Abstract
Background
Hypovolemic shock reduces oxygen delivery and compromises energy dependent cell volume control. Consequent cell swelling compromises microcirculatory flow, which reducing oxygen exchange further. The importance of this mechanism is highlighted by the effectiveness of cell impermeants in low volume resuscitation (LVR) solutions in acute studies. The objective of this study was to assess impermeants in survival models and compare them to commonly used crystalloid solutions.
Methods
Adult rats were hemorrhaged to a pressure of 30–35 mm Hg, held there until the plasma lactate reached 10 mM, and given an LVR solution (5–10% blood volume) with saline alone (control), saline with various concentrations of Polyethylene glycol-20k (PEG-20k), hextend or albumin. When lactate again reached 10 mM following LVR, full resuscitation was started with crystalloid and red cells. Rats were either euthanized (acute) or allowed to recover (survival). The LVR time, which is the time from the start of the LVR solution until the start of full resuscitation was measured as was survival and diagnostic labs. In some studies, the capillary oncotic reflection coefficient was determined for PEG-20k to determine its relative impermeant and oncotic effects.
Results
PEG-20k (10%) significantly increased LVR times relative to saline (8 fold), hextend, and albumin. Lower amounts of PEG-20k (5%) were also effective but less so than 10% doses. PEG-20k maintained normal arterial pressure during the low volume state. Survival of a 180 minute LVR time challenge was 0% in saline controls and 100% in rats given PEG-20k as the LVR solution. Surviving rats had normal labs 24 hours later. PEG-20k had an oncotic reflection coefficient of 0.65, which indicates that the molecule is a hybrid cell impermeant with significant oncotic properties.
Conclusions
PEG-20k based LVR solutions are highly effective for inducing tolerance to the low volume state and for improving survival.
Keywords: Cell Swelling, Shock, Polyethylene Glycol
Background
Deaths due to injury in the US reached over 190,000 and costs over $400 billion a year in health care costs and lost productivity in 20121. Deaths from trauma are the number 1 cause of death for people under 44 years of age in the US and the third leading cause of death overall for all age groups. Trauma accounts for about 30% of all life years lost in the US, compared to cancer (16%), heart disease (12%), and HIV (2%)2. For all traumatic injuries, hemorrhagic shock is responsible for over 35% of pre-hospital deaths and over 40% of all deaths within the first 24 hours. This is second only to trauma deaths induced by severe CNS injury3. Finally, hemorrhagic hypotension exposes the patient to immediate complications of life threatening infections, coagulopathies, and multiple organ failure4, 5.
Early resuscitation strategies include the use of low volumes of intravenous blood products to increase oxygen delivery and to replace lost coagulation and clotting factors (coagulation proteins and platelets). While this approach is fine for hospital emergency departments, it is not currently practical in pre-hospital settings where early intervention may be the key to preventing future complications following more definitive resuscitation. Crystalloids are available for pre-hospital use because they can be safely transported and stored but they are generally limited in their effectiveness. Attempts to modify basic intravenous crystalloids for pre-hospital resuscitation by adding hypertonic NaCl or starch (Hextend) as a volume expander have had disappointing results6, 7. The future use of effective spray dried blood products will be a valuable tool in pre-hospital settings since they replace chemical coagulation precursors and factors. The use of fresh frozen plasma in the field, which is currently being tested at many centers, will also be useful but it too is limited by the need for refrigeration8. There remains a need for a better crystalloid to resuscitate patients with severe hemorrhagic shock, especially in a pre-hospital setting. The successful design of such a solution is highly dependent on understanding the pathophysiological mechanisms that lead to injury during hemorrhagic hypotension and subsequent resuscitation. The optimal solution will likely be an effective new stable crystalloid that targets these mechanisms used together with reconstituted dried plasma products for the replacement and reconstitution of coagulation potential.
