Significance
In highly socialized animals such as humans or songbirds, individuals postnatally develop their skills to communicate with conspecifics under the social influence. Both genetic and environmental influences play a crucial role in the development of such abilities, but dissection of the influences has been difficult, because genetic manipulation of avian species is still a challenging issue. In this study, we applied transgenic technology to songbirds along with an experimental song-training paradigm to separately manipulate both genes and social environment, and found that appropriate activity of cAMP response element-binding protein (CREB) is necessary for the postnatal song learning in songbirds.
Keywords: songbird, CREB, transgenic animal, vocal learning, postnatal development
Abstract
Songbirds postnatally develop their skill to utter and to perceive a vocal signal for communication. How genetic and environmental influences act in concert to regulate the development of such skill is not fully understood. Here, we report the phenotype of transgenic songbirds with altered intrinsic activity of cAMP response element-binding protein (CREB) transcription factor. By viral vector-mediated modification of genomic DNA, we established germ line-transmitted lines of zebra finches, which exhibited enhanced or suppressed activity of CREB. Although intrinsically acquired vocalizations or their hearing ability were not affected, the transgenic birds showed reduced vocal learning quality of their own songs and impaired audio-memory formation against conspecific songs. These results thus demonstrate that appropriate activity of CREB is necessary for the postnatal acquisition of learned behavior in songbirds, and the CREB transgenic birds offer a unique opportunity to separately manipulate both genetic and environmental factors that impinge on the postnatal song learning.
The development of behavioral traits in animals is influenced both by intrinsic and extrinsic factors. The contributions of the genetic and environmental factors on the development of such behaviors have often attracted public interest, i.e., the “nature versus nurture” debate; however, tangible dissection of the magnitude of the contributions of such factors has been difficult. The songbird’s skill to vocalize and to perceive a birdsong, a vocal signal for intraspecies communication, is one of the prominent skills that require both genetic and environmental factors for the development (1, 2). During postnatal developmental periods of zebra finch (Taeniopygia guttata), juvenile birds hear songs of their conspecifics and store this information inside their brain to acquire the knowledge to utter and to perceive vocal signals (3–5). Inadequate auditory experience during the postnatal development results in abnormalities of their songs (3, 6–8), which often results in a reduced communication or mating performance (9–11). On the other hand, even the songs of birds reared in acoustic isolation contain species-specific syllable elements (7, 12), and such birds prefer the songs of conspecifics over those of different songbird species (13, 14). Moreover, it has been reported that some characteristics of vocal traits are heritable (15, 16). Hence, both genetic and environmental influences are necessary for developing the functional neural network required for the proper song vocalization and perception in songbirds.
Neural activity-dependent gene transcription is one of the key mechanisms by which postnatal experience can affect the expression of genes in the neural systems (17, 18). Among the transcription factors that regulate the activity-dependent gene transcription in neurons, the cAMP response element-binding protein (CREB) is one of the most well studied (19–22). Various kinds of external stimuli induce phosphorylation of CREB, the modification of which is required for the transcription of target genes (19, 20). CREB functions as a molecular hub to regulate neuronal gene transcription depending on the neuronal activity (23) and is known to play a pivotal role in neuronal plasticity and memory formation in various species (24–27). In songbirds, however, a previous histological study has found that activation of CREB occurs in the brain regions responsible for vocalization and discrimination of songs in adult zebra finches after they hear songs (28), but its role in vocal learning or auditory discrimination of songs had not been analyzed.
Because the postnatal environment can be controlled experimentally, songbirds have been an ideal experimental animal to study how one’s ability develops according to postnatal experiences (1, 29–31). Furthermore, they are one of the rare species that exhibits imitation of vocal signals, a behavior that is thought to be important for the acquisition of languages in humans (32). Moreover, the skill to communicate with learned vocal patterns are culturally transmitted through social interaction (33, 34). These features make songbirds as a rare and promising experimental system to study not only the requirement of genetic and environmental factor, but also how social or cultural influence stimulates the individual development of a behavior. Nevertheless, the lack of efficient methods to manipulate the genome of songbirds has hampered the research needed to reveal the contribution of the genetic factor to the development of communicative ability. Recently, however, transgenic expression of exogenous genes became possible by the development of virus-mediated transgenic technology in songbirds (35). This technology has allowed us to study in detail the genetic and environmental influences on the song acquisition of zebra finches. Taking this approach, we have established germ line-transmitted lines of zebra finch that express mutant forms of CREB molecules. Here, we show that formation of auditory memories against conspecific songs and the acquisition of own song is impaired in those transgenic birds, although the basal hearing ability or the acoustic quality of intrinsic vocalizations are not altered. These birds demonstrate that appropriate activity of CREB is necessary for song learning and offer an opportunity to separately manipulate both genetic and environmental factor influence in the acquisition of postnatally learned behaviors.
Results
Generation of the Transgenic Zebra Finches with Modified CREB Activity.
