Significance
The mechanism of incorporation of the HIV envelope glycoprotein (Env) into a developing particle is not well understood. We used a previously identified cellular trafficking factor, Rab11-FIP1C, as a probe to identify a key motif in the Env cytoplasmic tail that is essential for Env incorporation into particles. We show that this motif governs the cell-type–specific incorporation of Env into particles and the appearance of Env at the particle budding site. Our results provide key insights into how HIV Env is incorporated into budding particles and support an important role for FIP1C in this process.
Keywords: HIV assembly, HIV envelope, pseudotyping, FIP1C, Rab coupling protein
Abstract
Lentiviruses such as HIV-1 encode envelope glycoproteins (Env) with long cytoplasmic tails (CTs) that include motifs mediating interactions with host-cell–trafficking factors. We demonstrated recently that Rab11-family interacting protein 1C (FIP1C) is required for CT-dependent incorporation of Env into HIV-1 particles. Here, we used viruses bearing targeted substitutions within CT to map the FIP1C-dependent incorporation of Env. We identified YW795 as a critical motif mediating cell-type–dependent Env incorporation. Disruption of YW795 reproduced the cell-type–dependent particle incorporation of Env that had previously been observed with large truncations of CT. A revertant virus bearing a single amino acid change near the C terminus of CT restored wild-type levels of Env incorporation, Gag–Env colocalization on the plasma membrane, and viral replication. These findings highlight the importance of YW795 in the cell-type–dependent incorporation of Env and support a model of HIV assembly in which FIP1C/RCP mediates Env trafficking to the particle assembly site.
Lentiviruses such as HIV encode envelope glycoproteins (Envs) with long cytoplasmic tails (CTs) of 150 amino acids or more, whereas avian and murine retroviruses generally encode CTs of 20–30 residues. The reasons for this difference are not entirely clear, but may be attributable to interactions with host-trafficking pathways that define the specificity of Env incorporation into viral particles. A large number of tyrosine- and dileucine-based motifs are present in the HIV-1 Env CT, some of which have been shown to interact with factors involved in vesicular trafficking. The membrane-proximal Yxxϕ motif (Y712) has been well studied and serves as a docking site for the μ-subunit of the clathrin adaptor AP-2 (1, 2). Disruption of this motif enhances cell-surface Env concentration, yet somewhat paradoxically reduces Env incorporation into particles and particle infectivity (3–6). Disruption of YW802 has also been shown to reduce Env incorporation and infectivity (5, 7). The C-terminal dileucine LL855 motif interacts with the AP-1 (8) or AP-2 (9) clathrin adaptor proteins and plays a role in endocytosis and in determining the cell-surface levels of Env. We recently performed a systematic mutagenesis of tyrosine- and dileucine-based motifs in the Env CT that confirmed the importance of Y712 on cell-surface levels of Env (3). This study also illuminated an important region of the CT-spanning residues 795–803, in which disruption of YW or LL motifs had dramatic effects on viral replication.
In an important advance to our understanding of the role of the Env CT, Murakami and Freed demonstrated that the incorporation of Env into viral particles was cell-type–dependent and that this incorporation in most T-cell lines and macrophages requires an intact long cytoplasmic tail (10). They demonstrated that Env incorporation in 293T cells did not require the long CT, nor was the CT absolutely required for incorporation in particles produced from HeLa or MT-4 cells. However, Env incorporation into particles produced from other T-cell lines and macrophages was severely impaired by CT truncation, and productive replication of virus bearing an Env with a truncated tail was possible only in MT-4 cells. This study strongly implicated host factors in the CT-dependent incorporation of Env.
We recently reported that Rab11-FIP1C (FIP1C) (also known as Rab coupling protein or RCP) and Rab14 are required for Env incorporation and that the effect of FIP1C was dependent upon the Env CT (11). This suggested to us that the cell-type–dependent findings reported by Murakami and Freed (10) may be related to FIP1C-mediated transport of Env mediated through motifs on the Env CT. To test this hypothesis, we examined a panel of viruses bearing mutations of tyrosine- and dileucine-based motifs for their ability to redistribute FIP1C to the plasma membrane. We identified YW795 as a critical motif that is required for CT-dependent FIP1C redistribution out of the endosomal recycling compartment. Remarkably, the disruption of YW795 completely recreated the pattern of cell-type dependence on Env incorporation previously observed with CT truncation, and FIP1C depletion had no effect on the level of incorporation of this mutant Env. A downstream second-site revertant was derived that restored Env incorporation and dependence on FIP1C for particle incorporation, suggesting that YW795 and FIP1C mediate Env incorporation in a cell-type–specific manner.
Results
YW795 Motif in the gp41 CT Is Required for HIV-1 Env-Mediated Redistribution of FIP1C and for Env Incorporation into Virions.