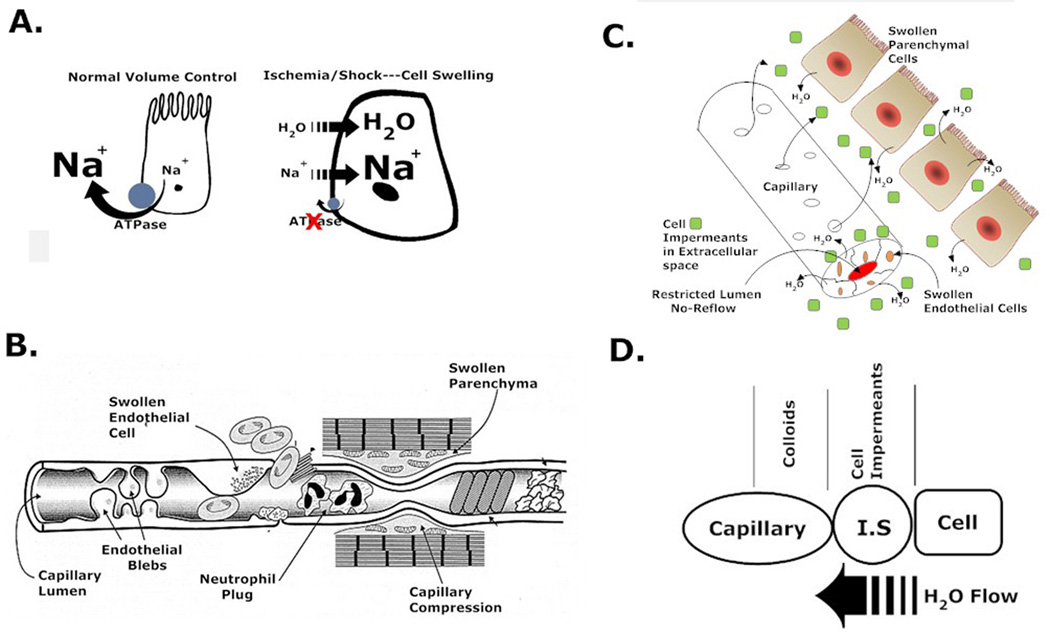
The predominant root mechanism of injury in hemorrhagic shock is energy failure. While global ischemia and reperfusion injury are causally based at many levels, they all arise from changes that occur when the cell energetics drops because of a loss of adequate microvascular oxygen transport and subsequent loss of aerobically produced high energy adenine nucleotides9–11. One mechanism of cell, tissue, and organ injury is cell swelling that occurs from the loss of ATP-dependent cell volume regulatory control mechanisms. In most cells, the single highest energy consuming process is the running of the Na/K ATPase pumps in the cell membrane. These pumps actively transport sodium ions out of the cell to maintain membrane potentials and to run numerous Na+-dependent facilitated membrane transport processes such as calcium, glucose, amino acids, and organic cation transporters. In the absence of ATP to run those pumps, as occurs in ischemia following hemorrhagic shock, the Na/K ATPase turns off and sodium enters the cell as it runs back down its electrochemical gradient. The elevated intracellular sodium futilely stimulates the sodium pump that can’t run because of loss of ATP12. Chloride then enters the cell down an electrical gradient and water follows the sodium chloride down a developing osmotic gradient, which causes the cell to swell. Hydropic degeneration from energy failure damages membrane and mitochondrial structures13, which may lead to cell death. Swelling of parenchymal cells can also compress local capillaries leading to further reduced capillary flow and oxygen delivery causing a self-amplifying cycle. Figure 1 shows how this mechanism occurs and how novel cell impermeant molecules can passively reverse this dangerous water flow.
Figure 1.
Proposed mechanism of action of cell impermeants in the non-energetic rebalancing of water movements during low volume shock states. A. The original defect is caused by the energy dependent collapse of the Na/K ATPase activity during shock due to low oxygen delivery and loss of ATP. As the pump fails, Na+ enters the cell followed by water. B. Swollen parenchymal cells compress local capillary networks in the tissue that increase the resistance to capillary blood flow and further impede microcirculatory oxygen delivery. This allows local lactates to rise. C. Loading the interstitial space with cell impermeants like gluconate or raffinose prevents ischemia-induced water movement (swelling) by osmotically holding water in the interstitial space. This prevents capillary compression and preserves local exchange capacity, even under low volume conditions. D. The inclusion of an oncotic molecule with an impermeant establishes an osmotic-oncotic gradient between the intracellular-interstitial-capillary compartments, which promotes further the energy independent flow of water from the cell (where it shouldn’t be) into the capillary (where it should be). The movement of capillary water with oncotic agents then increases capillary pressures that promote capillary flow even under low volume states.. The sum effect is to promote effective and efficient capillary transport and oxygen delivery in the low volume state.
