forward primer, 5′-ACAGAAATACGGTCCGAAACG-3′; c-Myb reverse primer, 5′-CCAATTCTCCCCTTTAAGTGC-3′; NFE4 forward primer, 5′-CCAGAAAGCAGGCCACAGCA-3′; NFE4 reverse primer, 5′-AGGGCCCCAGTAGGTGAGAT-3′; CP2 forward primer, 5′-ACAAACTTCTCAGGGGCAGA-3′; CP2 reverse primer, 5′-GTTAACCTTGGACGCACCAT-3′; β-actin forward primer, 5′-CCTGAACCCCAAGGCCAACC-3′; β-actin reverse primer, 5′-CAGGGATAGCACAGCCTGGA-3′.
Flow cytometry analysis.
Primary human adult erythroid cells were labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD71 (BD Biosciences; catalog no. 555536) and phycoerythrin (PE)-conjugated anti-CD235a (BD Biosciences; catalog no. 555570) in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) at 25°C for 20 min in the dark. Then, the cells were washed twice in PBS with 0.1% BSA, followed by resuspension with 1 ml PBS with 0.1% BSA and filtration by cell strainer. The cells were analyzed on a BD LSRII-18P flow cytometer within 1 h after staining. Unstained cells were used as a negative control.
Western blot analysis and antibodies.
For whole-cell extract preparation, the cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing a complete protease inhibitor cocktail (Roche) for 30 min on ice. For isolation of the total cellular histones, the primary human adult erythroid cells were lysed in histone extraction buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT], 1.5 mM phenylmethylsulfonyl fluoride [PMSF], and 0.2 N HCl) at 4°C overnight and then neutralized by adding 0.375 M Tris-HCl (pH 8.0). Twenty micrograms of the whole-cell extract or histone extract was separated on SDS-PAGE gels, transferred onto nitrocellulose paper, and incubated with different primary antibodies. Anti-histone H3 (catalog no. 05-499), anti-acetylated histone H3 (catalog no. 16-599), anti-histone H4 (catalog no. 05-858), anti-acetylated histone H4 (catalog no. 06-866), anti-dimethyl-histone H3 (Lys4) (catalog no. 07-030), anti-dimethyl-histone H3 (Lys9) (catalog no. 17-681), and anti-trimethyl-histone H3 (Lys9) (catalog no. 07-442) were from Millipore. The mouse anti-BCL11A monoclonal antibody (ab-19487) was from Abcam. Rabbit anti-p38 MAPK antibody (catalog no. 9212s) and rabbit anti-phospho-p38 MAPK (T180/Y182) antibody (catalog no. 92118) were from Cell Signaling. Anti-β globin chain antibody (sc-21757) and anti-γ globin chain antibody (sc-21756) were purchased from Santa Cruz.
ChIP assay.
The chromatin immunoprecipitation (ChIP) assay followed the general protocol (30). The cell extracts from cross-linked primary human adult erythroid cells were immunoprecipitated with the addition of purified rabbit IgG and antibodies against BCL11A, H3K4me2, H3K9me2 and H3K9me3, respectively. The immunocomplexes were subjected to a reversal of cross-links at 67°C for 4 h and treated with proteinase K at 45°C overnight. The DNA samples recovered were analyzed by quantitative PCR (qPCR) in the ABI 7500 real-time system. All experiments were performed in triplicate. The primer sequences used in the ChIP assay are as follows: HS3 forward primer,5′-ATAGACCATGAGTAGAGGGCAGAC-3′; HS3 reverse primer, 5′-TGATCCTGAAAACATAGGAGTCAA-3′; Aγ promoter forward primer, 5′-TTACTGCGCTGAAACTGTGG-3′; Aγ promoter reverse primer, 5′-CAGTGGTTTCTAAGGAAAAAGTGC-3′; Aγ (+3)kb forward primer, 5′-GAACAGAGCAGCACACTT-3′; Aγ (+3)kb reverse primer, 5′-TGAACAATAGTCCATGTCAAATCCT-3′; δ (−1)kb forward primer, 5′-GCAACAGAAGCCCAGCTATT-3′; δ (−1)kb reverse primer, 5′-GTGGCATGGTTTGATTTGTG-3′; β promoter forward primer, 5′-TGGTATGGGGCCAAGAGATA-3′; β promoter reverse primer, 5′-TGCTCCTGGGAGTAGATTGG-3′.
Statistical analysis.
All data are from 3 to 7 independent experiments, and they are presented as means ± standard errors of the means (SEM). Statistical significance of the difference between experimental and control groups was determined by the two-tailed Student t test.
RESULTS
Setup of a high-throughput screening system to survey embryonic/fetal globin gene expression-inducing chemical compounds.
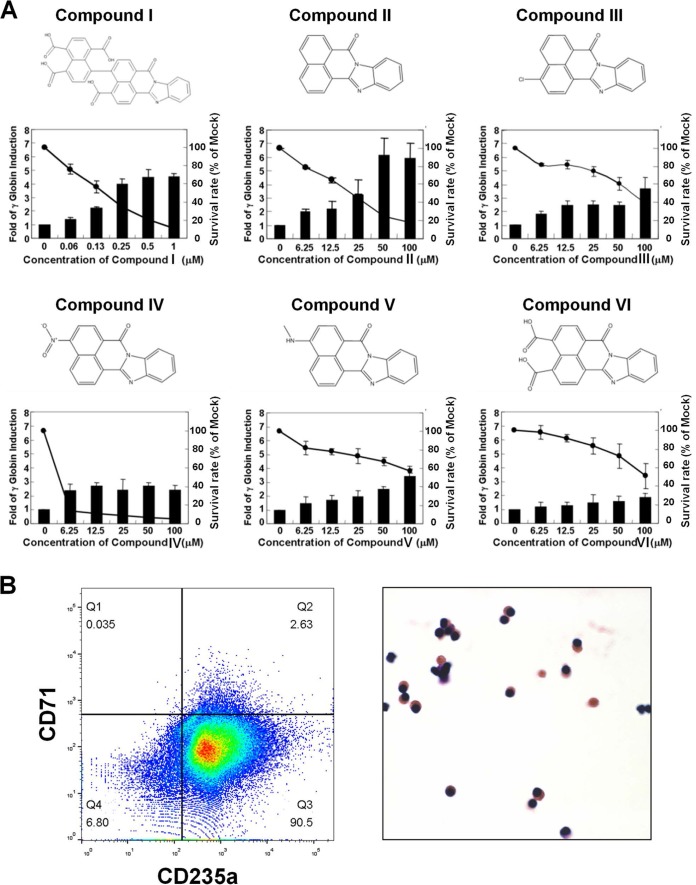
To identify novel compounds capable of inducing the expression of the fetal γ globin gene, we constructed a dual-fluorescence reporter as a screening tool for high-throughput screening (Fig. 1A). Consistent with previous findings (31, 32), the expression of the γ globin promoter-directed DsRed fluorescence was detectable only in the human fetal/embryonic erythroid K562 cells but not in mouse adult erythroid MEL cells (top two rows of panels, Fig. 1B). Moreover, reactivation of the γ globin promoter, as revealed by the induction of DsRed fluorescence, could be achieved by treating MEL cells with the HbF-inducing agent NaB (third row of panels, Fig. 1B). These data suggest that MEL cells carrying the dual-fluorescence reporter could be used to screen for novel HbF-inducing compounds. A total of 10,000 compounds were tested for their ability to induce γ globin promoter-directed DsRed fluorescence in MEL cells by following the high-throughput screening flow chart in Fig. 2. Six heterocyclic compounds, compounds I to VI (Fig. 3A), induced DsRed fluorescence in MEL cells, as exemplified by compound I (bottom row of panels, Fig. 1B). Consistent with the reporter assay, RT-qPCR analysis showed that the levels of the endogenous mouse embryonic/fetal globin genes (βh1 and Εy) were induced by 7-fold and 50-fold, respectively, in compound I-treated MEL cells (data not shown).