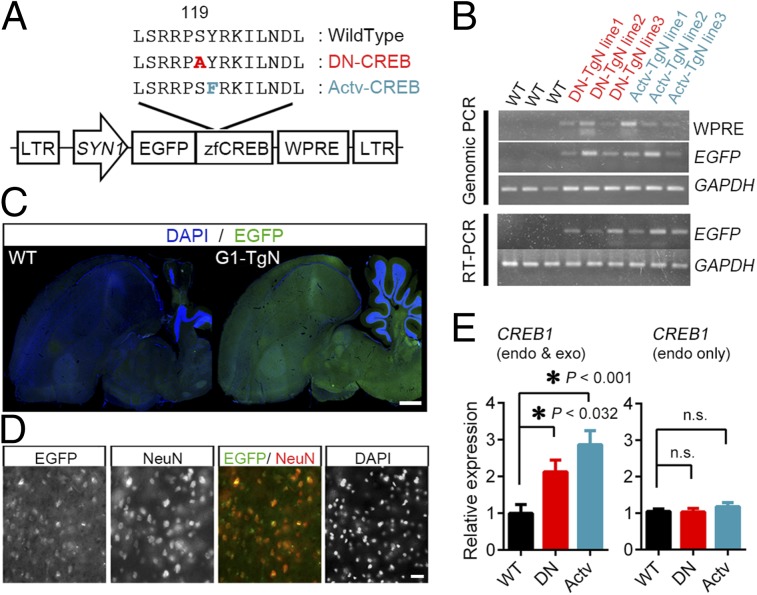
By regulating the expression of multiple genes, transcription factors that show neural activity-dependent gene transcription, including CREB, can alter the proteomic landscape depending on environmental influences (24, 36). To substantiate the link between CREB-mediated gene transcription and the quality of the postnatally acquired communicative ability of songbirds, we manipulated the activity of CREB in zebra finches by transgenically expressing mutant CREB molecules. Introduction of transgenes was performed by injecting lentiviral vectors bearing the transgene into the early embryo in fertilized zebra finch eggs (35) (SI Materials and Methods). Exogenously expressed mutant CREB1 genes harboring the amino acid substitution affect the activity of CREB-mediated gene transcription by forming heterodimer with the endogenous CREB molecule (37). In the present study, we tried to express these mutant zebra finch CREB1 under the control of a human SYN1 (synapsin I) promoter, which restricts the transgene expression to neurons (38). Transgenic lines expressing the phosphorylation-deficient form of CREB (DN) (S119A, equivalent to mouse CREB-S133A) and the constitutively active form of CREB (Actv) (Y120F, equivalent to mouse CREB-Y134F) were established (Fig. 1 and Fig. S1). Germ line transmission and the expression of the transgenes (EGFP-CREB) in their offspring were observed in 20 out of 1,473 virus-injected eggs. By crossing these 20 founder birds (11 for DN and 9 for Actv) with wild-type (WT) birds, a total of 116 DN and 103 Actv transgenic G1 offspring were obtained. Integration of the transgene into genome (Fig. 1B and Fig. S1B), and brain expression of transgenes were observed in the G1 offspring (Fig. 1C and Fig. S1A). As observed in the transgenic quails (38), the expression of transgene was observed throughout the NeuN-expressing cells (Fig. 1D). These birds express exogenous CREB mutants in addition to the endogenous CREB (Fig. 1E).
Fig. 1.
Generation of the transgenic zebra finches with modified CREB activity. (A) Schematics of the transgenes used to generate transgenic zebra finches. SYN1, human synapsin 1 promoter; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element. (B) PCR analysis of genome integration and expression of transgenes. Genome DNA (Upper) and total RNA (Lower) of WT and transgenic line (TgN) were collected from an adult bird from three transgenic lines for each. (C) Images of sagittal sections from WT and G1-TgN birds immunostained against EGFP showing the expression of transgene in G1-TgN birds. (Scale bar, 1 mm.) See Fig. S1A for the anatomical profiles. (D) High-magnification image of nidopallium, showing signals of EGFP (green), and Neu-N (red; neuronal nucleus marker), and DAPI. (Scale bar, 20 μm.) (E) Quantitative RT-PCR analysis of RNA collected from WT and the transgenic birds. Data from primer sets that amplify endogenous CREB1 (Right) and endogenous and exogenous CREB (Left) are shown. See Table S1 for used primers. Bar graph shows the mean ± SEM of relative expression values normalized to the WT. *P < 0.05, n.s. P > 0.5, Dunnett’s post hoc test.
G1 offspring of neither genotype showed any significant difference in their body weight compared with that of the WT control birds (Fig. S2A). Also, their brain morphology looked unchanged, and the size of their song nuclei (HVC, Area X, robust nucleus of the arcopallium) did not show any significant difference (Fig. S2 B and C). We next asked whether these birds show any difference in their hearing ability or behavioral reactions to stimuli, by observing the reactions against the increasing volume of sounds (Fig. S3). When the change in behavioral reaction was analyzed by calculating the frequency of vocalization [“calls,” a short vocalization different from “songs” (39)], birds of all genotypes equally discriminated the increased volume of white noise between 45 and 47 dB (Fig. S3B). Notably, we found that, whereas WT and DN birds suppressed calling behaviors in response to the volume change of 45 to 47 dB, Actv birds tended to increase them (Fig. S3C). These observations indicate that, although the basal hearing ability needed to discriminate the sounds was unchanged between the transgenic birds, Actv birds tend to show a differential reaction in response to stimuli compared with WT and DN birds.