This basic mechanism of cell ischemic injury has been well described in organ preservation associated with transplantation14–16. Effective modern organ preservation solutions were developed around this concept and contain high concentrations of cell impermeants17. These are classes of non-toxic molecules, usually saccharides and small organic cations and anions, which are small enough to freely egress the capillary space in the microcirculation but are too large or too charged to cross the cell membrane. As such, they preferentially load into the interstitial space where they create an osmotic force that prevents the movement of water into the cell as the sodium concentrations rise during ischemia. They prevent lethal cell swelling. Cell impermeant, as a class of agents, are one of the most effective components of organ preservation solutions used today18. The University of Wisconsin solution contains high amounts of raffinose, lactobionic acid, sulfate, and phosphate, which all act as cell impermeants to prevent water movement. The Belzer-UW MPS solution uses gluconate and HTK solution uses both high concentrations of histidine and mannitol as impermeants. Water movement in organ preservation is slower than ischemia at normal mammalian temperatures because hypothermia is used to preserve organs, which slows down the process. Since cell swelling during ischemia induced by hemorrhagic hypotension also occurs19 and at a much faster rate than in organ preservation because of the warmer temperatures, it was hypothesized that loading the interstitial space with nontoxic cell impermeants during the low volume period would prevent lethal cell swelling and increase the tolerance of the patient to the low volume state and improve outcomes at resuscitation. In fact, acute studies in rodents with severe hemorrhagic shock indicate that small cell impermeants double the tolerance of animals to the low volume state20. This study further found that one particular molecule, polyethylene glycol-20k (PEG-20k) increased the tolerance to the low volume state 6 fold, compared to saline controls. It was hypothesized that PEG-20k superiority was due to the molecule behaving as hybrid where some escapes the capillary space to act as an impermeant to prevent water movement into the cell and a large portion of the molecule stays behind in the capillary to exert oncotic force that draws the interstitial water into the capillary. This was supported by observations that PEG-20k in LVR solutions also normalizes the arterial blood pressure in the low volume state immediately after administration20. This previous study did not assess the effects of PEG-20k based LVR solutions in a survival model nor did it compare them to standard crystalloid solutions used today. This was the objective of the current study. We hypothesize that PEG-20k added to LVR solutions possesses both impermeant and colloidal properties that greatly improves outcomes in a low volume resuscitation model of severe hemorrhagic shock.
Methods
All animal work was conducted under a protocol approved by the VCU Institutional Animal Care and Use Committee, which is governed by the rules and regulations set forth in the NIH guide and the USDA.
Rodent Shock Model
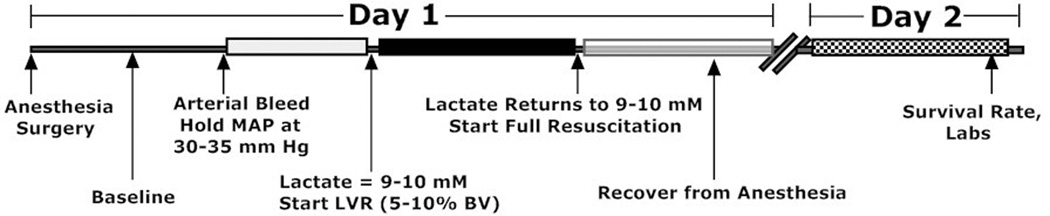
A low volume resuscitation (LVR) model was used in adult rats to test the impermeant based LVR solution used for pre-hospital resuscitation of rats with severe hemorrhagic shock. Adult Sprague Dawley rats were anesthetized with isofluorane and maintained in a light surgical plane of anesthesia during the study. Polyethylene catheters were placed in both femoral arteries for blood pressure monitoring and blood sampling and a catheter was placed in one femoral vein for administration of fluids. The animals were allowed to ventilate on their own to establish normal arterial blood gas (ABG) values. A 1-cm midline incision was created to induce soft tissue injury and for the placement of a temperature probe in the abdomen. The animals were kept at 38 C using a heating pad and an incandescent light source above them. Arterial blood pressure, heart rate, and temperature were continuously recorded using a PowerLab (ADInstruments, Boston, MA). After a 30 min stabilization period, heparin was given (500 U/kg) and arterial blood was slowly removed at 1 ml/min into a syringe to maintain blood pressure at 30–35 mm Hg. This hypotension was maintained until the plasma lactate reached a value between 9–10 mM, as measured every 15 minutes with a hand held lactate analyzer (Lactate Plus, Nova Biomedical, Waltham, MA) and every hour with a blood gas analyzer (Radiometer 800). In preliminary studies, 9–10 mM was the highest plasma lactate level achievable without mortality during the LVR period. Once the target lactate was reached, a low volume resuscitation equal to 5–10% of the calculated blood volume21 of saline was administered I.V. over a 10 min period using a syringe infusion pump. When the blood lactate again reached 9–10 mM, full resuscitation was started, which consisted of a volume of saline equal to the volume of the blood loss (about 55–60% of total blood volume) plus 30% of the removed red blood cells (washed) infused I.V. over 10 minutes. After 1 hour of full resuscitation, the animals were euthanized by an anesthetic overdose and terminal blood was removed for analysis. The time from the start of the LVR period until the start of full resuscitation is called the LVR time and it represents the tolerance of the animal to the low volume state or the maximum amount of time that a shocked subject can safely remain in the low volume state until more definitive resuscitation is required. This was a major outcome used in the study. In some experiments, survival from severe shock was studied with impermeant based LVR and compared to saline controls. In these studies, the animals were held in the low volume state for 180 min receiving either 10% saline as a control or 10% saline containing 10% PEG-20k impermeant. After 180 min, the animals were given full resuscitation and were awaken from anesthesia after the catheters were removed. These surviving subjects were studied the following day (24 hours) to determine the rate of survival, blood pressure, lactates, base excess, PaO2 (A-a gradient), and other blood lab values. The shock and LVR protocol is illustrated in Figure 2.
Figure 2.
