Abstract
Diabetes and depression occur together approximately twice as frequently as would be predicted by chance alone. Comorbid diabetes and depression are a major clinical challenge as the outcomes of both conditions are worsened by the other. Although the psychological burden of diabetes may contribute to depression, this explanation does not fully explain the relationship between these 2 conditions. Both conditions may be driven by shared underlying biological and behavioral mechanisms, such as hypothalamic-pituitary-adrenal axis activation, inflammation, sleep disturbance, inactive lifestyle, poor dietary habits, and environmental and cultural risk factors. Depression is frequently missed in people with diabetes despite effective screening tools being available. Both psychological interventions and antidepressants are effective in treating depressive symptoms in people with diabetes but have mixed effects on glycemic control. Clear care pathways involving a multidisciplinary team are needed to obtain optimal medical and psychiatric outcomes for people with comorbid diabetes and depression.
Keywords: Depression, Diabetes, Screening, Treatment, Mechanisms
Introduction
With diabetes and mental illness affecting approximately 8.3 % and 10 % of the total world’s population, respectively [1, 2], a degree of comorbidity between diabetes and depression is to be expected. However, epidemiologic studies have shown consistently that the 2 conditions occur together approximately twice as frequently as would be predicted by chance alone [3]. The combination of diabetes and depression present a major clinical challenge as the outcomes of both conditions are worsened by the presence of the other. Quality of life is worse, diabetes self-management is impaired, the incidence of complications is increased and life expectancy is reduced [4]. The costs of treatment increase significantly for both individual patients and health economies but these costs do not necessarily result in significant improvements in disease or quality of life outcomes [5].
The association between mental illness and diabetes has been recognized for many years [6]. In the 17th century, Thomas Willis, the famous anatomist and founding member of the Royal Society, described how “diabetes is a consequence of prolonged sorrow” [7]. Nevertheless, it is a frequently ignored yet vital component of holistic diabetes care. This review will explore the association between the 2 conditions, highlighting the epidemiology, pathogenesis and treatment options.
Methods
The authors prepared this review from literature searches in PubMed and data presented at the International Diabetes and Depression Conference held in Washington, DC in October 2012, which was hosted by the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) in collaboration with the National Institute of Mental Health and the Dialogue on Diabetes and Depression [8, 9].
Epidemiology of Diabetes and Depression
Significant depressive symptoms affect approximately 1 in 4 adults with type 1 and type 2 diabetes, whereas a formal diagnosis of depressive disorders is made in approximately 10 %–15 % of people with diabetes [3]. The prevalence estimates vary widely because of methodological differences in the definition of depression. In some studies, the term ‘depression’ means self-reported high depressive symptom scores, whereas in others it reflects a formal diagnosis by psychiatric interview. In addition, the construct of ‘diabetes-related distress’ captures the emotional distress associated with diabetes self-management, social support, and health care [10]. This construct has been found to be modestly correlated with depressive symptoms with approximately 30 % overlapping variance but remains distinct from depression in its association with adherence and glycemic control [10, 11].
A recent meta-analysis of 11 studies including nearly 50,000 people with type 2 diabetes but without depression at baseline has indicated that the incidence of depression is also 24 % higher in people with diabetes [12]. Once depressive symptoms occur or a diagnosis of depression is made, the symptoms appear to be persistent. For example, Peyrot and Rubin found self-reported depressive symptoms persisted in 73 % of people 12 months after a diabetes education program [13]. Furthermore, Lustman and colleagues observed a relapse rate for diagnosed major depressive disorder of 79 % over a 5-year period [14]. These data are in contrast to general population studies that suggest a depressive episode usually lasts 8–12 weeks indicating that in people with diabetes depressive episodes are more long-lasting and more likely recurrent.
There have been few studies of depression in children and adolescents but these suggest that rates of depression are also elevated in either type 1 or type 2 diabetes with prevalence rates ranging from 9 %–26 % [15].
As observed by Thomas Willis, epidemiologic studies have demonstrated that the association between depression and diabetes is bi-directional [16, 17]. A meta-analysis of 9 cohort studies found that adults with depression had a 37 % increased risk of developing type 2 diabetes [18] after accounting for factors common to both disorders including sex, body mass index, and poverty. There was considerable heterogeneity across studies with the risk varying between a nonsignificant increased relative risk of 1.03 to 2.50. A further meta-analysis of 13 studies found incident depression was increased by 15 % (OR 1.15 (95 % CI 1.02–1.30)) in people with diabetes at baseline [16].
General population risk factors for depression, including female sex, marital status, childhood adversity, and social deprivation also apply to people with diabetes. In addition, there are a number of diabetes specific risk factors associated with depression. In people with type 2 diabetes, the rates of depression are higher amongst those using insulin compared with noninsulin medications or dietary and lifestyle interventions alone [19, 20]. This does not imply that the insulin itself is causative but may reflect disease progression and the increased treatment demands made on an individual when insulin is initiated. The development of diabetes complications, particularly sexual dysfunction and painful peripheral neuropathy, also predict the development of depression [21]. In a specialized outpatient clinic, the presence of 2 or more complications was associated with a greater than 2-fold increase in the risk of depression in people with type 2 diabetes, with neuropathy and nephropathy showing the strongest association with depression [22]. Other diabetes specific risk factors include recurrent hypoglycemia and poor glycemic control. Two trials have reported that intensive self-monitoring of blood glucose has an adverse effect on depression rates in people with type 2 diabetes although other studies found no effect [20].