FIG 1.

High-throughput screening to find compounds capable of inducing the fetal globin gene. (A) Physical map of the dual-fluorescence reporter. (B) Phase-contrast and DsRed florescence images of cells stably transfected with the dual-fluorescence reporter plasmid. Top row, K562 cells; second row, MEL cells; third row, MEL cells treated with NaB; bottom row, MEL cells treated with compound I for 3 days.
FIG 2.

Experimental procedures for the high-throughput screening. In step 1, ∼10,000 heterocyclic compounds were tested for their ability to activate the γ globin promoter in MEL cells cultured on 96-well plates. In steps 2 and 3, elevation of DsRed fluorescence was detected by a fluorescence reader and further confirmed by a digital image detector. In step 4, the activation of the endogenous mouse embryonic and/or fetal globin genes as induced by the compound(s) was verified by RT-qPCR analysis. HU and NaB were used as reference compounds.
FIG 3.
Induction of γ globin gene expression by six heterocyclic compounds with a common core structure. (A) Six heterocyclic compounds with identical core structures (benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one) were examined with respect to their effects on the induction of γ globin gene expression and survival rates of the primary human adult erythroid cells. The relative fold induction of the γ globin gene (bars) by these compounds was analyzed by RT-qPCR (the mock control was set as 1). The relative rates of cell survival (the curves) were derived from use of the alamarBlue reagent (with the mock control set as 100%). (B) Flow cytometry and morphological analyses of compound II-treated cells. (Left panel) Differentiated primary human adult erythroid cells were treated with compound II for 3 days and then labeled with FITC-conjugated anti-CD71 and PE-conjugated anti-CD235a monoclonal antibodies. The percentage of cells in the Q3 region (CD71low CD235ahigh) representing late orthochromatophilic erythroblasts and reticulocytes was determined by flow cytometry. (Right panel) The morphology of compound II-treated cells was analyzed by a Liu staining assay.
Induction of endogenous γ globin gene expression and modulation of globin gene regulators in primary human adult erythroid cells by heterocyclic compounds.
We set up primary human erythroid cultures from adult normal donors and confirmed the globin gene expression pattern over time throughout the differentiation process (data not shown). The data are consistent with the profiles reported by Xu et al. (33). The dosage dependence of the heterocyclic compounds I to VI on γ globin gene activation in the primary human erythroid cells and their inhibitory effects on cell survival are shown in Fig. 3A. Interestingly, these heterocyclic compounds shared an identical core structure, i.e., that of compound II (benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one). As shown in Fig. 3B, 90% of the compound II-treated primary erythroid cells remained orthochromatic erythroblasts, as validated by flow cytometry and Liu staining analyses. Relative fold inductions of the γ and β globin genes in the primary human adult erythroid cells by compounds I, II, and III at their half-maximal inhibitory concentrations (CC50), as estimated by RT-qPCR analysis, were compared to those by HU and NaB. As seen in Fig. 4A, compounds I, II, and III were more potent γ globin inducers than either HU or NaB. The ratios of γ/(γ + β) globin mRNAs were calculated from the absolute quantification and are shown in Fig. 4B. HU and compound I to III treatment significantly increased the ratio of γ/(γ + β) globin mRNAs in comparison to that of the mock control. Although compound II had the highest γ globin gene induction among the tested compounds, it could only modestly increase the ratio of γ/(γ + β) globin mRNAs due to the nonspecific activation of the β globin mRNA expression. Notably, although NaB treatment failed to increase the γ globin mRNA expression at its CC50 (222 μM) (Fig. 4A), it could elevate the level of the γ globin mRNA at a higher concentration, e.g., 500 μM (data not shown). Finally, while cells treated with a combination of 22 μM compound II and 147 μM HU did not show better γ globin gene induction than the cells treated with compound II or HU alone, combination treatment with 22 μM compound II and 500 μM NaB resulted in additive elevation of the γ globin mRNA expression (data not shown).
FIG 4.
Comparison of the γ and β globin levels in the primary human adult erythroid cells treated with different γ globin-inducing agents. (A) The cells were treated with different compounds at the their CC50s, HU (147 μM), NaB (222 μM), and heterocyclic compounds I (0.2 μM), II (22 μM), and III (75 μM), respectively, for 3 days. The total mRNAs were extracted, and the relative fold inductions of the β and γ globin mRNAs were determined by relative RT-qPCR analysis. (B) For calculation of the ratio of γ/(γ + β) globin mRNAs in the compound-treated primary human adult erythroid cells, the amounts of γ and β globin mRNAs were estimated by using an absolute RT-qPCR method. All data are presented as means ± SEM (n = 3) (*, P < 0.05; **, P < 0.01, by t test). (C) Hemolysates were prepared from mock control primary erythroid cells (left panel) or compound II-treated cells (right panel) on day 10 of differentiation, and the presence of HbF and HbA was revealed by hemoglobin HPLC. Hemoglobins and proteins in hemolysates were separated by HPLC, and the proportions of each peak are shown. The position of the HbF peak is labeled with an arrow in the chromatogram. The table below the chromatogram shows the raw data for the retention time, height, area, and area percentage of each peak: F (hemoglobin F/HbF), LA1c/CHb-1 (labile A1c), A1c (glycated hemoglobin), A0 (hemoglobin A0/HbA0), and A2 (hemoglobin A2/HbA2).
The induction level of HbF in compound II-treated primary erythroid culture was also analyzed by hemoglobin high-performance liquid chromatography (HPLC). As seen in Fig. 4C, a substantially elevated fraction representing HbF (increasing from 2.5% to 11.8%) was detected, indicating that the induction of γ globin mRNA was accompanied by an increase in HbF level. The proportion of hemoglobin A2 (HbA2 [A2]) was also increased (from 3% to 7.9%), whereas the proportion of the adult hemoglobin (HbA0 [A0]) was significantly decreased from 80% to 64% upon compound II treatment (Fig. 4C).
A number of transcription factors have been identified to serve as either activators or repressors of globin gene transcription, including GATA1 (34), NF-E2 (35), EKLF (36), YY1 (37), TR2/TR4 (38), NF-E4 (39), RREB1 (40), and BCL11A (20). Among these factors, BCL11A has been suggested to be a critical repressor of γ globin gene expression, and its downregulation in primary adult erythroid cells has been suggested to lead to the activation of HbF expression (20). Moreover, inactivation of BCL11A in SCD transgenic mice was shown to correct the pathological defects of SCD through inducing a high level of HbF (41). As shown in Fig. 5A, the mRNA levels of BCL11A and c-Myb were diminished, while that of NF-E4 was upregulated in a dosage-dependent manner upon treatment of the primary erythroid culture with compound I, II, or III. On the other hand, in NaB-treated erythroid cells, there was a decrease in only the BCL11A mRNA level. Furthermore, HU treatment did not cause a change in the mRNA level of BCL11A, c-Myb, or NF-E4 (top 3 panels, Fig. 5A). No significant change in the mRNA level of CP2 (bottom panel of Fig. 5A), TR2, TR4, or GATA-1 (data not shown) could be observed upon treatment by any of the heterocyclic compounds tested.