Diagram showing the shock, resuscitation, and recovery protocol used for these studies. The low volume resuscitation (LVR) time is the time from the start of the low volume resuscitation (after the lactate during hemorrhagic shock reaches 9–10 mM) until the time after the LVR infusion when the lactate rises back up to 9–10 mM again. This immediately precedes full resuscitation. The LVR time is a measure of the tolerance to the low volume state and is a function of the microcirculatory effectiveness since it is dependent on the rate of change of the plasma lactate.
Oncotic reflection coefficient
The oncotic reflection coefficient (σd) of PEG-20k in rodent capillaries was determined to characterize the biophysical characteristics of this impermeant in capillary networks. The σd describes the relative convective solvent drag transport of a molecule across capillary pores. This characteristic is diffusion independent and is measured by determining the ratio of a compounds lymph concentration to the plasma concentration at high lymph flow rates. In these studies, rats were anesthetized as before and a PE10 cannula was introduced into the thoracic duct as previously described22 to direct the lymphatic flow into a collection tube. Heavy cream (5-ml) was injected into the stomach to help visualize the duct after the lipid was absorbed. A saline infusion (I.V.) was started at 0.25 ml/min to accelerate lymph flow. Then, a single bolus injection of 5 mg FITC-labeled PEG-20k (Nanocs, New York, NY) in saline was given. Blood plasma and lymphatics were collected every 10 minutes for an hour. FITC-PEG was then quantitated by direct measurement of the FITC fluor using a fluorescence plate reader (Biotek FL-800) with an excitation wavelength at 485 nM and an emission wavelength at 520 nM. The σd was estimated as 1-L/P of FITC-PEG-20k as previously described23. The values for σd are from 0–1 where 0 means no reflection into the capillary or complete freedom of passage through the capillary pores (impermeant characteristics). A σd of 1.0 means total reflection back into the capillary or complete oncotic properties.
Statistical evaluations of mean values were performed using parametric ANOVA with multiple comparisons corrections using the Dunnett or Bonferroni test for more than 2 groups or an unpaired T-Test for comparison of data with only two values (control and test group). Fisher’s exact test was used for survival testing and A P value of 0.05 was set as a cut-off for statistical significance.
Results
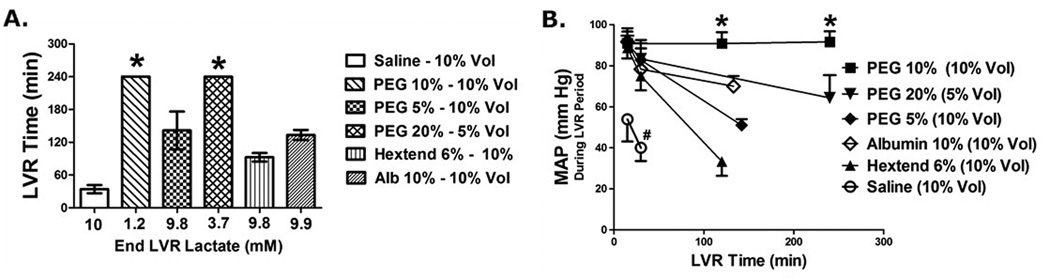
The effects of LVR solutions on the low volume resuscitation time are shown in Figure 3A. The LVR time in this model is an index measuring the tolerance of the individual to the low volume state. It is the length of time that a patient can safely remain in the low volume state until definitive medical care and resuscitation is needed (golden hour), as indexed by the accumulation of a critical level of oxygen debt (lactate). Using normal saline (10% blood volume) as a control base crystalloid, the LVR time was determined to be about 30 min. Specifically, the time from the start of the low volume infusion (triggered when the patient accumulated a lactate of 10 mM) until the time when the patient re-accumulated the same lactate level, was determined to be an average of 30 minutes. This was significantly increased to 240 min (8 fold increase) when the same volume of saline contained 10% PEG-20k. Compared to PEG-20k, the LVR times for traditional resuscitation solutions such as 10% Hextend or 10% albumin were significantly lower. The plasma lactate at the end of the LVR period was close to 10 mM in all groups because this level of oxygen debt triggered the end of the LVR period by definition. However, the plasma lactate in the PEG-20k group at the end of 240 min was only 1.2 mM, which is far lower than the 10 mM trigger. Thus, the LVR time in the PEG-20k group (10%) was arbitrarily cut off and is a significant underestimation of its true value.
Figure 3.
