Abstract
Mutations in the gene that encodes α-synuclein (αS) are a known cause of Parkinson's disease. αS is also the major component of pathological inclusions that characterize this disorder and a spectrum of other neurodegenerative diseases termed synucleinopathies. The effects of the most recently identified αS mutation, A53E, on αS aggregation were studied in vitro and in cell culture models. The A53E mutation in αS impedes the formation of aggregated, amyloid protein in vitro compared to wild-type αS. Under certain conditions, A53E αS can still form elongated amyloid fibrils with similar morphology, but with thinner width compared to wild-type αS. Using amyloid seeding of αS in cell culture studies, we demonstrate that significantly less A53E αS could be induced to aggregate compared to wild-type αS, although the mutant protein was still able to form mature inclusions within some cells. Furthermore, expression of A53E αS enhanced toxicity in cells experiencing mitochondrial stress. These findings indicate that the A53E mutation in αS reduces the propensity of αS to aggregate both in vitro and in the cellular environment, and may lead to cellular toxicity through other mechanisms.
Keywords: α-synuclein, Parkinson's disease, SNCA mutation, fibrillization kinetics, inclusion formation
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by resting tremor, rigidity, slowed movement and postural instability. Pathological hallmarks of PD include depigmentation of the substantia nigra indicating death of nigral dopaminergic neurons, and the presence of α-synuclein (αS) containing Lewy bodies [1,2]. The αS protein has been further implicated in the pathogenesis of PD, as missense mutations in, or increased copy number of the gene that encodes αS (SNCA), can cause familial PD [reviewed in 3 plus 4–8]. Although all of the functions of αS have yet to be fully described, its abundance within the presynaptic terminal lends credibility to its proposed role in synaptic transmission [9]. The natively unfolded αS is able to adopt secondary structure for example, it becomes partially α-helical upon binding to lipids [10], which appears to be an important property of the protein. Alternatively it can adopt a β-sheet secondary structure, termed amyloid, which occurs under disease conditions [2, 11]. This amyloid structure is prone to aggregate, forming oligomers and fibrils which ultimately combine to produce Lewy pathology [2].
The exact cause or causes of PD are still under question within this field of research. The sequestration of functional αS into the aggregates could be reducing the amount of available αS for normal cellular function, which may lead to death of the dopaminergic neurons. Alternatively the presence of the large intracellular aggregates could be causing degeneration of the cells or the production of an oligomeric species of αS during polymerization could be toxic to the cells [1,2]. Furthermore, αS interacting proteins may be sequestered into αS aggregates, removing them from the functional pool and potentially causing additional neuronal stress. The presence of αS positive Lewy bodies in the brains of not only SNCA mutation harboring PD patients, but also sporadic PD patients shows that multiple insults can lead to the same neurodegenerative phenotype and study of αS and its mutants may provide valuable clues to its involvement in disease [1,2].
The PD-causing missense mutations in SNCA have been shown to alter fibrillization kinetics of αS. The A53T, E46K and H50Q mutations have been consistently shown to accelerate fibril formation [12–16]. However, we and others reported that the G51D mutation attenuates fibril formation in vitro, which was the first time that this was shown consistently for an SNCA mutation [16,17]. This finding suggests that there is a different disease mechanism for this mutation, compared to the others, which indicates the importance of investigating the effects of each newly identified SNCA mutation.
The novel A53E SNCA mutation was recently identified in a PD family [8]. One report showed that this mutation may attenuate αS fibril formation, similar to G51D [18]. In this study, we extended these findings and investigated the ability of this mutant αS to form inclusions and confer cellular toxicity in cultured cells.
