Abstract
Background
The transition from no coronary artery calcium (CAC) to detectable CAC is important, as even mild CAC is associated with increased cardiovascular events. We sought to characterize the anatomical distribution and burden of newly detectable CAC over 10-years follow-up.
Methods
We evaluated 3112 participants (mean age 58, 64% female) with baseline CAC=0 from the Multi-Ethnic Study of Atherosclerosis (MESA). Participants underwent repeat CAC testing at different time intervals (between 2–10 years after baseline) per MESA protocol. Among participants who developed CAC on a follow-up scan, we used logistic regression and marginal probability modeling to describe the coronary distribution and burden of new CAC by age, gender, and race/ethnicity after adjustment for cardiovascular risk factors and time-to-detection.
Results
A total of 1125 participants developed detectable CAC during follow-up with mean time-to-detection of 6.1 ± 3 years. New CAC was most commonly isolated to one vessel (72% of participants), with the left anterior descending (44% of total) most commonly affected followed by the right coronary (12%), left circumflex (10%) and left main (6%). These patterns were similar across age, gender, and race/ethnicity. In multivariable models, residual predictors of multi-vessel CAC (28% of total) included male gender, African-American or Hispanic race/ethnicity, hypertension, obesity, and diabetes. At the first detection of CAC>0, burden was usually low with median Agatston CAC score of 7.1, and <5% with CAC scores >100.
Conclusion
New onset CAC most commonly involves just one vessel, occurs in the left anterior descending artery, has low CAC burden. New CAC can be detected at an early stage when aggressive preventive strategies may provide benefit.
Keywords: Coronary artery calcium, Left anterior descending artery, Right coronary artery, Left main artery, Left circumflex artery
Introduction
Coronary artery calcium (CAC) is an imaging marker that is nearly pathognomonic for the presence of coronary atherosclerosis1, 2, and can be detected using non-contrast cardiac computed tomography (CT). Indeed, CAC testing is specific for the presence of coronary atherosclerosis, and highly sensitive test for increasing burden of obstructive atherosclerotic coronary artery disease3.
CAC is also highly effective for risk stratification of selected asymptomatic patients. For example, elevated CAC>300 is associated with a nearly 10-fold increased risk of adverse coronary events after multivariable adjustment4. Equally important, the absence of CAC in asymptomatic adults is associated with a low mortality rate of 1% over 10 years5–7. Even mildly elevated CAC denotes risk, as patients with CAC scores between 1–10 have a 2 to 3-fold increased risk of cardiovascular adverse events and death as compared to those with CAC=06, 8, 9.
Given these prognostic differences, a clinical finding of zero vs. non-zero CAC has important implications for clinical decision making, including the decision to treat risk conditions with lifestyle vs. pharmacotherapy10. Thus, there is a great deal of interest in studying the transition from zero to non-zero CAC. However, little is known about the characteristics of newly detected CAC, including its typical coronary distribution and burden.
To fill this gap, we used longitudinal CAC data from the Multi-Ethnic Study of Atherosclerosis (MESA) to describe the imaging characteristics related to the transition from zero to non-zero CAC. In particular, we asked: 1) At the first detection of new CAC, does CAC occur more commonly in one vessel vs. multiple vessels? 2) Does new CAC preferentially occur in particular coronary arteries? 3) At the first detection of new CAC, what is the CAC score burden? 4) Does the coronary distribution and burden of new CAC vary by age, gender, or race/ethnicity?
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA)
The Multi-Ethnic Study of Atherosclerosis (MESA) was designed to study the prevalence, risk factors, and progression of subclinical cardiovascular disease in individuals without known cardiovascular disease11. Between July 2000 and September 2002, MESA enrolled 6,814 individuals at six field centers in the United States (Baltimore; Chicago; Forsyth County, North Carolina; Los Angeles; New York; and St. Paul, Minnesota) as part of a prospective cohort study that has now spanned 5 in-person visits. Participants included women and men aged 45–84 years who identified themselves as White, Chinese, Black, or Hispanic. The institutional review board at all participating centers approved the study, and all participants gave written informed consent.
Patient Population
The study population for this analysis consisted of 3,112 participants who had CAC=0 at baseline and had at least 1 additional CT scan (scored on a per-vessel basis) during MESA follow-up.
Following the initial baseline scan, participants underwent repeat CT scans during different follow-up visits as per the pre-specified MESA protocol. MESA visits 2, 3, 4 and 5 included subjects who had follow-up CT scans at mean 1.7 ± 0.3, 3.2 ± 0.4, 4.9 ± 0.5, and 9.7 ± 0.6 years respectively following the initial baseline scan. In MESA, not all participants had follow up scan at each of the visits. As part of the study design, approximately half (n=1522) of the participants received repeat scan at visit 2 and the other half (n=1425) at visit 3. The visit 4 prioritized participants without scan at visit 3, and included 677 participants with CAC=0 on prior visits. During visit 5, a total of 1461 participants with CAC=0 on prior visits received repeat scan and preferentially included participants with scan from visit 3 and 4 (Supplemental Figure 1). Repeat CT scanning in MESA was unrelated to specific individual participant characteristics. All participants with CAC=0 at baseline received at least 1 repeat scan (100%), while 47% had 2 total scans, 42% had 3 total scans, and 11% had 4 total scans over MESA follow-up.
