Abstract
Maintenance of chromosomal ends (telomeres) directly contributes to cancer cell immortalization. The telomere protection enzymes belonging to the tankyrase (Tnks) subfamily of poly(ADP-ribose) polymerases (PARPs) have recently been shown to also control transcriptional response to secreted Wnt signaling molecules. Whereas Tnks inhibitors are currently being developed as therapeutic agents for targeting Wnt-related cancers and as modulators of Wnt signaling in tissue-engineering agendas, their impact on telomere length maintenance remains unclear. Here, we leveraged a collection of Wnt pathway inhibitors with previously unassigned mechanisms of action to identify novel pharmacophores supporting Tnks inhibition. A multifaceted experimental approach that included structural, biochemical, and cell biological analyses revealed two distinct chemotypes with selectivity for Tnks enzymes. Using these reagents, we revealed that Tnks inhibition rapidly induces DNA damage at telomeres and telomeric shortening upon long-term chemical exposure in cultured cells. On the other hand, inhibitors of the Wnt acyltransferase Porcupine (Porcn) elicited neither effect. Thus, Tnks inhibitors impact telomere length maintenance independently of their affects on Wnt/β-catenin signaling. We discuss the implications of these findings for anticancer and regenerative medicine agendas dependent upon chemical inhibitors of Wnt/β-catenin signaling.
INTRODUCTION
Tankyrase proteins (Tnks1 and -2) belong to the superfamily of poly(ADP-ribose) polymerases (PARPs) that catalyze the addition of poly(ADP-ribose) onto substrates, thereby influencing the activity and stability of the modified proteins (1, 2). Tnks proteins are expressed in nearly every tissue and control a broad range of cellular processes that include DNA damage repair, Wnt signaling, and telomere length maintenance (2–4). Deletion of both TNKS genes results in embryonic lethality, thus revealing redundant but essential roles during development (5).
In Wnt signaling, Tnks enzymes establish a cellular threshold of response to ligands by controlling the abundance of axin, a protein that promotes the destruction of the transcriptional coactivator β-catenin (6). Thus, loss of Tnks activity results in accelerated destruction of β-catenin and loss of Wnt-dependent transcriptional responses mediated by the TCF/LEF family of DNA binding proteins. The tumor suppressor adenomatous polyposis coli (APC) scaffolds a destruction complex that promotes β-catenin turnover and is mutated in >80% of colorectal cancer (CRC) cases. The sensitivity of β-catenin turnover to Tnks activity even in the absence of normal APC function suggests that Tnks inhibitors could be useful against CRC (6, 7).
Despite the abundance of evidence that disabling Tnks activity can achieve specific anti-Wnt/β-catenin signaling effects (6, 7), the consequences stemming from Tnks inhibition for other Tnks-associated cellular processes remain unclear (4, 8–11). Indeed, Tnks1 was initially identified as a regulator of telomeric repeat binding factor (Terf1/Trf1), a member of a protein family now recognized as essential to telomere replication (12–14). At the same time, disruption of Tnks function has been shown to induce telomere cohesion (15). A greater understanding of the cellular impact of Tnks inhibition should reveal novel uses of Tnks inhibitors and at the same time potential liabilities associated with achieving anti-Wnt pathway effects with such chemicals.
Here, we used biochemical approaches to identify selective Tnks inhibitors from a small-compound collection enriched for Wnt pathway antagonists. We then used this newly assembled chemical panel to evaluate the effects of Tnks inhibition on telomere length maintenance. We demonstrate that loss of Wnt/β-catenin signaling induced by Tnks inhibitors is coupled with rapid DNA damage response at telomere ends and telomeric shortening in cells subjected to long-term chemical exposure. Thus, our findings delineate a chemical approach for disabling two cancer-associated cellular processes with a single agent, as well as an approach for targeting Wnt signaling without compromising telomeric integrity using Porcn inhibitors.
MATERIALS AND METHODS
Reagents.
Antibodies were purchased from the following sources: BD Biosciences (Ctnnb1), Sigma (β-actin and acetylated tubulin), Santa Cruz Biotechnology (Tnks and glutathione S-transferase [GST]), Cell Signaling Technology (axin1, axin2, and Dvl2), Millipore (phospho-histone H2A.X [Ser139]/gamma H2A.X), and Epitomics (Terf2). HeLa, DLD-1, and SW480 cells were acquired from ATCC. Inhibitor of Wnt response 1 (IWR-1) and inhibitor of Wnt production 2 (IWP-2) were synthesized as previously described (16). The sources of chemicals were as follows: IWR-3 to -8 (ChemDiv or ChemBridge), XAV-939 (Maybridge), and LGK-974 (ActiveBiochem) (see Fig. S1 in the supplemental material for IWR-8 synthesis).
Biochemical assays.
For Western blot analysis, cell lysates were generated with either phosphate-buffered saline (PBS)–NP-40 buffer (PBS–1% NP-40), or Tris–Triton X-100 buffer (50 mM Tris [pH 8], 200 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10% glycerol, 1 mM dithiothreitol [DTT], 0.5 mM deoxycholate). Both buffers were supplemented with protease inhibitor cocktail (Sigma). For telomeric oligonucleotide pulldown assays, cell lysates were generated with Tris–Triton X-100 buffer supplemented with a protease inhibitor cocktail, 5 μM ADP (hydroxymethyl)pyrrolidinediol (ADP-HPD), and 1 μM IWR-1 (to prevent Tnks parsylation postlysis). The lysates were cleared by centrifugation at 15,000 rpm for 10 min. Terf1 was incubated with either biotinylated telomeric (TTAGGG)5 or control (TGAGGG)5 oligonucleotides and streptavidin-Sepharose for 4 h, followed by washing with the same buffer.
RNA interference (RNAi) and flow cytometry.
Control, Tnks1 (Dharmacon MU-004740-01-0002), Tnks2 (Dharmacon MU-004741-01-0002), or Tnks1 and -2 small interfering RNA (siRNA) pools were cotransfected with a green fluorescent protein (GFP) expression construct into HeLa cells using Effectene (Qiagen). After 24 h, GFP+ cells were sorted with a MoFlo-XDP flow cytometer (Beckman Coulter). Approximately 5,000 GFP+ cells were plated on coverslips and incubated for 24 h and then processed for telomere dysfunction-induced focus (TIF) quantification (see below). For Western blot analysis, ∼2,500 GFP+ cells were plated in 24-well tissue culture plates and incubated with IWR-1 (10 μM) for 24 h prior to cell lysis.
Luciferase reporter assays.
Assays were executed as described previously using a Dual Luciferase kit (Promega) and SuperTopFlash and control simian virus 40 (SV40)-driven Renilla luciferase reporters (SV40-Ren luc) (16).
TIF assay.
Cells were treated with chemicals for 24 h before fixation (2% formaldehyde with permeabilization in 0.5% [vol/vol] NP-40) and then incubated with gamma H2A.X and Terf2 antibodies and secondary antibodies (mouse fluorescein isothiocyanate-conjugated or Alexa Fluor 488-conjugated antibodies). The primary and secondary antibodies were diluted in PBS, 0.2% fish gelatin, and 0.5% bovine serum albumin (BSA). Cells were imaged using a Zeiss LSM 780 confocal/multiphoton microscope, and three-dimensional (3D) colocalization was assessed using Bitplane Imaris software. The TIF index was determined by assessment of colocalization of pH2A.X and Terf2 for 90 to 100 cells in 10 random areas within each slide.
TRF telomere length assay.