FIG 5.
Changes in the levels of γ globin gene regulators in primary human adult erythroid cells treated with compound I, II, or III in comparison to HU and NaB. (A) Dosage dependence of the effects of HU, NaB, and compounds I to III on the mRNA levels of different γ globin gene regulators. The primary human adult erythroid cells were treated with different dosages of the indicated compounds for 3 days. The relative fold inductions of BCL11A, c-Myb, NF-E4, and CP2 mRNAs were then determined by relative RT-qPCR analysis. The expression levels of these genes in the mock control cells were set as 1. (B) Western blotting of BCL11A, β globin, γ globin, and β-actin in primary human adult erythroid cells. The total proteins were extracted from the cells, and the expression patterns of BCL11A, the β globin chain, and the γ globin chain were determined by Western blotting (upper panels). The expression of β-actin was used as a loading control. The intensities of the Western blot signals of the BCL11A, β globin chain, and γ globin chain of different samples were quantified using densitometry analysis software and compared in the bar graph. (C) ChIP analysis of BCL11A binding on the human β-like globin locus in primary human adult erythroid cells before (Mock) and after (II) treatment with compound II. ChIP of the primary human adult erythroid cells was carried out using the BCL11A antibody. IgG was used as the control. The PCR-assayed regions of the β-like globin locus included HS3, the Aγ globin promoter, a region at 3 kb downstream of Aγ, a region at 1 kb upstream of the δ globin gene, and the β globin promoter. All data are presented as means ± SEM (n = 3) (*, P < 0.05; **, P < 0.01, by t test).
Western blotting of BCL11A in the different compound-treated erythroid cultures (Fig. 5B) was consistent with the RNA data in Fig. 5A. In particular, the amount of the BCL11A protein was drastically reduced in the primary human adult erythroid cells treated with compounds I to III and, to a lesser extent, NaB (Fig. 5B). In interesting contrast, similar to the RT-qPCR analysis in Fig. 5A, no obvious reduction in the level of the BCL11A protein could be detected in the primary human adult erythroid cells upon HU treatment (Fig. 5B).
Regarding the NaB effect, downregulation of BCL11A by RNA interference (RNAi) (20) or NaB (42) was shown to elevate the level of γ globin mRNA. However, we did not see a significant change of the γ globin mRNA level upon treatment with NaB at its CC50 (Fig. 4A), despite downregulation of the level of BCL11A protein (Fig. 5B). This inconsistency could be due to the differences of the assay conditions, e.g., the different amounts of the chemicals used, the different cell types, the different time points and lengths of the NaB treatment, etc. In particular, 600 μM NaB was used to treat K562 cells by Chen et al. (42), while we used 222 μM NaB in the analyses described for Fig. 4A and 5B. Notably though, as mentioned above, the level of γ globin mRNA in the primary erythroid culture could be elevated by a higher concentration, i.e., 500 μM, of NaB (data not shown).
Also, NaB did not change the level of γ globin mRNA at its CC50 (222 μM), but it elevated the level of the γ globin chain (compare Fig. 4A and 5B). This observation was consistent with a previous report that NaB could increase the efficiency of γ globin chain translation without change of the γ globin mRNA level (43).
Decreased binding of BCL11A in the human β globin gene locus in compound II-treated primary human adult erythroid cells.
The binding profile of the BCL11A factor within the human β-like globin gene locus was studied previously (20, 33). As seen in Fig. 5C, the chromatin immunoprecipitation (ChIP) assay showed that BCL11A indeed bound to 5′ HS3, a region 3 kb downstream of the Aγ globin gene, and a region 1 kb upstream of the δ globin gene of the human β-like globin gene locus in the primary human adult erythroid cells (white bars, Fig. 5C). However, BCL11A binding at these regions was significantly reduced upon compound II treatment (black bars, Fig. 5C). These data suggest that a lower level of BCL11A in the compound II-treated erythroid cells also results in inefficient recruitment of this γ globin repressor to the β-like globin locus, thus relieving the γ globin gene from transcriptional repression.
No change in the global acetylation levels of histones H3 and H4 in primary human adult erythroid cells treated with compound I or II.
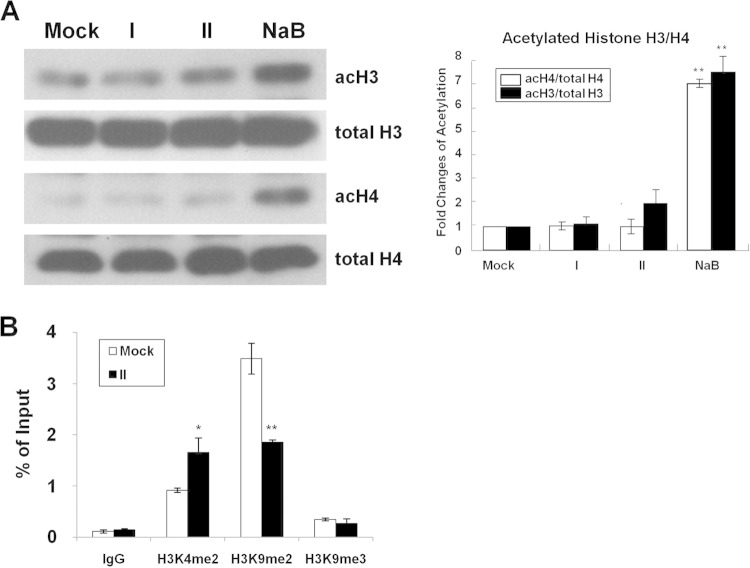
Treatment of cells with TSA or NaB leads to global changes in the histone modifications, in particular in acetylation, and subsequently induces γ globin gene expression (12). We thus next examined and compared the acetylation status of histones H3/H4 in the primary human adult erythroid cells treated with compound I or II or NaB, which is a class I and II HDACi. The global levels of acetylated histone H3 (acH3) and acetylated histone H4 (acH4) were significantly enhanced in the NaB-treated cells but not upon treatment with compounds I and II (Fig. 6A) or HU (data not shown), suggesting that compounds I and II and HU are not HDACi and do not globally change the acetylation status of histones H3 and H4. Possible changes of the methylation statuses of lysine 4 on histone H3 (H3K4) and lysine 9 on histone H3 (H3K9) around the Aγ globin promoter were also examined. As expected, the level of H3K4me2 was enriched on the Aγ globin promoter upon activation of the γ globin gene by compound II. On the other hand, the level of histone H3 with dimethylated lysine 9 (H3K9me2) was significantly decreased after compound II treatment (Fig. 6B).
FIG 6.