A. Low Volume Resuscitation (LVR) times for rodents in acute studies comparing the effectiveness of LVR solutions containing polyethylene glycol 20,000 (PEG-20k), Hextend, albumin, and saline controls. The numbers on the x-axis are the corresponding lactate concentrations in the plasma at the end of the LVR period. Most are close to 10 mM because that was the definition of the end of the LVR period. All agents were at a concentration of 5–20% by weight and were delivered at a volume equal to 10% or 5% of the calculated blood volume. * P<0.05 relative to all other values, all treated groups are significantly different from saline (control), all values are mean ± SD, n = 6–10 per group. B. Mean Arterial (blood) Pressure (MAP) measured at 15 min, 30 min, and at the end of the low volume resuscitation period for (6) groups of rats treated with various amounts of PEG-20k, Albumin, Hextend, or Saline as the low volume resuscitation solution. * P<0.05 relative to the other corresponding groups, # P<0.05 relative to all other corresponding values, n= 6–10 per group, all values are mean ± SD.
During the low volume state after the LVR solution is administered, mean arterial blood pressure was measured for the duration of the LVR period for a variety of LVR solutions (Figure 3B). Saline solution (10%) resulted in low MAP values over the 30 min LVR time with values below 60 and 40 mmHg at 15 and 30 minutes, respectively. These pressures were improved with Hextend and albumin and completely normalized with PEG-20k (90–100 mm Hg).
Survival studies were conducted in a series of animals to determine the long term survival effects of impermeant based LVR solutions after a severe blood loss (55–60%) and a high accumulation of oxygen debt (lactate 10 mM). These results are shown in the table. All animals were required to undergo a controlled 180 minute LVR time with a 10% LVR solution following the hemorrhagic shock protocol (Figure 2). When saline was used, 0% survived for 24 hours and most died within 30–45 minutes after the LVR solution was administered. By comparison to the control, survival was 100% with the same volume of saline containing 10% PEG-20k. Furthermore, all of the animals that survived 24 hours after full resuscitation had normal blood pressure and arterial blood gas values, both during the 180 min low volume resuscitation period and after 24 hours of recovery. Saline treated animals had very low pressures during the LVR period, they demonstrated aberrant ABG values that were characteristic of severe metabolic acidosis, and they did not report 24 hour values since they all died during the LVR period.
Table 1.
The effects of PEG-20k LVR solution on hemorrhagic shock values during the LVR period and after full resuscitation in survival animals
| During LVR | |||||
| LVR Time (min) | MAP (mm Hg) | Lactate (mM) | HCO3− (mM) | PaO2 (mm Hg) | |
| Saline (10%) | 34 (18) | 49.3 (11) | 9.53 (2.1) | 11.9 (2.1) | 389 (72) |
| PEG-20k (10%) | 180 (0)* | 95.0 (3.5)* | 1.42 (0.6)* | 25.3 (3.4)* | 465 (31) |
| Next Day Survival | |||||
| Survival (%) | MAP (mm Hg) | Lactate (mM) | HCO3− (mM) | PaO2 (mm Hg) | |
| Saline (10%) | 0 (0) | NA | NA | NA | NA |
| PEG-20k (10%) | 100 (0)* | 85.6 (6.2)* | 1.2 (0.1)* | 25.6 (2.6)* | 475 (80)* |
Values are Mean (SD), *P<0.05, Relative to corresponding saline values, n= 5, PaO2 measured with an FiO2 of 0.9.
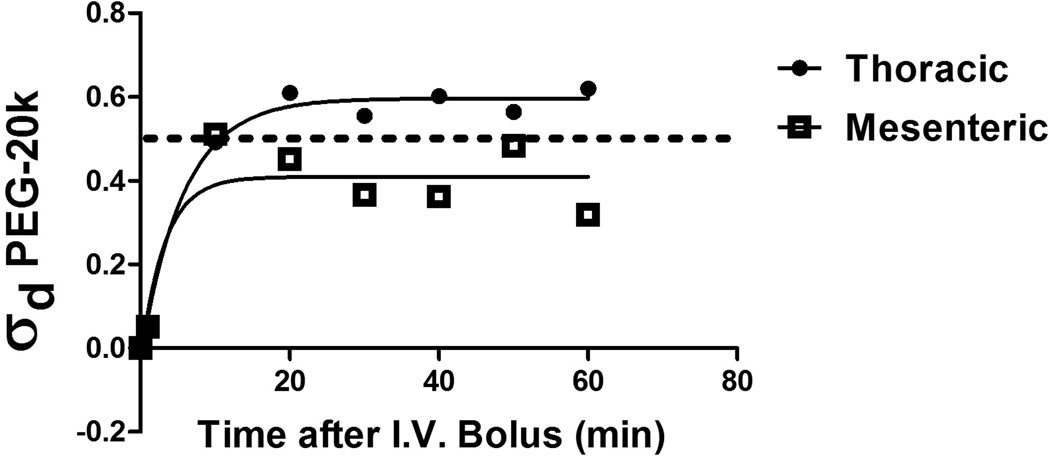
The capillary oncotic reflection coefficient for PEG-20k was measured in rats (Figure 4). The reflection coefficient was determined to be about 0.65, which indicates that some of the fluorescently labeled PEG-20k marker was pushed across the capillary into the interstitial space and lymphatics while much of the label was also clearly detected in the capillary space (plasma). The oncotic reflection coefficients were measured under high lymphatic flow rates by administering an intravenous infusion of saline during the 1 hour study. These conditions unmask the convective solvent drag transport potential of the tracer rather than the diffusional transport characteristics24.