The Mechanisms and Pathogenesis Underlying the Association Between Diabetes and Depression
A variety of explanatory theoretical models have been proposed to explain the comorbidity of diabetes and depression.
Clinical Burden of Disease
Traditionally it was widely believed that depression was an understandable reaction to the difficulties resulting from living with a demanding and life-shortening chronic physical illness that is associated with debilitating complications. This model is supported by a systematic review of 11 studies that found no difference in the rates of depression between those with undiagnosed diabetes, those with impaired glucose metabolism, and people with normal glucose metabolism [23••]. An increased rate of depression was only found in those with diagnosed diabetes suggesting that the knowledge of the diagnosis and the burden of managing the condition and its complications are associated with depressive symptoms rather than biological mechanisms such as hyperglycemia. The implication of this finding is that healthcare professionals may have an important role in moderating the psychological burden associated with diabetes by considering the way in which the diagnosis of diabetes is conveyed and the psychosocial support that is given at and after diagnosis.
Lifestyle Factors and Adherence
Lifestyle factors are hypothesized to play a role in priming or reinforcing the comorbidity of depression and diabetes. For example, people with depression are more likely to be sedentary and eat diets that are rich in saturated fats and refined sugars while avoiding fruit and vegetables, which may contribute to the increased risk of developing type 2 diabetes [24–26].
Nonadherence to self-care management in those already diagnosed with diabetes and experiencing depressive symptoms has also been found [27, 28]. A meta-analysis of 47 independent samples found that depression was significantly associated with nonadherence to diabetes treatment recommendations including missed medical appointments, diet, exercise, medication use, glucose monitoring, and foot care [27]. In a primary care study of 879 people with type 2 diabetes, a 1-point increase in depressive symptoms scores was found to result in a 10 % increased risk of nonadherence to fruit and vegetable intake and foot care [28]. This suggests the possibility that there may be a mutually-reinforcing phenomenon that poorer adherence to self-care may increase blood glucose, which in turn may contribute to depressive symptoms and consequently contribute to decreased adherence to self-care behaviors.
Antidepressant Medications
It is also possible that the use of antidepressants contributes to the risk of diabetes. Case reports, and observational studies have shown a consistent association between people receiving antidepressant medications and diabetes but whether this relationship is causative remains unproved [29•]. Randomized controlled trials have emphasized that antidepressants vary considerably in their association with weight gain and both hyperglycemic and hypoglycemic effects have been observed [29•].
Brain Structure and Function
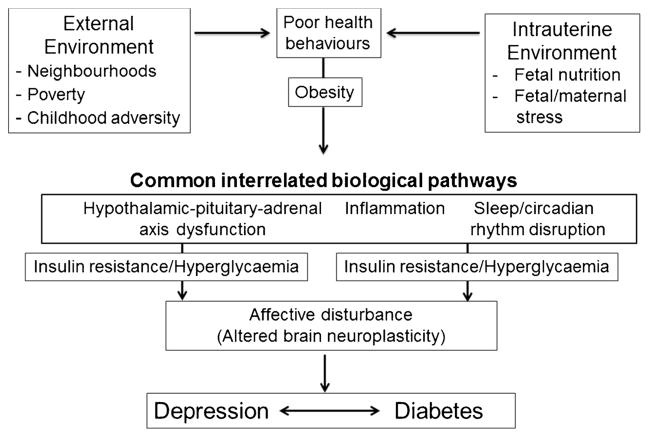
In addition to the psychosocial models described above, there are a number of shared biological changes that occur in diabetes and depression that may increase the risk of the other condition (Fig. 1). These mechanisms provide a novel perspective in considering the association between depression and diabetes by focusing more on common pathogenic mechanisms rather than focusing on the direction of association.
Fig. 1.
Shared biological mechanisms that may underlie both diabetes and depression
It is well recognized that both hypoglycemia and hyperglycemia can have major effects on brain function in areas of cognition and mood. MRI scanning of the brains of people with type 1 diabetes has shown that prefrontal glutamate-glutamine-gamma-aminobutyric acid levels are higher than in healthy control subjects, and these levels correlate with mild depressive symptoms [30]. Furthermore, animal models have shown that diabetes negatively affects hippocampal integrity and neurogenesis, which may interact with other aspects of neuroplasticity and contribute to mood symptoms in diabetes [31]. In humans, hippocampal neurogenesis can be indirectly assessed via MRI and there is hippocampal atrophy in diabetes [31].
Hypothalamic-Pituitary Adrenal (HPA) Axis Dysfunction
Both depression and diabetes are associated with dysfunction of the hypothalamic-pituitary adrenal (HPA) axis, which manifests as subclinical hypercortisolism, blunted diurnal cortisol rhythm, or hypocortisolism with impaired glucocorticoid sensitivity, and increased inflammation [32].
Sleep
Disrupted sleep patterns are associated with depression [33] and recently, poor sleep quality and altered circadian rhythms have been shown to increase insulin resistance and risk of type 2 diabetes [34]. In addition, a recent meta-analysis showed that depressive symptoms are weakly associated with insulin resistance, providing a potential link to incident type 2 diabetes [35].