Global acetylation patterns of histones H3 and H4 in primary human adult erythroid cells treated with compound I or II or NaB. (A) Primary human adult erythroid cells were treated with compound I or II or NaB at its CC50 for 3 days, and the total cellular histones were prepared by the acid extraction method. The acetylation patterns of the histones were analyzed by Western blotting using antibodies against histone H3, histone H4, acetylated histone H3 (acH3), and acetylated histone H4 (acH4) (left panels). The changes of the acetylation of H3 and H4 are shown on the right. (B) Methylation statuses of H3K4 and H3K9 around the Aγ globin promoter. ChIP analysis with use of anti-H3K4me2, anti-H3K9me2, or anti-H3K9me3 was carried out to examine the distributions of H3K4me2, H3K9me2, and H3K9me3 on the human Aγ globin promoter in the primary human adult erythroid cells before (Mock) and after (II) compound II treatment. IgG was used as the control. All data are presented as means ± SEM (n = 3) (*, P < 0.05; **, P < 0.01, by t test).
Therapeutic potential of compounds I to VI in comparison with NaB and HU.
To evaluate the therapeutic potential of compounds I to VI in comparison with other HbF inducers, we determined the half-maximal inhibitory concentration (CC50) and the effective concentration (EC) of each compound. The CC50 value is the concentration of a compound at which the cell survival rate is reduced by 50%. The EC value was defined as the concentration of a compound that induced the γ globin gene expression by 1.9-fold, the fold of the γ globin gene induction by HU at its CC50. The CC50/EC ratio was used to determine whether the compound tested has better therapeutic potential than that of HU, the CC50/EC ratio of which was set as 1. As shown in Table 1, compounds I to V exhibited better therapeutic potentials (CC50/EC) than HU. It should be noted that compounds I to V also induced γ globin gene expression to higher levels than HU at their CC50 (Table 1). Among all the compounds, compound II had superior therapeutic potential, with a CC50/EC ratio of 9.7 and a relatively higher γ globin fold induction (3.0) at its CC50 (Table 1).
TABLE 1.
Comparison of CC50 and γ globin gene-inducing abilities of different compoundsc
| Compound | CC50 (μM)a | EC (μM)b | CC50/EC | γ globin induction fold (CC50) (mean ± SEM) |
|---|---|---|---|---|
| I | 0.17 | 0.12 | 1.4 | 2.7 ± 0.4* |
| II | 22 | 2.3 | 9.7 | 3.0 ± 0.2** |
| III | 75 | 9.3 | 8.1 | 2.6 ± 0.8* |
| IV | 3.6 | 1.9 | 1.9 | 2.2 ± 0.7 |
| V | 135 | 25.8 | 5.2 | 3.4 ± 1.4 |
| VI | 103 | 106 | 1.0 | 1.9 ± 0.4 |
| Butyric acid | 222 | NAd | NA | 1.3 ± 0.2 |
| Hydroxyurea | 147 | 147 | 1.0 | 1.9 ± 0.3* |
CC50, half-maximal inhibitory concentration; the concentration of a compound at which the cell survival rate was reduced by 50%.
EC, effective concentration; the concentration of a compound that induces the γ globin gene expression by 1.9-fold (the fold induction of the γ globin gene by HU at its CC50).
The primary human erythroid cells were treated with NaB, HU, or compounds I to VI individually. The CC50, the EC, and the ratio of CC50 to EC (CC50/EC) of compounds I to VI were calculated and compared together with those of NaB and HU. Data are presented as the means from three independent experiments. Statistical significance of the difference between experimental and control groups was determined by the two-tailed Student t test (*, P < 0.05; **, P < 0.01).
NA, not available.
To evaluate the specificities of globin gene activation by compounds I to III, the amounts of the individual β-like globin mRNAs were measured by absolute RT-qPCR. As shown in Table 2, the proportions of the ε and γ globin mRNAs were both significantly increased by these compounds. On the other hand, the proportions of the β globin mRNA were notably reduced after drug treatment. In comparison to HU and NaB, compounds I to III were more effective γ globin inducers, increasing the proportions of the γ globin mRNA from 8.0% to between 10.7% and 19.6% in the drug-treated primary human adult erythroid cells while reducing the β globin mRNA proportions from 87.2% to between 75.2% and 81.4% (Table 2).
TABLE 2.
Relative expression levels of individual β-like globin genes in primary human erythroid cells treated with different compoundsa
| Compound | % expression of gene (mean ± SEM) (n = 3) for: |
|||
|---|---|---|---|---|
| ε globin | γ globin | δ globin | β globin | |
| Mock | 0.1 ± 0.0 | 8.0 ± 0.7 | 4.7 ± 0.2 | 87.2 ± 1.0 |
| HU | 0.2 ± 0.0 | 9.9 ± 0.8 | 6.8 ± 0.4 | 83.1 ± 1.3 |
| NaB | 0.3 ± 0.0 | 8.8 ± 1.1 | 3.2 ± 0.2 | 87.8 ± 1.2 |
| Compound I | 0.6 ± 0.2 | 19.6 ± 1.3 | 4.7 ± 0.1 | 75.2 ± 0.9 |
| Compound II | 0.3 ± 0.0 | 10.7 ± 1.0 | 7.6 ± 0.6 | 81.4 ± 1.2 |
| Compound III | 0.3 ± 0.1 | 15.6 ± 1.2 | 7.6 ± 0.4 | 76.5 ± 1.0 |
Primary human erythroid cells were treated with HU (147 μM), NaB (222 μM), compound I (0.2 μM), compound II (22 μM), or compound III (75 μM), respectively, for 3 days, and the expression level of each globin mRNA was estimated by absolute RT-qPCR. The proportions of the individual β-like globin mRNAs among the total β-like globin mRNAs are shown.
Induction of γ globin gene expression in HU-resistant erythroid cells by compound II.
Previous studies have suggested that the increase in HbF levels in primary erythroid cultures treated with HU was comparable to the HU responsiveness of patients who received HU treatment (44). To evaluate whether compound II could induce the γ globin gene in HU-resistant cells, we established primary human erythroid cell cultures from the peripheral blood samples of 10 different normal blood donors and divided them into two groups according to their HU responsiveness based on pretested culture response. The dose responses of the two groups of primary human erythroid cells to HU were compared in Fig. 7A. Out of the 10 cultures, the γ globin gene expression in 7 of them, including those shown already in Fig. 3 to 5, could be upregulated by HU (Fig. 7B, HU-responsive). However, the other 3 erythroid cultures were resistant to HU induction of the γ globin gene (Fig. 7B, HU-resistant). Remarkably, compound II could induce γ globin gene transcription in all of these 10 primary human erythroid cell cultures (Fig. 7B). Notably, similarly to the HU-responsive erythroid cultures (Fig. 5A), the mRNA levels of BCL11A and NF-E4 in the HU-resistant primary human erythroid cultures were decreased and increased, respectively, upon treatment with compound II, and they were unaltered by HU (Fig. 7C).
FIG 7.