Cells (1 × 106) were collected and washed with PBS. DNA was isolated following the manufacturer's instructions (Qiagen). The DNA (2.5 μg) was digested with six different restriction enzymes (HhaI, HinfI, MspI, HaeIII, RsaI, and AluI) (New England BioLabs) and incubated at 37°C overnight. The digested DNA was separated on a 0.7% agarose gel overnight at 70V. The terminal restriction fragment (TRF) gel was denatured for 20 min in denaturing solution (0.5 M NaOH, 1.5 M NaCl, pH 13.2) and dried on Whatman 3MM paper under vacuum for 3 h at 56°C. The gel was neutralized for 15 min in neutralization buffer (1.5 M NaCl, 0.5 M Tris-HCl, pH 8.0) and then probed with a radiolabeled telomeric probe (C rich) for 16 h at 42°C in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer, 5× Denhardt's solution, 10 mmol/liter Na2HPO4, and 1 mmol/liter Na2H2P2O7. The gel was washed once with 2× SSC, 0.1% SDS; twice with 0.5× SSC, 0.1% SDS; and then twice with 0.5× SSC, 1% SDS at room temperature for 15 min. The gels were exposed to a PhosphorImager screen overnight and analyzed using a Typhoon PhosphorImager scanner system (Molecular Dynamics). The radioprobe hybridization density relative to vertical distance was plotted using ImageJ software, with an average of 25 data points generated for each lane.
Protein expression and purification.
The catalytic domain of human Tnks1 (residues 1105 to 1313) was expressed by using a modified pET28 vector, which encodes an N-terminal His6 tag and a cleavage site for the tobacco etch virus (TEV) protease. Protein expression was induced by 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 15°C when the culture of the bacterial strain BL21(DE3) transformed with the plasmid reached an optical density (OD) of 1.5. Cells were harvested 12 h after induction and lysed by French pressing. The lysate was cleared by centrifugation at 15,000 rpm for 1 h. The protein was purified by Ni-nitrilotriacetic acid (NTA)-based affinity chromatography and ion-exchange chromatography. The N-terminal His6 tag was removed by overnight TEV protease treatment at 4°C. The purified protein was concentrated and stored at −80°C.
Crystallization, data collection, and structure determination.
The purified Tnks1 catalytic domain at 5 mg/ml (0.22 mM) mixed with IWR-1 (the exoform), IWR-3, and IWR-8 at 0.25 mM was subjected to crystallization trials using sitting-drop 96-well plates. Following initial hits of crystallization, large crystals were obtained by hanging-drop vapor diffusion under conditions optimized based on the initial conditions. Crystals of the Tnks1/IWR-1 complex were grown at 20°C in 0.1 M Bis-Tris (pH 5.3), 0.15 M MgCl2, and 20 to 23% polyethylene glycol (PEG) 3350. Crystals of the Tnks1/IWR-3 complex were grown at 20°C in 0.1 M Tris (pH 7.5), 0.2 M sodium acetate, and 30% PEG 4000. Crystals of the Tnks1/IWR-8 complex were grown at 20°C in 0.1 M Bis-Tris propane (pH 8.0), 0.2 M sodium bromide, and 25% PEG 3350. The Protein Data Bank (PDB) identifiers (IDs) for the structures are as follows: Tnks1/IWR-1 (4OA7), Tnks1/IWR-3 (4TOS), and Tnks1/IWR-8 (4TOR).
Crystals were cryoprotected in the crystallization buffer supplemented with 25% glycerol and flash-cooled in liquid nitrogen. Diffraction data were collected at 100 Kelvin on beamline 19ID at the Advanced Photon Source (Argonne National Laboratory). The data were indexed, integrated, and scaled by using HKL2000. The structure of the Tnks1 catalytic domain in the apo state (PDB ID, 2RF5) (17) was used as the search model for molecular replacement by using the Phaser module in the Phenix package (18). Iterative model building and refinement were performed by using the Phenix and Coot programs, respectively (19, 20). The IWR compounds were placed when the Rfree was below 32% and the position of the compound was well defined by the electron density map. Comprehensive model validation was performed by using MolProbity (21). Detailed statistics of data collection and refinement are listed in Table 1. Structure figures were rendered in PyMOL (PyMOL Molecular Graphics System; Schrodinger).
TABLE 1.
Data collection and refinement statistics for IWR compound/Tnks1 crystal structure
| Statistic | Valuea |
||
|---|---|---|---|
| TNKS1/IWR-1 | TNKS1/IWR-3 | TNKS1/IWR-8 | |
| Data collection | |||
| Space group | P 62 | P 212121 | P 62 |
| Cell dimensions | |||
| a, b, c (Å) | 107.94, 107.94, 121.92 | 48.20, 81.17, 114.16 | 108.38, 108.38, 122.22 |
| a, β, γ (°) | 90, 90, 120 | 90, 90, 90 | 90, 90, 120 |
| Resolution (Å) | 50.0–2.3 (2.34–2.30) | 31.0–1.8 (1.87–1.80) | 37.4–1.5 (1.55–1.50) |
| Rsym | 11.2 (51.3) | 7.3 (>100) | 8.3 (95.1) |
| I/σI | 15.7 (2.4) | 38.8 (1.5) | 26.3 (1.3) |
| Completeness (%) | 99.7 (94.7) | 99.3 (94.5) | 99.1 (92.0) |
| Redundancy | 7.3 (5.2) | 7.0 (6.2) | 6.2 (3.0) |
| CC1/2b at the highest-resolution shell | 0.86 | 0.84 | 0.60 |
| Refinement | |||
| Resolution (Å) | 2.30 | 1.80 | 1.50 |
| No. of reflections | 34,662 | 41,860 | 128,406 |
| Completeness (%) | 97 | 99 | 99 |
| Rwork/Rfreec (%) | 22.6/28.3 | 18.1/21.1 | 17.7/20.7 |
| No. of atoms | 7,048 | 3,522 | 7,804 |
| Protein | 6,568 | 3,262 | 6,820 |
| Ligand/ion | 128 | 44 | 163 |
| Water | 352 | 216 | 821 |
| B-factors | |||
| Protein | 43.7 | 45.7 | 22.0 |
| Ligand/ion | 40.6 | 37.3 | 26.2 |
| Water | 39.5 | 49.9 | 33.7 |
| R.m.s. deviations | |||
| Bond length (Å) | 0.004 | 0.007 | 0.007 |
| Bond angle (°) | 0.77 | 1.01 | 1.08 |
| Ramachandran plot | |||
| Favored (%) | 98.2 | 99.0 | 99.0 |
| Allowed (%) | 1.8 | 1.0 | 1.0 |
| Disallowed (%) | 0 | 0 | 0 |
The highest-resolution shell is shown in parentheses.
Pearson correlation coefficient between random half data sets.
Rwork/Rfree = Σ|Fobs − Fcalc|/Σ|Fobs|, where Fobs and Fcalc are observed and calculated structure factor amplitudes, respectively. Rwork was calculated from data used for the refinement, whereas Rfree was calculated from the test set.
qPCR.
Total RNA was purified using an RNeasy Plus minikit (Qiagen) and reverse transcribed with a ProtoScript First Strand cDNA synthesis kit (New England BioLabs) according to the manufacturer's protocol. Quantitative PCR (qPCR) was performed using FastStart SYBR green Master (Roche) on a Light Cycler 480 System. The primers used for reverse transcription (RT)-PCR were as follows: hAXIN2-F, CCACACCCTTCTCCAATCC; hAXIN2-R, TGCCAGTTTCTTTGGCTCTT; Actin-β-F, GGATGCAGAAGGAGATCACTG; Actin-β-R, CGATCCACACGGAGTACTTG.
In vitro Tnks activity assays.