Through maintaining the transgenic lines, we noticed that, although the transgenic birds look normal, they tend to die abruptly. We next analyzed the difference of survival ratios among the genotypes. Being kept in our rearing condition, in which all birds were separated from the parents after the fledge and were kept in a soundproof chamber through ∼30–140 d post hatch (dph), G1 offspring of both genotypes lived significantly shorter compared with WT birds (Fig. S4A). After they become sexually mature, we tried to obtain G2 birds by crossing G1-TgN to WT birds. However, we found that the fertilization rates of G1-TgN were remarkably lower in both sexes (Fig. S4B). Even for the pairs that succeeded to leave G2 offspring, more days were needed to obtain chicks compared with WT (Fig. S4C). Because we were unable to obtain sufficient numbers of G2 offspring, further behavioral analysis was performed using the male G1 offspring from multiple transgenic lines.
Transgenic Zebra Finches Showed Altered Intrinsic Activity of CREB.
The activity of CREB-mediated gene transcription was assayed by a lentivirus-based transcription reporter construct (Fig. 2A). In this construct, a constitutive human phosphoglycerate kinase 1 (PGK1) promoter expresses an infection reference gene [flag-tagged Histone-2B (H2B-flag)], whereas in the other direction, a minimal promoter expresses a reporter gene [turboGFP (tbGFP)]. The expression of the reporter gene is influenced by the CREB binding sequence (CRE), inserted just upstream of the minimal promoter in LV-CREB-reporter constructs. By normalizing the quantified number of the transcribed reporter mRNAs using those of internal infection reference, these constructs can reliably reflect the activity of CREB irrespective of the transfected number or the genome-inserted locus of the reporter constructs (Fig. 2B). Injecting these lentivirus-based transcription reporter into the brains of transgenic birds, we compared the intrinsic activity of CREB between WT, DN, and Actv birds. CREB-mediated gene transcription was significantly suppressed in DN birds and augmented in Actv birds, compared with WT birds (Fig. 2C). Quantitative RT-PCR analysis of the relative expression of endogenous genes in G1 offspring further revealed the effect of CREB-mediated gene transcription in these transgenic finches. As observed with the transgenic mice expressing mutant CREBs (23), the expression of many genes was increased or decreased in DN and Actv birds compared with WT birds (Fig. S5). Specifically, in both DN and Actv birds, the expression levels of genes of which human homologs have CREs in the promoter region (CRE+ genes) were significantly changed, compared with those genes without CRE (CRE– genes) (Fig. 2D and Fig. S5). Collectively, these results demonstrate that CREB-mediated gene transcription was misregulated in both DN- and Actv-CREB–expressing transgenic finches.
Fig. 2.
Transgenic zebra finches show misregulated CREB-mediated gene transcription. (A) Schematics of the lentivirus (LV)-based transcription reporter constructs. A constitutive human phosphoglycerate kinase (PGK1) promoter expresses an infection reference gene [flag-tagged Histone-2B (H2B-flag)]. In the other direction, a minimal promoter expresses a reporter gene [turboGFP fused to PEST sequence (tbGFP-PEST)], whose expression is influenced by the presence of CREB binding sequence (CRE), in the LV-CREB-reporter. (B) HEK293T cells transfected either with LV-CREB-reporter or LV-Control-reporter at multiplicities of infection (MOIs) of 1, 0.1, and 0.01, and were treated with vehicle (0.1% DMSO) or 100 μM forskolin to stimulate the cAMP-dependent activation of CREB. Each reporter activity was quantified by dividing the amount of tbGFP-PEST by the amount of H2B-flag, both of which were quantified by quantitative RT-PCR. Stimulus-dependent changes in CREB activity were calculated by dividing the reporter activity of LV-CREB-reporter by those of LV-Control-reporter. *P < 0.0001 against each vehicle-treated cell. Bar graph shows mean ± SEM; n = 4 independent experiments. (C) Activity of CREB-mediated gene transcription in transgenic birds. LV-CREB-reporter and LV-Control-reporter were injected into the striatum and the reporter activities were quantified for each subject. WT, n = 10; DN, n = 6; Actv, n = 5 birds. Bar graphs indicate mean ± SEM. *P < 0.05, Dunnett’s post hoc test. (D) Quantitative RT-PCR analysis of endogenous RNA collected from WT and transgenic birds (n = 11 birds for each genotype). Unpaired t test; bar graph shows mean ± SEM of the absolute log2 value of relative amount of expression against WT, comparing gene with (n = 47) and without (n = 32) CREs. See Fig. S5 and Table S1 for details.
Deficit of Memory Formation in Zebra Finch with Mutated CREB.
