Abstract
For nephronophthisis (NPHP), the primary genetic cause of chronic renal failure in young adults, three loci have been mapped. To identify a new locus for NPHP, we here report on total-genome linkage analysis in seven families with NPHP, in whom we had excluded linkage to all three known NPHP loci. LOD scores >1 were obtained at nine loci, which were then fine mapped at 1-cM intervals. Extensive total-genome haplotype analysis revealed homozygosity in one family, in the region of the PCLN1 gene. Subsequent mutational analysis in this gene revealed PCLN1 mutations, thereby allowing exclusion of this family as a phenocopy. Multipoint linkage analysis for the remaining six families with NPHP together yielded a maximum LOD score (Zmax) of 8.9 (at D1S253). We thus identified a new locus, NPHP4, for nephronophthisis. Markers D1S2660 and D1S2642 are flanking NPHP4 at a 2.9-cM critical interval. In one family with NPHP4, extensive genealogical studies were conducted, revealing consanguinity during the 17th century. On the basis of haplotype sharing by descent, we obtained a multipoint Zmax of 5.8 for D1S253 in this kindred alone. In addition, we were able to localize to the NPHP4 locus a new locus for Senior-Løken syndrome, an NPHP variant associated with retinitis pigmentosa.
Introduction
Nephronophthisis (NPHP) comprises a group of autosomal recessive cystic renal disorders that constitute the most common genetic cause of end-stage renal disease (ESRD) during the first 2 decades of life (Smith and Graham 1945; Fanconi et al. 1951; Hildebrandt 1999). Following the symptoms of polyuria, polydipsia, anemia, and growth retardation, ESRD inevitably develops during childhood or young adulthood, requiring renal-replacement therapy for survival. Three gene loci have been mapped: those for (i) juvenile nephronophthisis (NPHP1 [MIM 256100]), on chromosome 2q13 (Antignac et al. 1993; Hildebrandt et al. 1993b); (ii) infantile nephronophthisis (NPHP2 [MIM 602088]), on 9q22-q31 (Haider et al. 1998); and (iii) adolescent nephronophthisis, on 3q21-q22 (NPHP3 [MIM 604387]) (Omran et al. 2000). Although there is genetic-locus heterogeneity, disease variants are indistinguishable by renal histology, which exhibits a triad of interstitial-cell infiltrates, renal tubular-cell atrophy with cysts arising from the cortico-medullary junction of the kidneys, and renal interstitial fibrosis (Waldherr et al. 1982). Clinically, there is a statistically different age at onset at ESRD: terminal renal failure develops at median ages of 1 year, 13 years, and 19 years, in NPHP2, NPHP1, and NPHP3, respectively (Omran et al. 2000). We recently identified the causative gene, NPHP1, for juvenile nephronophthisis (Hildebrandt et al. 1997). The gene product nephrocystin contains an SH3 protein-protein–interaction domain (Otto et al. 2000). Since nephrocystin interacts with the focal-adhesion components p130CAS, tensin, and focal adhesion kinase 2 (Benzing et al. 2001), defects in focal-adhesion signaling are most likely involved in the pathogenesis of NPHP1 (Hildebrandt and Otto 2000). Associations of NPHP with autosomal recessive retinitis pigmentosa (RP) have been described as the so-called Senior-Løken syndrome (SLS [MIM 266900]) (Løken et al. 1961; Senior et al. 1961). A late-onset form of RP occurs in a small percentage of patients with NPHP1 mutations without an apparent genotype/phenotype correlation (Caridi et al. 1998). Interestingly, another locus for early-onset SLS (i.e., Leber congenital amaurosis) colocalizes to the NPHP3 locus (Omran et al. 2002). We here report chromosomal localization of a fourth gene locus, NPHP4, for NPHP. In addition, we localize to the new NPHP4 locus a novel locus for SLS, termed “SLSN4.”
Patients and Methods
Blood samples and clinical data for seven families with NPHP were obtained after informed consent was given by patients and their parents and siblings. Ethnic origins of these families were as follows: families F10, F30, and F158 are German; family F344 is Lebanese; family F444 is Finnish; family F461 is French; and family F698 is Belgian. The diagnosis of NPHP was made by a pediatric nephrologist who was the primary physician for these patients. Diagnostic criteria were (i) development of ESRD following a history of polyuria, polydipsia, and anemia; (ii) renal ultrasound compatible with NPHP; (iii) a kidney-biopsy result consistent with NPHP; and (iv) absence of extrarenal symptoms, such as RP, Cogan syndrome, liver fibrosis, and cone-shaped epiphyses. For family F461, kidney-biopsy results were not available. Since there was clearly no doubt regarding the diagnosis of NPHP in this family, an exception was made, a priori, to include this family, despite the lack of biopsy data. In these patients, ESRD commenced within a wide age range, 11–34 years.