The HT Universal Color PARP assay kit with histone-coated strip wells (Trevigen) was used to monitor the activity of purified Tnks1 SAM-PARP protein (1 μg/96-well reaction; provided by Herwig Schüler) according to the manufacturer's protocol. The Tnks-Fc construct was generated by PCR-based cloning of human Tnks1 sequence in frame into the pcDNA3-hIgG-Fc vector using BglII restriction sites (22). Overexpressed Tnks-Fc protein immobilized on protein A-Sepharose was incubated with human Terf1-GST protein (Abnova) and NAD biotin (2.5 μM; Trevigen) for 30 min at room temperature prior to Western blot analysis. Parsylated Terf1 protein was detected using streptavidin-horseradish peroxidase (HRP). Terf1-GST and Tnks1-Fc proteins were detected by Western blotting with anti-GST and anti-Fc antibodies, respectively.
Digital droplet PCR.
The digital droplet TRAP (20 mM Tris-HCl, pH 8.3, 1.5 mM MgCl2) (ddTRAP) assay was performed as previously described (23). Briefly, pellets of 100,000 fresh sample cells were lysed on ice for 30 min in NP-40 lysis buffer [10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1 mM MgCl2, 1% (vol/vol) NP-40, 0.25 mM sodium deoxycholate, 150 mM NaCl, 10% (vol/vol) glycerol, 5 mM β-mercaptoethanol, 0.1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride) (AEBSF)]. A total of 1,250 cell equivalents of lysate was added to the telomerase extension reaction mixture in TRAP buffer with 0.4 mg/ml BSA, the telomerase extension substrate (TS) (200 nM high-performance liquid chromatography [HPLC]-purified 5′-AATCCGTCGAGCAGAGTT), and 2.5 mM deoxynucleoside triphosphates (dNTPs). The extension reaction mixture was incubated at 25°C for 40 min, followed by heat killing at 95°C for 5 min. The PCR was performed in 1× EvaGreen ddPCR Supermix v2.0 (Bio-Rad) with 50 nM TS primer, 50 nM ACX reverse primer, and 50 cell equivalents of the extension reaction mixture as input. Droplets were produced following the manufacturer's instructions in the droplet generator (Bio-Rad), and the emulsions were transferred to a 96-well plate for amplification (95°C for 5 min; 40 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 30 s; hold at 12°C). Fluorescence was quantitated using a QX200 droplet reader (Bio-Rad) in the 6-carboxyfluorescein (FAM) channel. Thresholds for quantitation were determined by comparison to a telomerase-negative fibroblast line and no-cell-input negative-control samples and untreated HeLa cell positive-control samples.
PARP profiling.
PARP profiling was performed by BPS Bioscience Inc., San Diego, CA, following the BPS PARP or TNKS assay kit protocol. The enzymatic reactions were conducted in duplicate at room temperature for 1 h in a 96-well plate coated with histone substrate. Fifty microliters of reaction buffer (Tris-HCl, pH 8.0) contained NAD+, biotinylated NAD+, activated DNA, recombinant PARP enzyme, and 10 μM test compound. Olaparib (BPS Biosciences) was used at 20 nM concentration. After enzymatic reactions, 50 μl of streptavidin-horseradish peroxidase was added to each well, and the plate was incubated at room temperature for an additional 30 min. One hundred microliters of developer reagents was added to the wells, and the luminescence was measured using a BioTek Synergy 2 microplate reader.
RESULTS
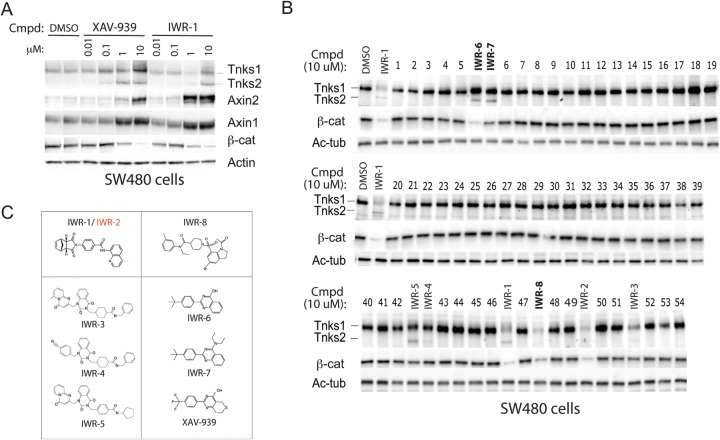
By screening a 200,000-small-molecule library in cells with autonomous Wnt signaling, we previously identified ∼60 compounds that disrupted cellular response to Wnt ligands (termed inhibitors of Wnt response [IWR] compounds) (16). The identification of the Tnks enzymes as the targets of two IWR compounds (IWR-1 and -2) suggested that other IWR compounds may also disable Wnt signaling by the same mechanism (7, 24). Inhibition of Tnks-mediated autoparsylation, a biochemical trigger for its proteasome-mediated destruction, results in changes in Tnks abundance in cultured cells, thus affording a straightforward screen for potentially novel Tnks inhibitors (Fig. 1A) (6). The loss of β-catenin protein additionally serves as a useful marker of chemically induced Tnks inhibition. After subjecting all of the IWR compounds to these biochemical tests for Tnks-inhibitory activity in cultured cells, we uncovered six additional Tnks antagonists (IWR-3 to -8) (Fig. 1B). Thus, a total of four chemotypes supporting Tnks inhibition emerged from our original Wnt pathway antagonist screen (Fig. 1C). We note that IWR-6 and -7 resemble the previously identified Tnks inhibitor XAV-939, whereas IWR-8 represents a novel chemotype with such activity.
FIG 1.
Identification of novel Tnks inhibitors from the IWR chemical collection. (A) Biochemical markers of Tnks inhibition associated with the Wnt/β-catenin pathway. The Tnks inhibitors XAV-939 and IWR-1 induce stabilization of both Tnks enzymes and axin proteins in SW480 cells (expressing truncated APC protein) but promote destruction of β-catenin (β-cat). Cmpd, compound; DMSO, dimethyl sulfoxide. (B) Biochemical screen to identify additional Tnks inhibitors from the IWR collection of Wnt pathway inhibitors. The effects of the IWR compounds on Tnks1 and -2, β-catenin, and acetylated tubulin (Ac-Tub) (loading control) expression levels were determined in SW480 cells by Western blotting. IWR-1 and -2 are known Tnks inhibitors, whereas IWR-3 to -5 were previously shown to induce β-cat destruction but not assigned as Tnks inhibitors. Novel compounds that induced changes in Tnks and β-catenin levels identified by this screen were renamed IWR-6 to -8 (in boldface). (C) Chemotype-based grouping of potential Tnks inhibitors identified from the IWR collection. IWR-6 and -7 are similar to the previously reported XAV-939 compound.
We confirmed that molecules representative of each chemotype found in this chemical set were able to directly target Tnks using a recombinant Tnks protein assay that utilizes histone parsylation as a readout (Fig. 2A). Given the intense interest in developing Tnks inhibitors as anticancer drugs, we reasoned that the novel chemical scaffold supporting Tnks inhibition revealed by IWR-8 may provide new opportunities for achieving a drug-like molecule. Indeed, similar to other established Tnks inhibitors, IWR-8 disrupted deviant Wnt signaling and cell growth in cells lacking normal APC activity in a dose-dependent fashion (Fig. 2B and C). Thus, despite the absence of resemblance to other, previously described Tnks inhibitors, IWR-8 exhibits both in vitro and in vivo activities associated with a bona fide Tnks inhibitor.
FIG 2.