Cardiac CT Protocol and CAC scoring
Baseline cardiac CT was performed at 3 sites using a cardiac-gated electron-beam CT scanner and at 3 sites using 4-slice multi-detector CT. Each participant was scanned twice consecutively, and the images were interpreted at the MESA CT reading center at Harbor-UCLA Medical center, Los Angeles, California. The results of the 2 scans were averaged to provide a more accurate point estimate of the amount of calcium present. Carr et al12 have reported details of the methods used by MESA for CT scanning and interpretation. The amount of calcium was quantified with the Agatston scoring method13. When CAC was detected on CT images, its location was ascertained to left main (LM), left anterior descending (LAD), left circumflex (LCX) or right coronary arteries (RCA). Data regarding segmental distribution of CAC within individual coronary arteries was not available for our analysis. The kappa statistic for agreement on presence of any CAC was 0.92.
Risk Factor Assessment
As part of the baseline examination, study teams at each of the six centers collected information on cardiovascular risk factors. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglyceride, and glucose levels were measured in blood samples obtained after a 12-hour fast. The low-density lipoprotein cholesterol (LDL-C) level was determined with the Friedewald equation14. Hypertension (HTN) status was classified according to the Seventh Report of the Joint National Committee on the Detection, Evaluation, and Treatment of High Blood Pressure15. Diabetes mellitus (DM) status was classified according to American Diabetes Association (ADA) 2003 criteria16. Obesity (BMI ≥ 30 kg/m2) was classified according to World Health Organization (WHO) classification. Medication use was determined by questionnaire. Smoking status was classified as never smoker, former smoker and current smoker. Never smoker was defined as lifetime consumption of less than 100 cigarettes and current smoker was defined as smoking within 30 days as per National Cholesterol Education Program – Adult Treatment Panel III (NCEP-ATP III)17.
Statistical Analysis
Baseline characteristics of the overall study population (N=3112) are presented in aggregate and by incident CAC status (follow-up CAC=0 vs. follow-up CAC>0). Continuous variables are presented as mean +/− standard deviation while categorical variables are presented as total number and the proportion of the total. Differences between two groups were compared using chi-square analysis for categorical variables, and using two sample t-tests for normally distributed continuous variables. Kruskal-Wallis equality of population rank tests were used to compare distributions between groups of non-normally distributed continuous variables.
The remainder of the analyses focused on patients with newly detected CAC during follow-up (N=1125). To characterize the anatomic distribution of new-onset CAC over time in MESA, we described new onset CAC as occurring in a single vessel (LM, RCA, LAD, LCX) or in multiple vessels (2, 3, 4 vessels). We then calculated the adjusted marginal probabilities of each CAC distribution pattern using the “margins” command in STATA. Probabilities were post-estimated following a multivariable logistic regression model adjusted for the following variables: age, race, gender, time-to-CAC>0 detection, hypertension status, household income (a measure of socio-economic status), smoking status, diabetes status, LDL-C>160 mg/dl, use of lipid lowering medications, and obesity (BMI >30 kg/m2). Time-to-CAC>0 detection was included in the models to account for the effect of the differential time between scans of individual participants in MESA. Marginal probabilities should not be considered the absolute prevalence of CAC by particular attribute (i.e. race), but rather the residual differences in new onset CAC that remain within that attribute after adjusting for other risk factors.
To characterize the burden of newly detected CAC over time in MESA, we assigned individuals to distinct CAC score categories (1–10, 11–100, and >100) as well as summarizing the median Agatston score at the first detection of CAC. Using chi-square analysis and Kruskal-Wallis testing, we assessed for differences in CAC burden by anatomic distribution (individual vessel involvement in those with single vessel CAC), as well as for differences in CAC burden between single vessel and multi-vessel CAC. Statistical analysis was performed using STATA 13 (College Station, TX, USA). A two-sided p-value of <0.05 was used to indicate statistical significance.
Results
Baseline Characteristics
Table 1 presents the characteristics of our overall study sample. A total of 3112 subjects were included in the study. Average age was 57.9 ± 9 years and approximately 64% were female. The race/ethnicity distribution of our sample includes 34% White, 12% Chinese-American, 31% Black, and 23% Hispanic.
Table 1.