Materials and Methods
Bacterial (pRK172) and mammalian (pcDNA3.1) expression plasmids containing full-length wild-type αS or N-terminally truncated (21-140) αS (pRK172 only) were previously described [19,20]. To generate constructs encoding the A53E mutation, site-directed mutagenesis was performed using primers designed to contain the cytosine to adenosine base change at cDNA position 158 on these constructs and PCR with Pfu Turbo DNA Polymerase AD (Agilent) followed by Dpn I restriction enzyme digestion to degrade the original plasmid. Following transformation in DH10 E. coli, individual clones were screened for the presence of only the desired nucleotide variant by DNA sequencing provided as a service by the Interdisciplinary Center for Biotechnology Research at the University of Florida. Wild-type, 21-140 and A53E recombinant αS proteins were produced by expression in, and purification from BL21 (DE3/RIL) E.coli (Agilent Technologies) as previously described [14,19].
To assemble fibrils, proteins were incubated at 37°C with constant shaking at 1050rpm (Thermomixer; Eppendorf) at 1, 2.5 and 5mg/ml in 100mM sodium acetate pH7.5 or PBS. The amount of amyloid structure and the proportion of insoluble protein in the samples was measured by K114 fluorometric assay and sedimentation analyses respectively, as described in [16], at 1, 2, and 4 days (all concentrations), and also at 8 and 16 days (1mg/ml), in quadruplicate. Coomassie stained gels and Western blots were quantified using Image J software (National Institutes of Health).
Fibrils produced in PBS were adsorbed to 300-mesh carbon-coated copper grids, washed, stained with 1% uranyl acetate and imaged at 100,000x magnification using a Hitachi H7600 transmission electron microscope (Hitachi) for assessment of fibril morphology. Fibril diameters were determined by loading micrograph images into Image-Pro Plus software (Media Cybernetics) and the widths of >1000 fibrils per protein were measured.
Neuro2A cells, maintained in Dulbecco's modified Eagle's medium (Invitrogen)/10% fetal bovine serum/ 100U/ml penicillin/ 100µg/ml streptomycin at 37°C with 5% CO2, were plated into 6 well plates or onto poly-D-lysine coated coverslips (6 well or 24 well). At ~25% confluency, cells were transfected with 0.8μg (6 well) or 0.2μg (24 well) pcDNA3.1 containing wild-type or A53E αS, or pEGFP-CI (Clontech), using lipofectamine 2000 according to the manufacturer's protocol (Invitrogen). Inclusion formation was induced by treating the cells with exogenous 21-140 αS fibrils as previously described [16]. After 40 hours, cells underwent biochemical fractionation, to determine the proportion of αS that became aggregated, and immunofluorescence staining to identify mature αS inclusions as described in [16]. To evaluate the kinetics of inclusion formation, immunofluorescent staining was performed on transfected, fibril treated cells at 24, 36 and 48 hours post-transfection. For double immunofluorescence analyses, the antibodies used were SNL4, which recognizes residues 2-12 of αS [21] and pSer129/81A [22], which detects αS phosphorylated at serine129 and is a marker of pathological αS. The percentage of cells containing αS inclusions (pSer129/81A-positive) relative to the total number of cells overexpressing human αS (SNL4-positive) was determined by analyzing 10 images (20x magnification) per experimental condition.
Cell viabilities were determined using the LIVE/DEAD Fixable Red Dead Cell Stain kit, for 488nm excitation (Life Technologies) as previously described [16], using MPP dihydrochloride hydrate (MPP+; Sigma) to introduce mitochondrial stress.
Graphs were made, and statistical analyses were performed using GraphPad Prism v5.03 software (GraphPad). Unpaired two-tailed t-tests were used to compare K114 values, percentage of insoluble protein and fibril widths of wild-type and A53E αS. One-way analysis of variance with post-hoc Dunnett's multiple comparison test, using wild-type αS as the control, were used to test the difference between the MPP+ treated, EGFP or wild-type or A53E αS transfected Neuro2A cells.