Figure 4.
The oncotic reflection coefficient (σd) for PEG-20k was measured in six rats to determine the impermeant and the oncotic effects of this molecule in both the mesenteric vascular bed and the thoracic bed. FITC-labeled PEG-20k was used as a tracer molecule and the reflection coefficient was determined by measuring the lymph (L) to plasma (P) concentrations of FITC-PEG after an IV injection of the tracer under conditions of high lymph flow induced by volume loading with I.V. saline infusions (0.25 ml/min). Lymph was sampled from a cannula placed into the thoracic duct to drain either the thoraces or the mesentery. FITC-labelled PEG-20k was measured by excitation-emission spectrofluorometry. The oncotic reflection coefficient was calculated as 1-L/P for PEG-20k at high lymph flow rates to make transport across the capillary totally dependent on convective solvent drag transfer and independent of diffusion. A coefficient of 1.0 indicates complete reflection back into the capillary and describes a pure oncotic agent. A reflection coefficient of 0 indicates no reflection at high lymph flow rates and describes a pure impermeant molecule (providing it is impermeant to cell membranes). The actual measured σd for PEG-20k was about 0.60 in the thoracic tissues and 0.40 in the mesenteric tissues, which suggest the molecule is in fact behaving as a hybrid where some escapes into the interstitial space to act as an impermeant and a large amount of the material stays in the capillary where it behaves as an oncotic agent. The lower values in the mesentery is consistent with the known fenestrated “leaky” capillaries in the gut. The hybrid behavior is consistent with its physiological effects on blood pressure and low volume tolerance following shock. This property also explains why PEG-20k is effective by itself without classic impermeants (like gluconate) added with it. The values of σd at each time point represent the average of 3 independent values from 3 animals for each vascular bed.
Discussion
Pre-hospital resuscitation of patients in the field with severe trauma and hypovolemic shock is challenging since the first responders are often forced to work with low volumes of simple crystalloid solutions that are both transportable and stable under field conditions. A recently described advancement in this area uses high concentrations of cell impermeant molecules in saline solutions as low volume resuscitation solutions for pre-hospital management of severely hypovolemic shock patients. In acute studies, these solutions prevent ischemia-induced cell swelling, which alleviates both the harmful effects on cell and mitochondrial membranes and greatly improves microcirculatory capillary flow and exchange by preventing the occlusion of the microcirculation by swollen parenchymal and endothelial cells. This study extends those findings by testing the effects in survival shock models and compares these effects to crystalloid solutions that are considered standard of care in the field today. Finally, this study explores further the unique mechanism of action of PEG-20k, which has been determined to be the most superior impermeant molecule yet tested for low volume resuscitation in severe hypovolemic shock.
Cell impermeants are useful in severe shock because they load the interstitial space with osmotically active molecules that are impermeant to the cell membrane, but freely escape the capillary space. The increased osmotic force generated outside of the cell prevents intracellular water accumulation, cell swelling, and secondary capillary compression (Figure 1). The addition of an oncotic agent to a cell impermeant solution was hypothesized to potentiate the effect of the impermeant alone by establishing a second oncotic gradient between the capillary space and the interstitial compartment, thereby augmenting the translocation of water accumulated in the interstitial space by the impermeants into the capillary space. This non-energetic movement of water into the capillary raises capillary pressure and increases capillary perfusion by both reducing the resistance to capillary flow (by preventing diameter changes from compression) and by increasing the capillary pressure gradient for flow. To our surprise, the addition of the oncotically active impermeant, PEG-20k, to LVR solutions containing simple impermeants like gluconate geometrically potentiated the impermeant effect. The total response of the two components was reproduced by the PEG-20k component alone and much less than a pure oncotic agent alone (albumin). This suggested that PEG-20k may have a hybrid effect where the molecule acts both as a capillary permeable cell impermeant and as a traditional oncotic agent. Therefore, this study focused solely on the PEG-20k molecule.
An LVR solution containing 10% by weight of PEG-20k (given at 10% blood volume) has been shown to be optimal in our shock models. This was the gold standard to compare other solutions to size up their clinical potential. A 10% PEG solution given at 10% of the calculated blood volume (about 500-ml for an adult patient) produced the longest tolerance to the low volume state as compared to 6% Hextend and 10% albumin solutions. Since clinical formulations of albumin are generally about half strength (6% by weight), the values observed in this study probably are over-estimations of the effects observed clinically. Furthermore, the effect of the PEG-20k group has been significantly underestimated in this study since the LVR times were cut off at only 240 minutes, which is lower than the true LVR time because the trigger of 10 mM was never achieved in this group. Had the LVR time been increased until the lactate in the PEG-20k group reached 10 mM, the final LVR time would have been much greater than 240 minutes. Therefore, the true limits of PEG-20k based LVR solutions, regarding tolerance to the low volume state, are not yet known.