Demographics and Prevalence of Risk Factors
| Baseline CAC=0 (N=3112) | Follow up CAC=0 (N=1987) | Follow up CAC>0 (N=1125) | P-value | |
|---|---|---|---|---|
| Age, years | 57.9 ± 9.1 | 56.9 ± 9.0 | 59.6 ± 8.9 | 0.0001 |
|
| ||||
| Female | 1960, 64 % | 1315, 66% | 645, 57% | <0.0001 |
|
| ||||
| Race | 0.022 | |||
| • White | 1059, 34% | 654, 33% | 405, 36% | |
| • Chinese | 364, 12% | 256, 13% | 108, 10% | |
| • Black | 963, 31% | 624, 31% | 339, 30% | |
| • Hispanic | 726, 23% | 453, 23% | 273, 24% | |
|
| ||||
| Smoking status | 0.042 | |||
| • Never Smoker | 1740, 56% | 1142, 58% | 598, 53% | |
| • Former Smoker | 960, 31% | 584, 29% | 376, 34% | |
| • Current Smoker | 401, 13% | 252, 13% | 149, 13% | |
|
| ||||
| BMI, kg/m2 | 28.3 ± 5.6 | 27.8 ± 5.6 | 29.1 ± 5.7 | <0.001 |
|
| ||||
| Hypertension | 1077, 35% | 587, 30% | 490, 44% | <0.001 |
|
| ||||
| Diabetes | 274, 9% | 138, 7% | 136, 12% | <0.001 |
|
| ||||
| Systolic Blood Pressure, mm Hg | 124.1 ± 19.8 | 121.7 ± 19.5 | 128.4 ± 19.7 | 0.0001 |
|
| ||||
| Diastolic Blood Pressure, mm Hg | 72.5 ± 10 | 71.5 ± 10 | 74.3 ± 9.7 | <0.0001 |
|
| ||||
| Cholesterol, mg/dl | 198.1 ± 35.1 | 195.7 ± 34.6 | 202.3 ± 35.6 | 0.0001 |
|
| ||||
| HDL-C, mg/dl | 52.6 ± 15 | 53.8 ± 15.4 | 50.4 ± 14 | 0.0001 |
|
| ||||
| LDL-C, mg/dl | 116.3 ± 30.7 | 114.6 ± 30.6 | 119.3 ± 30.6 | 0.0001 |
|
| ||||
| Anti-hypertensive Medication | 876, 28% | 478, 24% | 398, 35% | <0.001 |
|
| ||||
| Lipid-lowering Medication | 330, 11% | 163, 8% | 167, 15% | <0.001 |
|
| ||||
| Framingham Risk Score (%) | 8 ± 6 | 7 ± 6 | 10 ± 7 | 0.0001 |
|
| ||||
| CVD Risk Score (%) | 7 ± 9 | 6 ± 8 | 9 ± 10 | 0.0001 |
|
| ||||
| Aspirin Usage | 421, 14% | 221, 12% | 200, 19% | <0.001 |
|
| ||||
| Number of Follow-up CAC Scans | 1.78 ± 0.68 | 1.65 ± 0.69 | 2.01 ± 0.60 | <0.001 |
N= numbers of subjects, HDL-C= High-density lipoprotein cholesterol, LDL-C= low-density lipoprotein cholesterol, BMI= body mass index, CVD= Cardiovascular disease.
Approximately 36 % (1125 subjects) of our study sample developed new CAC with a mean time-to-detection of 6.1 ± 3.4 years. The percentage of subjects who developed new CAC at visit 2, 3, 4 and 5 were 11%, 21%, 22% and 34% respectively. The mean time to detection of new CAC was shorter in males (5.9 ± 3.4) compared to females (6.3 ± 3.4) and did not differ among subjects from different race/ethnicity groups.
New CAC was more prevalent among males and the mean age of these subjects was higher when compared to subjects who did not develop CAC on follow up scans. There was higher prevalence of hypertension, diabetes mellitus and smoking among subjects with new CAC compared to those with CAC=0. Subjects with new CAC demonstrated significantly higher systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), cholesterol, LDL-C, Framingham risk score, Cardiovascular disease risk score and lower HDL-C compared to CAC=0 group. Baseline use of lipid lowering and anti hypertensive medications was significantly higher in subjects with new CAC. Participants with new CAC also had more number of follow-up CAC Scans compared to CAC=0 group (Table 1).