Results
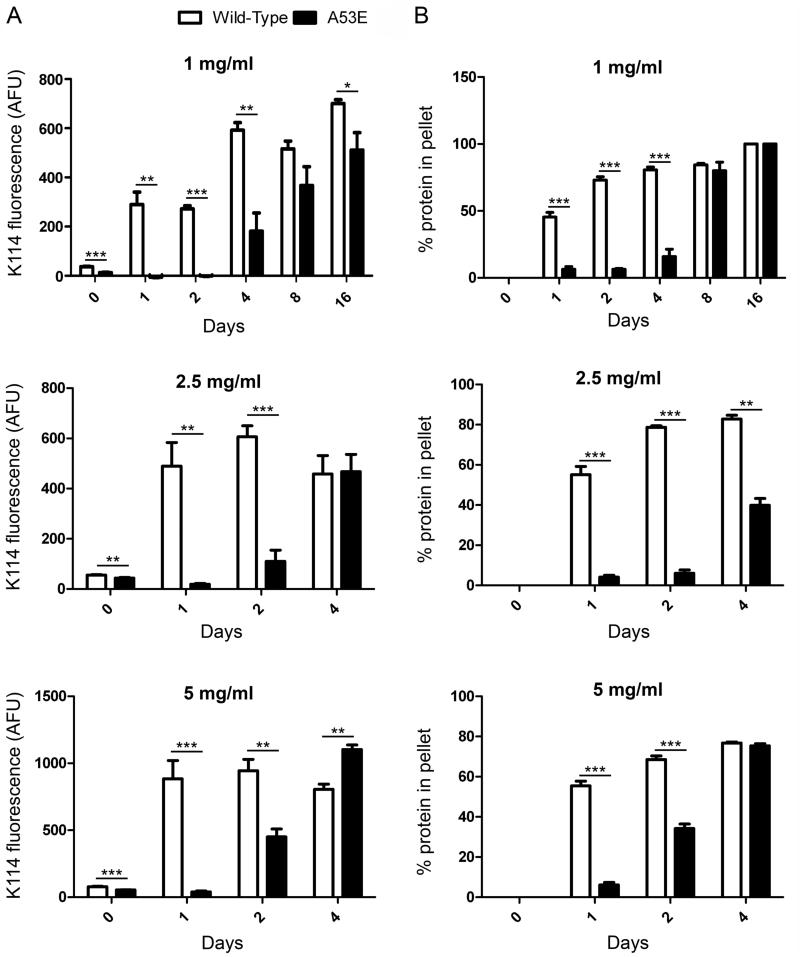
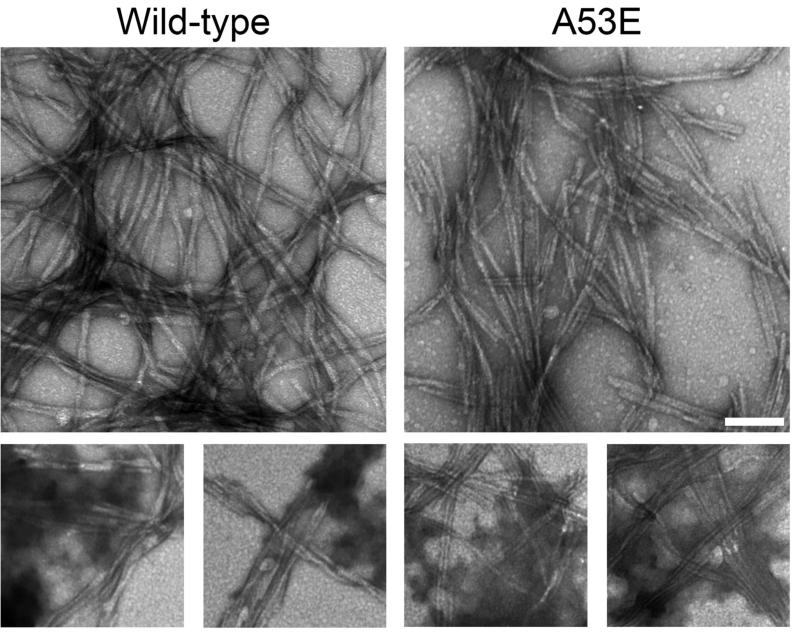
To investigate the effect of the A53E mutation on the rate of αS fibril formation, we performed fibril assembly and tracked the formation of amyloid structure and aggregated protein that could be sedimented over time (Figure 1). The K114 amyloid-detecting assay (Figure 1A) revealed a delay in the formation of amyloid structure of A53E αS at all concentrations. Similarly, formation of sedimented αS was slower for A53E αS compared to wild-type αS (Figure 1B). Therefore, the A53E mutation reduced the propensity of αS to aggregate into amyloid. Examination of the morphology of fibrils produced by the A53E αS protein by negative-staining electron microscopy (Figure 2) revealed similar elongated fibrils to wild-type αS fibrils. The staining of the A53E αS fibrils appeared slightly lighter than for wild-type αS. We also observed diffuse, amorphous material surrounding some of the fibrils in both cases, although this appeared more often for the A53E αS fibrils. Measurement of fibril widths (>1000 fibrils per protein) revealed that A53E αS fibrils (9.22 ± 2.00nm) were slightly, but significantly thinner than wild-type αS fibrils (10.09 ± 1.83nm; t(2061)=10.28, p<0.0001).