In a dose de-escalation trial, we compared 10% PEG-20k given at either a lower volume (5% blood volume) of the same concentration (10%) or a lower volume (5%) at twice the concentration (20%). The lower total PEG-20k dose was less effective while the same dose but given at the lower volume was still very effective. This suggests that even lower volumes of LVR solutions can be achieved down to 5% of calculated blood volume. This is approximately 250-ml for an adult patient and may find use in combat casualty care on the battlefield where carry volumes of intravenous fluids for resuscitation are more of a concern.
The previous trials of impermeant based LVR solutions in shock were acute studies. The effects on survival are an important consideration for possible clinical use. When the LVR time was controlled to 180 minutes, all of the animals resuscitated with 10% PEG-20k as the LVR solution survived 24 hours compared to 0% survival in the saline control group. Furthermore, the surviving rodents were perfectly normal both in terms of physiological lab values and behaviorally. There were no apparent side effects of the PEG-20k LVR solutions except a temporary diuresis immediately after administration of the solution and a temporary metabolic alkalosis. Since it is believed that PEG-20k acts as a hybrid molecule, it is likely that some of the material passes across Bowman’s space in the glomerulus where it acts as an impermeant in the tubules to increase osmotic water clearance and cause a diuresis, similar to a mannitol effect. Furthermore, the increased excretion of water and likely electrolytes too, could prevent hydrogen ion reabsorption and increase renal acid excretion thereby causing a metabolic alkalosis. This is a favorable effect in shocked patients that are experiencing severe metabolic lactacidosis and obviates the requirement of bicarbonate administration to correct acidosis during resuscitation.
The impermeant effect in LVR solutions is greatly augmented when a colloid is also present. Since the putative colloidal agent, PEG-20k, works as well by itself as it does with typical small molecule impermeants like gluconate, it was hypothesized that this size PEG polymer may act as a hybrid and possess both impermeant and colloidal properties. To support this hypothesis, the capillary oncotic reflection coefficient for PEG-20k was measured in rats (Figure 4). The reflection coefficient was determined to be about 0.65, which clearly suggests that some of the material escapes into the interstitial space (impermeant characteristics) while a large portion of the material stays behind in the capillary to act oncotically. This strongly indicates a hybrid nature of PEG-20k, which supports the direct observations of its superior utility alone as an LVR solution and its apparent ability to cross the glomerulus to cause a diuresis. Further studies are needed to characterize the renal handling of PEG-20k but its combined impermeant and colloid effects are now well supported.
There are three major limitations of the study. First, the model was for a controlled hemorrhage event and needs to be evaluated also in an uncontrolled model since many clinical conditions have an uncontrolled component. The current rodent model needs to be translated to a large animal pre-clinical study, and finally, the toxicological effects of PEG-20k need more evaluation, especially any possible platelet and coagulation responses (nothing has been identified to date in survival studies). Human trials are pending an FDA regulatory pathway decision.
In conclusion, PEG-20k used at 10% weight and administered at 10% calculated blood volume during severe hypovolemic shock produces striking salutary benefits. These effects dramatically prolong survival in a controlled hemorrhage model and are likely due to the molecules hybrid impermeant and oncotic properties.
Acknowledgments
Disclosure: This work was supported by grants from the National Institutes of Health R01 DK087737 and The Department of Defense W81XWH-12-1-0599 to Dr. Mangino
Footnotes
Conflict of Interest: There were no conflicts of interest to report
This work was presented, in part, at the 28th annual meeting of the Eastern Association for the Surgery of Trauma, January 13–17, 2015, in Lake Buena Vista, FL
Authorship Statement: Each author contributed significantly to, and is willing to take public responsibility for, one or more aspects of the study: its design, data acquisition, and analysis and interpretation of data.
Contributor Information
Dan Parrish, Email: dparrish@mcvh-vcu.edu.
Valerie Plant, Email: vplant@mcvh-vcu.edu.
Susanne L. Lindell, Email: slindell@mcvh-vcu.edu.
Ashley Limkemann, Email: ajlimkemann@mcvh-vcu.edu.
Heather Reichstetter, Email: hmbass@vcu.edu.
Michel Aboutanos, Email: maboutanos@mcvh-vcu.edu.
Martin J. Mangino, Email: mjmangino@vcu.edu.
REFERENCES
- 1.National Center for Injury Prevention and Control. Web–based Injury Statistics Query and Reporting System (WISQARS) 2013 Ref Type: Online Source.