The diagnosis of SLS was made according to the same criteria, with regard to renal symptoms, as were used for NPHP. In SLS, these renal symptoms are associated with RP. Clinical data for family F3 with SLS have been published previously (Polak et al. 1983): All three affected siblings had RP suggestive of Leber amaurosis congenita. Individual F3-II-1 had only central areas of vision and died in 1993, because of complications from chronic renal failure following renal transplantation. Individual F3-II-3 had received a cadaveric renal transplant before the age of 28 years. In patient F3-II-7, reexamination for this study revealed, at age 30 years, long-standing flat electroretinographic results and central tunnel vision of 10°, in both eyes. At age 35 years, visual acuity was reduced to only light perception by the right eye and hand-movement perception by the left eye. At the same time, there was ESRD, with a serum creatinine level raised to 751 μmol/liter.
Genomic DNA was isolated, by standard methods (Maniatis et al. 1987), either directly from blood samples or after Epstein-Barr–virus transformation of peripheral blood lymphocytes (Steel et al. 1977). Individuals were genotyped in a genomewide linkage analysis using 384 microsatellite markers with an average spacing of 11 cM. For haplotyping at fine resolution, novel microsatellite markers were generated by running the program BLAST with sequences of di-, tri-, and tetranucleotides as query against genomic sequence of the respective regions. Semiautomated genotyping was performed by a MegaBACE-1000 analysis system, as described elsewhere (Saar et al. 1997). Data were analyzed by Genetic Profiler Software, version 1.1. For two-point and multipoint analyses, genetic maps and allele numbers were taken from the Généthon data (Dib et al. 1996), with equal allele frequencies being assumed. For marker D1S253, which yielded the maximum LOD score (Zmax), allele frequency was 0.2. Two-point LOD-score calculations were performed by the LINKAGE program package (Lathrop and Lalouel 1984), with the help of the newly developed LINKRUN computer program (Saar et al. 1997), using an autosomal recessive model with 100% penetrance, a gene frequency of NPHP, and SLS frequency set at 0.00001 (Potter et al. 1980). For computation of multipoint LOD scores, the programs VITESSE (O’Connell and Weeks 1995) and SIMWALK (Barnes et al. 2001) were used. Haplotyping was performed by GENEHUNTER (Kruglyak et al. 1996) and, in a total-genome synopsis of all examined families in tabular form, by Excel 5.0 (Microsoft). The “LODmax − 1 support interval” was defined as the genetic-map positions intersecting the LOD-score curve at Zmax − 1 (Conneally et al. 1985). For display of genomewide two-point LOD-score data, the programs MAKESCAN and LODVIEW (Hildebrandt et al. 1993a) were used. Graphic display of pedigree structure was accomplished by CYRILLIC 2.1 (Cherwell Scientific) and, for multipoint LOD scores, by Excel 5.0 (Microsoft).
Results
During the past 10 years we ascertained, worldwide, 61 multiplex families with NPHP. In 26 of them, we excluded NPHP1, on the basis of either absence of mutations in NPHP1 or lack of linkage to this locus (Hildebrandt et al. 2001). After exclusion both of families with extrarenal symptoms and of families compatible with linkage to either NPHP2 or NPHP3, seven multiplex families remained, indicating the presence of an additional, unknown locus for NPHP, with exclusive renal involvement. A total-genome search for linkage was performed in these seven families. Suggestive LOD scores >2 were obtained on chromosomes 1q and 6q, and LOD scores >1 were obtained on chromosomes 1p, 3p, 9q, 12p, 16, 17p, and 22q. To extract maximum information from the total-genome search, total-genome haplotypes generated by the GENEHUNTER program were evaluated in tabular form, on the basis of physical-map order, for regions of compatibility with linkage in at least four of the seven families with NPHP4. In these regions, additional markers were haplotyped at fine resolution of ∼1 cM, partially on the basis of newly generated microsatellite markers. This led to the identification, in family F344, of a 62-cM region of homozygosity on 3q29, in the region of the paracellin-1 (PCLN1) gene (Simon et al. 1999). Further scrutiny revealed that recessive loss-of-function mutations were present in this gene, thereby identifying this family, on a molecular genetic basis, as a phenocopy actually representing primary hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHN [MIM 603959]). Thus, we were able, a posteriori, to exclude family F344 from the study.