Figure 1. Comparison of aggregated amyloid formation by wild-type and A53E αS proteins following in vitro incubation.
K114 fluorometry (A) and sedimentation analysis (B) of wild-type (white) and A53E (black) αS following in vitro incubation at 1, 2.5 and 5 mg/ml for the number of days indicated. Error bars represent SEM. Significant differences were determined by t-tests. AFU; arbitrary fluorescence units, * p<0.05, ** p<0.005, *** p<0.001.
Figure 2. Electron microscopy analysis of fibrils formed by wild-type and A53E αS proteins.
Representative images of uranyl acetate stained fibrils formed by incubating proteins at 1mg/ml for 4 days. The panels on the bottom show diffuse, amorphous staining. Scale bar represents 100 nm.
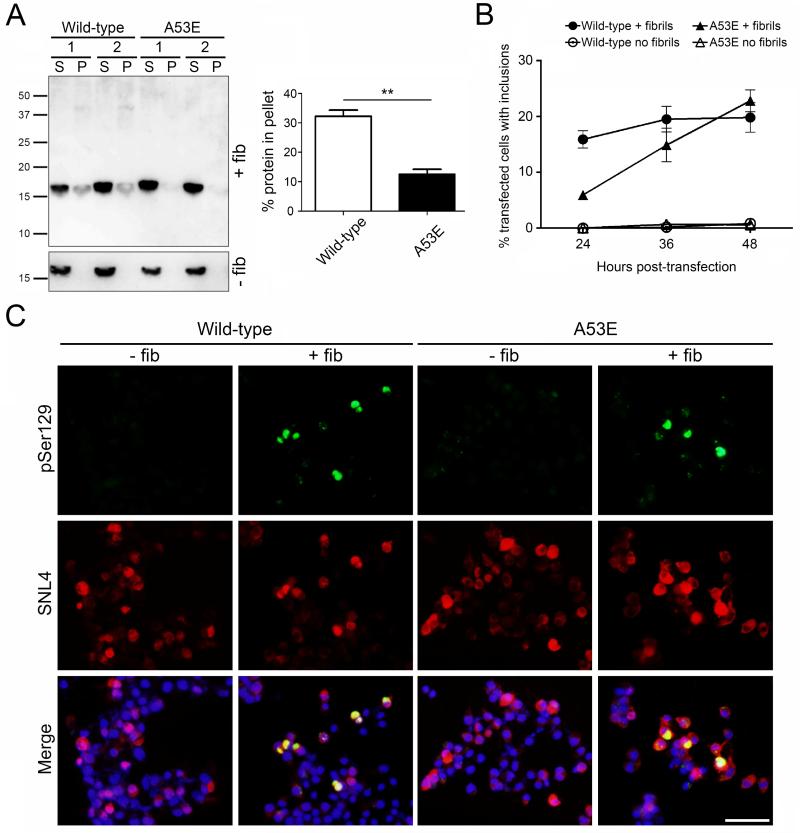
To examine the behavior of the A53E αS in a cellular environment, we transfected Neuro 2A cells with plasmids encoding wild-type or A53E αS and added exogenous 21-140 αS fibrils to induce inclusion formation (Figure 3). The use of 21-140 αS fibrils allows us to track only the expressed protein, and not the exogenous fibrils, through the use of an antibody to the N-terminus of αS (SNL4). Fractionation analysis showed that some A53E αS was induced to aggregate within the cells when 21-140 αS fibrils were added, however this was significantly less than in the wild-type αS transfected cells (Figure 3A; t(4)=7.455, p=0.0017). Double immunofluorescence of the cells using a general αS antibody (SNL4) and an antibody to αS phosphorylated serine129 (a marker of mature αS inclusions) revealed that A53E αS is able to form mature inclusions with morphology similar to those formed by wild-type αS (Figure 3C). Examining the percentage of αS transfected cells which contained pSer129-positive inclusions that filled the cell over a 48 hour time course, we found that cells overexpressing A53E αS formed mature inclusions more slowly than wild-type αS overexpressing cells, when treated with exogenous αS fibrils (Figure 3B). However, the percentage of A53E αS transfected cells with inclusions reached approximately the same number as wild-type αS overexpressing cells at 48 hours post-transfection. The formation of inclusions was not induced in cells overexpressing either protein, when fibrils were not present.