CREB plays a pivotal role in neuronal activity-dependent gene regulation and neural plasticity in various species (24–26, 40). For example, disrupting CREB function is known to suppress the formation of long-term memory in fear-conditioned mice (41). To assess whether the transgenic manipulation of CREB activity has any effect on the memory formation in songbird, we analyzed the process of associative auditory memory formation in WT and the transgenic birds. To this end, a standard classical auditory conditioning paradigm was used to assess formation of memory after training. For using the classical fear memory conditioning in zebra finches, we developed an auditory song-conditioning test for songbirds (Fig. 3A; SI Materials and Methods). In this test, a subject finch was isolated in a soundproof chamber, and five songs of zebra finches, recorded from five different individuals unfamiliar to any of the subjects, were played through a speaker in a random order; after one particular song [conditioned song stimulus (CS)], calls of a crow were presented [unconditioned stimulus (US)], whereas the other songs [control song stimulus (Cont)] were followed only by an interval of silence. The presentation of a crow’s call caused freezing behavior (conditioned response), which was reflected in a significant decrease in their behavior (such as calling) throughout the training blocks (TBs) (Fig. 3B). These freezing behaviors to the crow’s call seemed to be intrinsic, because the zebra finches used in this study had never heard such calls before. At the fourth training block (TB4), WT birds began to decrease call behavior in response to CS, indicating that they began to associate the appearance of US with CS (Fig. 3C). By contrast, DN birds did not show any significant difference in their change in behavioral responses to CS compared with those to the control songs (DN; TB1, P = 0.21; TB4, P = 0.67; Student’s paired t test; Fig. 3C). Actv birds did not show significant change in behavior either (Actv; TB1, P = 0.45; TB4, P = 0.60; Fig. 3C). At the training blocks on the next day, the WT birds showed the conditioned response even at the beginning session (TB5), indicating that the memory was retained to the next day. In contrast, the DN birds failed to show conditioned responses, even at the final training block (DN; TB5, P = 0.22; TB8, P = 0.92; Fig. 3C); and the Actv birds showed conditioned responses only at the final block (Actv; TB5, P = 0.12; TB8, P < 0.001; Fig. 3C). Similar results were obtained when the presented song stimulus was changed to another set of songs, to exclude the possibility that such behaviors were specific to a particular song (Fig. S6 A and B). The decrease in the learning in DN and Actv-TgN birds was not due to motor defects or impaired auditory perception, because significant behavioral responses to US were observed for each genotype in every training block (Fig. 3B), indicating that the memory formation associating CS with US was specifically impaired in DN and Actv-TgN birds.
Fig. 3.
Deficits in auditory memory formation in transgenic zebra finches. (A) Experimental timeline (Upper) and the schematics of the experiment of one training block (Lower). (B and C) Results of the auditory conditioning experiments. Behavioral reaction against control song stimulus (Cont) (dotted lines) and unconditioned stimulus (US) (solid lines; B) or conditioned song stimulus (CS) (solid lines; C) are shown. Change in call behavior number after the presentation of stimuli (Cont, US, and CS) are normalized and shown for each genotype (WT, Left; DN, Middle; Actv, Right). Mean ± SEM are shown. Asterisks indicate a significant difference in the call response before and after the presentation of each stimulus; P < 0.05, Student’s paired t test; WT, n = 25; DN, n = 25; Actv, n = 25. See also Fig. S6 for the raw number of the call behavior, and the experiment performed with another set of song stimuli.
Although both DN and Actv birds displayed difficulties of song memory formation, we noticed a trend that DN and Actv birds react differently to US. Although presentation of US caused a significant reduction in call behavior (Fig. 3B) to DN and Actv birds, Actv birds showed significantly more number of calling, suggesting that they are behaviorally more active after the presentation of US compared with WT and DN-TgN birds [Fig. S6C; WT, P < 0.0016; DN, P < 0.014; Tukey’s post hoc analysis; two-way ANOVA, F(2,288) = 5.27, genotype factor, P < 0.0058]. Together, these data show that transgenic manipulation of the CREB transcriptional activity altered the memory formation in adult birds, consistent with a reported role of CREB in memory formation in other animal models (24–26).
At the beginning of the fourth training block (TB4), when the behavioral association between CS and US was observed, phosphorylation of CREB at serine 119 (equivalent to serine 133 in mouse CREB), which activates its transcriptional activity (42), was detected in the brains of auditory conditioned WT birds. We observed pCREB signal in various brain regions. One of the brain regions that showed differential phosphorylation of CREB was the basal ganglia including Area X, the nucleus essential for song learning (43, 44) (Fig. S7A). During the conditioning, we did not observe the singing of the subjects; this phosphorylation was similarly observed when the subjects were auditory conditioned in a dark chamber, indicating that such phosphorylation was not caused by singing (45) (Fig. S7B). Rather, these signals may be caused by perception of auditory stimuli (28), or by motor behavior of subjects such as vocalization of calls (46), or by neuromodulators such as dopamine (47). This phosphorylation was suppressed by injection of STO609 (20 μM), a selective inhibitor of calmodulin-dependent protein kinase kinase (CaMKK), which is known to suppress the activity-dependent phosphorylation of CREB through suppressing the activity of calcium calmodulin-dependent protein kinase IV (48), into the basal ganglia before the trainings (Fig. S7C).
The basal ganglia is known to play an important role in the learning of sequential motor behavior or in selecting the action in classical conditioning (49), not only in rodents, but also in avian species (50, 51). We therefore investigate the role of CREB activation in basal ganglia to the auditory conditioning formation. We injected vehicle or STO609 bilaterally into the basal ganglia of WT birds before the auditory conditioning task, and analyzed the effect of these manipulations on the memory formation (Fig. S7 D–F). The injected drug seems to spread to parts of Area X and the surrounding striatum (Fig. S7C; SI Materials and Methods). The injection of vehicle or STO609 did not affect the freezing response against the presentation of the unconditioned stimulus in the next day sessions (Fig. S7E). However, injection of STO609 before the conditioning training abolished the conditioned response in the next day (Fig. S7F; STO609; TB5, P = 0.12; TB8, P = 0.41, Student’s paired t test), whereas the vehicle-treated birds showed normal conditioned responses (Fig. S7F; Vehicle). Thus, the pharmacological method to suppress CREB activation in a local brain structure reproduced the results of CREB-transgenic animals, indicating that CREB was involved in the formation of the conditioned response in this experiment.