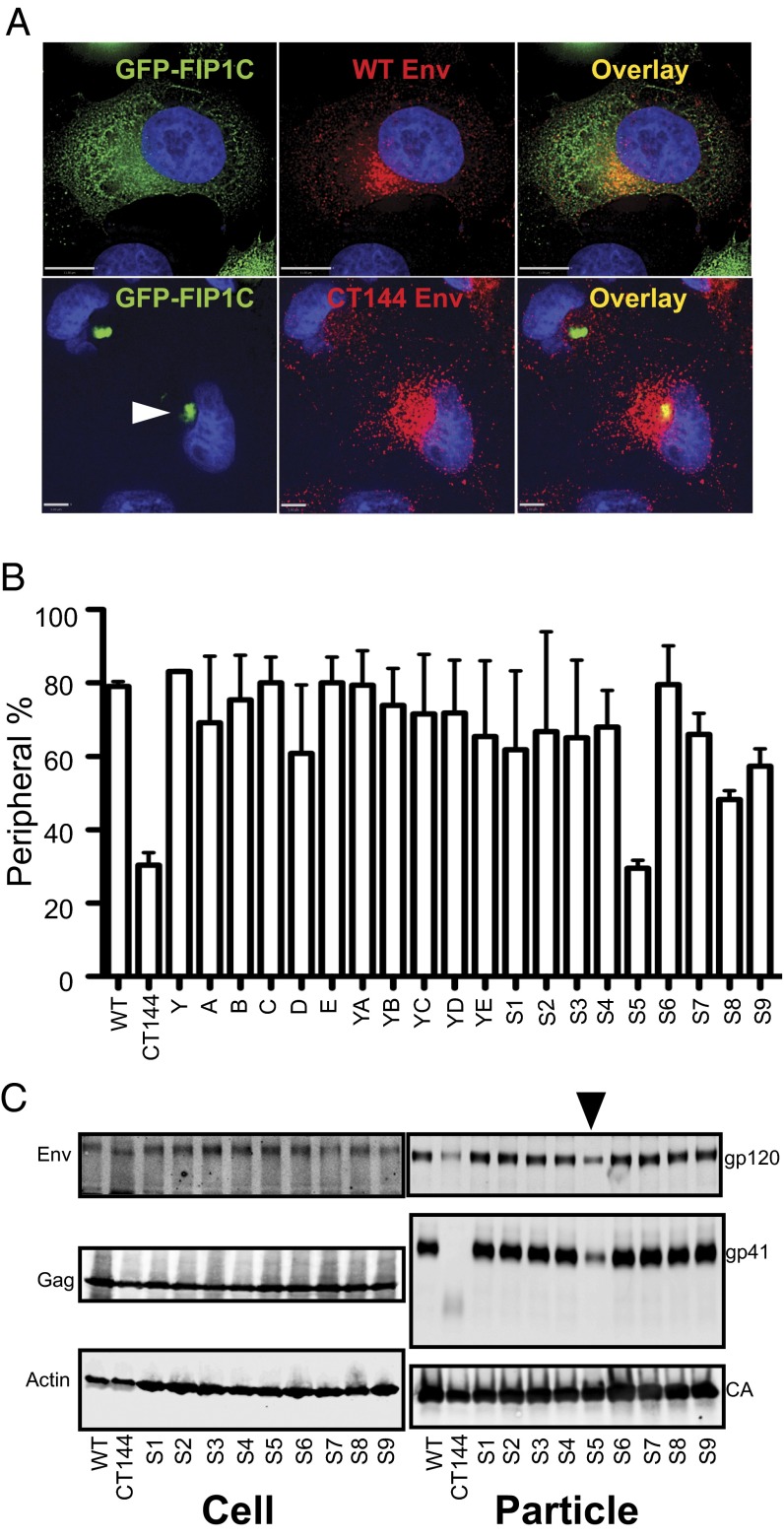
We recently demonstrated that FIP1C is required for HIV-1 Env incorporation in a gp41 CT-dependent manner (11). We noted in that study that the subcellular distribution of GFP–FIP1C was substantially altered upon expression of wild-type Env, whereas expression of a tail-truncated Env (CT144) failed to redistribute GFP-FIP1C from a predominant perinuclear location in HeLa cells. We hypothesized that this redistribution assay could be used to map important motifs in the CT that are involved in FIP1C-dependent trafficking of Env. We used a previously characterized panel of Env constructs bearing mutations in tyrosine- and dileucine-based motifs for this analysis (3) (Fig. S1) and collected images of GFP–FIP1C subcellular distribution that were categorized as predominantly “redistributed” or “perinuclear” as shown for wild-type Env and CT144 Env, respectively, in Fig. 1A. Images obtained from 100 cells were characterized by three independent investigators in a blinded fashion to generate the data in Fig. 1B. To be clear, we were not scoring colocalization of Env and FIP1C in this semiquantitative assay, but rather the ability of Env to induce subcellular redistribution of FIP1C out of the perinuclear endosomal recycling compartment. We found that most of the tyrosine and dileucine mutants redistributed FIP1C out of the perinuclear location in a manner similar to that of wild-type Env (Fig. 1B). One mutant, however, was noted to affect FIP1C redistribution only minimally, similar to the phenotype observed with CT144. This was mutant S5 in Fig. 1, a YW795/SL substitution within a nine-amino-acid stretch (Y795WWNLLQYW802) of alpha helix 2 of the gp41 CT previously found to be important for Env incorporation and for viral replication in T-cell lines (3, 7). Next, we tested nine individual mutants of tyrosine- and dileucine-based motif mutants for incorporation of Env into HIV-1 particles in the H9 T-cell line. Notably, S5 (YW795/SL) demonstrated a significant defect in Env incorporation (Fig. 1C), whereas no significant defect was observed following disruption of the other eight motifs. We conclude that disruption of the YW795 motif prevents redistribution of GFP–FIP1C and greatly diminishes Env incorporation, suggesting that it may play a role in FIP1C-mediated Env incorporation into particles.
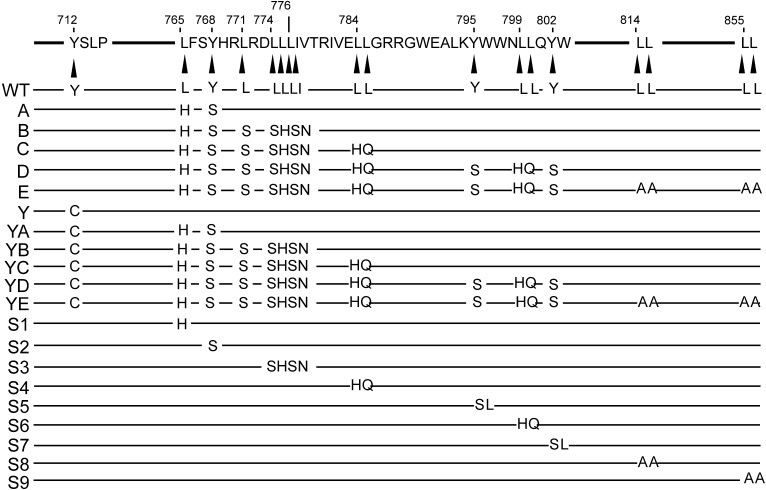
Fig. S1.
Panel of Env CT mutants used in this study (3). WT sequence and numbering from NL4-3 is shown at top. Amino acids targeted for mutagenesis are represented by AA designation on WT line, and each mutant construct below WT shows altered residues.
Fig. 1.