Figure 3. Formation of insoluble inclusions in seeded cultured cells overexpressing wild-type or A53E αS.
(A, left) Western blot probed with anti-N-terminal αS antibody SNL4 showing soluble (supernatant; S) and insoluble (pellet; P) αS extracted from wild-type or A53E αS overexpressing Neuro 2A cells with or without the addition of 21-140 αS fibrils (+/− fib). (A, right) Quantification of western blots showing average percent of αS in the insoluble fraction. N = 3 per group. Error bars represent SEM. Significant difference was determined by t-tests. ** p<0.005. (B) and (C) Quantification of inclusions over time (B) and representative immunofluorescent images at 40 hours post-transfection (C) of cultured Neuro2A cells overexpressing wild-type or A53E αS with or without the addition of 21-140 αS fibrils (+/− fib). Cells were double stained with anti-αS antibodies pSer129/81A (green) and SNL4 (red), and overlaid with DAPI (Merge). Scale bar represents 50μm.
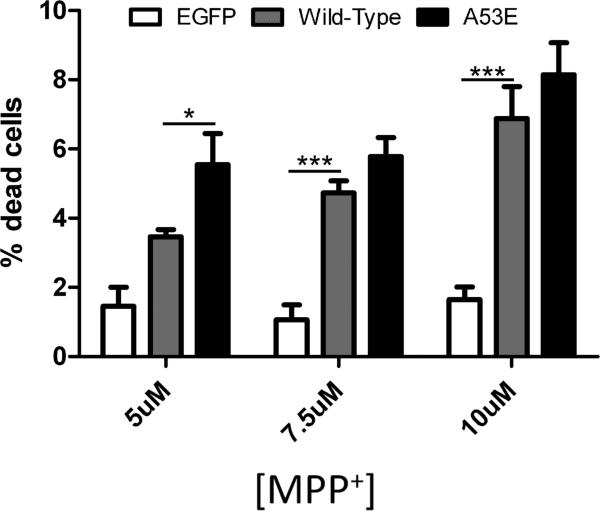
To assess whether A53E αS confers toxicity under cellular stress due to mitochondrial impairment that can model Parkinsonism, we treated neurons overexpressing A53E αS with MPP+. We found that overexpression of wild-type or A53E αS similarly promoted MPP+ toxicity at higher concentrations (7.5 or 10 [.proportional]M). In addition, A53E αS significantly increased cell death at the lowest MPP+ concentration (5μM) tested compared to wild-type.