Impaired Vocal Learning in Transgenic Zebra Finches.
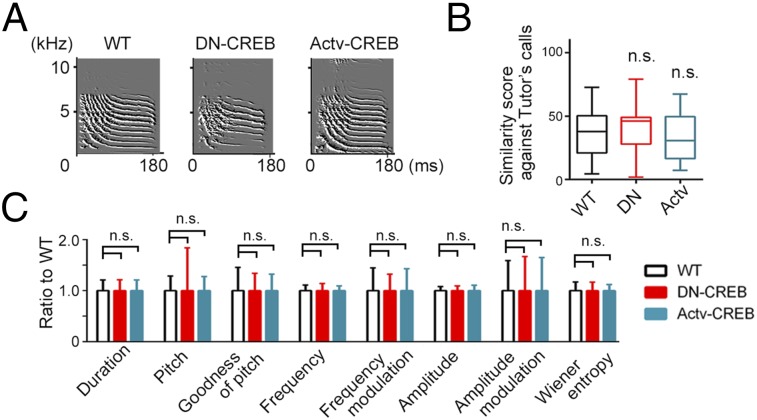
Next, we analyzed how the genetic manipulation of CREB activity affects song development, which requires social learning during the postnatal period. Postnatal song acquisition was assayed by use of our song-training paradigm, which allows us to compare the accuracy with which song of a tutor (used repeatedly to different juveniles) can be copied by individuals of different genetic backgrounds (Fig. 4A; SI Materials and Methods). We found that the relative qualities of acquired songs (52) between the transgenic birds and WT birds, tutored by a common male tutor, were strongly affected by the genotype (Fig. 4 B–F and Fig. S8A). Birds with DN-CREB expression developed songs with severely reduced similarity scores compared with WT controls [Fig. 4C; one-way ANOVA: F(2,85) = 4.10, P < 0.0004; DN, P < 0.0008, Dunnett’s post hoc test]. On the other hand, birds with Actv-CREB expression developed songs that showed no significant difference compared with those of WT birds (Fig. 4C; Actv, P = 0.87). Both WT and Actv birds, but not the DN birds (P = 0.11), showed an increase in song quality during development (between 60 and 140 dph) (Fig. 4D). Song similarity analysis based on the similarity of an entire motif (52), a stereotyped temporal sequence of syllables, also yielded a similar result (Fig. 4 E and F and Fig. S8B). In contrast to songs, call is known to be acquired mainly intrinsically (15, 39), although some feature of calls are modified postnatally through learning (53). Notably, we did not observe any significant genotype effect on the similarity of tutee’s calls against the tutor’s calls (Fig. 5 A and B and Fig. S8C; Kruskal–Wallis test, P > 0.50), nor the acoustic quality of calls (Fig. 5C). These results indicate that postnatally acquired behavior was specifically affected in the mutant CREB-transgenic birds. Collectively, the findings demonstrate that genetic manipulation of intrinsic factor, the activity of CREB, differentially affected the postnatal song development even within shared environmental conditions.
Fig. 4.
Acquisition of tutor’s song in transgenic zebra finches. (A, Top) Experimental timeline. (Bottom) Schematics of the experiment. Juvenile male birds were moved from their home cages and kept in a soundproof chamber with a live male finch (tutor). The same tutor bird was used multiple times for comparisons. (B) Examples of the sonograph of the tutor bird’s song (Tutor’s song) and that of the 140-dph birds of different genotype, reared with the same tutor (Tutee’s song). (C) Similarity score of tutee’s songs at 140 dph, calculated from the similarity of each syllable. (D) Developmental changes in the similarity score. (E and F) Similarity score of tutee’s songs calculated from the similarity of the entire motif. *P < 0.001, one-way ANOVA, Dunnett’s post hoc test, n.s., P > 0.67 against WT. Summarized values from 85 tutees (WT, n = 39; DN, n = 23; Actv, n = 23) tutored by five tutors are shown. Boxes and whiskers show the respective median and the 25th to 75th and 10th to 90th percentiles. Line graphs shows mean ± SD.
Fig. 5.
Call vocalization in transgenic zebra finches. (A) Examples of the sonograph of the calls of the 140-dph birds of different genotype. (B) Similarity scores between tutor’s and tutee’s call. (C) The differences of acoustic features of calls between the genotype. Summarized values from 85 tutees (WT, n = 39; DN, n = 23; Actv, n = 23) tutored by five tutors are shown. Boxes and whiskers show the respective median and the 25th to 75th and 10th to 90th percentiles. Line graphs shows mean ± SD; n.s., P > 0.15 against WT, Kruskal–Wallis test.