Coronary Distribution of New CAC
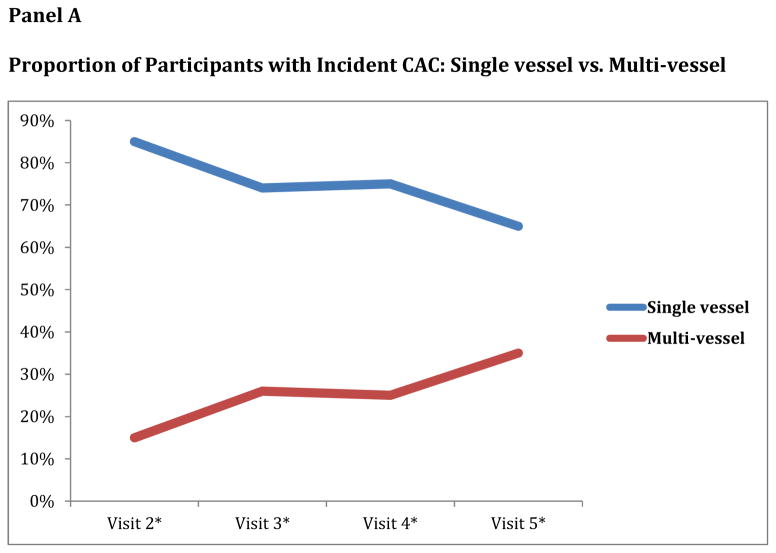
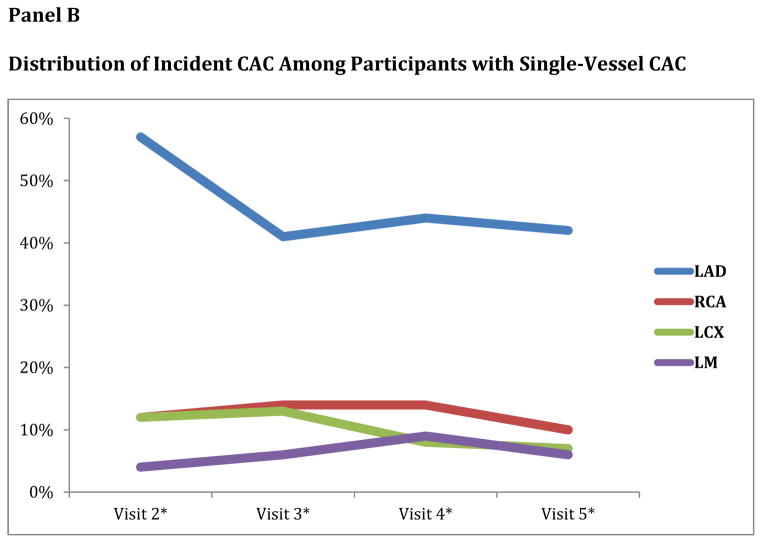
New CAC most commonly involved one vessel (unadjusted probability of 72%). Over the entire range of time from the baseline scan, one vessel involvement remained most common presentation of new CAC (Figure 1, Panel A). Among subjects with one vessel involvement, the LAD was the most common vessel affected and this pattern continued over the entire range of time from baseline scans. LAD involvement was followed in order of frequency by the RCA, LCX and LM coronary arteries (Table 2) (Figure 1, Panel B).
Figure 1.
Panel A. Proportion of Participants with Incident CAC: Single vessel vs. Multi-vessel
*Visit 2, 3, 4 and 5 were at mean 1.7 ± 0.3, 3.2 ± 0.4, 4.9 ± 0.5 and 9.7 ± 0.6 years respectively following baseline scan.
Panel B. Distribution of Incident CAC Among Participants with Single-Vessel CAC
*Visit 2, 3, 4 and 5 were at mean 1.7 ± 0.3, 3.2 ± 0.4, 4.9 ± 0.5 and 9.7 ± 0.6 years respectively following baseline scan.
Table 2.
CAC distribution at the first detection of CAC>0
| Distribution of New Onset CAC | All visits (N=1125) | Visit 2* (N=170) | Visit 3* (N=305) | Visit 4* (N=150) | Visit 5* (N=500) |
|---|---|---|---|---|---|
| Single Vessel | 72% (807) | 85% (144) | 74% (226) | 75% (113) | 65% (324) |
| • LAD | 44% | 57% | 41% | 44% | 42% |
| • RCA | 12% | 12% | 14% | 14% | 10% |
| • LCX | 10% | 12% | 13% | 8% | 7% |
| • LM | 6% | 4% | 6% | 9% | 6% |
| Multi-vessel | 28% (318) | 15% (26) | 26% (79) | 25% (37) | 35% (176) |
| • 2 vessel | 20% | 12% | 20% | 18% | 24% |
| • 3 or 4 vessel | 8% | 3% | 6% | 7% | 11% |
Note:
Visit 2, 3, 4 and 5 include subjects who had detectable CAC on a follow up scan at mean 1.7 ± 0.3, 3.2 ± 0.4, 4.9 ± 0.5 and 9.7 ± 0.6 years respectively following baseline scan.
Abbreviations: N= number of subjects, LAD= Left anterior descending artery, RCA= Right coronary artery, LCX= Left circumflex artery, LM= left main artery, CAC= coronary artery calcium.
Multi-vessel involvement of new CAC was seen in 28% of subjects. With increased time from baseline scan, there was increased frequency of multi-vessel involvement of new CAC (15% at visit 2 vs 35% at visit 5). However one vessel involvement of new CAC was the most common pattern even at Visit 5, which occurred nearly 10 years after baseline scan (Table 2) (Figure 1, Panel A).
Association between Risk Factors and Distribution of New CAC
Among all age, gender, and race/ethnicity groups, single vessel CAC with LAD involvement was the most common phenotype. With increase in age there was increased multi-vessel involvement of new CAC, which peaked in the age group of 65–74, however single vessel CAC with LAD involvement remained the most common presentation even in this age group (Table 3). Males compared to females and Hispanic and Black compared to White and Chinese were associated with higher residual probability of multi-vessel new CAC involvement after multi-variable adjustment (Table 3).