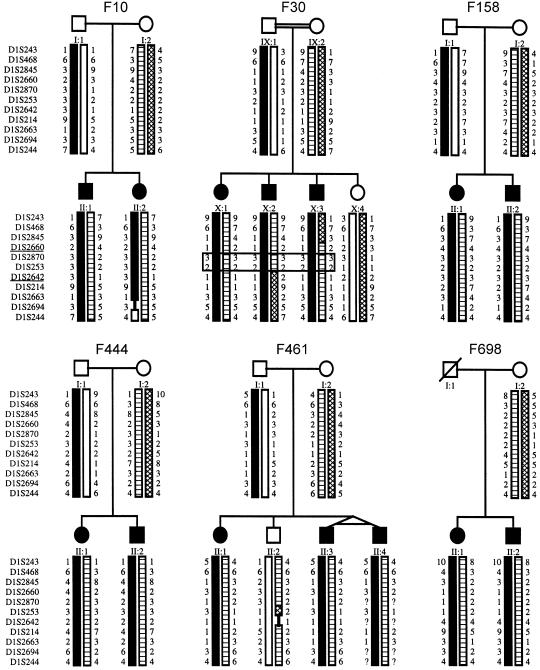
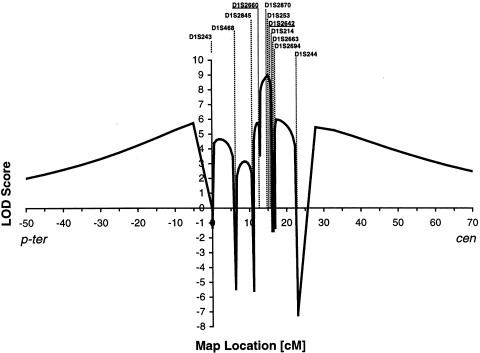
By further high-resolution haplotyping, we detected a chromosome 1p36.31 interval compatible with linkage for all other six NPHP families (fig. 1). Multipoint linkage analysis of 11 markers at the NPHP4 locus, which were calculated together for all six remaining families with NPHP4, yielded a Zmax of 8.9, for marker D1S253 at relative position 15.1 (fig. 2). The 95% CI at Zmax − 1 (Conneally et al. 1985) extends over a 2.9-cM interval between markers D1S2660 and D1S2642 (which are at relative positions 13.1 and 16.0, respectively) (fig. 2).
Figure 1.
Haplotypes on chromosome 1p36 in six families with NPHP4. Eleven microsatellites, from p-ter to cen (top to bottom), are shown to the left of the pedigrees. Circles denote females; squares denote males; filled symbols indicate affected status. Haplotypes are indicated by as differently shaded bars. In family F30, the double line indicates consanguinity, and genotypes homozygous by descent in affected individuals are boxed. Markers flanking the NPHP4 locus, as defined by lack of homozygosity in family F30, are underlined.
Figure 2.
Multipoint LOD scores for the NPHP4 locus, versus the 11 markers shown in figure 1, calculated in all six families with NPHP4. Positions (in cM) are according to the Généthon map relative to the position of D1S243 (Dib et al. 1996). The two markers—D1S2660 and D1S2642—that flank the NPHP4 region (see fig. 1) are underlined. p-ter = p-terminal orientation; cen = centromeric orientation.