- 2.Finkelstein EA, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. USA: Oxford University Press; 2006. [Google Scholar]
- 3.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 4.Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–549. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Franklin GA, Boaz PW, Spain DA, Lukan JK, Carrillo EH, Richardson JD. Prehospital hypotension as a valid indicator of trauma team activation. J Trauma. 2000;48:1034–1037. doi: 10.1097/00005373-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Riha GM, Kunio NR, Van PY, Hamilton GJ, Anderson R, Differding JA, Schreiber MA. Hextend and 7.5% hypertonic saline with Dextran are equivalent to Lactated Ringer's in a swine model of initial resuscitation of uncontrolled hemorrhagic shock. J Trauma. 2011;71:1755–1760. doi: 10.1097/TA.0b013e3182367b1c. [DOI] [PubMed] [Google Scholar]
- 7.Riha GM, Kunio NR, Van PY, Kremenevskiy I, Anderson R, Hamilton GJ, Differding JA, Schreiber MA. Uncontrolled hemorrhagic shock results in a hypercoagulable state modulated by initial fluid resuscitation regimens. J Trauma Acute Care Surg. 2013;75:129–134. doi: 10.1097/ta.0b013e3182984a9b. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon's perspective. Hematology Am Soc Hematol Educ Program. 2013;2013:656–659. doi: 10.1182/asheducation-2013.1.656. [DOI] [PubMed] [Google Scholar]
- 9.Chaudry IH, Sayeed MM, Baue AE. Depletion and restoration of tissue ATP in hemorrhagic shock. Arch Surg. 1974;108:208–211. doi: 10.1001/archsurg.1974.01350260062014. [DOI] [PubMed] [Google Scholar]
- 10.Gomez H, Mesquida J, Hermus L, et al. Physiologic responses to severe hemorrhagic shock and the genesis of cardiovascular collapse: can irreversibility be anticipated? J Surg Res. 2012;178:358–369. doi: 10.1016/j.jss.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudry IH. Use of ATP following shock and ischemia. Ann N Y Acad Sci. 1990;603:130–140. doi: 10.1111/j.1749-6632.1990.tb37667.x. [DOI] [PubMed] [Google Scholar]
- 12.Barlet-Bas C, Khadouri C, Marsy S, Doucet A. Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J Biol Chem. 1990;265:7799–7803. [PubMed] [Google Scholar]
- 13.Petit PX, Goubern M, Diolez P, Susin SA, Zamzami N, Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 1998;426:111–116. doi: 10.1016/s0014-5793(98)00318-4. [DOI] [PubMed] [Google Scholar]
- 14.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–247. doi: 10.1146/annurev.med.46.1.235. [DOI] [PubMed] [Google Scholar]
- 15.Southard JH, Beltzer FO. Principles of Organ Preservation Part I. Surgical Rounds. 1993:353–360. [Google Scholar]
- 16.Southard JH, Beltzer FO. Principles of Organ Preservation Part II. Surgical Rounds. 1993:443–448. [Google Scholar]
- 17.Southard JH, Belzer FO. Control of canine kidney cortex slice volume and ion distribution at hypothermia by impermeable anions. Cryobiology. 1980;17:540–548. doi: 10.1016/0011-2240(80)90068-1. [DOI] [PubMed] [Google Scholar]
- 18.Southard JH, van Gulik TM, Ametani MS, Vreugdenhil PK, Lindell SL, Pienaar BL, Belzer FO. Important components of the UW solution. Transplantation. 1990;49:251–257. doi: 10.1097/00007890-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Mees N, Southard JH, Belzer FO. Inhibition of ischemic induced cellular swelling in kidney cortex tissue by lactobionate anions. J Trauma. 1982;22:118–120. doi: 10.1097/00005373-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Parrish D, Lindell S, Reichstetter H, Aboutanos M, Mangino MJ. Cell impermeant based low volume resuscitation in hemorrhagic shock: A biological basis for injury involving cell swelling. Ann. Surg. 2014 doi: 10.1097/SLA.0000000000001049. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora TK, Malhotra AK, Ivatury R, Mangino MJ. L-arginine infusion during resuscitation for hemorrhagic shock: impact and mechanism. J Trauma Acute Care Surg. 2012;72:397–402. doi: 10.1097/ta.0b013e3181d039fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ionac M. One technique, two approaches, and results: thoracic duct cannulation in small laboratory animals. Microsurgery. 2003;23:239–245. doi: 10.1002/micr.10136. [DOI] [PubMed] [Google Scholar]
- 23.Reed RK, Townsley MI, Taylor AE. Estimation of capillary reflection coefficients and unique PS products in dog paw. Am J Physiol. 1989;257:H1037–H1041. doi: 10.1152/ajpheart.1989.257.3.H1037. [DOI] [PubMed] [Google Scholar]
- 24.Mortillaro NA, Granger DN, Kvietys PR, Rutili G, Taylor AE. Effects of histamine and histamine antagonists on intestinal capillary permeability. Am J Physiol. 1981;240:G381–G386. doi: 10.1152/ajpgi.1981.240.5.G381. [DOI] [PubMed] [Google Scholar]