Table 3.
Multivariable adjusted residual probability (%) of newly detected CAC distribution patterns by age, gender, and race/ethnicity
| Single vessel
|
Multi-vessel | P-value* | ||||
|---|---|---|---|---|---|---|
| LAD | RCA | LCX | LM | |||
| Age | ||||||
| 45–54 | 48 | 14 | 9 | 5 | 24 | Reference |
| 55–64 | 45 | 14 | 7 | 5 | 29 | 0.11 |
| 65–74 | 39 | 9 | 10 | 8 | 34 | 0.01 |
| 75–84 | 53 | 6 | 13 | 11 | 17 | 0.29 |
| Gender | ||||||
| Female | 49 | 11 | 9 | 6 | 25 | Reference |
| Male | 41 | 13 | 8 | 7 | 31 | 0.049 |
| Race | ||||||
| White | 50 | 11 | 8 | 7 | 24 | Reference |
| Chinese | 54 | 10 | 7 | 5 | 24 | 0.99 |
| Black | 41 | 13 | 9 | 6 | 31 | 0.05 |
| Hispanic | 39 | 12 | 11 | 5 | 33 | 0.02 |
P-value is for single vessel vs multi-vessel involvement of CAC. Refer to table 1 for sample size of each category.
Predicted probabilities adjusted for: Age categories, Race categories, Gender, Time-to-CAC>0 detection, Hypertension, Household income, Smoking status, use of any lipid lowering medications, Diabetes, LDL-C >160, and obesity (BMI>30).
Abbreviations: LAD= Left anterior descending artery, RCA= Right coronary artery, LCX= Left circumflex artery, LM= left main artery.
The residual probability of multi-vessel new CAC involvement was significantly higher in subjects with hypertension, diabetes mellitus, and obesity after adjustment for the remaining risk variables. In current smokers and in subjects with LDL-C ≥ 160 mg/dL and total cholesterol/HDL-C ratio>3, there was no increased residual probability of multi-vessel new CAC involvement (Table 4).
Table 4.
Multivariable adjusted residual probability (%) of newly detected CAC distribution patterns by individual risk factors
| Risk factor | Single vessel
|
Multi-vessel | P-value* | |||
|---|---|---|---|---|---|---|
| LAD | RCA | LCX | LM | |||
| No Hypertension | 46 | 13 | 10 | 6 | 25 | Reference |
| Hypertension | 44 | 11 | 7 | 6 | 32 | 0.03 |
| Non Smoker | 45 | 12 | 9 | 6 | 28 | Reference |
| Smoker | 44 | 11 | 10 | 6 | 29 | 0.88 |
| No Diabetes | 47 | 11 | 9 | 6 | 27 | Reference |
| Diabetes | 35 | 16 | 8 | 5 | 36 | 0.048 |
| LDL-C <160 | 45 | 12 | 9 | 6 | 28 | Reference |
| LDL-C≥160 | 46 | 10 | 8 | 8 | 28 | 0.91 |
| TC/HDL-C ratio≤3 | 52 | 9 | 7 | 5 | 27 | Reference |
| TC/HDL-C ratio>3 | 44 | 13 | 9 | 6 | 28 | 0.39 |
| BMI <30 | 49 | 11 | 8 | 7 | 25 | Reference |
| BMI ≥30 | 38 | 13 | 11 | 5 | 33 | 0.02 |
P-value is for single vessel vs multi-vessel involvement of CAC. Refer to table 1 for sample size of each category.
Predicted probabilities adjusted for: Age categories, Race categories, Gender, Time-to-CAC>0 detection, Hypertension, Household income, Smoking status, use of any lipid lowering medications, Diabetes, LDL >160 and obesity (BMI>30).
Abbreviations: LDL-C= low density lipoprotein cholesterol, HDL-C= high-density lipoprotein cholesterol, TC= total cholesterol, BMI= body mass index.
Burden of New CAC
Among subjects with new CAC, 52% had CAC scores 1–10, 44% had CAC scores 11–100 and 4% had CAC scores >100. CAC burden was higher among subjects who developed CAC in visit 4 and 5 compared to those in visit 2 and 3 (supplemental table 1). The burden of new onset CAC was significantly higher in subjects with multi-vessel CAC compared to those with one vessel involvement. Of those subjects with new CAC in a single vessel, 65% had CAC scores 1–10, 34% had CAC scores 11–100 and 1% had CAC scores >100. Of those subjects with multi-vessel involvement at the time of new CAC detection, 20% had CAC scores 1–10, 68% had CAC scores 11–100 and 12% had CAC scores >100 (Table 5).
Table 5.