Molecular basis of inducibility of γ globin gene activation in HU-responsive and HU-resistant erythroid cells. (A) HU dosage responses of the HU-responsive and HU-resistant primary human erythroid cells. HU responses of the primary human adult erythroid cells from different normal blood donors were pretested first as described in Materials and Methods, and the cells were divided into HU-responsive and the HU-resistant groups. The 2 groups of the primary human adult erythroid cells were then treated with different dosages of HU for 3 days. After the HU treatment, the total mRNAs were extracted for RT-qPCR analysis and the relative induction levels of the γ globin genes in the 2 groups of cells were calculated and compared. The expression level of the γ globin gene in the mock control cells was set as 1. All data are presented as means ± SEM (n = 3). (B) Average fold induction of the γ globin mRNA in 7 independent HU-responsive and 3 independent HU-resistant primary human erythroid cell cultures treated with HU, NaB, or compound II. Note that the level of γ globin mRNA in the HU-resistant erythroid cells could be enhanced by treatment with compound II but not HU or NaB. Mock, no treatment. (C) Relative levels of BCL11A and NF-E4 mRNAs in HU-responsive and HU-resistant primary erythroid cells with or without drug treatment. The primary erythroid cells were treated with HU or compound II for 3 days, and the expression levels of BCL11A and NF-E4 mRNAs were determined by relative RT-qPCR. The expression levels of these genes in the mock control cells were set as 1. (D) Phosphorylation status of p38 MAPK in HU-responsive and HU-resistant primary erythroid cells with or without drug treatment. Upper panels, Western blot patterns of phosphorylated p38 MAPK (P-p38) and total p38. Bottom panels, effects of HU, NaB, and compound II on the relative levels of P-p38. Mock, no treatment. (E) Inducibility of the γ globin gene activation in HU-responsive primary human adult erythroid cell cultures treated with the p38 MAPK inhibitor SB (SB203580). The cultured erythroid cells were preincubated with or without SB (10 μM) for 1 h before treatment with HU, NaB, or compound II at its CC50 for 3 days. The fold induction of γ globin mRNAs was then analyzed by relative RT-qPCR. Each bar is the average of data from 3 independent HU-responsive primary human erythroid cell cultures. All data are presented as means ± SEM (n = 3 or 7) (**, P < 0.01, by t test).
The activation of the p38 MAPK signaling pathway has been suggested to mediate the fetal γ globin gene expression in response to NaB or TSA treatment (23, 24). To see whether the p38 MAPK signaling pathway was involved in the reactivation of the γ globin gene expression by the compounds described above, we examined the phosphorylation status of p38 MAPK in the primary human adult erythroid cells. HU, NaB, and compound II all significantly increased the phosphorylation level of p38 MAPK in the HU-responsive erythroid cells (left panel of Fig. 7D). In interesting contrast, the phosphorylation level of p38 MAPK was not notably changed in the HU-resistant erythroid cells upon treatment with these compounds (right panel of Fig. 7D). To further explore the possible role of p38 MAPK activation in relation to the HU responsiveness of the erythroid cells, the HU-responsive erythroid cells were pretreated with a selective p38 MAPK inhibitor, SB203580 (SB), and then the fold induction of γ globin by HU, NaB, and compound II, respectively, was analyzed. As shown in Fig. 7E, after inactivation of the p38 MAPK signaling by SB203580, HU failed to elevate the γ globin mRNA level whereas compound II could still induce γ globin expression (compare the right 4 bars of Fig. 7E). Similar results were observed in the HU-resistant cells upon pretreatment with SB203580 (data not shown). Taken together, the data in Fig. 7 show that the HU resistance of erythroid cells with respect to γ globin gene activation is associated with a lack of activation of p38 MAPK. Since treatment with compound II but not HU leads to significant decrease of BCL11A and increase of NF-E4 in all of the primary human erythroid cultures (Fig. 5A and 7C), the former agent could still induce the γ globin gene in the HU-resistant erythroid cells.
DISCUSSION
Several fluorescence-based (or luciferase-based) reporter systems have been used previously for the assessment of the activation of γ globin promoter as affected by HbF-inducing agents to explore new therapeutic agents for treating severe β-thalassemia and SCD (31, 32, 45–47). Several short-chain fatty acid (SCFA) derivatives that are able to induce γ globin have been identified by one of these studies (48). Also, the capability of SCFAs to induce γ globin gene expression and F-reticulocytes has been evaluated in transgenic mice and baboons (48–50). Among them, 2,2-dimethylbutyrate (HQK-1001) has been found to be the most potent oral γ globin gene inducer and is now being tested for use in SCD, β-thalassemia intermedia, and HbE/β-thalassemia in phase II clinical trials (15, 51–54). However, the therapeutic effects of HQK-1001 in patients who are irresponsive to HU treatment are presently unknown. In the present study, we have used a simple MEL cell-based dual-fluorescence reporter system (Fig. 1) to carry out high-throughput screening for novel γ globin-inducing compounds (Fig. 2). Six asymmetrical heterocyclic benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one derivatives (compounds I to VI) capable of inducing the γ globin gene have been identified (Fig. 3A). Taking compound II (core structure of the six) as an example, we have investigated the mode of action of this series of compounds on γ globin gene activation by using primary human erythroid cultures. HPLC analysis shows that compound II is very stable after 3 days of culture (data not shown). Most interestingly, we have found that compound II is able to activate γ globin gene expression even in HU-resistant cells.
Several experiments have provided interesting insights into the possible underlying mechanisms of γ globin gene induction by the heterocyclic compounds in relation to the modulation of the phosphorylation status of p38 MAPK and the expression levels of γ globin transcription regulators (Fig. 5A and 7D). First, we have found that the phosphorylation level of p38 MAPK is significantly increased by compound II as well as HU and NaB in the primary human erythroid cells that are HU responsive (left panel of Fig. 7D). Previous studies have suggested that HU induces γ globin transcription through elevation of the level of circulating nitric oxide (NO), which stimulates p38 MAPK activation (55–57). Furthermore, inhibition of p38 MAPK activation prevents HU-induced γ globin gene expression in K562 cells (58). Interestingly, NaB and TSA also increase the γ globin synthesis through activation of p38 MAPK signaling (59). Since many HbF-inducing agents stimulate the stress-activated p38 MAPK, a cell stress signaling model for fetal globin induction has been proposed (60). Compound II thus may induce the γ globin gene in part through this cell stress signaling pathway as well.
Second, as shown in Fig. 5A, in addition to p38 MAPK, the induction of γ globin gene expression by the currently identified compounds is also accompanied by a significant increase of the activator NF-E4 and decreases of the repressors BCL11A and c-Myb, respectively. The levels of these γ globin regulators have been previously demonstrated by others to modulate γ globin gene expression in either K562 cells or primary human erythroid cells (20–22, 41). Among them, BCL11A appears to be the primary repressor of the γ globin gene, and its downregulation elevates the fetal γ globin gene expression in vitro in primary human adult erythroid cell culture and in vivo in genetically engineered mice (20, 61). The requirement for BCL11A in chromatin loop formation and modulation of γ globin gene silencing has been studied by chromosome conformation capture (3C) assay (33). Several erythroid regulators, including GATA1, FOG1, and SOX6, and the nucleosome remodeling/deacetylase complex (NuRD) have been found to interact with BCL11A and may be involved in regulation of the hemoglobin switch (20, 33). In addition, KLF1 regulates the expression of BCL11A and modulates the γ to β globin gene switching (62). Interestingly, compound II decreases the levels of BCL11A mRNA and BCL11A protein in the primary human erythroid cells (Fig. 5A and B). Significantly, the decrease of BCL11A upon compound II treatment is associated with the lower binding of the repressor at several regions of the human β-like globin locus (Fig. 5C), which has been suggested to play an important role in γ globin gene regulation (20). Thus, reactivation of the γ globin gene expression in adult erythroid cells by compound II appears to involve the modulations of two different pathways, i.e., regulation of the mRNA levels of γ globin transcription regulators and activation of the cellular p38 MAPK signaling to phosphorylate p38. Notably, decrease of BCL11A gene expression upon compound II treatment does not decrease the level of β globin gene expression (Fig. 5), which is consistent with a previous report that the promoter activity of the β globin gene remains unchanged in cells with RNAi-mediated knockdown of BCL11A (32). Thus, transient knockdown of BCL11A expression appears to affect the expression of only the γ, but not the β, globin gene.