Mapping of HIV-1 gp41 cytoplasmic tail for GFP-FIP1C redistribution and Env incorporation. HeLa cells were transfected with GFP-FIP1C and proviral constructs as indicated in individual panels. Twenty-four hours after transfection, cells were fixed with 4% paraformaldehyde and stained with human monoclonal antibody 2G12 and fluorescent dye-conjugated secondary antibody. Coverslips were then mounted and images acquired for classification of “perinuclear” vs. “peripheral” pattern of GFP-FIP1C. (A) Example of FIP1C pattern when cells were transfected with wild-type NL4-3 provirus (Top) or with provirus encoding CT144 (Bottom). White arrowhead indicates typical ERC-localized GFP-FIP1C in this cell type. [Scale bars: 11 μm (Top) and 5 μm (Bottom ).] (B) Quantitation of patterns for panel of Env CT mutants from ref. 3. For each Env tail mutant, 100 cells were examined by three independent researchers, and the GFP-FIP1C distribution was categorized into either a perinuclear or peripheral redistributed pattern. Scoring was not performed to measure Env and FIP1C colocalization, but rather scoring reflects the pattern of FIP1C seen upon the expression of each Env construct. The bar graph shows the percentage of FIP1C redistribution for each mutant, with SD representing comparison of means derived from three different observers. Env mutants are designated below each bar; sequence of these mutants is presented in Fig. S1. (C) Env incorporation for selected Env CT mutants from ref. 3. H9 cells were infected with VSV-G–pseudotyped viruses as labeled below the blot. HIV particles were harvested, pelleted, and examined for Env incorporation. Arrowhead indicates S5 or YW795/SL mutant that was selected for further evaluation.
YW/SL795 Virus Replicates Poorly in T-Cell Lines.
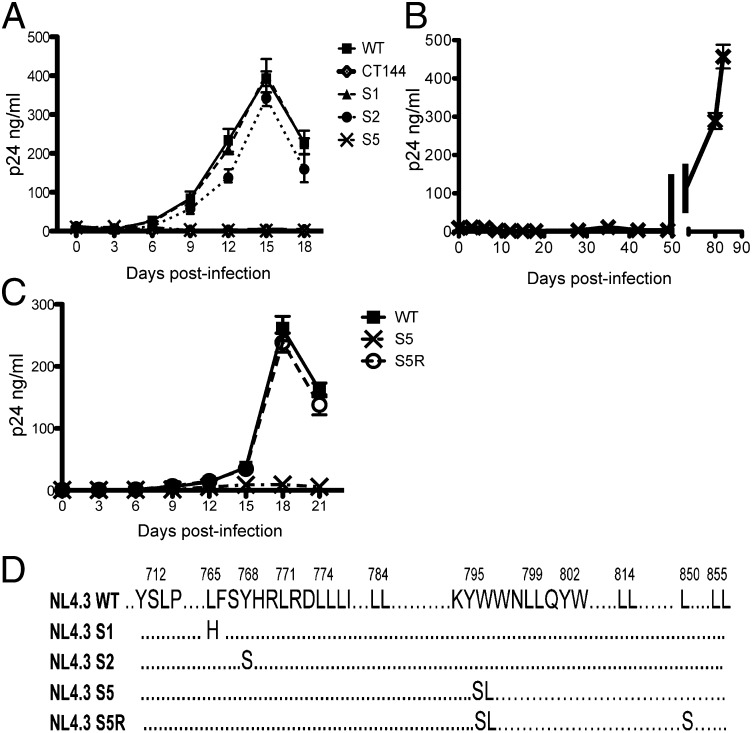
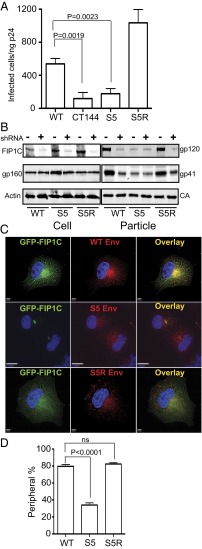
Murakami and Freed have shown that truncation of the HIV-1 Env CT blocks viral replication in CEM, Jurkat, and MT-2 T-cell lines, as well as in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cell (PBMCs) and in monocyte-derived macrophages (MDMs) (10). We hypothesized that S5 may recapitulate these results in the absence of tail truncation. We therefore infected H9 T cells with vesicular stomatitis virus glycoprotein (VSV-G)–pseudotyped NL4-3, CT144, S1, S2, or S5 viruses (sequences depicted in Fig. S1 and Fig. 2D) at a multiplicity of infection (MOI) of 0.1 and monitored p24 production in the supernatants over a 3-wk period. As shown in Fig. 2A, S5 and CT144 replicated very poorly in H9 cells, whereas NL4-3, S1, and S2 viruses demonstrated a similar pattern of viral spread, with peak production of p24 around day 18. A very similar pattern of replication was seen for this panel of viruses when introduced into CEM T cells (Fig. S2). This suggested to us that the YW795 motif is required for efficient cell-to-cell spread in nonpermissive cells, similar to that previously documented for CT144.
Fig. 2.
Growth curve of S5 mutant in H9 cell culture and revertant analysis. (A) H9 cells were infected with WT, CT144, and three selected Env CT mutants from the CT panel. A total of 50,000 H9 cells were infected with VSV-G–pseudotyped virus at MOI 0.1. Culture supernatants were collected for each virus every 3 d for p24 quantitation. Note that CT144 and S5 virus production is not detected on this scale. (B) S5 was cultured for an extended time; note break in x axis timescale. (C) Revertant S5R virus and comparator viruses were introduced into H9 cells for growth curve generation as in A. (D) Diagram depicting relevant WT and mutant CT sequences including S5 and S5R changes.
Fig. S2.
Growth curve of S5 mutant in CEM culture. CEM cells were infected with WT, CT144, and three selected Env CT mutants from the CT panel as described in the legend to Fig. 2. P24 antigen released into supernatants is shown on the y axis.
S5 Revertant Incorporates Wild-Type Levels of Env and Replicates Well in H9 Cells.