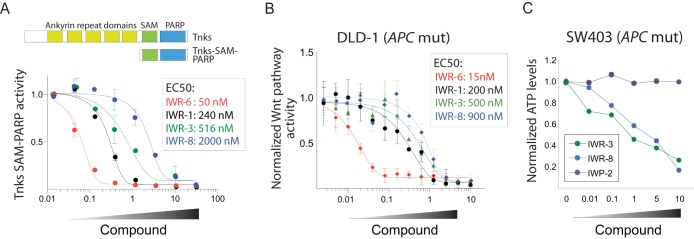
The novel Tnks inhibitor chemotype IWR-8 directly inhibits Tnks enzymes and disrupts β-catenin-dependent transcriptional activity induced by loss of APC function. (A) IWR-3, -6, and -8 directly target Tnks. Representative IWRs from each chemotype were evaluated for the ability to inhibit purified recombinant Tnks1 protein (Tnks1 SAM-PARP protein). Parsylation of immobilized histone protein was determined by colorimetric detection of incorporated biotinylated NAD+ in a 96-well format. (B) IWR-8 inhibits β-catenin activity induced by loss of APC function. The 50% effective concentrations (EC50s) of IWR-6, as well as IWR-1, IWR-3, and IWR-8, were measured in DLD-1 cells using a Wnt/β-catenin-specific luciferase reporter. (C) IWR-8 inhibits cell growth in a cancer cell line with compromised APC function. SW403 cells previously shown to exhibit Wnt/β-catenin pathway-dependent cell growth in culture (58) were treated with either IWR-3, IWR-8, or IWP-2 (an inhibitor of the Wnt acyltransferase Porcupine [16]; a negative control).
Tnks enzymes accommodate their ADP-ribose donor substrate, NAD (NAD+), in two subpockets—one binds the nicotinamide (NI) and the other binds the adenosine (AD) moiety. IWR-1 was previously shown to be a first-in-class inhibitor that targets the AD-binding pocket rather than the NI-binding pocket (25). We selected representative compounds from each group and determined their modes of attack using structural analyses. First, we confirmed observations that IWR-1 engages the AD-binding pocket, unlike prototypical PARP inhibitors, represented here by the NI-binding-pocket inhibitor XAV-939 (Fig. 3A and Table 1). IWR-2, a derivative of IWR-1, also binds Tnks1 in this mode (26). Despite their chemical dissimilarity, IWR-8, like IWR-1, exploits a histidine uniquely found in the D-loop of Tnks enzymes (His1201 in Tnks1; His1048 in Tnks2) to engage the AD pocket while concomitantly inducing a change in the D-loop conformation (Fig. 3B). Instead of a quinolone group found in IWR-1 that mediates aromatic stacking with His1201, IWR-8 achieves a similar chemical-protein interaction using an indoline group. We noted from the crystallographic and nuclear magnetic resonance (NMR)-based evidence that the structure of IWR-8 is actually a regioisomer of the structure that was described in the first report of its synthesis (see Fig. S1 in the supplemental material). Perhaps not surprisingly, given its size, IWR-3 engages both NI- and AD-binding pockets (Fig. 3C). In the end, these Tnks antagonists can be organized based on their pocket-binding preferences (Fig. 3D).
FIG 3.
Crystallographic studies reveal distinct modes of chemical attack by members of the IWR collection of Tnks inhibitors. (A) Crystal structure of IWR-1/Tnks1. The structure of XAV-939, a prototypical PARP inhibitor, bound to Tnks2 (PDB ID, 3KR8) is superimposed for comparison with respect to chemical binding pocket preference and ability to induce conformational change in the D loop. (B) Crystal structure of IWR-8/Tnks1. (C) Crystal structure of IWR-3/Tnks1. The D loops of Tnks1 (1196 to 1211) and Tnks2 (1043 to 1058) are shown in turquoise and cyan, respectively. His1201, which interacts with all three IWR compounds and facilitates chemical-specific D-loop conformational changes, is highlighted. AD- and NI-binding sites are shown in pink and green, respectively. (D) Summary of binding pocket preferences of IWR compounds that target Tnks enzymes. Given the structural similarity of IWR-6 and -7 to XAV-939, these novel molecules likely engage the NI pocket of Tnks.
The higher level of conservation with the NI-binding pocket in PARPs than with the AD-binding pocket likely imposes greater challenges to achieving specificity with NI-binding pocket inhibitors (27). Indeed the ∼100-fold-greater specificity of IWR-1 compared to XAV-939 for Tnks enzymes over other PARPs has been attributed to the difference in their modes of attack (25, 26). At the same time, the discovery of structurally distinct chemicals that are both capable of binding the AD-binding pocket in similar fashions (IWR-1 and IWR-8) afforded an opportunity to evaluate the strength of this hypothesis. We profiled two AD-binding-pocket compounds (IWR-1 and IWR-8), one NI-binding pocket compound (IWR-6), and one dual-pocket inhibitor (IWR-3) against a panel of recombinant PARP proteins that covers 70% of the protein family (Table 2). In support of the hypothesis, the AD-binding chemicals exhibited greater specificity than the NI-binding chemical. Surprisingly, despite having to engage both pockets, IWR-3 exhibited poor selectivity. In the end, our overall effort to glean novel Tnks antagonists from our IWR chemical collection identified two highly specific Tnks inhibitors—IWR-1 and IWR-8—with on-target activities confirmed using biochemical, structural, and cell biological analyses.
TABLE 2.
Specificities of novel IWR Tnks inhibitorsa
| Protein | % inhibition |
||||
|---|---|---|---|---|---|
| AD pocket inhibitor |
NI pocket inhibitor (IWR-6) | Dual-pocket inhibitor (IWR-3) | Control (olaparib) | ||
| IWR-1 | IWR-8 | ||||
| PARP1 | 0 | 0 | 44 | 0 | 100 |
| PARP2 | 5 | 3 | 71 | 27 | 100 |
| PARP3 | 6 | 11 | 60 | 11 | 100 |
| TNKS1 | 100 | 99 | 100 | 98 | N/A |
| TNKS2 | 100 | 99 | 100 | 100 | N/A |
| PARP6 | 0 | 0 | 0 | 6 | 100 |
| PARP7 | 7 | 8 | 9 | 20 | 87 |
| PARP8 | 1 | 0 | 0 | 21 | 96 |
| PARP10 | 18 | 9 | 8 | 49 | 99 |
| PARP11 | 2 | 12 | 32 | 17 | 96 |
| PARP12 | 11 | 14 | 36 | 31 | 84 |
| PARP15 | 0 | 0 | 0 | 28 | 82 |
The indicated IWR compounds (10 μM) and the control pan-PARP inhibitor olaparib (20 nM) were incubated with the indicated recombinant proteins, and parsylation of immobilized histone was measured.
Terf1 (also known as Trf1) is essential to telomere integrity due to binding double-stranded TTAGGG telomere repeats and is a well-established target of Tnks enzymatic activity (4). Each round of cell doubling engenders loss of some telomeric sequence due to the inability of the DNA replication machinery to achieve complete chromosomal-end duplication (the “end replication” problem) (28). Loss of Terf1 has been shown to induce cohesion of telomere ends and the formation of G quadruplexes in the lagging strand, thus promoting telomere replication stalling and fragility (14). When telomeric sequences become sufficiently shortened, chromosomal ends become “uncapped” and are unable to properly engage the shelterin protective components, such as Terf1, thereby resulting in a DNA damage response and cellular senescence or apoptosis. Cancerous cells overcome this cell growth braking mechanism largely by invoking telomerase expression (29).