The beneficial effects of HU treatment, including reductions in the rate of pain crisis and acute chest syndrome, seem to be attributable to the increase in HbF levels in patients (63–65). The requirement for HbF induction for clinical benefits during HU therapy is also supported by studies of the sickle cell mouse model (66). However, many hemoglobinopathic patients are poorly responsive or unresponsive to HU therapy, but the molecular mechanisms underlying this resistance to HU have been unclear. In particular, HU can increase HbF in only approximately half of SCD patients (18). In addition, around 30% of β-thalassemia patients are found to be nonresponders to HU treatment (19). Although multiple single nucleotide polymorphisms (SNPs) have been found to be associated with the HbF response to HU, the regulatory pathway(s) responsible for the HU-resistance of γ globin gene activation has remained unknown. We have investigated the activation of p38 MAPK signaling and its correlation with HU responsiveness of the primary erythroid cells (Fig. 7D). It appears that either of two intracellular changes contributes to the reactivation of the γ globin gene in compound-treated adult erythroid cells: (i) activation of p38 MAPK and (ii) alterations in the amounts of the γ globin gene regulators, i.e., reduction of the repressor BCL11A and increase of the activator NF-E4 (Fig. 5 and 7C). In HU-responsive erythroid cells, HU induces the first change but not the second. On the other hand, compound II induces both changes (Fig. 7C and D). Thus, the level of the γ globin gene expression in adult erythroid cells treated with compound II is higher than that in HU-treated cells (Fig. 7B). In HU-resistant erythroid cells, HU does not induce either of the two changes (Fig. 7C and D). Although compound II is not able to activate the p38 MAPK pathway in HU-resistant cells either (right panel of Fig. 7D), it can still induce alterations of the amounts of the γ globin regulators (Fig. 7C) and consequently reactivate the γ globin gene in these cells (right panel of Fig. 7B), albeit to a lesser extent than in the HU-responsive cells (compare the 2 panels of Fig. 7B). However, whether the lower sensitivity of p38 MAPK signaling to stress plays a causative role in the irresponsiveness of HU-resistant erythroid cells to γ globin gene induction needs to be further investigated.
In summary, our high-throughput screening and the following analyses have identified a series of new compounds that can induce γ globin gene expression in the primary human erythroid cells derived from normal adult blood donors. These compounds appear to be excellent lead compounds for further development of universal drugs for treating SCD and severe β-thalassemias. First, compound II shows a better therapeutic potential (CC50/EC) as well as a higher γ globin-inducing capability at its CC50 than the other HbF-inducing agents tested (Table 1). Second, this series of compounds displays a higher activity of the γ globin gene induction than the other compounds tested (Table 2). Third, compound II does not change the global acetylation status of histones H3 and H4 (Fig. 6A), while it modulates the levels of H3K4me2 and H3K9me2 on the γ globin promoter (Fig. 6B). Fourth, compound II induces the γ globin gene transcription in HU-resistant as well as HU-responsive primary human erythroid cells through their capability to modulate the expression levels of several γ globin regulators, including the repressor BCL11A. Finally, combined use of compound II or its derivatives with a reagent(s) capable of activating the p38 MAPK pathway in HU-resistant erythroid cells would be of great therapeutic potential for treatment of SCD and severe β-thalassemias. However, further studies are warranted to evaluate the therapeutic effects of compound II and its derivatives in patients with SCD and severe β-thalassemias.
ACKNOWLEDGMENTS
We thank Szu-Huei Wu of the Institute of Biotechnology and Pharmaceutical Research, NHRI, for preparation of the heterocyclic compound library used in our screening. We appreciate the technical assistance in HPLC analysis by Liang-in Lin and Su-Cheng Tseng in the National Taiwan University. The pLAR-β plasmid was a generous gift from M. Groudine (Fred Hutchinson Cancer Research Center). We also thank Miranda Loney for correcting grammatical errors and improving the quality of the manuscript.
This research was supported by a National Health Research Institute grant (NHRI-EX100-10011SI) and the Academia Sinica (AS), Taipei, Taiwan. C.-K.J.S. is an AS Senior Investigator Awardee.
Y.-C.C., R.-L.C., and Z.-S.L. designed and performed the experiments and analyzed the data; J.-S.S. and Y.-S.C provided critical compounds and helpful discussions during the course of this work; Y.-C.C. and C.-K.J.S. developed the project and wrote the manuscript.
We declare no conflict of interest.