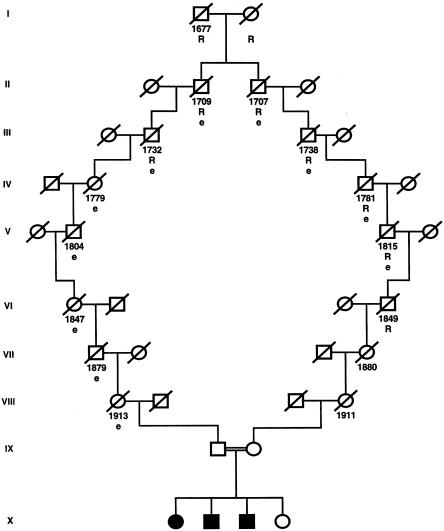
In family F30, there were two markers found to be homozygous in affected individuals (fig. 1). We therefore inverted the paradigm of homozygosity mapping (Lander and Botstein 1987), by postulating homozygosity by descent for these individuals (Omran et al. 2002). On both the paternal and the maternal sides of family F30, we identified an ancestor with a rare surname that occurs only 96 times among 40 million entries in the German online telephone registry. The fact that both the paternal and the maternal ancestors came from a small township facilitated the tracing of the postulated consanguinity loop (fig. 3). With the help of church records, we were able to demonstrate consanguinity in family F30, through a common ancestor born in 1677 (fig. 3). On the basis of consanguinity by descent, the LOD score for family F30 alone was Zmax=5.8 (recombination fraction 0) for marker D1S253. The existence of shorter loops of consanguinity, however, cannot be excluded in this kindred. Closer chains of kinship, as well as the use of higher allele frequencies for markers, would lower the Zmax attainable, as has been discussed elsewhere (Omran et al. 2002).
Figure 3.
Discovery of consanguinity in family F30. Year of birth is indicated below the symbol for each individual (but, in the last two generations, is omitted, to ensure anonymity). Note that consanguinity in the 17th century was detected; tracing of the consanguinity loop was facilitated by the occurrence of a rare name (“R”) and residence in a small township (“e”). Symbols are as in figure 1. A slash through a symbol indicates that the individual is deceased.
We thus identified a new gene locus, NPHP4, for a new disease entity of nephronophthisis, NPHP4. Since markers D1S2660 and D1S2642 are lacking homozygosity by descent in family F30 (fig. 1), they delimit, as flanking markers, the NPHP4 critical region, to within a 2.9-cM critical interval of genetic distance. On the Project Ensembl physical map, this interval measures ∼2.0 Mb (relative marker positions are 4,950,934 and 6,876,371, respectively).
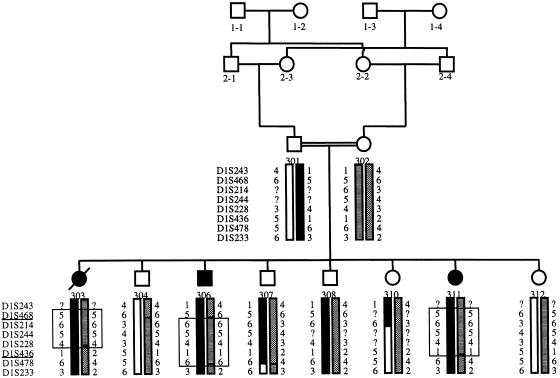
A gene locus for SLS—that is, the association of NPHP with RP—has been described in patients with homozygous deletions in the NPHP1 gene (Caridi et al. 1998). In addition, an SLS locus has been colocalized to NPHP3 (Omran et al. 2002), raising the question of whether pleiotropic genes at both loci are responsible for NPHP as well as for SLS phenotypes. We therefore tested the hypothesis that a gene locus for SLS might colocalize to the newly identified, NPHP4 locus. In family F3, a consanguineous family with SLS (fig. 4) and with three individuals affected with late-onset RP, pedigree structure was sufficient to yield a LOD score >2. This is sufficient proof of linkage if only a few candidate regions are examined, thus avoiding the problem of multiple testing, a problem that is inherent in a total-genome search for linkage (Ott 1991). In family F3, haplotype analysis of eight markers at the NPHP4 locus was compatible with homozygosity by descent in all three affected children (fig. 4). Multipoint linkage analysis for these markers yielded a Zmax of 2.7, for marker D1S214 (data not shown). This marker is positioned, on a physical map, only 0.3 Mb from marker D1S2642, which flanks the NPHP4 region on the centromeric side (fig. 2). We thus identified a new locus, SLSN4, for SLS. Lack of homozygosity defined markers D1S468 and D1S436 as flanking markers delimiting a 36.8-cM interval that overlaps with the critical NPHP4 interval delimited by markers D1S2660 and D1S2642. The critical regions for NPHP4 and SLSN4 thus overlap each other.
Figure 4.
Haplotypes on chromosome 1p36.31 of family F3 with SLS. Eight microsatellite markers, from p-ter to cen (top to bottom), are shown to the left of the pedigrees. Symbols are as in figure 1. In each of the affected individuals, the genotypes homozygous by descent are boxed. The two markers—D1S468 and D1S436—that flank the SLSN4 region are underlined.