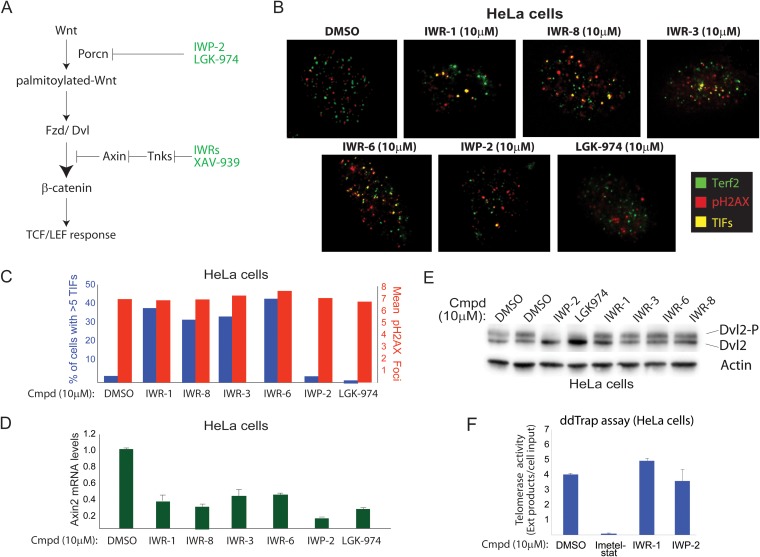
We exploited the chemical dissimilarity of the IWR compounds to identify high-confidence on-target effects of disrupting Tnks catalytic activity on telomere length maintenance. As controls, we employed chemicals targeting Porcn, an acyltransferase essential for Wnt-instructed β-catenin activation, as a means of influencing β-catenin without directly disabling Tnks (Fig. 4A). TIF are markers of telomeric damage scored by the appearance of the colocalizing shelterin component Terf2/Trf2 and phosphorylated H2A.X (gamma H2A.X), a biochemical marker of damaged DNA (30). Whereas the Tnks inhibitors markedly induced TIF formation in a cervical carcinoma cell line (HeLa cells), the Porcn inhibitors failed to do the same (Fig. 4B and C). However, true to the previously assigned activities of Tnks and Porcn enzymes in Wnt signaling, all the compounds tested disrupted the expression of Axin2, a well-validated target gene of the Wnt/β-catenin pathway (Fig. 4D). At the same time, Porcn, but not Tnks, inhibitors blocked Wnt ligand-dependent Frizzled receptor activity in these cells, as measured by phosphorylation of the cytoplasmic signaling molecule Dishevelled 2 (Dvl2) (Fig. 4E) (31, 32). Finally, we ruled out inadvertent direct inactivation of telomerase by Tnks inhibitors as the cause of TIF using IWR-1 in an in vitro assay for telomerase activity (Fig. 4F). Thus, the chemical induction of telomeric damage is independent of the effects stemming from the dampening of Wnt/β-catenin pathway activity.
FIG 4.
Tnks inhibitors induce telomeric stress in human cells independently of Wnt/β-catenin pathway inhibition. (A) Porcn inhibitors afford a Tnks-independent approach to disrupting Wnt/β-catenin signaling. Chemicals targeting the Wnt acyltransferase Porcn abrogate Wnt palmitoylation, which in turn results in loss of activity of the Frizzled (Fzd) family of Wnt receptors. Fzd receptors directly induce activation of the cytoplasmic signaling molecule Dishevelled (Dvl) in a ligand-dependent manner, which in turn results in activation of β-catenin and transcription mediated by the TCF family of DNA binding proteins. IWP-2 and the clinical candidate LGK-974 represent two structurally distinct Porcn inhibitors. (B) Tnks, but not Porcn, inhibitors induce DNA damage at telomeres. The appearance of colocalized Terf2 and pH2A.X (an indicator of DNA damage at the telomere ends, TIF) was scored using indirect immunofluorescence in HeLa cells (expressing wild-type APC protein). (C) Quantification of results represented in panel B. Cells with >5 TIF were scored as positive for telomere damage. The mean total phosphorylated H2A.X (gamma H2A.X) was used to measure general DNA damage responses. (D) Tnks and Porcn inhibitors disrupt β-catenin-dependent transcription in HeLa cells. qPCR analysis of Axin2, a target gene of Wnt/β-catenin signaling, reveals that both classes exert influence on β-catenin activity. The error bars indicate standard deviations. (E) IWP-2 and LGK-974, but not Tnks, inhibitors block ligand-dependent phosphorylation of Dvl2, a cytoplasmic signaling molecule regulated by the Frizzled family of Wnt receptors. (F) IWR compounds do not inhibit telomerase. Digital droplet-based telomerase extension analysis of genomic DNA isolated from HeLa cells treated for 24 h with the indicated compounds.
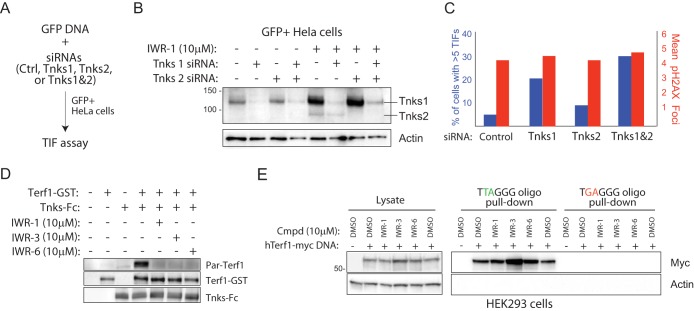
Using a cell-sorting strategy to isolate cells transfected with pooled siRNAs targeting one or both Tnks transcripts (Fig. 5A and B), we demonstrated, using RNAi in HeLa cells, that decreased Tnks1 and -2 protein is associated with an increased number of TIF (Fig. 5C). Consistent with a previous report that both Tnks proteins contribute to Terf1 regulation, simultaneous knockdown of both transcripts gave rise to a greater induction of TIF than from individual-transcript-targeting experiments (3). Next, we directly evaluated the effects of the IWR compounds on Tnks-mediated Terf1 parsylation using an in vitro reconstitution assay. Three Tnks inhibitors with distinct chemotypes blocked Terf1 parsylation under these conditions, thus providing evidence that both adenosine- and nicotinamide-binding pocket inhibitors likely disrupt Tnks regulation of Terf1 in cells (Fig. 5D).
FIG 5.
Chemical disruption of Tnks likely results in increased parsylation-dependent Terf1 binding to telomeric DNA. (A) Strategy for isolating HeLa cells transfected with Tnks1 and/or Tnks2 siRNA. A plasmid supporting constitutive expression of GFP is cotransfected with a pool of siRNAs (4 individual siRNAs per gene) targeting either Tnks1, Tnks2, or both into HeLa cells. GFP+ HeLa cells isolated by flow cytometry were analyzed for levels of TIF 24 h later. (B) Biochemical evidence for on-target effects of Tnks siRNA pools in GFP+ HeLa cells. GFP+ cells presumably harboring the indicated Tnks siRNA pools were isolated by fluorescence-activated cell sorting (FACS) and then subjected to Western blot analysis following culturing in the presence/absence of IWR-1 for 24 h. IWR-1 was used here to aid in the visualization of relative Tnks protein abundance in siRNA-treated/untreated cells. (C) Quantification of TIF in GFP+ HeLa cells transfected with the indicated siRNA pools as described for panel B. (D) IWR-1, -3, and -6 inhibit Tnks-mediated parsylation of Terf1 in vitro. Tnks1-Fc fusion protein purified, using protein A-Sepharose, from HEK293 cells transiently transfected with Tnks1-Fc DNA was incubated with recombinant Terf1-GST protein, NAD-biotin (2.5 μM), and the indicated compounds (5 μM). (E) Tnks inhibitors increase Terf1 binding to telomeric repeat sequences. HEK293 cells transfected with a human Terf1-myc (hTerf1-myc) or control DNA were treated with the indicated compounds for 36 h prior to lysing. The lysate was incubated with biotinylated oligonucleotides containing 5 copies of Terf1-binding or mutated telomeric repeat sequence and streptavidin-coated Sepharose. The starting material and oligonucleotide-bound protein were subjected to Western blot analysis.