Discussion
The transgenic technology has been applied to a wide variety of animal species to study the effect of genetic involvement on animal behaviors and development. However, because the early embryogenesis of avian species has specific features different from other animals, generating transgenic avian species is still challenging (38, 54). Recently, transgenic lines of zebra finch that express GFP was generated by injecting lentiviral vectors into the early embryos (35). Using this approach, we obtained several lines of zebra finches expressing mutated CREBs. In addition to the existing methods, the new transgenic technology in songbirds will be a strong tool to study how specific genes influence the acquisition of behaviors. For example, by generating transgenic zebra finches with suppressed CREB activity, we showed that birds expressing DN-CREB develop songs with poor copying quality of tutor’s songs (Fig. 4). Transgenic manipulation of the genome allows a uniform expression of transgenes in the entire population of cells involved in executing or learning certain behaviors. In a certain situation, this method is better than other methods such as local injection of pharmacological reagents or viral vectors into the brain, which are unable to control the extent of diffusion or the efficiency of transfection among the cell population. For example, the transgenic strategy seems to be particularly advantageous in the present study because manipulations of the activity of CREB in a subset of the neuronal population has been shown to lead compensation of the disturbed function by the surrounding population that is not affected by the treatment (55). One caveat that should be mentioned about our present study is the transgenic strategy used in this study results in the expression of transgenes in a wide population of neurons throughout development, owing to the activity of the synapsin promoter used to express the transgene. Another concern is the possibility of disrupting the expression of endogenous genes especially near the integration locus of transgenes, which may cause some difference of phenotypes among the transgenic lines. Further technical refinements using transgenic strategies already applied in other animals, such as the application of cell type-specific promoters or inducible promoters, or knock-in of transgene into a specific locus, may specify the neuronal circuitries or the time window of plasticity involved in the development of the postnatally learned behaviors in songbirds.
Although the effect on the intrinsic phenotypes still needs to be analyzed, the results presented here indicate that activation of the CREB signaling pathway is essential for the proper song learning in postnatal periods. In other animal models, CREB has been known to function as a positive regulator of memory formation by regulating the activity-dependent gene transcription in neurons (24, 26, 27, 40, 56). Transgenic rodent models have shown that expression of dominant-negative mutant of CREB suppresses whereas constitutive active mutants enhance some forms of memory acquisition (40, 56). Our results are basically in line with the idea that CREB is a key molecule to regulate postnatal learning in animals, and provide evidence that songbirds also use CREB in auditory and vocal learning of birdsongs. We observed that the transgenic zebra finches expressing DN-CREB (DN; S119A) developed songs with severely reduced copying accuracy. On the other hand, the birds expressing the active form of the CREB mutant (Actv; Y120F) showed songs with comparable copying accuracy to those of WT birds (Fig. 4). Because the song learning may be well optimized through the evolutionary processes of songbirds, it is possible that our experimental methods did not allow the enhancement of song learning in Actv birds. Whether the chronic enhancement of CREB activity actually shows enhanced or accelerated learning of songs or auditory memory in a different context remains to be determined in further studies. In contrast to song development in juvenile birds, we observed impaired auditory memory formation in adult Actv birds, similarly to DN birds (Fig. 3). Genetic manipulation of CREB function in rodents sometimes causes discrepancies in the evaluation of behavioral studies, because enhancement of CREB phosphorylation in mice does not necessarily result in the enhancement of memory formation, depending on the expression level of proteins or the testing context (40, 57–59). In our hearing threshold analysis (Fig. S3), although Actv birds showed normal auditory ability to discriminate the change of sound volume, we noted that the reaction to the auditory signal presentation was different; i.e., whereas WT and DN birds showed a reduction in their number of calling behavior, Actv birds showed a significant increase in it (Fig. S3C). Similarly, Actv birds were more active in their response to the US in the auditory conditioning test (Fig. S6C). The difference in the reaction to sound stimuli may be partly attributable to the disturbed formation of conditioned responses in the Actv-CREB birds.
Although the efficiency is still low, the transgenic technology has opened the door to perform molecular genetic studies on songbirds (35). Additional technologies, such as gene knockout or conditional expression of transgenes, will clarify the contribution of genes and environments, as well as how these are intermingled, to the development of the sophisticated ability for song acquisition in songbirds. Because we can manipulate both genetic and environmental factors, as shown in this study, songbirds may provide valuable knowledge as to how environments affect the development or disorders of an animal’s behaviors.
Materials and Methods
Also see SI Materials and Methods for detailed descriptions.
Animal Care and Treatment.
All animal experiments were performed with the approval of The Animal Care and Use Committee of Kyoto University. To generate transgenic zebra finches, freshly laid eggs were collected from nests, and lentiviral vectors were microinjected around the central portion of the embryos as described earlier (35). Germ line transmission of transgene was analyzed by performing PCR-mediated genotyping of the offspring that were produced by crossing the virus-injected bird with WT birds (G1 generation). Transgene expression was further checked by RT-PCR and by immunostaining of brain sections.
Song Similarity Analysis and Behavioral Analysis.