Despite the apparent lack of replication shown in Fig. 2A, low levels of p24 could be detected in H9 culture supernatants, suggesting that there was ongoing replication at a very reduced level. We continually passed the S5-inoculated H9 T cells until we observed a significant rise in p24 output at 80–83 d postinfection (Fig. 2B). Sequencing of this suspected revertant virus revealed conservation of the YW795/SL substitution and a single L850S second-site mutation (depicted in Fig. 2D). Sequencing of the matrix (MA) region of the revertant revealed no changes in this region (wild-type NL4-3 MA sequence). We then reintroduced the L850S in combination with the YW795/SL change into the wild-type NL4-3 background to ensure that this was the relevant change and compared growth of the revertant (termed “S5R”) to that of the S5 virus. As shown in Fig. 2C, S5R replicated well in H9 cells with a growth cure almost identical to that of wild type. This confirmed that the L850S change is a second-site reversion regulating HIV-1 spread in H9 T cells.
S5 Recreates the Cell-Type–Specific Restriction of CT144 Env Incorporation, Whereas S5R Restores Env Incorporation in All Cell Types.
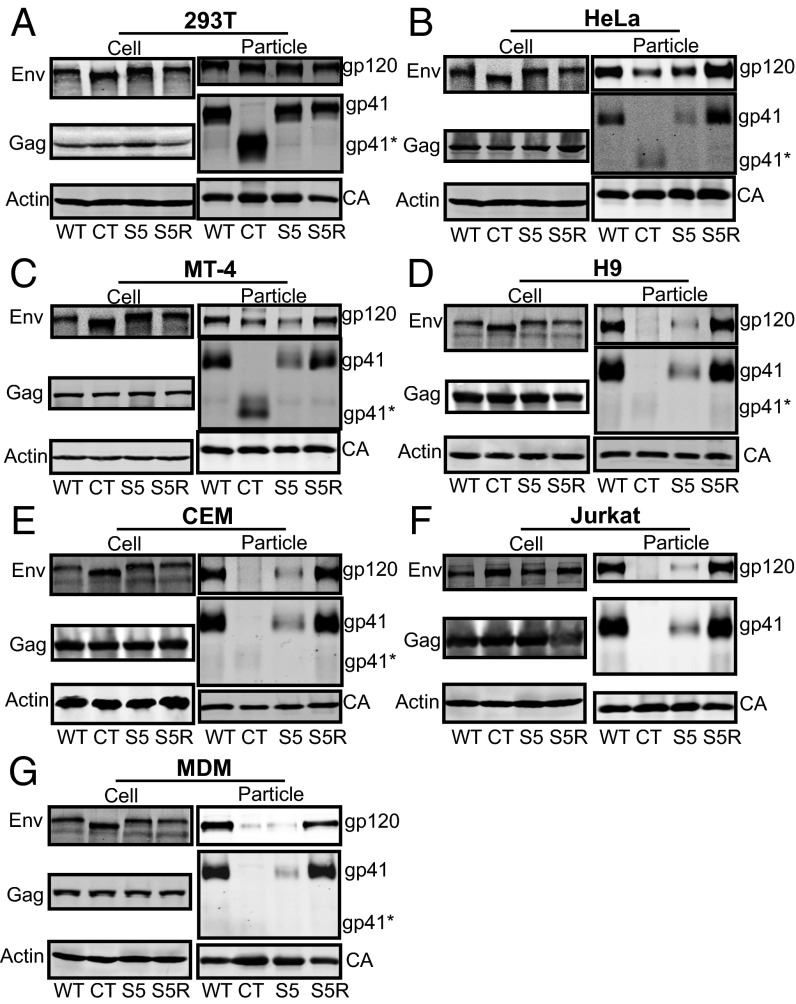
We suspected from the results above that the L850S change in the Env CT had restored efficient Env incorporation in H9 T cells. We next asked whether S5 could completely recapitulate the permissive (293T), semipermissive (HeLa, MT-4), and nonpermissive (H9, CEM, Jurkat, MDM) phenotype with regard to Env incorporation that had been previously established for CT144 (10) and if S5R consistently reversed this restriction of Env incorporation. To test this idea, each cell type was infected with VSV-G–pseudotyped wild-type NL4-3, CT144, S5, or S5R viruses, and Env incorporation into released viral particles was evaluated by Western blot analysis. As shown in Fig. 3A, Env incorporation for each of the four viruses was equal in permissive 293T cells. Both CT144 and S5 Env levels were reduced compared with wild-type NL4-3 in semipermissive HeLa and MT-4 cells, whereas S5R restored Env incorporation to wild-type levels (Fig. 3 B and C). Remarkably, infection of nonpermissive cell lines and MDMs demonstrated that S5 Env incorporation was restricted similarly to that of CT144 (Fig. 3 D–G). In each case, S5R restored particle Env incorporation to wild-type levels. These results indicated to us that the YW795/SL substitution in the CT completely reproduced the cell-type–specific pattern of Env incorporation seen with a drastic truncation of the CT, whereas the L850S second-site revertant restored wild-type Env incorporation in all cell types tested.
Fig. 3.
Cell-type–dependent incorporation of WT and CT mutant HIV envelope glycoprotein. A panel of permissive (A) (293T), semipermissive (B and C) (HeLa, MT-4), and restrictive (D–G) (H9, CEM, Jurkat, MDM) cells for envelope incorporation was used to characterize S5 and S5R viral Env incorporation into particles. VSV-G–pseudotyped viruses were used to infect each indicated cell type at an MOI of 1.0. Cell and virus-associated proteins were analyzed 2 d postinfection for all cell types except for MDMs, where cells and viruses were harvested following 8 d of infection. “gp41*” indicates the faster-migrating form of gp41 in the CT144 particle lanes.
S5R Restores FIP1C-Dependent Env Incorporation and Particle Incorporation.
To further evaluate the significance of the levels of Env incorporation exhibited by S5 and S5R viruses in restrictive cells, we measured particle infectivity from supernatants of infected H9 cells. Particles released from CT144- and S5-infected cells were significantly less infectious than wild type, whereas S5R-infected cells released particles that were somewhat more infectious than wild type (Fig. 4A). We previously established that wild-type Env incorporation is dependent upon FIP1C and that FIP1C-dependent Env incorporation requires the intact CT (11). We therefore next asked if the CT revertant mutation in S5R restored FIP1C-dependent Env incorporation. Depletion of FIP1C greatly diminished wild-type Env incorporation in H9 cells as had been previously shown (Fig. 4B). Remarkably, although we could see no effect of FIP1C depletion on the low level of Env incorporation of S5 virus, S5R regained sensitivity to FIP1C depletion (Fig. 4B). In other words, the restoration of cell-type–dependent Env incorporation seen with S5R restored particle infectivity, and the restoration of Env particle incorporation correlated with a dependence on the cellular trafficking factor FIP1C.