Inflammation
Chronic inflammation may also underlie the association as cytokines and other inflammatory markers, such as increased C-reactive protein, TNF-α and proinflammatory cytokines, are increased in diabetes and the metabolic syndrome and are implicated in causing sickness behavior in animal models and depression in humans [36, 37].
Environmental Factors
These shared biological mechanisms provide a model by which environmental factors, ranging from the intra-uterine environment to neighborhood surroundings may affect the risk of comorbidity.
There is strong evidence that intrauterine environment and birth weight can predispose individuals to type 2 diabetes [38]. The studies of the relationship between adverse intra-uterine environment and risk for adult depression are less conclusive, but some studies suggest a positive association, whereas others have null findings [39–42]. However, in human studies, programming of the HPA axis and elevated cortisol reactivity in childhood, adolescence, and adulthood, which may predispose the individual to stress-related and metabolic disorders, have been seen following both low birth weight and fetal overexposure to cortisol secondary to maternal stress [43].
Several environmental factors, including childhood adversity, neighborhood environment and poverty influence the predisposition to depression and diabetes. Poor physical (eg, physical disorder, traffic, noise, decreased walkability) and social environments (eg, lower social cohesion and social capital, increased violence, decreased residential stability) are associated with worse diet and lower physical activity patterns, obesity, diabetes, hypertension, and depression [44]. The current studies do not permit a determination of causality but adverse neighborhood environments have also been associated with HPA axis dysfunction and a blunted circadian rhythm [45–49] as well as enhanced inflammation [50, 51].
Clinical Consequences of Comorbid Depression and Diabetes
Comorbid depression adversely affects diabetes outcomes and decreases quality of life [52–54]. A postal survey of 4168 people with diabetes found that, compared with those without depression, the 487 people with major depression reported significantly more diabetes symptoms and the number of depressive symptoms correlated with the overall number of diabetes symptoms [55].
Studies of the relationship between depression and glycemic control in adults have yielded discrepant findings with some showing that depression is associated with a small deterioration in glycated hemoglobin whereas others have shown no effect [56–58]. In children and adolescents, there is a clearer relationship between depressive symptoms and poorer glycemic control [15].
Nevertheless, depression is associated with worsened severity across the full range of diabetes complications although the direction and mechanisms that underlie this association are not fully understood [21].
Depression, even if mild, is also associated with premature mortality through a range of physical conditions [59•]. The excess mortality is not wholly explained by an increase in known risk factors because in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, clinically significant depressive scores were an independent risk factor for all-cause mortality after adjustment for blood pressure, glycated hemoglobin, lipids, body mass index, aspirin use, tobacco, alcohol, living alone, and educational level [60].
Treatment of Diabetes and Depression
Diagnosis of Depression
The first step to the effective management of depression is its recognition and diagnosis. A formal diagnosis of depression requires a validated interview but this is time-consuming and requires the healthcare professional to consider the diagnosis [61, 62]. Consequently, quick and cheaper methods are needed to screen people in primary and secondary care settings [63]. Many short questionnaires have been used to screen for depression in the general population but only a few have been evaluated adequately in people with diabetes (Table 1) [64•]. A number of symptoms, such as lethargy, irritability, or weight change, are common to both conditions and so questionnaires that rely heavily on these symptoms may overestimate the probability of depression. Table 1 describes the most well validated depression screening questionnaires for people with diabetes [64•] and includes the Beck Depression Inventory [65], the Centre for Epidemiologic Studies Depression Scale [66], the Patient Health Questionnaire (PHQ) [67], and the Hospital Anxiety and Depression Scale (HADS) [68]. The most widely used and validated questionnaire in people with type 2 diabetes is the PHQ-9 [67], which is also the shortest, containing 9 questions and can be completed by the patient. Consequently, it is easy to administer in primary care as well as population settings. The cut-off for major depression is ≥10 in primary care populations but it has been suggested that increasing the cut-off by 2 points to ≥12 points in people with diabetes may improve the discrimination between diabetes related symptoms and depressive symptoms [69]. The PHQ-9 has also been extensively used in primary care to assess the success of depression interventions using established cut-off scores for remission and clinical treatment response.
Table 1.
Most well validated depression screening questionnaires for people with diabetes
| Assessment tool | Type of diabetes (n) | Type of validation studies in diabetes (n) | ||
|---|---|---|---|---|
| Primary Care Health Questionnaire (PHQ-9) [67] | ||||
| Self-report measure of depressive symptoms that assessed DSM-IVTR diagnostic criteria for Major Depressive Disorder | Type 1 | 6 | Incidence/prevalence | 8 |
| Type 2 | 11 | Observational | 16 | |
| Not reported | 16 | RCT | 1 | |
| Beck Depression Inventory (BDI-II) [65] | ||||
| 21-item self-report measure of depressive symptom severity in the prior 2 wk period. | Type 1 | 25 | Incidence/prevalence | 11 |
| Type 2 | 17 | Observational | 34 | |
| Not reported | 19 | Case–control | 4 | |
| Experimental | 6 | |||
| Center for Epidemiologic Studies – Depression | ||||
| Scale (CESD) [66] | ||||
| 20-item self-report questionnaire assessing depressive symptom severity. | Type 1 | 10 | Incidence/prevalence | 17 |
| Type 2 | 28 | Observational | 43 | |
| Not reported | 20 | Case–control | 4 | |
| Validity | 3 | |||
| Hospital Anxiety and Depression Scale (HADS) [68] | ||||
| Brief self-report questionnaire assessing symptoms of both anxiety (7 items) and depression (7 items) during the past wk | Type 1 | 9 | Incidence/prevalence | 6 |
| Type 2 | 11 | Observational | 20 | |
| Not reported | 12 | RCT | 3 | |
Adapted from: Roy T, Lloyd CE, Pouwer F, Holt RI, Sartorius N. Screening tools used for measuring depression among people with Type 1 and Type 2 diabetes: a systematic review. Diabetes Med. 2012;29:164–75) [64•].