CAC burden at the first detection of CAC>0
| Vessel Affected | CAC 1-10 | CAC 11-100 | CAC >100 | P-value* | Median CAC Score (IQR) | P-value** |
|---|---|---|---|---|---|---|
| Total population (N=1125) | 52% | 44% | 4% | 9.4 (3.7 – 22.9) | ||
|
| ||||||
| Single vessel (N=807) | 65% | 34% | 1% | 0.01 | 5.9 (2.6 – 14.3) | <0.001 |
| • LAD (N=500) | 61% | 38% | 1% | 6.8 (2.8 – 14.9) | ||
| • RCA (N=133) | 68% | 31% | 1% | 5.6 (2.3 – 12.6) | ||
| • LCX (N=107) | 80% | 20% | 0% | 3.7 (1.9 – 8.8) | ||
| • LM (N=67) | 66% | 34% | 0% | 7.0 (2.8 – 16.8) | ||
|
| ||||||
| Multi-vessel (N=318) | 20% | 68% | 12% | 26.2 (12.6 – 54.7) | ||
|
| ||||||
| Comparison of Single vessel vs. Multi-vessel | <0.001 | <0.001 | ||||
P-value is for comparison of CAC burden among individual coronary arteries (LAD vs RCA vs LCX vs LM) as well as between single vessel vs multi-vessel involvement.
P-value is for comparison of median CAC among individual coronary arteries (LAD vs RCA vs LCX vs LM) as well as between single vessel vs multi-vessel involvement.
Abbreviations: N= number of subjects, LAD= Left anterior descending artery, RCA= Right coronary artery, LCX= Left circumflex artery, LM= left main artery, CAC= coronary artery calcium, IQR= Interquartile range.
The median CAC score in subjects with single vessel and multi-vessel involvement was 5.9 and 26.2 respectively. Among subjects with one vessel involvement, CAC was significantly higher in LAD and LM followed by RCA and then LCX (Table 5). There was no difference in CAC burden by age, gender, and race/ethnicity groups. Subjects with new-onset advanced CAC (CAC>100) were equally distributed among all age, gender, and race/ethnicity groups (supplemental table 2).
Discussion
While CAC=0 is associated with excellent prognosis, presence of even mild CAC is associated with an increased risk of adverse events, and little is known about the transition from CAC=0 to initially detectable CAC>0. In MESA, we found that new CAC was most commonly found in a single vessel at first detection, with this pattern persisting among subjects of all age, gender, and race/ethnicity groups. Among the individual coronary arteries, the LAD was the most likely location for new CAC. Multi-vessel involvement of new CAC was more likely among males, Hispanics, Blacks, and in subjects with hypertension, diabetes mellitus and obesity. At the first detection of new CAC, the CAC burden was low with <5% of subjects having a CAC score of greater than 100. This is the first community-based study to describe the CAC distribution and CAC score burden at the initial detection of new CAC.
Prior studies on distribution of coronary atherosclerosis
The prevalent distribution of atherosclerosis in the coronary arteries has been described in prior cross sectional studies. Pathological studies have shown that the prevalence and burden of atherosclerosis was higher in LAD, followed by RCA and then LCX18, 19. However, the differences have not been dramatic and some studies even observed roughly equal frequency of atherosclerosis in the LAD and the RCA, and lower frequency in the LCX20, 21. Tuzcu et al, using intravascular ultrasound, showed that there was no statistically significant difference in distribution of lesions within different coronary arteries, although there was a trend toward higher prevalence in LAD and lower prevalence within LCX. It has been hypothesized that the preferential development of atherosclerosis in LAD can be due to difference in the flow patterns in various anatomic locations of coronary arteries22. To our knowledge, there are no longitudinal studies describing the geographical incidence of atherosclerosis in the coronary arteries. Using CAC as a marker of atherosclerosis, we showed that incidence of new CAC is higher in LAD compared to RCA and LCX. However, we cannot rule out the possibility that calcium deposition occurs earlier in atherosclerotic lesions in LAD compared to lesions in other coronary arteries (RCA, LCX and LM).
Comparison to prior CAC studies
In our analysis, we observed that traditional coronary heart disease (CHD) risk factors including hypertension, diabetes mellitus, smoking, high BMI, and high LDL levels are independently associated with development of new CAC. Similar results were seen in previous studies23–25. The percentage of subjects who developed new CAC (36%) was higher in our study compared to previous studies24, 25, which is likely due to the older patient population, higher risk factor burden, and longer follow-up of subjects in our study. While a prior study by Min et al had the advantage of yearly CAC scanning, this study did not investigate the distribution of new CAC upon first detection23.
Detection of CAC is an excellent method of assessing atherosclerotic plaque presence, and it is important to note that the amount of calcium correlates with the overall magnitude of atherosclerotic plaque burden26. Previous cross sectional studies have suggested that quantity of CAC was significantly higher in LAD than in RCA and LCX27–29. However, to our knowledge, there are no longitudinal studies defining the origin and demographic distribution of new CAC in coronary arteries. Therefore we have extended prior work by demonstrating that new CAC most commonly involves the LAD across all age, gender, and race/ethnicity groups.