Previous studies using overexpressed Tnks protein demonstrated changes in total Terf1 abundance with increasing Tnks protein levels (33, 34). In cells exposed to one of several IWR compounds, we did not detect a change in total Terf1, suggesting that the majority of Terf1 protein is not subject to endogenous Tnks protein regulation (Fig. 5E). Nevertheless, we did observe a general increase in Terf1 binding to oligonucleotides with telomere repeat sequences in cells treated with one of three IWR compounds tested (see Fig. 5E). These results are consistent with a previous report that Terf1 parsylation prevents its binding to telomeric DNA (4). Notably, despite having the weakest anti-Tnks activity of the three IWR compounds evaluated, IWR-3 induced the greatest increase in Terf1 binding to telomeric repeat sequences (Fig. 2A and B). Given the promiscuity of IWR-3 for other PARP family members (Table 2), we reason that, conceivably, other PARP family members may also participate in Terf1 regulation. Nevertheless, taking together the existing biochemical and genetic evidence that Tnks-Terf1 interaction is essential to telomere regulation, our observations demonstrate that IWR-dependent induction of TIF is likely a consequence of impaired Tnks regulation of Terf1.
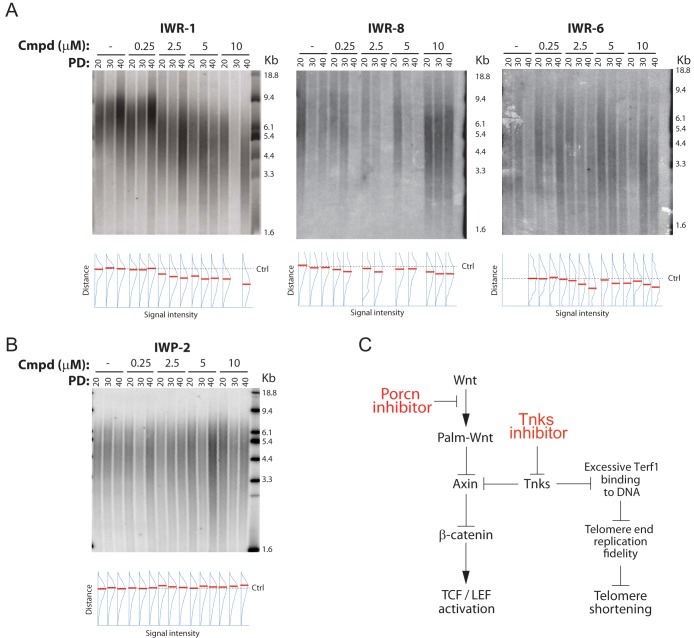
We next evaluated the effects of Tnks inhibition on telomere length maintenance in human HeLa cells treated for an extended period with IWR-1 (up to 40 population doublings). Whereas IWR-1, IWR-8, and IWR-6 induced shortened telomere length in a dose-dependent manner, the Porcn inhibitor IWP-2 did not (Fig. 6A and B). Our data are consistent with Tnks inhibitor-induced telomeric shortening independent of changes to the Wnt signaling status in this cell line. We note that another study using XAV-939 observed similar effects on telomere length maintenance in a neuroblastoma cell line, thereby extending the relevance of our findings to other cell types (35). Taken together, our findings reveal a single-agent strategy for inducing telomerase shortening that is distinct from current efforts devoted to directly targeting telomerase. At the same time, we uncovered an inseparable biological impact on Wnt/β-catenin signaling and telomere length maintenance stemming from the chemical attack of Tnks enzymes (Fig. 6B).
FIG 6.
Tnks inhibitors induce telomeric shortening. (A) IWR-1, IWR-8, and IWR-6 induce telomere shortening. HeLa cells were incubated with IWR-1, IWR-8, or IWP-6 at various concentrations for the indicated number of population doublings (PD), and telomere length was measured by TRF analysis. The density of radioprobe hybridization relative to the vertical distance from the largest molecular size marker was quantified using ImageJ, and the point of highest density in each lane is marked in red. The position of the control telomere-restricted fragment signal (Ctrl) is indicated with a dashed line. Lanes with insufficient strength in labeling or that were disrupted with blemishes during experimental processing were not quantified. (B) Inhibition of β-catenin transcription does not induce telomere shortening. The same assay described in the legend to panel A was used to test the Porcn inhibitor IWP-2. (C) Model of Tnks-dependent regulation of Wnt/β-catenin transcriptional and telomere length maintenance in human cells. Excessive Terf1 binding to DNA in the absence of Tnks-mediated Terf1 parsylation results in replication fork stalling in telomeres (59, 60), which then presumably results in telomere shortening.
DISCUSSION
Chemically based efforts to disable deviant Wnt/β-catenin signaling in cancer have converged on two major strategies that target either the Tnks or Porcn enzyme (36). Our findings reveal that suppression of telomere lengthening and suppression of Wnt/β-catenin signaling are intextricable responses in human cells to highly selective Tnks inhibitors. In the context of cancer, Tnks inhibitors may afford a single-agent synthetic-lethal strategy for targeting cancer-initiating cells that rely on mechanisms for maintaining stem cell-like characteristics. At the same time, loss of Wnt/β-catenin signaling invoked by short or prolonged chemical attack of Porcn spares telomeres, suggesting that previous observations linking Wnt/β-catenin-mediated transcription to telomere regulation may not be universal (37–39) (Fig. 6B).
Tnks and Porcn inhibitors, including IWR-1 and IWP-2, are now widely used for the in vitro engineering of various tissues, including cardiomyocytes, retinal pigmented epithelial cells, pneumocytes, and dopaminergic neurons (40–47). Given the transient nature of the chemical exposure in these protocols (typically 2 to 3 days) and similar efficacies of Tnks and Porcn inhibitors for directing cell fate in a direct comparison (46), we assume the chemical induction of these cell types is due to suppression of Wnt/β-catenin transcriptional responses and not telomeric stress or shortening. Further studies will be required to address whether concomitant induction of telomeric stress associated with Tnks inhibition has any adverse affects on tissue-engineering agendas.
The recent identification of specific Tnks inhibitors is timely, given that the development of Imetelstat (Geron), an oligonucleotide-based inhibitor representing the only small-molecule-targeting telomerase to advance in clinical testing, has stalled due to hematological and hepatoxic dose-limiting side effects (48, 49). Whereas PARP inhibitors have been proposed to be useful for sensitizing telomeres for chemical attack using other agents (34, 50), our study using selective Tnks inhibitors suggests a single-agent strategy can achieve the same endpoint. Moreover, the AD-binding pocket of Tnks can accommodate diverse pharmacophores, as demonstrated here and by other efforts, thus providing a more versatile starting point than imetelstat with respect to medicinal-chemistry goals (51). Recent advances in genetic testing have also uncovered new patient cohorts associated with long telomeric length that may benefit from a Tnks inhibitor, including those with mutations in the shelterin component POT1 in familial melanoma and chronic lymphocytic leukemia (52–54), the catalytic subunit of telomerase Tert in familial and sporadic melanoma (55), and single nucleotide polymorphisms or mutations near the promoters for Tert in glioma and urothelial cancers (56, 57). Thus, the clinical development path for Tnks inhibitors as anticancer agents should include not only consideration of the status of Wnt/β-catenin signaling in various diseases but also the potential contribution of telomere-associated genetic alterations to drug sensitivity.