The question of whether the phenotypes of NPHP4 with isolated kidney involvement and of SLS4 arise (i) from the pleiotropic action of one and the same gene or (ii) from a large homozygous deletion of two neighboring genes can only be answered once the responsible gene has been identified. In projects of total-genome analysis for linkage of recessive traits, for which a large number of pedigrees is difficult to ascertain, the approach described here might be useful. It includes extraction of complete linkage information by total-genome haplotyping in tabular form, with consecutive fine mapping of all regions, in which linkage is not excluded for at least half of the pedigrees. If fine mapping reveals homozygosity in affected individuals, then consanguinity, even if remote, should be actively sought.
Acknowledgments
We are indebted to all the patients and their families, for their participation in the study, and are especially indebted to family F30, for outstanding support. For contribution of clinical information and blood samples, we are very grateful to I. van den Born, Amsterdam (for family F3); Ch. Feldhoff and K.-E. Bonzel, Essen (for family F10); T. Felten and Dr. K. Kuehn, Karlsruhe, and H.P.H. Neumann, Freiburg (for family F30); J. Muscheites, Rostock (for family F158); S. Fründ, Muenster (for family F344); U. Vossmerbäumer, Würzburg (for family F461); and K. Devriendt, Antwerp (for family F698). We wish to thank Anita Imm for excellent technical assistance. We greatly appreciate the expertise and essential contribution of C. Lauer and G. Stüber, in studying the genealogy of family F30, at the Evangelische Zentralarchiv, Speyer. F.H. is a Heisenberg Scholar of the German Research Foundation and is receipient of its grant Hi 381/7-2 and also was supported by State of Baden-Württemberg grant FSP1-2000 and DFG Sonderforschungsbereich grant SFB 592. H.O. was supported by German Research Foundation grant Om 6/1-2.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Généthon, http://www.genethon.fr/ (for map positions)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for NPHP1 [MIM 256100], NPHP2 [MIM 602088], NPHP3 [MIM 604387], SLS [MIM 266900], and FHHN [MIM 603959])
- Project Ensembl, http://www.ensembl.org
References
- Antignac C, Arduy C, Beckmann JS, Benessy F, Gros F, Medhioub M, Hildebrandt F, Dufier JL, Kleinknecht C, Broyer M (1993) A gene for familial juvenile nephronophthisis (recessive medullary cystic disease) maps to chromosome 2p. Nat Genet 3:342–345 [DOI] [PubMed] [Google Scholar]
- Barnes KC, Mathias RA, Nickel R, Freidhoff LR, Stockton ML, Xue X, Naidu RP, Levett PN, Casolaro V, Beaty TH (2001) Testing for gene-gene interaction controlling total IgE in families from Barbados: evidence of sensitivity regarding linkage heterogeneity among families. Genomics 71:246–251 [DOI] [PubMed] [Google Scholar]
- Benzing T, Gerke P, Hildebrandt F, Kim E, Walz G (2001) Nephrocystin forms a multimeric protein complex with Pyk2, p130cas and tensin, and triggers phosphorylation and activation of Pyk2. Proc Natl Acad Sci USA 98:9784–9789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridi G, Murer L, Bellantuono R, Sorino P, Caringella DA, Gusmano R, Ghiggeri GM (1998) Renal-retinal syndromes: association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am J Kidney Dis 32:1059–1062 [DOI] [PubMed] [Google Scholar]
- Conneally PM, Edwards JH, Kidd KK, Lalouel JM, Morton NE, Ott J, White R (1985) Report of the Committee on Methods of Linkage Analysis and Reporting. Cytogenet Cell Genet 40:356–359 [DOI] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Fanconi G, Hanhart E, Albertini A, Uhlinger E, Dolivo G, Prader A (1951) Die familiäre juvenile Nephronophthise. Helv Paediatr Acta 6:1–49 [PubMed] [Google Scholar]
- Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D (1998) A bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am J Hum Genet 63:1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F (1999) Juvenile nephronophthisis. In: Avner E, Holliday M, Barrat T (eds) Pediatric nephrology. Williams & Wilkins, Baltimore [Google Scholar]