Burden of new onset CAC has been described in some of the studies mentioned above24, 25, 30. In our study, 52% had CAC score of 1–10, 44% had CAC score of 11–100 and 4% had CAC score of >100. Our study has a higher burden of new onset CAC compared to the study by Gopal et al30 (approximately 69% had CAC score of 1–9, 26% had CAC score of 10–50 and 5% had CAC score >50) and the burden is even less in the study by Koulaouzidis et al25 (approximately 84% had CAC score of 1–9, 14% had CAC score of 10–50 and 2% had CAC score >50). Our study has an older patient population and higher traditional risk factor burden compared to prior studies, perhaps also explaining our observed burden of new onset CAC. In addition, lack of annual scanning resulting in increased lag period between the scans can be a contributing factor for the higher CAC burden at first detection in our study.
Cross-sectional burden of CAC among different age, gender, and race/ethnicity groups has been described in previous studies31–34. However, no studies have investigated the demographic variation of newly detected CAC. A striking finding from our study is that there is no difference in the burden of new CAC by age, gender, and race/ethnicity group.
Clinical Implications
To our knowledge, there are no formal guidelines on repeat CAC testing for routine quantification of CAC progression35, however prior data suggests that a repeat scan in 4–5 years appears reasonable in individuals with a baseline score of CAC=024, 25. We have shown that at the first detection of CAC>0, the burden is usually low (less than 100). This suggests that people rarely convert from CAC=0 to high CAC scores, which is important because high scores are associated with the increased event rates36. Therefore, repeat scans to identity subjects with CAC>0 may allow detection while scores are still low and with adequate time to initiate aggressive preventive therapies. Whether this strategy can reduce cardiac events warrants further study. Our data also have implications for readers of cardiac CT scans, allowing readers to be familiar with the most common patterns of new onset CAC.
Limitations
The principal limitation of this study is that annual CAC scanning was not performed. The timing of CAC scans were pre-specified per the MESA protocol, which did not call for every participant to have scans at each follow-up visit. Therefore, it is impossible to know when exactly new CAC was developed, and our data should be considered time-to-first-CAC-detection rather than strict time-to-CAC-incidence. However, the patterns and implications we have described should still hold, as lack of annual scanning would result in overestimation of the incidence of multi-vessel CAC and of CAC score burden at the first CAC detection.
In addition, we did not model change in risk factor or change in medication use over the course of the study. It is possible that changing behaviors or therapies may have had an impact on new-onset CAC. As the Agatston method was used to quantify CAC in our study, there is a possibility that certain lesions, especially those with low-density calcium and small areas of calcium (<1 mm2) can be missed which could influence our data. In our study, we do not have data regarding CAC distribution in different segments with in individual coronary arteries. CAC analysis between coronary artery segments of similar length can provide better picture of the segments with high chances of developing new CAC. Finally, although we demonstrated the coronary distribution and burden of new CAC, we did not study their prognostic implications. Papers on the prognostic significance of coronary distribution in MESA are forthcoming37.
Conclusion
Distinguished from referenced work by Min et al and Kronmal et al, this is the first study to describe the distribution of new CAC at its first detection. New onset CAC most commonly affects one vessel and occurs in the LAD. The probability of multi-vessel involvement of new CAC is higher in certain high-risk groups. Importantly, CAC scores are usually low at onset of new CAC suggesting adequate time to increase aggressiveness of preventive therapies.
Supplementary Material
Highlights.
New onset CAC most commonly affects one vessel and occurs in the LAD.
Multi-vessel involvement of new CAC is higher in certain high-risk groups.
New CAC can be detected at an early stage when preventive strategies may provide benefit.
Acknowledgments
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. In addition, this publication was developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the U.S Environmental protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations
- CAC
Coronary artery calcium
- LAD
Left anterior descending artery
- RCA
Right coronary artery
- LM
Left main artery
- LCX
Left circumflex artery
- MESA
Multi-Ethnic Study of Atherosclerosis
- BMI
Body mass index
- LDL-C
Low density lipoprotein-cholesterol
- HDL-C
High density lipoprotein cholesterol
Footnotes
Conflicts of interest:
Atherotech Diagnostics Lab, Scientific Advisory Board, Atherotech Diagnostics Lab, Grant support (in-kind, diagnostic testing), Sanofi - Regeneron, consulting for Dr. Steven Jones. Other authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nasir K, Clouse M. Role of nonenhanced multidetector ct coronary artery calcium testing in asymptomatic and symptomatic individuals. Radiology. 2012;264:637–649. doi: 10.1148/radiol.12110810. [DOI] [PubMed] [Google Scholar]
- 2.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 3.Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: A bayesian approach. Circ Cardiovasc Qual Outcomes. 2011;4:253–256. doi: 10.1161/CIRCOUTCOMES.110.958496. [DOI] [PubMed] [Google Scholar]