Another straightforward method that can be used by diabetes healthcare professionals is to ask 2 simple questions [70]:
“During the past month, have you been bothered by having little interest or pleasure in doing things?”
During the past month, have you been bothered by feeling down, depressed, or hopeless?”
If the answer to either is “yes,” the patient should be asked if they want help with this problem. If the answer to this is also “yes,” then the patient should be formally assessed by a diagnostic interview and offered appropriate referral and treatment.
Although a necessary first step, screening alone is insufficient to improve clinical outcomes and should be coupled to defined care pathways to allow more intensive depression management when required [63]. The importance of linking the screening to on-going care was demonstrated in a Dutch randomized controlled trial, which investigated the benefits of depression screening. Following screening, written feedback was provided to both patient and doctor [71•] but this did not change utilization of mental health services or improve depression scores. A further study found that physician recognition and treatment behavior was unchanged by screening per se [72].
Treatment
Both psychological therapies and antidepressant medication should be determined on an individual basis. As poor metabolic control, low rates of blood glucose self-monitoring, and diabetes complications all predict inadequate response to depression treatment, an equal emphasis on both diabetes and depression is needed.
Until recently, people with diabetes were specifically excluded from trials of the depression treatment and so consequently, there are relatively few studies examining antidepressant and psychotherapy treatment of depression in this population. Nevertheless studies published over the last decade have clearly indicated that treating depression is effective [73].
Psychological Interventions
The majority of psychotherapy protocols have used cognitive behavioral therapy delivered individually by mental health providers [74] or trained nurse case managers [75] but other common psychological interventions used in people with diabetes include problem solving, interpersonal therapy, motivational interviewing, counselling, and psychodynamic therapy [73]. The few published randomized controlled trials have included mixed populations of people with type 1 and type 2 diabetes, despite the fact that these are distinct diseases with differing ages of onset that affect the life course differently. The trials have a preponderance of people with type 2 diabetes and no trials have been conducted to date with only people with type 1 diabetes. There is preliminary evidence to suggest the web-based psychological therapies may also be effective in treating depression in people with diabetes but may have limited effects on glycemic outcomes [76, 77].
The best response to psychological interventions occurs when these are combined with diabetes education, to provide diabetes self-management skills as well as the psychological support to use these effectively [73]. As diabetes education often uses psychological components, there is the potential to combine the 2 in a seamless way.
Antidepressants
Investigations of antidepressant medications in people with either type 1 or type 2 diabetes have shown short-term amelioration of depressive symptoms as long as the medications are used in adequate doses, with a response similar to that observed in the general population [73]. However, efficacy trials in people with diabetes have been highly heterogeneous in terms of study designs (eg, ranging from single case studies to randomized controlled trials) and limited to a subset of commonly prescribed antidepressant medications (eg, nortriptyline [78], fluoxetine [79–81], bupropion [82], sertraline [83, 84], paroxetine [80], citalopram [81, 85]). Consequently, there remain substantial gaps in the evidence for effectiveness in treating depressive symptoms, effects on glycemic control, and the interaction of side effects on diabetes outcomes for other commonly prescribed antidepressants, including venlafaxine, desvenlafaxine, milnacipran, levomilnacipran, mirtazapine, nefazodone, and escitalopram that have not been investigated. All antidepressants appear to have similar efficacy in terms of depression outcomes and so the treatment of choice depends largely on the side effect profile, patient preference, and individual response [86]. Selective serotonin re-uptake inhibitors are less cardiotoxic than tricyclic antidepressants and are safer in overdose and, thus, are the treatment of choice for most people with diabetes. Some antidepressants, including mirtazapine, paroxetine and some tricyclic antidepressants, are associated with significant weight gain and are less suitable because of worsening insulin resistance and potentially glycemic control [87]. By contrast, bupropion, which is available in the USA, is associated with weight loss. Furthermore, unlike SSRIs, it does not appear to worsen sexual function and, therefore, may have advantages for people with diabetes [88].
There are mixed effects on glycemic control ranging from hyperglycemic effects with tricyclic antidepressant medications to euglycemic or slightly hypoglycemic effects with selective serotonin reuptake inhibitors and serotonin–nor-adrenaline reuptake inhibitors [73]. Sertraline may have specific advantages for glycemic control [73].