At each developmental time point, birds were isolated in a soundproof chamber; and their vocalizations were recorded with a microphone. The songs and calls were analyzed with Sound Analysis Pro-2011 (52) (SAP2011), using the similarity batch mode. Behavioral analysis was done as described previously (34). Only male birds were used for the behavioral analysis. For the auditory conditioning, birds were isolated in a soundproof chamber and their responses against song presentation were video recorded. For normalization of the call behavior to the CS, the number of call responses during the 1-min period before the CS presentation was subtracted from the number of call responses of the 1-min period after (and during) the CS, and divided by the sum of the values before and after. For the US, the number of call behaviors during the 1-min period before the CS presentation was subtracted from the number of call responses of the 1-min period after (and during) the US, and divided by the sum of the values. Statistical analysis was performed using the paired t test on raw values before and after the stimulus presentation (without normalization).
Supplementary Material
Acknowledgments
We thank N. Yokoi, M. Wajima, and F. Ageta for maintenance of animals, and members of our laboratory as well as H. Fujimoto, N. Fujishiro, T. Imai, and S. Kida for fruitful help and suggestions. This work was supported by Japan Society for the Promotion of Science KAKENHI Grant 22700335 (to K.A.), 25115717 (to K.A.), and 24220009 (to D.W.); Precursory Research for Embryonic Science and Technology Program (K.A.) by Japan Science and Technology Agency; and by scientific research grants from the Sumitomo Foundation (to K.A.) and the Inamori Foundation (to K.A.). DNA sequencing analysis was performed at the Medical Research Support Center at Kyoto University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413484112/-/DCSupplemental.
References
- 1.Doupe AJ, Kuhl PK. Birdsong and human speech: Common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 2.Clayton DF. The genomics of memory and learning in songbirds. Annu Rev Genomics Hum Genet. 2013;14:45–65. doi: 10.1146/annurev-genom-090711-163809. [DOI] [PubMed] [Google Scholar]
- 3.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22(7):770–783. [PubMed] [Google Scholar]
- 4.Marler P. A comparative approach to vocal learning: Song development in white-crowned sparrows. J Comp Physiol Psychol. 1970;71(2, Pt.2):1–25. [Google Scholar]
- 5.Slater PJB. Bird song learning: Causes and consequences. Ethol Ecol Evol. 1989;1:19–46. [Google Scholar]
- 6.Thorpe WH. The learning of song patterns by birds, with especial reference to the song of the chaffinch Fringilla coelebs. Ibis. 1958;100(4):535–570. [Google Scholar]
- 7.Price PH. Developmental determinants of structure in zebra finch song. J Comp Physiol Psychol. 1979;93(2):260–277. [Google Scholar]
- 8.Eales LA. Song learning in zebra finches: Some effects of song model availability on what is learnt and when. Anim Behav. 1985;33(4):1293–1300. [Google Scholar]
- 9.Williams H, Kilander K, Sotanski ML. Untutored song, reproductive success and song learning. Anim Behav. 1993;45(4):695–705. [Google Scholar]
- 10.Nowicki S, Searcy WA. Song function and the evolution of female preferences: Why birds sing, why brains matter. Ann N Y Acad Sci. 2004;1016:704–723. doi: 10.1196/annals.1298.012. [DOI] [PubMed] [Google Scholar]
- 11.Lauay C, Gerlach NM, Adkins-Regan E, DeVoogd TJ. Female zebra finches require early song exposure to prefer high-quality song as adults. Anim Behav. 2004;68(6):1249–1255. [Google Scholar]
- 12.Marler P, Sherman V. Innate differences in singing behaviour of sparrows reared in isolation from adult conspecific song. Anim Behav. 1985;33(1):57–71. [Google Scholar]
- 13.Marler P, Peters S. Selective vocal learning in a sparrow. Science. 1977;198(4316):519–521. doi: 10.1126/science.198.4316.519. [DOI] [PubMed] [Google Scholar]
- 14.Braaten RF, Reynolds K. Auditory preference for conspecific song in isolation-reared zebra finches. Anim Behav. 1999;58(1):105–111. doi: 10.1006/anbe.1999.1134. [DOI] [PubMed] [Google Scholar]
- 15.Forstmeier W, Burger C, Temnow K, Derégnaucourt S. The genetic basis of zebra finch vocalizations. Evolution. 2009;63(8):2114–2130. doi: 10.1111/j.1558-5646.2009.00688.x. [DOI] [PubMed] [Google Scholar]
- 16.Fehér O, Wang H, Saar S, Mitra PP, Tchernichovski O. De novo establishment of wild-type song culture in the zebra finch. Nature. 2009;459(7246):564–568. doi: 10.1038/nature07994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3(12):921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 18.Abe K. Neural activity-dependent regulation of gene expression in developing and mature neurons. Dev Growth Differ. 2008;50(4):261–271. doi: 10.1111/j.1440-169X.2008.00999.x. [DOI] [PubMed] [Google Scholar]
- 19.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2(8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 20.Shaywitz AJ, Greenberg ME. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 21.Bito H, Deisseroth K, Tsien RW. Ca2+-dependent regulation in neuronal gene expression. Curr Opin Neurobiol. 1997;7(3):419–429. doi: 10.1016/s0959-4388(97)80072-4. [DOI] [PubMed] [Google Scholar]