Fig. 4.
S5R restores infectivity and dependence on FIP1C for Env incorporation. (A) Virus harvested from H9 cells was normalized for p24 content and evaluated for infectivity on TZM-bl cells. Infectivity is reported as blue cells/nanogram of p24 input. (B) shRNA knockdown of FIP1C was performed in H9 cells, followed by infection with the indicated viruses. “+” indicates FIP1C shRNA treatment; “−” indicates control shRNA delivery. After 48 h, cells and virus-containing supernatants were harvested, viral particles pelleted, and Western blotting performed. (C) Representative images of redistribution of GFP-FIP1C by WT Env (Top), S5 Env (Middle), and S5R Env (Bottom). HeLa cells were cotransfected with GFP-FIP1C and the indicated proviral DNA, and cells were harvested at 24 h for immunostaining and imaging. [Scale bars: 5 μm (Top and Bottom) and 11 μm (Middle). (D) Quantitation of “peripheral” vs. “perinuclear” patterns, with percentage of peripheral pattern images ±SD presented. A total of 100 cells were counted by three investigators to derive these data as in Fig. 1.
We first identified the S5 virus from a panel of mutants in the CT by its loss-of-function phenotype in a GFP–FIP1C redistribution screen (Fig. 1). To complete this analysis using revertant Env, we asked if S5R Env regained the ability to redistribute GFP–FIP1C in HeLa cells. Indeed, although S5 Env was significantly impaired in this redistribution assay, S5R restored a level of FIP1C redistribution equivalent to wild-type Env (Fig. 4D). These data provide additional weight to the idea that FIP1C-dependent trafficking of Env is involved in the cell-type–dependent Env incorporation.
S5R Restores Env–Gag Colocalization at the Plasma Membrane.
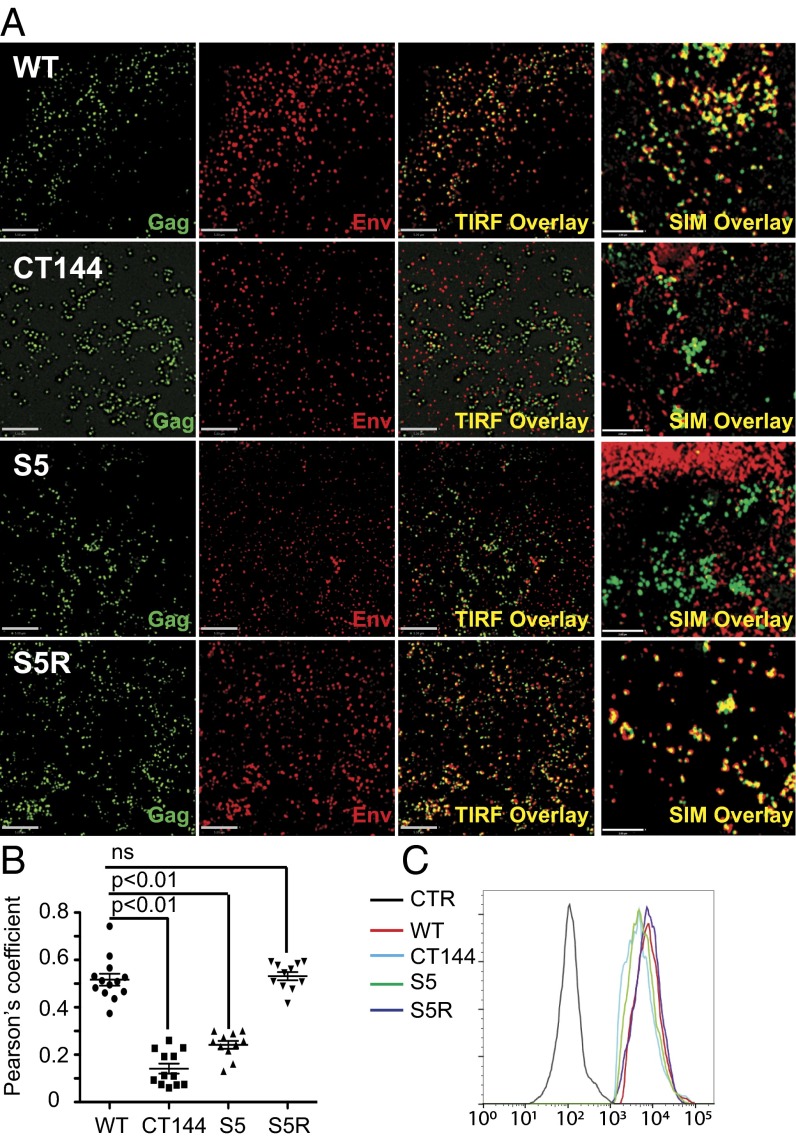
The poor incorporation of S5 Env into virions could be a result of a general disruption of trafficking to the plasma membrane or a disruption of trafficking to specific sites of particle budding. To begin to address this, we infected HeLa cells with VSV-G–pseudotyped viruses at MOI 1.0 and used total internal reflection fluorescence (TIRF) microscopy to analyze Gag and Env distribution on plasma membrane. Both Gag and Env were present in a punctate distribution on the cell surface (Fig. 5). However, the colocalization of Env with Gag puncta was markedly different when comparing wild type with CT144 Env. We noted that Gag particle puncta largely colocalized with Env staining for wild-type virus, whereas colocalization was much diminished with CT144 Env. Using a thresholded Pearson’s coefficient algorithm to quantify colocalization (12), we found a correlation value of 0.52 (±0.09) of Env to Gag for wild-type virus, whereas CT144 colocalization with Gag puncta was reduced to 0.14 (±0.07) (Fig. 5 A and B) (P < 0.01). S5 Env/Gag colocalization was also significantly below that seen for WT at 0.24 (±0.06) (Fig. 5 A and B) (P < 0.01). Notably, S5R Env restored the observed wild-type level of colocalization with Gag puncta (0.53 ± 0.06). The roughly threefold difference in colocalization between WT and CT144 or S5 Env with Gag is consistent with the level of Env incorporation seen from immunoblotting (Fig. 3). To further examine the surface distribution of Gag and Env, we performed superresolution microscopy at the level of cell attachment to the coverslip. Results were similar to those seen by TIRF, with marked colocalization of Gag and Env for wild-type and S5R virus and much reduced colocalization for CT144 and S5 (Fig. 5A, rightmost overlay panels).