Models of Care
At present, many healthcare systems are becoming increasingly fragmented and specialized and are ill equipped to manage comorbidity. This disadvantages individuals with comorbid physical and mental illness as indicated by the UK Disability Rights Commission, which has highlighted the concept of “overshadowing” where healthcare professionals focus solely on the mental disorder and fail to take note of physical health needs, despite the need for this care being greater [89]. Within the field of diabetes, this translates into poorer diabetes care; those with mental illness are less likely to be screened for diabetes leading to higher rates of undiagnosed diabetes [90]. People with comorbid mental illness are less likely to be examined for eye or foot complications despite more clinic visits, less likely to be offered blood tests to measure glycated hemoglobin or cholesterol, less likely to receive a statin, and less likely to receive diabetes education [90]. By contrast, depressive and other mental disorders are not sufficiently recognized and treated when they happen to people with diabetes or other physical illness [91, 92]. These types of systematic deficiencies within healthcare systems may contribute significantly to the poorer health outcomes seen in those with comorbid diabetes and depression.
Multidisciplinary team approaches to the identification and treatment of depression within primary care settings that incorporate identification of high risk cases, problem-solving therapy delivered by trained nurse case managers, and psychotropic medications using a stepped-care approach have been shown to deliver the most effective clinical outcomes [75]. Early data from the PATHWAYS study indicated positive improvements in depression outcomes among adults with type 1 and type 2 diabetes but no changes in glycemic control [75]. The subsequent TEAMcare approach combined behavioral and psychotropic depression intervention strategies with diabetes management in collaboration with primary care and endocrinology teams; the study demonstrated improved depressive symptoms as well as glycemic and blood pressure control [93].
Although adults with comorbid diabetes and depression have increased direct and indirect health care costs [94•], these models of care appear cost-effective as well as clinically effective, leading to fewer days with depression and less out-patient costs [95–97].
Around the world, this level of collaboration is sadly lacking. A recent survey by Diabetes UK found that only approximately a third of services had access to specialist psychological services [98]. Similarly 81 % of expert providers felt under-resourced to meet patient psychological needs because of the demand. More concerning, unlike many other aspects of care that have improved in the UK over the last 10 years, the provision of psychological support has reduced over the same time [4]. The implication of this is clear; most psychological care will be provided by primary care or diabetes teams and extra training and awareness are needed to give the health care professionals the necessary skills to achieve this. Furthermore, there is an urgent need to improve access to psychological services, not least because nonspecialists can provide better care if expert psychological support is available. Work is needed to adapt the US collaborative care model to other health services.
Conclusions
Comorbid diabetes and depression is a challenging and under-recognized clinical problem. Depressive symptoms affect up to one-third of people with diabetes and not only impair quality of life but also add to the difficulties experienced in diabetes self-management. It is incumbent on healthcare professionals to identify depression in people with diabetes when present and then treat this rapidly and effectively in order to achieve the best clinical outcomes for these individuals. Most health services are poorly equipped to deal with comorbidity and, therefore, novel care pathways are needed to address this important public health problem.
Acknowledgments
We would like to acknowledge the other members of the Planning Committee of the NIDDK conference: Christine Hunter, Wayne Katon, Irwin Lucki, Paul Muehrer, Norman Sartorius and Larry Cimino
Footnotes
This article is part of the Topical Collection on Diabetes and Other Diseases-Emerging Associations
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Richard IG Holt has acted as an advisory board member and speaker for Novo Nordisk, and as a speaker for Sanofi-Aventis, Eli Lilly, Otsuka, and Bristol-Myers Squibb. He has received grants in support of investigator trials from Novo Nordisk. Mary de Groot and Sherita Hill Golden declare that they have no conflict of interest.
Contributor Information
Richard I. G. Holt, Email: R.I.G.Holt@soton.ac.uk, Human Development and Health Academic Unit, Faculty of Medicine, The Institute of Developmental Sciences (IDS Building), MP887, Southampton General Hospital, University of Southampton, Tremona Road, Southampton SO16 6YD, UK
Mary de Groot, Department of Medicine and the Diabetes Translational Research Center, Indiana University School of Medicine, Indianapolis, IN, USA.