Supplementary Material
ACKNOWLEDGMENTS
We thank Herwig Schüler (Structural Genomics Consortium, Karolinska Institutet, Stockholm, Sweden), Susan Smith (NYU Langone Medical Center), and Ugur Eskiocak (UT Southwestern Medical Center) for providing reagents and Ilgen Mender and Rubina Tuladhar for technical help. We also thank Jon Bohmann for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health grants R01CA168761 (L.L.), R01GM053163 (Z.O.), and P50-CA70907 (L.L.); Simmons Cancer Center Support Grant 5P30 CA142543 (J.W.S); Welch Foundation grants I-1665 (L.L.), I-1702 (X.Z.), and I-1596 (C.C.); and Cancer Prevention and Research Institute of Texas grant RP130212 (L.L. and C.C.) and grant RP140661 (X.Z.). X.Z. is a Virginia Murchison Linthicum Scholar in Medical Research at UTSW. J.W.S is the Southland Financial Corporation Distinguished Chair in Geriatric Research. This work was supported by the fund from the Japan Society for the Promotion of Science (JSPS) for the Institutional Program for Young Researcher Overseas Visits (K.Y.). The results in this report are derived from work performed at Argonne National Laboratory, Structural Biology Center, at the Advanced Photon Source. Argonne is operated by University of Chicago Argonne, LLC, for the U.S. Department of Energy Office of Biological and Environmental Research under contract DE-AC02-06CH11357. This work was performed in a space constructed with support from National Institutes of Health grant C06 RR30414.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00392-15.
REFERENCES
- 1.Lehtio L, Chi NW, Krauss S. 2013. Tankyrases as drug targets. FEBS J 280:3576–3593. doi: 10.1111/febs.12320. [DOI] [PubMed] [Google Scholar]
- 2.Riffell JL, Lord CJ, Ashworth A. 2012. Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov 11:923–936. doi: 10.1038/nrd3868. [DOI] [PubMed] [Google Scholar]
- 3.Cook BD, Dynek JN, Chang W, Shostak G, Smith S. 2002. Role for the related poly(ADP-ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol Cell Biol 22:332–342. doi: 10.1128/MCB.22.1.332-342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith S, Giriat I, Schmitt A, de Lange T. 1998. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 5.Chiang YJ, Hsiao SJ, Yver D, Cushman SW, Tessarollo L, Smith S, Hodes RJ. 2008. Tankyrase 1 and tankyrase 2 are essential but redundant for mouse embryonic development. PLoS One 3:e2639. doi: 10.1371/journal.pone.0002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. 2009. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 7.Dodge ME, Lum L. 2011. Drugging the cancer stem cell compartment: lessons learned from the hedgehog and Wnt signal transduction pathways. Annu Rev Pharmacol Toxicol 51:289–310. doi: 10.1146/annurev-pharmtox-010510-100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang W, Dynek JN, Smith S. 2005. NuMA is a major acceptor of poly(ADP-ribosyl)ation by tankyrase 1 in mitosis. Biochem J 391:177–184. doi: 10.1042/BJ20050885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho-Park PF, Steller H. 2013. Proteasome regulation by ADP-ribosylation. Cell 153:614–627. doi: 10.1016/j.cell.2013.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guettler S, LaRose J, Petsalaki E, Gish G, Scotter A, Pawson T, Rottapel R, Sicheri F. 2011. Structural basis and sequence rules for substrate recognition by Tankyrase explain the basis for cherubism disease. Cell 147:1340–1354. doi: 10.1016/j.cell.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki Y, Matsui H, Asou H, Nagamachi A, Aki D, Honda H, Yasunaga S, Takihara Y, Yamamoto T, Izumi S, Ohsugi M, Inaba T. 2012. Poly-ADP ribosylation of Miki by tankyrase-1 promotes centrosome maturation. Mol Cell 47:694–706. doi: 10.1016/j.molcel.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi MT, Cesare AJ, Fitzpatrick JA, Lazzerini-Denchi E, Karlseder J. 2012. A telomere-dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nat Struct Mol Biol 19:387–394. doi: 10.1038/nsmb.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarek G, Vannier JB, Panier S, Petrini JH, Boulton SJ. 2015. TRF2 recruits RTEL1 to telomeres in S phase to promote T-loop unwinding. Mol Cell 57:622–635. doi: 10.1016/j.molcel.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann M, Kibe T, Kabir S, de Lange T. 2014. TRF1 negotiates TTAGGG repeat-associated replication problems by recruiting the BLM helicase and the TPP1/POT1 repressor of ATR signaling. Genes Dev 28:2477–2491. doi: 10.1101/gad.251611.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MK, Smith S. 2014. Persistent telomere cohesion triggers a prolonged anaphase. Mol Biol Cell 25:30–40. doi: 10.1091/mbc.E13-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. 2009. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehtio L, Collins R, van den Berg S, Johansson A, Dahlgren LG, Hammarstrom M, Helleday T, Holmberg-Schiavone L, Karlberg T, Weigelt J. 2008. Zinc binding catalytic domain of human tankyrase 1. J Mol Biol 379:136–145. doi: 10.1016/j.jmb.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 18.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. 2002. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D 58:1948–1954. doi: 10.1107/S0907444902016657. [DOI] [PubMed] [Google Scholar]
- 20.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D 60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 21.Chen VB, Arendall WB III, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. 2010. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D 66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lum L, Reid MS, Blobel CP. 1998. Intracellular maturation of the mouse metalloprotease disintegrin MDC15. J Biol Chem 273:26236–26247. doi: 10.1074/jbc.273.40.26236. [DOI] [PubMed] [Google Scholar]
- 23.Ludlow AT, Robin JD, Sayed M, Litterst CM, Shelton DN, Shay JW, Wright WE. 2014. Quantitative telomerase enzyme activity determination using droplet digital PCR with single cell resolution. Nucleic Acids Res 42:e104. doi: 10.1093/nar/gku439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, He X. 2008. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol 20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narwal M, Venkannagari H, Lehtio L. 2012. Structural basis of selective inhibition of human tankyrases. J Med Chem 55:1360–1367. doi: 10.1021/jm201510p. [DOI] [PubMed] [Google Scholar]
- 26.Gunaydin H, Gu Y, Huang X. 2012. Novel binding mode of a potent and selective tankyrase inhibitor. PLoS One 7:e33740. doi: 10.1371/journal.pone.0033740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahlberg E, Karlberg T, Kouznetsova E, Markova N, Macchiarulo A, Thorsell AG, Pol E, Frostell A, Ekblad T, Oncu D, Kull B, Robertson GM, Pellicciari R, Schuler H, Weigelt J. 2012. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat Biotechnol 30:283–288. doi: 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- 28.Palm W, de Lange T. 2008. How shelterin protects mammalian telomeres. Annu Rev Genet 42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 29.Shay JW, Wright WE. 2011. Role of telomeres and telomerase in cancer. Semin Cancer Biol 21:349–353. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takai H, Smogorzewska A, de Lange T. 2003. DNA damage foci at dysfunctional telomeres. Curr Biol 13:1549–1556. doi: 10.1016/S0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AM. 2004. Wnt proteins induce dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol Cell Biol 24:4757–4768. doi: 10.1128/MCB.24.11.4757-4768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob LS, Wu X, Dodge ME, Fan CW, Kulak O, Chen B, Tang W, Wang B, Amatruda JF, Lum L. 2011. Genome-wide RNAi screen reveals disease-associated genes that are common to Hedgehog and Wnt signaling. Sci Signal 4:ra4. doi: 10.1126/scisignal.2001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang W, Dynek JN, Smith S. 2003. TRF1 is degraded by ubiquitin-mediated proteolysis after release from telomeres. Genes Dev 17:1328–1333. doi: 10.1101/gad.1077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seimiya H, Muramatsu Y, Ohishi T, Tsuruo T. 2005. Tankyrase 1 as a target for telomere-directed molecular cancer therapeutics. Cancer Cell 7:25–37. doi: 10.1016/j.ccr.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Tian X, Hou W, Bai S, Fan J, Tong H, Bai Y. 2014. XAV939 promotes apoptosis in a neuroblastoma cell line via telomere shortening. Oncol Rep 32:1999–2006. doi: 10.3892/or.2014.3460. [DOI] [PubMed] [Google Scholar]