Fig. 5.
Plasma membrane colocalization of Gag and Env. (A) HeLa cells were infected with the indicated VSV-G–pseudotyped viruses at an MOI of 1.0. Forty-eight hours after infection, cells were fixed, permeabilized, and stained with 2G12 for Env (red) and CA183 for Gag (green). TIRF microscopy was performed using the Deltavision Core instrument. The leftmost images for each virus represent TIRF images that are representative of more than 20 fields examined. The rightmost images for each virus are representative superresolution images obtained from the OMX Blaze (Applied Precision/Perkin-Elmer) at the level of the plasma membrane. (Scale bars: 5 μm for TIRF images and 2 μm for SIM images.) (B) The degree of colocalization as determined by TIRF imaging was measured with a thresholded Pearson’s correlation using Volocity 6.3 software (Perkin-Elmer). The points represent individual cells with means shown. P values were calculated using the Student’s unpaired t test. ns, nonsignificant. (C) HeLa cells were infected with viruses as before and were fixed and stained for Env using 2G12, followed by permeabilization and staining for Gag using antibody KC57 (Beckman Coulter). This allowed gating on only the infected cells and quantitation of cell-surface Env staining by flow cytometry. CTR: staining with secondary antibody only.
Cellular levels of Env were next examined on a population basis in infected HeLa cells using flow cytometry. As presented in Fig. 5C, all samples tested expressed high levels of Env protein at cell surface that are well above isotype control-stained cells. WT-, CT144-, S5-, and S5R-infected cells showed similar amounts of Env on the cell surface, with only a very slight shift downward in CT144 and S5 cell-surface levels. This result is generally consistent with a previous study (10), which observed similar levels of WT Env and CT144 Env on plasma membrane in CEM T cells. Thus, the marked differences observed in Env incorporation and in Gag–Env colocalization were not explained by similar reductions in cell-surface Env.
Discussion
The mechanism of specific incorporation of HIV-1 Env into particles remains incompletely understood. Small deletions or substitutions in the globular head of MA reduce or eliminate Env incorporation, a defect that can be rescued when the Env CT is truncated. This suggests that the Env CT and MA interact or, alternatively, that an intact MA and CT are both required for trafficking to specific sites of particle assembly on the plasma membrane. A critical insight into the mechanism of Env incorporation was gained from the work of Murakami and Freed, who showed that an intact CT was required in most T-cell lines, primary PBMCs, and MDMs for efficient particle incorporation and replication (10). This work implied that signals in the long CT regulate incorporation, and the presence of significant cell-type–specific differences in incorporation strongly supported a role for host cell factors that may differ between cell types. Permissive cells such as 293T cells and semipermissive cells such as HeLa and MT-4 may thus allow passive incorporation of Env in a CT-independent manner, whereas active, specific incorporation involving host factors is the dominant path in most T-cell lines and relevant primary cells.
Our findings support the CT-dependent incorporation of Env and highlight the importance of the YW795 motif on alpha helix 2 of the CT. This motif lies within a predicted alpha-helical segment of the tail that has previously been shown to be important for Env incorporation into particles through deletional mutagenesis (13), and mutations of dileucine- or tyrosine-based motifs in this region severely impair replication in T-cell lines (3). Findings presented here provide important clues to explain the importance of this segment of the CT. The YW795 mutant failed to redistribute GFP-FIP1C from a predominantly perinuclear location in HeLa cells, suggesting to us that this may be a FIP1C-interacting domain. Rab11-FIP1C is an adaptor protein that dimerizes and forms a heterotetrameric trafficking complex with two copies of Rab11, Rab14, or Rab4 (14–16). We previously showed that FIP1C and Rab14 are required for CT-dependent Env incorporation (11). Although the evidence remains indirect, we propose that a trafficking complex including FIP1C and Rab14 directs Env to the particle budding site through interactions with the Env CT and that YW795/SL disrupts this interaction. Following through with this model, a revertant near the end of the CT (L850S) restores FIP1C-dependent trafficking and Env incorporation. One model that could explain our findings is that helix 2 of the Env CT contains a discrete binding site for FIP1C that is disrupted by the YW795/SL mutation. A further tenet of this model would logically be that L850S is able to recreate the structural motif required for FIP1C binding and subsequent trafficking to the particle assembly site. We do not have direct binding data to support this model at present, but experiments are in progress to test this possibility. The trafficking model also predicts that we might observe partial colocalization between FIP1C and Env during transit of the complex to the particle budding site. Defining the colocalized populations was not the focus of the current study, and we note that detection of this Env population in static images is complicated by the many compartments in the cell where Env is found, including the endoplasmic reticulum, Golgi, plasma membrane, and endosomes. However, defining the complex of Env and FIP1C using dynamic imaging techniques should be possible and will be the focus of future studies.
A number of models for HIV-1 Env incorporation into particles have previously been proposed, including passive incorporation, cotrafficking of Env and Gag to a common membrane microdomain, direct interactions between Env and Gag, and indirect interactions through the action of a cellular adaptor molecule (17, 18). Results shown here fit with either the cotrafficking model, in which both Gag and Env traffic independently to a common microdomain on the plasma membrane, or with the indirect interaction model, in which FIP1C or its binding partners could serve as an adaptor directly linking Gag and Env. Our results argue strongly against the passive incorporation model as a relevant model for wild-type Env in most T-cell lines and macrophages, whereas passive incorporation of truncated Env may potentially explain the incorporation of CT144 in 293T or MT-4 cells. We note that the YW795 motif is highly conserved in clade B strains of HIV-1, whereas at this same position YL is more frequent in clade A and C isolates. A second highly conserved YW motif lies just C-terminal at position 802 and was previously implicated in Env incorporation and in linking Env to TIP47 (19). However, the connection between Env and TIP47 has not been supported in other studies (20).