- 22.Qiu Z, Ghosh A. A brief history of neuronal gene expression: Regulatory mechanisms and cellular consequences. Neuron. 2008;60(3):449–455. doi: 10.1016/j.neuron.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 23.Benito E, Valor LM, Jimenez-Minchan M, Huber W, Barco A. cAMP response element-binding protein is a primary hub of activity-driven neuronal gene expression. J Neurosci. 2011;31(50):18237–18250. doi: 10.1523/JNEUROSCI.4554-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 25.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 26.Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4(6):pii: a005751. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6(2):264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi H, Wada K, Maekawa M, Watsuji T, Hagiwara M. Song-induced phosphorylation of cAMP response element-binding protein in the songbird brain. J Neurosci. 1999;19(10):3973–3981. doi: 10.1523/JNEUROSCI.19-10-03973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clayton DF, Balakrishnan CN, London SE. Integrating genomes, brain and behavior in the study of songbirds. Curr Biol. 2009;19(18):R865–R873. doi: 10.1016/j.cub.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brainard MS, Doupe AJ. Translating birdsong: Songbirds as a model for basic and applied medical research. Annu Rev Neurosci. 2013;36:489–517. doi: 10.1146/annurev-neuro-060909-152826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woolley SMN. Early experience shapes vocal neural coding and perception in songbirds. Dev Psychobiol. 2012;54(6):612–631. doi: 10.1002/dev.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitch WT. The Evolution of Language. Cambridge Univ Press; Cambridge, UK: 2010. [Google Scholar]
- 33.Slater PJ. The cultural transmission of bird song. Trends Ecol Evol. 1986;1(4):94–97. doi: 10.1016/0169-5347(86)90032-7. [DOI] [PubMed] [Google Scholar]
- 34.Abe K, Watanabe D. Songbirds possess the spontaneous ability to discriminate syntactic rules. Nat Neurosci. 2011;14(8):1067–1074. doi: 10.1038/nn.2869. [DOI] [PubMed] [Google Scholar]
- 35.Agate RJ, Scott BB, Haripal B, Lois C, Nottebohm F. Transgenic songbirds offer an opportunity to develop a genetic model for vocal learning. Proc Natl Acad Sci USA. 2009;106(42):17963–17967. doi: 10.1073/pnas.0909139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du K, Asahara H, Jhala US, Wagner BL, Montminy M. Characterization of a CREB gain-of-function mutant with constitutive transcriptional activity in vivo. Mol Cell Biol. 2000;20(12):4320–4327. doi: 10.1128/mcb.20.12.4320-4327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott BB, Lois C. Generation of tissue-specific transgenic birds with lentiviral vectors. Proc Natl Acad Sci USA. 2005;102(45):16443–16447. doi: 10.1073/pnas.0508437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford Univ Press; Oxford: 1996. [Google Scholar]
- 40.Barco A, Marie H. Genetic approaches to investigate the role of CREB in neuronal plasticity and memory. Mol Neurobiol. 2011;44(3):330–349. doi: 10.1007/s12035-011-8209-x. [DOI] [PubMed] [Google Scholar]
- 41.Kida S, et al. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5(4):348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 43.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53(1):51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 44.Scharff C, Nottebohm F, Cynx J. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J Neurobiol. 1998;36(1):81–90. [PubMed] [Google Scholar]
- 45.Feenders G, et al. 2008. Molecular mapping of movement-associated areas in the avian brain: A motor theory for vocal learning origin. PLoS One 3(3):e1768.
- 46.Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol. 2007;97(6):4271–4283. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- 47.Liu FC, Graybiel AM. Spatiotemporal dynamics of CREB phosphorylation: Transient versus sustained phosphorylation in the developing striatum. Neuron. 1996;17(6):1133–1144. doi: 10.1016/s0896-6273(00)80245-7. [DOI] [PubMed] [Google Scholar]
- 48.Redondo RL, et al. Synaptic tagging and capture: Differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J Neurosci. 2010;30(14):4981–4989. doi: 10.1523/JNEUROSCI.3140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 50.Izawa E, Yanagihara S, Atsumi T, Matsushima T. The role of basal ganglia in reinforcement learning and imprinting in domestic chicks. Neuroreport. 2001;12(8):1743–1747. doi: 10.1097/00001756-200106130-00045. [DOI] [PubMed] [Google Scholar]
- 51.Marzluff JM, Miyaoka R, Minoshima S, Cross DJ. Brain imaging reveals neuronal circuitry underlying the crow’s perception of human faces. Proc Natl Acad Sci USA. 2012;109(39):15912–15917. doi: 10.1073/pnas.1206109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59(6):1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 53.Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990;10(5):1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sang H. Prospects for transgenesis in the chick. Mech Dev. 2004;121(9):1179–1186. doi: 10.1016/j.mod.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Han J-H, et al. Neuronal competition and selection during memory formation. Science. 2007;316(5823):457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 56.Kida S, Serita T. Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Res Bull. 2014;105:17–24. doi: 10.1016/j.brainresbull.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Viosca J, et al. Chronic enhancement of CREB activity in the hippocampus interferes with the retrieval of spatial information. Learn Mem. 2009;16(3):198–209. doi: 10.1101/lm.1220309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki A, et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011;31(24):8786–8802. doi: 10.1523/JNEUROSCI.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gruart A, Benito E, Delgado-García JM, Barco A. Enhanced cAMP response element-binding protein activity increases neuronal excitability, hippocampal long-term potentiation, and classical eyeblink conditioning in alert behaving mice. J Neurosci. 2012;32(48):17431–17441. doi: 10.1523/JNEUROSCI.4339-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.