Sherita Hill Golden, Departments of Medicine and Epidemiology, Johns Hopkins University Schools of Medicine and Public Health, Baltimore, MD, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Cross-national comparisons of the prevalences and correlates of mental disorders. WHO International Consortium in Psychiatric Epidemiology Bull World Health Organ. 2000;78:413–26. [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 4.Holt RI, Katon WJ. Dialogue on diabetes and depression: dealing with the double burden of comorbidity. J Affect Disord. 2012;142(Suppl):S1–3. doi: 10.1016/S0165-0327(12)00632-5. [DOI] [PubMed] [Google Scholar]
- 5.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25:464–70. doi: 10.2337/diacare.25.3.464. [DOI] [PubMed] [Google Scholar]
- 6.Holt RI. Undoing Descartes: integrating diabetes care for those with mental illness. Pract Diabetes. 2011;28:270–5. [Google Scholar]
- 7.Willis T. Pharmaceutice rationalis sive diabtriba de medicamentorum operantionibus in humano corpore. Oxford; 1675. [Google Scholar]
- 8.Sartorius N, Cimino L. The Dialogue on Diabetes and Depression (DDD): origins and achievements. J Affect Disord. 2012;142(Suppl):S4–7. doi: 10.1016/S0165-0327(12)70003-4. [DOI] [PubMed] [Google Scholar]
- 9.International Conference on Diabetes and Depression; Available at: http://www.niddk.nih.gov/news/events-calendar/Pages/international-conference-on-diabetes-and-depression.aspx. [Google Scholar]
- 10.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034–6. doi: 10.2337/dc09-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23–8. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53:2480–6. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peyrot M, Rubin RR. Persistence of depressive symptoms in diabetic adults. Diabetes Care. 1999;22:448–52. doi: 10.2337/diacare.22.3.448. [DOI] [PubMed] [Google Scholar]
- 14.Lustman PJ, Griffith LS, Clouse RE. Depression in adults with diabetes. Results of 5-yr follow-up study. Diabetes Care. 1988;11:605–12. doi: 10.2337/diacare.11.8.605. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds KA, Helgeson VS. Children with diabetes compared with peers: Depressed? Distressed? A meta-analytic review. Ann Behav Med. 2011;42:29–41. doi: 10.1007/s12160-011-9262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–9. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–45. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 19.Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. Affective and anxiety disorders in a German sample of diabetic patients: prevalence, comorbidity and risk factors. Diabetes Med. 2005;22:293–300. doi: 10.1111/j.1464-5491.2005.01414.x. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Ford ES, Strine TW, Mokdad AH. Prevalence of depression among U.S. adults with diabetes: findings from the 2006 behavioral risk factor surveillance system. Diabetes Care. 2008;31:105–7. doi: 10.2337/dc07-1154. [DOI] [PubMed] [Google Scholar]
- 21.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 22.van Steenbergen-Weijenburg KM, van Puffelen AL, Horn EK, Nuyen J, van Dam PS, van Benthem TB, et al. More co-morbid depression in patients with Type 2 diabetes with multiple complications. An observational study at a specialized outpatient clinic. Diabetes Med. 2011;28:86–9. doi: 10.1111/j.1464-5491.2010.03125.x. [DOI] [PubMed] [Google Scholar]
- 23••.Nouwen A, Nefs G, Caramlau I, Connock M, Winkley K, Lloyd CE, et al. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care. 2011;34:752–62. doi: 10.2337/dc10-1414. This meta-analysis compares the prevalence of depression in people with known and undiagnosed diabetes and impaired glucose tolerance. The higher rates in those with diagnosed diabetes suggest that the knowledge of the diagnosis and the burden of managing the condition and its complications are associated with depressive symptoms rather than biological mechanisms such as hyperglycemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMartin SE, Jacka FN, Colman I. The association between fruit and vegetable consumption and mental health disorders: evidence from five waves of a national survey of Canadians. Prev Med. 2013;56:225–30. doi: 10.1016/j.ypmed.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Payne ME, Steck SE, George RR, Steffens DC. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. 2012;112:2022–7. doi: 10.1016/j.jand.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weyerer S. Physical inactivity and depression in the community. Evidence from the Upper Bavarian Field Study. Int J Sports Med. 1992;13:492–6. doi: 10.1055/s-2007-1021304. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31:2398–403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222–7. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Barnard K, Peveler RC, Holt RI. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care. 2013;36:3337–45. doi: 10.2337/dc13-0560. This systematic review examines the evidence for a causal relationship between antidepressants and diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyoo IK, Yoon SJ, Musen G, Simonson DC, Weinger K, Bolo N, et al. Altered prefrontal glutamate-glutamine-gamma-aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Arch Gen Psychiatry. 2009;66:878–87. doi: 10.1001/archgenpsychiatry.2009.86. [DOI] [PubMed] [Google Scholar]
- 31.Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013;37:1346–62. doi: 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champaneri S, Wand GS, Malhotra SS, Casagrande SS, Golden SH. Biological basis of depression in adults with diabetes. Curr Diab Rep. 2010;10:396–405. doi: 10.1007/s11892-010-0148-9. [DOI] [PubMed] [Google Scholar]