- Hildebrandt F, Otto E (2000) Molecular genetics of the nephronophthisis-medullary cystic disease complex. J Am Soc Nephrol 11:1753–1761 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, Brandis M (1997) A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 17:149–153 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Pohlmann A, Omran H (1993a) LODVIEW: a computer program for the graphical evaluation of lod score results in exclusion mapping of human disease genes. Comput Biomed Res 26:592–599 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Rensing C, Betz RC, Sommer U, Birnbaum S, Imm A, Omran H, Leipoldt M, Otto E (2001) Establishing an algorithm for molecular genetic diagnostics in 127 families with juvenile nephronophthisis. Kidney Int 59:434–445 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Singh-Sawhney I, Schnieders B, Centofante L, Omran H, Pohlmann A, Schmaltz C, Wedekind H, Schubotz C, Antignac C, Brandis M (1993b) Mapping of a gene for familial juvenile nephronophthisis: refining the map and definition of flanking markers on chromosome 2. Am J Hum Genet 53:1256–1261 [PMC free article] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM (1984) Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- Løken AC, Hanssen O, Halvorsen S, Jølster NJ (1961) Hereditary renal dysplasia and blindness. Acta Paediatr 50:177–184 [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J (1987) Molecular cloning: a laboratory manual, 2d ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York [Google Scholar]
- O’Connell JR, Weeks DE (1995) The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recoding and fuzzy inheritance. Nat Genet 11:402–408 [DOI] [PubMed] [Google Scholar]
- Omran H, Fernandez C, Jung M, Häffner K, Fargier B, Waldherr R, Gretz N, Brandis M, Ruschendorf F, Reis A, Hildebrandt F (2000) Adolescent nephronophthisis: clinical, morphologic and genetic characterization of a novel disease entity. Am J Hum Genet 66:118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran H, Sasmaz G, Häffner K, Volz A, Olbrich H, Melkaoui R, Otto E, Wienker T, Korinthenberg R, Brandis M, Antignac C, Hildebrandt F (2002) Identification of a gene locus for Senior-Loken syndrome in the region of the nephronophthisis type 3 gene. J Am Soc Nephrol 13:75–79 [DOI] [PubMed] [Google Scholar]
- Ott J (ed) (1991) Analysis of human genetic linkage. Johns Hopkins University Press, Baltimore [Google Scholar]
- Otto E, Kispert A, Schaetzle S, Lescher B, Rensing C, Hildebrandt F (2000) Nephrocystin: gene expression and sequence conservation between human, mouse and C. elegans. J Am Soc Nephrol 11:270–282 [DOI] [PubMed] [Google Scholar]
- Polak BCP, van Lith FHM, Delleman JW, van Balen ATM (1983) Carrier detection in tapetoretinal degeneration in association with medullary cystic disease. Am J Ophthalmol 95:487–494 [DOI] [PubMed] [Google Scholar]
- Potter DE, Holliday MA, Piel CF (1980) Treatment of end-stage renal disease in children: a 15-year experience. Kidney Int 18:103–109 [DOI] [PubMed] [Google Scholar]
- Saar K, Chrzanowska KH, Stumm M, Jung M, Nuernberg G, Wienker TF, Seemanova E, Wegener RD, Reis A, Sperling K (1997) The gene for the ataxia-telangiectasia variant, Nijmegen breakage syndrome, maps to a 1-cM interval on chromosome 8q21. Am J Hum Genet 60:605–610 [PMC free article] [PubMed] [Google Scholar]
- Senior B, Friedmann AI, Braudo JL (1961) Juvenile familial nephropathy with tapetoretinal degeneration: a new oculo-renal dystrophy. Am J Ophthalmol 52:625–633 [DOI] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106 [DOI] [PubMed] [Google Scholar]
- Smith CH, Graham JB (1945) Congenital medullary cysts of the kidneys with severe refractory anemia. Am J Dis Child 69:369–377 [Google Scholar]
- Steel CM, Philipson J, Arthur E, Gardiner SE, Newton MS, McIntosh RV (1977) Possibility of EB virus preferentially transforming a subpopulation of human B lymphocytes. Nature 270:729–731 [DOI] [PubMed] [Google Scholar]
- Waldherr R, Lennert T, Weber HP, Fodisch HJ, Schärer K (1982) The nephronophthisis complex: a clinicopathologic study in children. Virchows Arch A Pathol Anat Histol 394:235–254 [DOI] [PubMed] [Google Scholar]