Results presented here do not yet address the role played by MA in CT-dependent Env incorporation. Tedbury and colleagues recently reported that the HIV-1 matrix mutant Q62R rescues Env incorporation of a wide range of matrix mutants (21). In the trimeric matrix structure, Q62 locates at the trimer interface, and thus changes in this region could modify the overall structure of the MA lattice at a particle budding site and rescue incorporation of the long CT on a purely structural basis. If steric exclusion of the long CT of Env trimers by mutant MA is the only mechanism at play, however, it is hard to understand how a CT mutant such as YW795/SL could lead to exclusion of incorporation into the normal MA lattice in T cells and macrophages, but not in 293T or HeLa cells. We suggest that a cellular trafficking complex including FIP1C plays a specific role in CT-dependent incorporation and that future studies will illuminate the role of components of this complex in linking MA with the Env CT.
Materials and Methods
Cells, Media, and Plasmids.
HeLa, 293T, and TZM-bl cells were maintained in DMEM containing 10% (vol/vol) FBS and antibiotics. H9, CEM, Jurkat, and MT-4 T-cell lines were cultured in RPMI 1640 supplemented with 10% FBS and 2 mM glutamine and penicillin/streptomycin. MDMs were prepared from human peripheral blood and cultured in media containing GM–CSF as described previously (22). pNL4-3CTdel-144–2 Env was kindly provided by Eric Freed at NCI (Frederick, MD) and referred to here as CT144. A panel of HIV-1 gp41 CT mutants created in the pNL4-3 plasmid backbone has been previously described (3). GFP-FIP1C has been previously described (11). VSV-G expression plasmid pHCMV-G was provided by J. Burns at the University of California, San Diego (23). See additional details in SI Materials and Methods.
shRNA-Mediated Depletion of FIP1C.
For depletion of FIP1C, human FIP1C/RCP shRNA (Sigma, TRCN0000145281) was packaged using the PKO.1 lentiviral vector system as previously described (11). Briefly, H9 cells were infected by vector viruses carrying FIP1C or control shRNA (Sigma, SHC002) overnight. The transduced cells were then pelleted and cultured in fresh media containing 1 μg/mL puromycin for 2 d. The surviving cells were subsequently infected with VSV-G–pseudotyped viruses and assayed for Env incorporation into particles.
Image Acquisition and Analysis.
Imaging was performed using the TIRF microscopy features of the Deltavision Core instrument (GE Healthcare) equipped with a 60× TIRF objective. Quantitation of colocalization was performed with Volocity 6.3 software (Perkin-Elmer), using the Costes Pearson’s Correlation algorithm. Superresolution microscopy was performed using the structured illumination microscopy technique on the OMX Blaze instrument (GE Healthcare). See additional details in SI Materials and Methods.
SI Materials and Methods
Viral Stocks and Infections.
VSV-G–pseudotyped NL4-3 wild-type and CT mutant viral stocks were generated by cotransfecting 293T cells with the indicated proviral constructs together with the VSV-G expression plasmid pHCMV-G using Lipofectamine (Life Technologies). Viruses were harvested from transfected 293T supernatants 48 h posttransfection and filter-sterilized, and infectivity was measured using TZM-bl indicator cells. For assessment of Env incorporation in T-cell lines and macrophages, cells were infected with the pseudotyped virus at an MOI of 1.0 overnight. Cells were then washed and supplied with fresh media for 24 h before harvesting of cells and supernatants. For all analyses of particle-associated proteins, supernatants were filtered through a 0.45-μm filter, pelleted through a 20% sucrose cushion, and then resuspended in loading buffer for analysis by SDS/PAGE and Western blotting.
Virus Release and Infectivity Assays.
Virion-containing culture supernatants were harvested, clarified by low-speed centrifugation, and filtered (0.45 µm). Infectious virus release was determined by inoculating TZM-bl indicator cells. Blue cells were counted and infectivity was calculated as blue cell numbers per nanogram of p24 inoculum. The remainder of the virion containing supernatant was layered onto 20% sucrose in PBS and centrifuged at 20,000 × g for 2 h at 4 °C. Virion pellets and corresponding virion-producing cells were dissolved in SDS/PAGE loading buffer. Virion and cell lysates were separated on 10% polyacrylamide gels and subjected to Western blotting using antibodies outlined above.
Antibodies for Immunoblotting and Immunostaining Procedures.
Goat polyclonal antibody AHP2204 from AbDSerotec was used for Western blotting of HIV-1 gp120 and gp160. Antibody used for immunoblotting of gp41 was murine monoclonal 5009 from BTI research reagents. HIV Gag detection was performed with mouse anti-p24 monoclonal CA-183 (provided by Bruce Chesebro and Kathy Wehrly through the NIH AIDS Research and Reference Reagent Program). Anti–VSV-G antibody was from Sigma (V5507). IRDye goat anti-mouse and IRDye goat anti-rabbit secondary antibodies used for Western blots were obtained from LI-COR Biosciences. All blots were developed using the LiCor Odyssey infrared detection system. Human anti-gp120 antibody IgG1 2G12 was used for immunofluorescence experiments; this recombinant antibody was synthesized from recombinant cDNA and provided by James Crowe, Vanderbilt University, Nashville, TN. Mouse anti–p24-FITC (KC57-FITC) was obtained from Beckman Coulter. Alexa Fluor goat anti-mouse and Alexa Fluor goat anti-rabbit secondary antibodies, as well as the DAPI nucleic acid stain, were obtained from Molecular Probes. Anti-CD9 antibody was from BD Pharmingen clone M-L13.
Image Acquisition and Analysis.
For immunofluorescence experiments, HeLa cells or MDMs were seeded in MatTek 35-mm poly-d-lysine–coated dishes (Brooke Knapp MatTek) overnight and then were infected as described above. Before staining, cells were washed with PBS and fixed in 4% paraformaldehyde for 10 min at room temperature. After fixation, cells were extensively washed. Cells were then permeabilized for 10 min with 0.2% Triton X-100 and block in Dako blocking buffer for 30 min. 2G12 for Env staining and KC57 Gag antibodies were diluted in Dako antibody diluent to 1:500. Fluorescent-labeled second antibodies were also diluted in Dako antibody diluent to 1:1,000. DAPI was used to stain the nuclei of the cells. The coverslips were mounted in Gelvatol overnight and examined directly the next day.