REFERENCES
- 1.Weatherall DJ. 2001. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet 2:245–255. doi: 10.1038/35066048. [DOI] [PubMed] [Google Scholar]
- 2.Patrinos GP, Grosveld FG. 2008. Pharmacogenomics and therapeutics of hemoglobinopathies. Hemoglobin 32:229–236. doi: 10.1080/03630260701680367. [DOI] [PubMed] [Google Scholar]
- 3.Schechter AN. 2008. Hemoglobin research and the origins of molecular medicine. Blood 112:3927–3938. doi: 10.1182/blood-2008-04-078188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer DE, Kamran SC, Orkin SH. 2012. Reawakening fetal hemoglobin: prospects for new therapies for the beta-globin disorders. Blood 120:2945–2953. doi: 10.1182/blood-2012-06-292078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgs DR, Engel JD, Stamatoyannopoulos G. 2012. Thalassaemia. Lancet 379:373–383. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- 6.Perrine SP, Castaneda SA, Chui DH, Faller DV, Berenson RJ, Siritanaratku N, Fucharoen S. 2010. Fetal globin gene inducers: novel agents and new potential. Ann N Y Acad Sci 1202:158–164. doi: 10.1111/j.1749-6632.2010.05593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natta CL, Niazi GA, Ford S, Bank A. 1974. Balanced globin chain synthesis in hereditary persistence of fetal hemoglobin. J Clin Invest 54:433–438. doi: 10.1172/JCI107779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. 1988. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 318:96–99. doi: 10.1056/NEJM198801143180207. [DOI] [PubMed] [Google Scholar]
- 9.Ley TJ, Nienhuis AW. 1985. Induction of hemoglobin F synthesis in patients with beta thalassemia. Annu Rev Med 36:485–498. doi: 10.1146/annurev.me.36.020185.002413. [DOI] [PubMed] [Google Scholar]
- 10.Humphries RK, Dover G, Young NS, Moore JG, Charache S, Ley T, Nienhuis AW. 1985. 5-Azacytidine acts directly on both erythroid precursors and progenitors to increase production of fetal hemoglobin. J Clin Invest 75:547–557. doi: 10.1172/JCI111731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivieri NF, Weatherall DJ. 1998. The therapeutic reactivation of fetal haemoglobin. Hum Mol Genet 7:1655–1658. doi: 10.1093/hmg/7.10.1655. [DOI] [PubMed] [Google Scholar]
- 12.McCaffrey PG, Newsome DA, Fibach E, Yoshida M, Su MS. 1997. Induction of gamma-globin by histone deacetylase inhibitors. Blood 90:2075–2083. [PubMed] [Google Scholar]
- 13.Witt O, Monkemeyer S, Ronndahl G, Erdlenbruch B, Reinhardt D, Kanbach K, Pekrun A. 2003. Induction of fetal hemoglobin expression by the histone deacetylase inhibitor apicidin. Blood 101:2001–2007. doi: 10.1182/blood-2002-08-2617. [DOI] [PubMed] [Google Scholar]
- 14.Constantoulakis P, Knitter G, Stamatoyannopoulos G. 1989. On the induction of fetal hemoglobin by butyrates: in vivo and in vitro studies with sodium butyrate and comparison of combination treatments with 5-AzaC and AraC. Blood 74:1963–1971. [PubMed] [Google Scholar]
- 15.Fucharoen S, Inati A, Siritanaratku N, Thein SL, Wargin WC, Koussa S, Taher A, Chaneim N, Boosalis M, Berenson R, Perrine SP. 2013. A randomized phase I/II trial of HQK-1001, an oral fetal globin gene inducer, in beta-thalassaemia intermedia and HbE/beta-thalassaemia. Br J Haematol 161:587–593. doi: 10.1111/bjh.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grigg A. 2007. Effect of hydroxyurea on sperm count, motility and morphology in adult men with sickle cell or myeloproliferative disease. Intern Med J 37:190–192. doi: 10.1111/j.1445-5994.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 17.Kinney TR, Helms RW, O'Branski EE, Ohene-Frempong K, Wang W, Daeschner C, Vichinsky E, Redding-Lallinger R, Gee B, Platt OS, Ware RE. 1999. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood 94:1550–1554. [PubMed] [Google Scholar]
- 18.Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ. 1997. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Multicenter study of hydroxyurea. Blood 89:1078–1088. [PubMed] [Google Scholar]
- 19.Atweh G, Fathallah H. 2010. Pharmacologic induction of fetal hemoglobin production. Hematol Oncol Clin North Am 24:1131–1144. doi: 10.1016/j.hoc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HK, Hirschhorn JN, Cantor AB, Orkin SH. 2008. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 21.Zhou W, Clouston DR, Wang X, Cerruti L, Cunningham JM, Jane SM. 2000. Induction of human fetal globin gene expression by a novel erythroid factor, NF-E4. Mol Cell Biol 20:7662–7672. doi: 10.1128/MCB.20.20.7662-7672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang J, Best S, Menzel S, Silver N, Lai MI, Surdulescu GL, Spector TD, Thein SL. 2006. cMYB is involved in the regulation of fetal hemoglobin production in adults. Blood 108:1077–1083. doi: 10.1182/blood-2006-01-008912. [DOI] [PubMed] [Google Scholar]
- 23.Pace B, Qian X, Sangerman J, Oforiacquah S, Baliga B, Han J, Critz S. 2003. p38 MAP kinase activation mediates γ-globin gene induction in erythroid progenitors. Exp Hematol 31:1089–1096. doi: 10.1016/S0301-472X(03)00235-2. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan V, Pace BS. 2011. Regulation of gamma-globin gene expression involves signaling through the p38 MAPK/CREB1 pathway. Blood Cells Mol Dis 47:12–22. doi: 10.1016/j.bcmd.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forrester WC, Novak U, Gelinas R, Groudine M. 1989. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A 86:5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodine DM, Ley TJ. 1987. An enhancer element lies 3′ to the human A gamma globin gene. EMBO J 6:2997–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer D, Cooper M, Maniatis GM, Marks PA, Rifkind RA. 1974. Erythropoietic differentiation in colonies of cells transformed by Friend virus. Proc Natl Acad Sci U S A 71:2668–2670. doi: 10.1073/pnas.71.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson LC, Nilsson K, Gahmberg CG. 1979. K562—a human erythroleukemic cell line. Int J Cancer 23:143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- 29.Yue QF, Xiong B, Chen WX, Liu XY. 2014. Comparative study of the efficacy of Wright-Giemsa stain and Liu's stain in the detection of Auer rods in acute promyelocytic leukemia. Acta Histochem 116:1113–1116. doi: 10.1016/j.acthis.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Daftari P, Gavva NR, Shen CK. 1999. Distinction between AP1 and NF-E2 factor-binding at specific chromatin regions in mammalian cells. Oncogene 18:5482–5486. doi: 10.1038/sj.onc.1202916. [DOI] [PubMed] [Google Scholar]
- 31.Vadolas J, Wardan H, Orford M, Voullaire L, Zaibak F, Williamson R, Ioannou PA. 2002. Development of sensitive fluorescent assays for embryonic and fetal hemoglobin inducers using the human beta-globin locus in erythropoietic cells. Blood 100:4209–4216. doi: 10.1182/blood-2001-12-0365. [DOI] [PubMed] [Google Scholar]
- 32.Chan KS, Xu J, Wardan H, McColl B, Orkin S, Vadolas J. 2012. Generation of a genomic reporter assay system for analysis of gamma- and beta-globin gene regulation. FASEB J 26:1736–1744. doi: 10.1096/fj.11-199356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Sankaran VG, Ni M, Menne TF, Puram RV, Kim W, Orkin SH. 2010. Transcriptional silencing of gamma-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev 24:783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orkin SH. 1992. GATA-binding transcription factors in hematopoietic cells. Blood 80:575–581. [PubMed] [Google Scholar]