- 33.Courtet P, Olie E. Circadian dimension and severity of depression. Eur Neuropsychopharmacol. 2012;22 (Suppl 3):S476–81. doi: 10.1016/j.euroneuro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. 2009;10 (Suppl 2):37–45. doi: 10.1111/j.1467-789X.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36:480–9. doi: 10.2337/dc12-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54:317–29. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- 37.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berends LM, Ozanne SE. Early determinants of type-2 diabetes. Best Pract Res Clin Endocrinol Metab. 2012;26:569–80. doi: 10.1016/j.beem.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–5. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- 40.Kajantie E, Raikkonen K. Early life predictors of the physiological stress response later in life. Neurosci Biobehav Rev. 2010;35:23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Colman I, Ataullahjan A, Naicker K, Van Lieshout RJ. Birth weight, stress, and symptoms of depression in adolescence: evidence of fetal programming in a national Canadian cohort. Can J Psychiatry. 2012;57:422–8. doi: 10.1177/070674371205700705. [DOI] [PubMed] [Google Scholar]
- 42.Vasiliadis HM, Gilman SE, Buka SL. Fetal growth restriction and the development of major depression. Acta Psychiatr Scand. 2008;117:306–12. doi: 10.1111/j.1600-0447.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 43.Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? J Intern Med. 2007;261:453–60. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- 44.de Vet E, de Ridder DT, de Wit JB. Environmental correlates of physical activity and dietary behaviours among young people: a systematic review of reviews. Obes Rev. 2011;12:e130–42. doi: 10.1111/j.1467-789X.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 45.Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol. 2011;23:1167–86. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenner AB, Zimmerman MA, Bauermeister JA, Caldwell CH. The physiological expression of living in disadvantaged neighborhoods for youth. J Youth Adolesc. 2013;42:792–806. doi: 10.1007/s10964-012-9838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karb RA, Elliott MR, Dowd JB, Morenoff JD. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: the Chicago Community Adult Health Study. Soc Sci Med. 2012;75:1038–47. doi: 10.1016/j.socscimed.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Do DP, Diez Roux AV, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, et al. Circadian rhythm of cortisol and neighborhood characteristics in a population-based sample: the Multi-Ethnic Study of Atherosclerosis. Health Place. 2011;17:625–32. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dulin-Keita A, Casazza K, Fernandez JR, Goran MI, Gower B. Do neighbourhoods matter? Neighbourhood disorder and long-term trends in serum cortisol levels. J Epidemiol Community Health. 2012;66:24–9. doi: 10.1136/jech.2009.092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Browning CR, Cagney KA, Iveniuk J. Neighborhood stressors and cardiovascular health: crime and C-reactive protein in Dallas, USA. Soc Sci Med. 2012;75:1271–9. doi: 10.1016/j.socscimed.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 51.Broyles ST, Staiano AE, Drazba KT, Gupta AK, Sothern M, Katzmarzyk PT. Elevated C-reactive protein in children from risky neighborhoods: evidence for a stress pathway linking neighborhoods and inflammation in children. PLoS One. 2012;7:e45419. doi: 10.1371/journal.pone.0045419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care. 2004;27:1066–70. doi: 10.2337/diacare.27.5.1066. [DOI] [PubMed] [Google Scholar]
- 53.Jacobson AM, de Groot M, Samson JA. The effects of psychiatric disorders and symptoms on quality of life in patients with type I and type II diabetes mellitus. Qual Life Res. 1997;6:11–20. doi: 10.1023/a:1026487509852. [DOI] [PubMed] [Google Scholar]
- 54.Carper MM, Traeger L, Gonzalez JS, Wexler DJ, Psaros C, Safren SA. The differential associations of depression and diabetes distress with quality of life domains in type 2 diabetes. J Behav Med. 2013 doi: 10.1007/s10865-013-9505-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski, et al. Depression and diabetes symptom burden. Gen Hosp Psychiatry. 2004;26:430–6. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 57.Hislop AL, Fegan PG, Schlaeppi MJ, Duck M, Yeap BB. Prevalence and associations of psychological distress in young adults with Type 1 diabetes. Diabetes Med. 2008;25:91–6. doi: 10.1111/j.1464-5491.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 58.Aikens JE, Perkins DW, Piette JD, Lipton B. Association between depression and concurrent Type 2 diabetes outcomes varies by diabetes regimen. Diabetes Med. 2008;25:1324–9. doi: 10.1111/j.1464-5491.2008.02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Park M, Katon WJ, Wolf FM. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen Hosp Psychiatry. 2013;35:217–25. doi: 10.1016/j.genhosppsych.2013.01.006. This meta-analysis shows that depression, even if mild, is also associated with premature mortality through a range of physical conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Connor PJ, Narayan KM, Anderson R, Feeney P, Fine L, Ali MK, et al. Effect of intensive vs standard blood pressure control on depression and health-related quality of life in type 2 diabetes: the ACCORD trial. Diabetes Care. 2012;35:1479–81. doi: 10.2337/dc11-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burk The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–77. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 62.Amorim P, Lecrubier Y, Weiller E, Hergueta T, Sheehan D. DSM-IH-R Psychotic disorders: procedural validity of the Mini International Neuropsychiatric Interview (MINI). Concordance and causes for discordance with the CIDI. Eur Psychiatry. 1998;13:26–34. doi: 10.1016/S0924-9338(97)86748-X. [DOI] [PubMed] [Google Scholar]
- 63.Holt RI, van der Feltz-Cornelis CM. Key concepts in screening for depression in people with diabetes. J Affect Disord. 2012;142(Suppl):S72–9. doi: 10.1016/S0165-0327(12)70011-3. [DOI] [PubMed] [Google Scholar]
- 64•.Roy T, Lloyd CE, Pouwer F, Holt RI, Sartorius N. Screening tools used for measuring depression among people with Type 1 and Type 2 diabetes: a systematic review. Diabetes Med. 2012;29:164–75. doi: 10.1111/j.1464-5491.2011.03401.x. This systematic review provides evidence of the validated screening tools for assessing depression in people with diabetes. [DOI] [PubMed] [Google Scholar]