- 36.Lum L, Clevers H. 2012. Cell biology. The unusual case of Porcupine. Science 337:922–923. doi: 10.1126/science.1228179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diala I, Wagner N, Magdinier F, Shkreli M, Sirakov M, Bauwens S, Schluth-Bolard C, Simonet T, Renault VM, Ye J, Djerbi A, Pineau P, Choi J, Artandi S, Dejean A, Plateroti M, Gilson E. 2013. Telomere protection and TRF2 expression are enhanced by the canonical Wnt signalling pathway. EMBO Rep 14:356–363. doi: 10.1038/embor.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R. 2012. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science 336:1549–1554. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Toh L, Lau P, Wang X. 2012. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/beta-catenin pathway in human cancer. J Biol Chem 287:32494–32511. doi: 10.1074/jbc.M112.368282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, Niklason LE. 2013. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest 123:4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez R, Lee JW, Schultz PG. 2011. Stepwise chemically induced cardiomyocyte specification of human embryonic stem cells. Angew Chem Int Ed Engl 50:11181–11185. doi: 10.1002/anie.201103909. [DOI] [PubMed] [Google Scholar]
- 42.Huang SX, Islam MN, O'Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. 2014. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. 2013. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc 8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Narytnyk A, Verdon B, Loughney A, Sweeney M, Clewes O, Taggart MJ, Sieber-Blum M. 2014. Differentiation of human epidermal neural crest stem cells (hEPI-NCSC) into virtually homogenous populations of dopaminergic neurons. Stem Cell Rev 10:316–326. doi: 10.1007/s12015-013-9493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren Y, Lee MY, Schliffke S, Paavola J, Amos PJ, Ge X, Ye M, Zhu S, Senyei G, Lum L, Ehrlich BE, Qyang Y. 2011. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol 51:280–287. doi: 10.1016/j.yjmcc.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Hao J, Hong CC. 2011. Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/beta-catenin signaling. ACS chemical biology 6:192–197. doi: 10.1021/cb100323z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams SCP. 2013. No end in sight for telomerase-targeted cancer drugs. Nat Med 19:6. doi: 10.1038/nm0113-6. [DOI] [PubMed] [Google Scholar]
- 49.Thompson PA, Drissi R, Muscal JA, Panditharatna E, Fouladi M, Ingle AM, Ahern CH, Reid JM, Lin T, Weigel BJ, Blaney SM. 2013. A phase I trial of imetelstat in children with refractory or recurrent solid tumors: a Children's Oncology Group Phase I Consortium Study (ADVL1112). Clin Cancer Res 19:6578–6584. doi: 10.1158/1078-0432.CCR-13-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerone MA, Burgess DJ, Naceur-Lombardelli C, Lord CJ, Ashworth A. 2011. High-throughput RNAi screening reveals novel regulators of telomerase. Cancer Res 71:3328–3340. doi: 10.1158/0008-5472.CAN-10-2734. [DOI] [PubMed] [Google Scholar]
- 51.Haikarainen T, Venkannagari H, Narwal M, Obaji E, Lee HW, Nkizinkiko Y, Lehtio L. 2013. Structural basis and selectivity of tankyrase inhibition by a Wnt signaling inhibitor WIKI4. PLoS One 8:e65404. doi: 10.1371/journal.pone.0065404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramsay AJ, Quesada V, Foronda M, Conde L, Martinez-Trillos A, Villamor N, Rodriguez D, Kwarciak A, Garabaya C, Gallardo M, Lopez-Guerra M, Lopez-Guillermo A, Puente XS, Blasco MA, Campo E, Lopez-Otin C. 2013. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat Genet 45:526–530. doi: 10.1038/ng.2584. [DOI] [PubMed] [Google Scholar]
- 53.Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, Pooley KA, Pritchard AL, Tiffen JC, Petljak M, Palmer JM, Symmons J, Johansson P, Stark MS, Gartside MG, Snowden H, Montgomery GW, Martin NG, Liu JZ, Choi J, Makowski M, Brown KM, Dunning AM, Keane TM, Lopez-Otin C, Gruis NA, Hayward NK, Bishop DT, Newton-Bishop JA, Adams DJ. 2014. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet 46:478–481. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi J, Yang XR, Ballew B, Rotunno M, Calista D, Fargnoli MC, Ghiorzo P, Bressac-de Paillerets B, Nagore E, Avril MF, Caporaso NE, McMaster ML, Cullen M, Wang Z, Zhang X, Bruno W, Pastorino L, Queirolo P, Banuls-Roca J, Garcia-Casado Z, Vaysse A, Mohamdi H, Riazalhosseini Y, Foglio M, Jouenne F, Hua X, Hyland PL, Yin J, Vallabhaneni H, Chai W, Minghetti P, Pellegrini C, Ravichandran S, Eggermont A, Lathrop M, Peris K, Scarra GB, Landi G, Savage SA, Sampson JN, He J, Yeager M, Goldin LR, Demenais F, Chanock SJ, Tucker MA, Goldstein AM, Liu Y, Landi MT. 2014. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet 46:482–486. doi: 10.1038/ng.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. 2013. TERT promoter mutations in familial and sporadic melanoma. Science 339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 56.Borah S, Xi L, Zaug AJ, Powell NM, Dancik GM, Cohen SB, Costello JC, Theodorescu D, Cech TR. 2015. Cancer TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 347:1006–1010. doi: 10.1126/science.1260200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh KM, Codd V, Smirnov IV, Rice T, Decker PA, Hansen HM, Kollmeyer T, Kosel ML, Molinaro AM, McCoy LS, Bracci PM, Cabriga BS, Pekmezci M, Zheng S, Wiemels JL, Pico AR, Tihan T, Berger MS, Chang SM, Prados MD, Lachance DH, O'Neill BP, Sicotte H, Eckel-Passow JE, van der Harst P, Wiencke JK, Samani NJ, Jenkins RB, Wrensch MR. 2014. Variants near TERT and TERC influencing telomere length are associated with high-grade glioma risk. Nat Genet 46:731–735. doi: 10.1038/ng.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, Woolfenden S, Yu KK, Markovits J, Killary K, Sonkin D, Yao YM, Warmuth M, Sellers WR, Schlegel R, Stegmeier F, Mosher RE, McLaughlin ME. 2011. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 108:17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munoz P, Blanco R, de Carcer G, Schoeftner S, Benetti R, Flores JM, Malumbres M, Blasco MA. 2009. TRF1 controls telomere length and mitotic fidelity in epithelial homeostasis. Mol Cell Biol 29:1608–1625. doi: 10.1128/MCB.01339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohki R, Ishikawa F. 2004. Telomere-bound TRF1 and TRF2 stall the replication fork at telomeric repeats. Nucleic Acids Res 32:1627–1637. doi: 10.1093/nar/gkh309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.