- 35.Bulger M, Sawado T, Schubeler D, Groudine M. 2002. ChIPs of the beta-globin locus: unraveling gene regulation within an active domain. Curr Opin Genet Dev 12:170–177. doi: 10.1016/S0959-437X(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 36.Bieker JJ. 2005. Probing the onset and regulation of erythroid cell-specific gene expression. Mt Sinai J Med 72:333–338. [PubMed] [Google Scholar]
- 37.Raich N, Clegg CH, Grofti J, Romeo PH, Stamatoyannopoulos G. 1995. GATA1 and YY1 are developmental repressors of the human epsilon-globin gene. EMBO J 14:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanabe O, McPhee D, Kobayashi S, Shen Y, Brandt W, Jiang X, Campbell AD, Chen YT, Chang C, Yamamoto M, Tanimoto K, Engel JD. 2007. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4 EMBO J 26:2295–2306. doi: 10.1038/sj.emboj.7601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallarda JL, Foley KP, Yang ZY, Engel JD. 1989. The beta-globin stage selector element factor is erythroid-specific promoter/enhancer binding protein NF-E4. Genes Dev 3:1845–1859. doi: 10.1101/gad.3.12a.1845. [DOI] [PubMed] [Google Scholar]
- 40.Chen RL, Chou YC, Lan YJ, Huang TS, Shen CK. 2010. Developmental silencing of human zeta-globin gene expression is mediated by the transcriptional repressor RREB1. J Biol Chem 285:10189–10197. doi: 10.1074/jbc.M109.049130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Peng C, Sankaran VG, Shao Z, Esrick EB, Chong BG, Ippolito GC, Fujiwara Y, Ebert BL, Tucker PW, Orkin SH. 2011. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 334:993–996. doi: 10.1126/science.1211053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, Luo HY, Steinberg MH, Chui DH. 2009. BCL11A represses HBG transcription in K562 cells. Blood Cells Mol Dis 42:144–149. doi: 10.1016/j.bcmd.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg RS, Ji X, Sutton M, Perrine S, Galperin Y, Li Q, Liebhaber SA, Stamatoyannopoulos G, Atweh GF. 2005. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood 105:1807–1809. doi: 10.1182/blood-2004-02-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang YM, Pace B, Kitchens D, Ballas SK, Shah A, Baliga BS. 1997. BFU-E colony growth in response to hydroxyurea: correlation between in vitro and in vivo fetal hemoglobin induction. Am J Hematol 56:252–258. doi:. [DOI] [PubMed] [Google Scholar]
- 45.Skarpidi E, Vassilopoulos G, Li Q, Stamatoyannopoulos G. 2000. Novel in vitro assay for the detection of pharmacologic inducers of fetal hemoglobin. Blood 96:321–326. [PubMed] [Google Scholar]
- 46.Migliaccio G, Di Baldassarre A, Di Rico C, Di Noia A, Nakamoto B, Cao H, Skarpidi E, Migliaccio AR. 2005. Spontaneous switch from Agamma- to beta-globin promoter activity in a stable transfected dual reporter vector. Blood Cells Mol Dis 34:174–180. doi: 10.1016/j.bcmd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Nam TG, Lee J, Walker JR, Brinker A, Cho CY, Schultz PG. 2011. Identification and characterization of small-molecule inducers of fetal hemoglobin. ChemMedChem 6:777–780. doi: 10.1002/cmdc.201000505. [DOI] [PubMed] [Google Scholar]
- 48.Pace BS, White GL, Dover GJ, Boosalis MS, Faller DV, Perrine SP. 2002. Short-chain fatty acid derivatives induce fetal globin expression and erythropoiesis in vivo. Blood 100:4640–4648. doi: 10.1182/blood-2002-02-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Constantoulakis P, Papayannopoulou T, Stamatoyannopoulos G. 1988. α-Amino-N-butyric acid stimulates fetal hemoglobin in the adult. Blood 72:1961–1967. [PubMed] [Google Scholar]
- 50.Liakopoulou E, Blau CA, Li Q, Josephson B, Wolf JA, Fournarakis B, Raisys V, Dover G, Papayannopoulou T, Stamatoyannopoulos G. 1995. Stimulation of fetal hemoglobin production by short chain fatty acids. Blood 86:3227–3235. [PubMed] [Google Scholar]
- 51.Inati A, Kahale M, Perrine SP, Chui DH, Taher AT, Koussa S, Abi Nasr T, Abbas HA, Ghalie RG. 2014. A phase 2 study of HQK-1001, an oral fetal haemoglobin inducer, in beta-thalassaemia intermedia. Br J Haematol 164:456–458. doi: 10.1111/bjh.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kutlar A, Reid ME, Inati A, Taher AT, Abboud MR, El-Beshlawy A, Buchanan GR, Smith H, Ataga KI, Perrine SP, Ghalie RG. 2013. A dose-escalation phase IIa study of 2,2-dimethylbutyrate (HQK-1001), an oral fetal globin inducer, in sickle cell disease. Am J Hematol 88:E255–E260. doi: 10.1002/ajh.23533. [DOI] [PubMed] [Google Scholar]
- 53.Patthamalai P, Fuchareon S, Chaneiam N, Ghalie RG, Chui DH, Boosalis MS, Perrine SP. 2014. A phase 2 trial of HQK-1001 in HbE-beta thalassemia demonstrates HbF induction and reduced anemia. Blood 123:1956–1957. doi: 10.1182/blood-2013-11-538470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reid ME, El Beshlawy A, Inati A, Kutlar A, Abboud MR, Haynes J Jr, Ward R, Sharon B, Taher AT, Smith W, Manwani D, Ghalie RG. 2014. A double-blind, placebo-controlled phase II study of the efficacy and safety of 2,2-dimethylbutyrate (HQK-1001), an oral fetal globin inducer, in sickle cell disease. Am J Hematol 89:709–713. doi: 10.1002/ajh.23725. [DOI] [PubMed] [Google Scholar]
- 55.Cokic VP, Smith RD, Beleslin-Cokic BB, Njoroge JM, Miller JL, Gladwin MT, Schechter AN. 2003. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest 111:231–239. doi: 10.1172/JCI16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lou TF, Singh M, Mackie A, Li W, Pace BS. 2009. Hydroxyurea generates nitric oxide in human erythroid cells: mechanisms for gamma-globin gene activation. Exp Biol Med (Maywood, NJ) 234:1374–1382. doi: 10.3181/0811-RM-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huwiler A, Pfeilschifter J. 1999. Nitric oxide stimulates the stress-activated protein kinase p38 in rat renal mesangial cells. J Exp Biol 202:655–660. [DOI] [PubMed] [Google Scholar]
- 58.Park JI, Choi HS, Jeong JS, Han JY, Kim IH. 2001. Involvement of p38 kinase in hydroxyurea-induced differentiation of K562 cells. Cell Growth Differ 12:481–486. [PubMed] [Google Scholar]
- 59.Witt O, Sand K, Pekrun A. 2000. Butyrate-induced erythroid differentiation of human K562 leukemia cells involves inhibition of ERK and activation of p38 MAP kinase pathways. Blood 95:2391–2396. [PubMed] [Google Scholar]
- 60.Mabaera R, West RJ, Conine SJ, Macari ER, Boyd CD, Engman CA, Lowrey CH. 2008. A cell stress signaling model of fetal hemoglobin induction: what doesn't kill red blood cells may make them stronger. Exp Hematol 36:1057–1072. doi: 10.1016/j.exphem.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Sankaran VG, Xu J, Ragoczy T, Ippolito GC, Walkley CR, Maika SD, Fujiwara Y, Ito M, Groudine M, Bender MA, Tucker PW, Orkin SH. 2009. Developmental and species-divergent globin switching are driven by BCL11A. Nature 460:1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou D, Liu K, Sun CW, Pawlik KM, Townes TM. 2010. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat Genet 42:742–744. doi: 10.1038/ng.637. [DOI] [PubMed] [Google Scholar]
- 63.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. 1991. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 325:11–16. [DOI] [PubMed] [Google Scholar]
- 64.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. 1994. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 330:1639–1644. [DOI] [PubMed] [Google Scholar]
- 65.Powars DR, Weiss JN, Chan LS, Schroeder WA. 1984. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 63:921–926. [PubMed] [Google Scholar]
- 66.Lebensburger JD, Pestina TI, Ware RE, Boyd KL, Persons DA. 2010. Hydroxyurea therapy requires HbF induction for clinical benefit in a sickle cell mouse model. Haematologica 95:1599–1603. doi: 10.3324/haematol.2010.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]