- 65.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 66.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 67.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345–59. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 69.van Steenbergen-Weijenburg KM, de Vroege L, Ploeger RR, Brals JW, Vloedbeld MG, Veneman TF, et al. Validation of the PHQ-9 as a screening instrument for depression in diabetes patients in specialized outpatient clinics. BMC Health Serv Res. 2010;10:235. doi: 10.1186/1472-6963-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12:439–45. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Pouwer F, Tack CJ, Geelhoed-Duijvestijn PH, Bazelmans E, Beekman AT, Heine RJ, et al. Limited effect of screening for depression with written feedback in outpatients with diabetes mellitus: a randomized controlled trial. Diabetologia. 2011;54:741–8. doi: 10.1007/s00125-010-2033-0. This randomized trial shows that depression screening per se does not improve clinical outcomes and therefore highlights the importance of linking screening to appropriate depression care pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178:997–1003. doi: 10.1503/cmaj.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Feltz-Cornelis CM, Nuyen J, Stoop C, Chan J, Jacobson AM, Katon W, et al. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2010;32:380–95. doi: 10.1016/j.genhosppsych.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;129:613–21. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 75.Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–9. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 76.van Bastelaar KM, Pouwer F, Cuijpers P, Riper H, Snoek FJ. Web-based depression treatment for type 1 and type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2011;34:320–5. doi: 10.2337/dc10-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Feltz-Cornelis CM. Comorbid diabetes and depression: do E-health treatments achieve better diabetes control? Diabetes Manag. 2013;3:379–88. [Google Scholar]
- 78.Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59:241–50. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 79.Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23:618–23. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 80.Gulseren L, Gulseren S, Hekimsoy Z, Mete L. Comparison of fluoxetine and paroxetine in type II diabetes mellitus patients. Arch Med Res. 2005;36:159–65. doi: 10.1016/j.arcmed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 81.Khazaie H, Rahimi M, Tatari F, Rezaei M, Najafi F, Tahmasian M. Treatment of depression in type 2 diabetes with Fluoxetine or Citalopram? Neurosciences. 2011;16:42–5. [PubMed] [Google Scholar]
- 82.Lustman PJ, Williams MM, Sayuk GS, Nix BD, Clouse RE. Factors influencing glycemic control in type 2 diabetes during acute- and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care. 2007;30:459–66. doi: 10.2337/dc06-1769. [DOI] [PubMed] [Google Scholar]
- 83.Goodnick PJ, Kumar A, Henry JH, Buki VM, Goldberg RB. Sertraline in coexisting major depression and diabetes mellitus. Psychopharmacol Bull. 1997;33:261–4. [PubMed] [Google Scholar]
- 84.Williams MM, Clouse RE, Nix BD, Rubin EH, Sayuk GS, McGill JB, et al. Efficacy of sertraline in prevention of depression recurrence in older vs younger adults with diabetes. Diabetes Care. 2007;30:801–6. doi: 10.2337/dc06-1825. [DOI] [PubMed] [Google Scholar]
- 85.Amsterdam JD, Shults J, Rutherford N, Schwartz S. Safety and efficacy of escitalopram in patients with comorbid major depression and diabetes mellitus. Neuropsychobiology. 2006;54:208–14. doi: 10.1159/000100369. [DOI] [PubMed] [Google Scholar]
- 86.National Collaborating Centre for Mental Health. Common mental health disorders: identification and pathways to care CG123. London: National Institute of Health and Clinical Excellence; 2011. [Google Scholar]
- 87.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–72. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 88.Sayuk GS, Gott BM, Nix BD, Lustman PJ. Improvement in sexual functioning in patients with type 2 diabetes and depression treated with bupropion. Diabetes Care. 2011;34:332–4. doi: 10.2337/dc10-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Disability Rights Commission. A formal investigation into physical health inequalities experienced by people with learning difficulties and mental health problems. London: Disability Rights Commission; 2006. Equal treatment: closing the gap. [Google Scholar]
- 90.Mitchell AJ, Malone D, Doebbeling CC. Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry. 2009;194:491–9. doi: 10.1192/bjp.bp.107.045732. [DOI] [PubMed] [Google Scholar]
- 91.Lawrence D, Coghlan R. Health inequalities and the health needs of people with mental illness. N S W Public Health Bull. 2002;13:155–8. doi: 10.1071/nb02063. [DOI] [PubMed] [Google Scholar]
- 92.Frayne SM, Halanych JH, Miller DR, Wang F, Lin H, Pogach L, et al. Disparities in diabetes care: impact of mental illness. Arch Intern Med. 2005;165:2631–8. doi: 10.1001/archinte.165.22.2631. [DOI] [PubMed] [Google Scholar]
- 93.Katon WJ, Lin EH, Von KM, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–20. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94•.Molosankwe I, Patel A, Jose GJ, Knapp M, McDaid D. Economic aspects of the association between diabetes and depression: a systematic review. J Affect Disord. 2012;14(Suppl):S42–55. doi: 10.1016/S0165-0327(12)70008-3. This review describes the economic burden of the comorbidity of diabetes and depression. [DOI] [PubMed] [Google Scholar]
- 95.Simon GE, Katon WJ, Lin EH, Rutter C, Manning WG, Von Korff M, et al. Cost-effectiveness of systematic depression treatment among people with diabetes mellitus. Arch Gen Psychiatry. 2007;64:65–72. doi: 10.1001/archpsyc.64.1.65. [DOI] [PubMed] [Google Scholar]
- 96.Katon W, Unutzer J, Fan MY, Williams JW, Jr, Schoenbaum M, Lin EH, et al. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29:265–70. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- 97.Hay JW, Katon WJ, Ell K, Lee PJ, Guterman J. Cost effectiveness analysis of collaborative care management of major depression among low-income, predominantly Hispanics with diabetes. Value Health. 2012;15:249–54. doi: 10.1016/j.jval.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nicholson TR, Taylor JP, Gosden C, Trigwell P, Ismail K. National guidelines for psychological care in diabetes: how mindful have we been? Diabetes Med. 2009;26:447–50. doi: 10.1111/j.1464-5491.2009.02701.x. [DOI] [PubMed] [Google Scholar]