- 4.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 5.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Shareghi S, Ahmadi N, Young E, Gopal A, Liu ST, Budoff MJ. Prognostic significance of zero coronary calcium scores on cardiac computed tomography. J Cardiovasc Comput Tomogr. 2007;1:155–159. doi: 10.1016/j.jcct.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JA, Blumenthal RS. Cardiovascular events with absent or minimal coronary calcification: The multi-ethnic study of atherosclerosis (mesa) Am Heart J. 2009;158:554–561. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: Mesa (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaha MJ, Silverman MG, Budoff MJ. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: Clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ Cardiovasc Imaging. 2014;7:398–408. doi: 10.1161/CIRCIMAGING.113.000341. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac ct in populationbased studies: Standardized protocol of multi-ethnic study of atherosclerosis (mesa) and coronary artery risk development in young adults (cardia) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 16.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 17.Berlin I, Lin S, Lima JA, Bertoni AG. Smoking status and metabolic syndrome in the multi-ethnic study of atherosclerosis. A cross-sectional study. Tobacco induced diseases. 2012;10:9. doi: 10.1186/1617-9625-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tejada C, Strong JP, Montenegro MR, Restrepo C, Solberg LA. Distribution of coronary and aortic atherosclerosis by geographic location, race, and sex. Lab Invest. 1968;18:509–526. [PubMed] [Google Scholar]
- 19.Crawford T, Dexter D, Teare RD. Coronary-artery pathology in sudden death from myocardial ischaemia. A comparison by age-groups. Lancet. 1961;1:181–185. doi: 10.1016/s0140-6736(61)91363-0. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez FL, Robbins SL, Banasiewicz M. Postmortem angiographic studies on the coronary arterial circulation: Incidence and topography of occlusive coronary lesions; relation to anatomic pattern of large coronary arteries. Am Heart J. 1964;68:490–499. doi: 10.1016/0002-8703(64)90150-4. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Zoll PM, Blumgart HL, Freiman DG. Location of coronary arterial occlusions and their relation to the arterial pattern. Circulation. 1963;28:35–41. doi: 10.1161/01.cir.28.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 23.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: Results from the multi-ethnic study of atherosclerosis (mesa) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 24.Min JK, Lin FY, Gidseg DS, Weinsaft JW, Berman DS, Shaw LJ, Rozanski A, Callister TQ. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: What is the “warranty period” for remaining normal? Journal of the American College of Cardiology. 2010;55:1110–1117. doi: 10.1016/j.jacc.2009.08.088. [DOI] [PubMed] [Google Scholar]
- 25.Koulaouzidis G, Charisopoulou D, Maffrett S, Tighe M, Jenkins PJ, McArthur T. Coronary artery calcification progression in asymptomatic individuals with initial score of zero. Angiology. 2013;64:494–497. doi: 10.1177/0003319712459213. [DOI] [PubMed] [Google Scholar]
- 26.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. Journal of the American College of Cardiology. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 27.Maher JE, Raz JA, Bielak LF, Sheedy PF, 2nd, Schwartz RS, Peyser PA. Potential of quantity of coronary artery calcification to identify new risk factors for asymptomatic atherosclerosis. Am J Epidemiol. 1996;144:943–953. doi: 10.1093/oxfordjournals.aje.a008864. [DOI] [PubMed] [Google Scholar]
- 28.Kajinami K, Seki H, Takekoshi N, Mabuchi H. Coronary calcification and coronary atherosclerosis: Site by site comparative morphologic study of electron beam computed tomography and coronary angiography. J Am Coll Cardiol. 1997;29:1549–1556. doi: 10.1016/s0735-1097(97)00090-9. [DOI] [PubMed] [Google Scholar]
- 29.Lubanski MS, Vanhecke TE, Chinnaiyan KM, Franklin BA, McCullough PA. Subclinical coronary atherosclerosis identified by coronary computed tomographic angiography in asymptomatic morbidly obese patients. Heart Int. 2010;5:e15. doi: 10.4081/hi.2010.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gopal A, Nasir K, Liu ST, Flores FR, Chen L, Budoff MJ. Coronary calcium progression rates with a zero initial score by electron beam tomography. Int J Cardiol. 2007;117:227–231. doi: 10.1016/j.ijcard.2006.04.081. [DOI] [PubMed] [Google Scholar]
- 31.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: Results from the multi-ethnic study of atherosclerosis (mesa) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 32.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: The multi-ethnic study of atherosclerosis (mesa) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 33.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Naydeck BL, Whittle J, Sutton-Tyrrell K, Edmundowicz D, Kuller LH. Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol. 2002;22:424–430. doi: 10.1161/hq0302.105357. [DOI] [PubMed] [Google Scholar]
- 35.McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: An important clinical measurement? A review of published reports. Journal of the American College of Cardiology. 2010;56:1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 36.Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, Ouyang P, Jones SR, Blumenthal RS, Budoff MJ, Nasir K. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: Implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman MG, Harkness JR, Blankstein R, Budoff MJ, Agatston AS, Carr JJ, Lima JA, Blumenthal RS, Nasir K, Blaha MJ. Baseline subclinical atherosclerosis burden and distribution are associated with frequency and mode of future coronary revascularization: Multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2014;7:476–486. doi: 10.1016/j.jcmg.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.