Flow Cytometry for HIV-1 Env Cell-Surface Levels.
HeLa cell-surface staining was performed with human monoclonal anti-gp120 antibody 2G12 at a final concentration of 0.1 μg/mL in PBS with 2% BSA and a second APC-conjugated anti-human antibody at 0.02 μg/mL. Mouse anti–p24-FITC (KC57-FITC, Beckman-Coulter) was used following permeabilization to allow gating on the infected population. 293T, HeLa, and MDM cells were harvested using Versene (Life Technologies). 293T, HeLa, and H9 cells were stained 2 d after infection, and MDMs were harvested at day 8 after infection. Assays were performed on a FACSCanto flow cytometer (BD Biosciences) and analyzed and presented using FlowJo software (Treestar, Inc.).
Supplementary Material
Acknowledgments
We thank James Goldenring for FIP1C plasmids. Flow Cytometry was performed using the Emory Children’s Pediatric Research Center Flow Cytometry Core, and the OMX Blaze was maintained in the Emory Integrated Cellular Imaging Core Laboratory. The Deltavision Core instrument and OMX Blaze instrument were purchased through a generous donation from the James B. Pendleton Charitable Trust. This work was supported by NIH Grant R01 GM111027 (to P.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504174112/-/DCSupplemental.
References
- 1.Boge M, Wyss S, Bonifacino JS, Thali M. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J Biol Chem. 1998;273(25):15773–15778. doi: 10.1074/jbc.273.25.15773. [DOI] [PubMed] [Google Scholar]
- 2.Ohno H, et al. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology. 1997;238(2):305–315. doi: 10.1006/viro.1997.8839. [DOI] [PubMed] [Google Scholar]
- 3.Bhakta SJ, Shang L, Prince JL, Claiborne DT, Hunter E. Mutagenesis of tyrosine and di-leucine motifs in the HIV-1 envelope cytoplasmic domain results in a loss of Env-mediated fusion and infectivity. Retrovirology. 2011;8:37. doi: 10.1186/1742-4690-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day JR, Münk C, Guatelli JC. The membrane-proximal tyrosine-based sorting signal of human immunodeficiency virus type 1 gp41 is required for optimal viral infectivity. J Virol. 2004;78(3):1069–1079. doi: 10.1128/JVI.78.3.1069-1079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambelé M, et al. Impact of natural polymorphism within the gp41 cytoplasmic tail of human immunodeficiency virus type 1 on the intracellular distribution of envelope glycoproteins and viral assembly. J Virol. 2007;81(1):125–140. doi: 10.1128/JVI.01659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West JT, et al. Mutation of the dominant endocytosis motif in human immunodeficiency virus type 1 gp41 can complement matrix mutations without increasing Env incorporation. J Virol. 2002;76(7):3338–3349. doi: 10.1128/JVI.76.7.3338-3349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami T, Freed EO. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J Virol. 2000;74(8):3548–3554. doi: 10.1128/jvi.74.8.3548-3554.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyss S, et al. The highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adaptor [correction of adapter] J Virol. 2001;75(6):2982–2992. doi: 10.1128/JVI.75.6.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byland R, Vance PJ, Hoxie JA, Marsh M. A conserved dileucine motif mediates clathrin and AP-2-dependent endocytosis of the HIV-1 envelope protein. Mol Biol Cell. 2007;18(2):414–425. doi: 10.1091/mbc.E06-06-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami T, Freed EO. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc Natl Acad Sci USA. 2000;97(1):343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi M, et al. Rab11-FIP1C and Rab14 direct plasma membrane sorting and particle incorporation of the HIV-1 envelope glycoprotein complex. PLoS Pathog. 2013;9(4):e1003278. doi: 10.1371/journal.ppat.1003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow AL, Macleod A, Noppen S, Sanderson J, Guérin CJ. Colocalization analysis in fluorescence micrographs: Verification of a more accurate calculation of Pearson’s correlation coefficient. Microsc Microanal. 2010;16(6):710–724. doi: 10.1017/S143192761009389X. [DOI] [PubMed] [Google Scholar]
- 13.Freed EO, Martin MA. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J Virol. 1996;70(1):341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin M, Goldenring JR. The Rab11-FIP1/RCP gene codes for multiple protein transcripts related to the plasma membrane recycling system. Biochim Biophys Acta. 2006;1759(6):281–295. doi: 10.1016/j.bbaexp.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Kelly EE, et al. 2010. Class I Rab11-family interacting proteins are binding targets for the Rab14 GTPase. Biol Cell 102(1):51–62.
- 16.Lindsay AJ, McCaffrey MW. Characterisation of the Rab binding properties of Rab coupling protein (RCP) by site-directed mutagenesis. FEBS Lett. 2004;571(1-3):86–92. doi: 10.1016/j.febslet.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 17.Checkley MA, Luttge BG, Freed EO. HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol. 2011;410(4):582–608. doi: 10.1016/j.jmb.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MC. Mechanisms for Env glycoprotein acquisition by retroviruses. AIDS Res Hum Retroviruses. 2011;27(3):239–247. doi: 10.1089/aid.2010.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Vergès S, et al. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc Natl Acad Sci USA. 2006;103(40):14947–14952. doi: 10.1073/pnas.0602941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Checkley MA, et al. Reevaluation of the requirement for TIP47 in human immunodeficiency virus type 1 envelope glycoprotein incorporation. J Virol. 2013;87(6):3561–3570. doi: 10.1128/JVI.03299-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tedbury PR, Ablan SD, Freed EO. Global rescue of defects in HIV-1 envelope glycoprotein incorporation: Implications for matrix structure. PLoS Pathog. 2013;9(11):e1003739. doi: 10.1371/journal.ppat.1003739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu H, et al. Tetherin/BST-2 is essential for the formation of the intracellular virus-containing compartment in HIV-infected macrophages. Cell Host Microbe. 2012;12(3):360–372. doi: 10.1016/j.chom.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: Concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]