Discussion
In this study we have demonstrated that the PD-causing A53E SNCA mutation attenuates αS fibrillization kinetics in vitro and shows reduced aggregation in a cell culture model of induced inclusion formation. Our in vitro fibrillization data indicate that, while A53E mutated αS is able to form fibrils, it does so at a slower rate than wild-type αS, with a 2-4 day lag period depending on the concentration of the protein. This finding is in agreement with a study recently published by Ghosh and colleagues [18]. The amorphous staining surrounding full length αS fibrils observed during electron microscopy, with an increased occurrence in A53E compared to wild-type αS, is suggestive of an oligomeric species of αS polymerization. Taking these data together, it is possible that A53E αS preferentially forms off-pathway oligomers and, while these oligomers can eventually be forced back on pathway, it takes time for this to occur. The A53E mutation occurs at the same position as the extensively studied A53T mutation, which shows accelerated fibril formation however, the opposite effect was seen here [12,13]. The introduction of a negative charge here, as opposed to the native non-polar alanine residue, may be interfering with the formation of fibrils through repulsive forces, slowing down fibrillization.
Biochemical fractionation of our cell culture studies showed a significantly reduced proportion of aggregated A53E αS compared to wild-type, which supports the notion that this αS mutant does not preferentially form fibrils. However, we did observe pSer129 positive αS inclusions in our cell culture model of inclusion formation that formed more slowly than wild-type, and the original paper reporting the discovery of the A53E mutation in a PD family described the presence of αS positive inclusions in both neurons and oligodendrocytes [8]. This proves that A53E αS is able to form mature inclusions. However, the A53E αS inclusions that are induced in our cell model are less advanced in aggregation within the time frame that our studies were conducted, since they are less detergent insoluble. Nevertheless, these cellular αS inclusions are phosphorylated at serine129 in this cell culture model, similar to the finding that some forms of aggregated serine129 phosphorylated αS are present in the soluble fraction of αS transgenic mouse brains (22). Our cell viability assay showed that the A53E αS mutation also can enhance cellular toxicity under conditions of mitochondrial stress.
In conclusion, we have shown that the A53E SNCA mutation attenuates αS fibril formation in vitro and reduces aggregation of αS in a cell culture model of inclusion formation. The A53E mutation appears to affect αS similarly to the G51D mutation, as both slow the rate of αS fibril formation [16,17]. Interestingly, both of these mutations introduce charged residues that may cause charge repulsion within the polymerizing protein. However, A53E αS demonstrated reduced aggregation in the seeded cell culture model whereas G51D αS showed similar levels of aggregated protein, when compared to wild-type indicating that A53E has a more robust effect at reducing inclusion formation. The A53E mutation will require further study to fully characterize its effects both in the cell and in vivo, and may provide potential clues of therapeutic relevance.
Highlights.
A53E mutated α-synuclein displays slowed fibril formation in vitro
A53E α-synuclein shows reduced aggregation in cultured cells
A53E α-synuclein enhances toxicity in mitochondrially impaired cells
Figure 4. Viability of cultured Neuro 2A cells overexpressing wild-type or A53E αS when subjected to mitochondrial impairment by treatment with MPP+.
Neuro2A cells were treated with MPP+ to induce mitochondrial stress. The percentage of dead transfected cells for the concentrations of MPP+ indicated was determined. Averages are shown with error bars representing SEM. Significant differences were determined by One-way ANOVA with post hoc Dunnett's multiple comparison tests. * p<0.05, ** p<0.005, *** p<0.001.
Acknowledgements
This work was supported by grants from the NINDS (NS089622) and the National Parkinson Foundation.
Abbreviations
- αS
α-synuclein
- E. coli
Escherichia coli
- EGFP
enhanced green fluorescent protein, K114, (trans,trans)-1-bromo-2,5-bis-(4-hydroxy)styrylbenzene
- MPP+
MPP dihydrochloride hydrate
- PBS
phosphate-buffered saline
- PD
Parkinson's disease
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997 Aug 28;388(6645):839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 2.Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 3.Houlden H, Singleton AB. The genetics and neuropathology of Parkinson's disease. Acta Neuropathol. 2012 Sep;124(3):325–38. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, et al. A novel α-synuclein missense mutation in Parkinson disease. Neurology. 2013 Mar 12;80(11):1062–4. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiely AP, Asi YT, Kara E, Limousin P, Ling H, Lewis P, et al. α-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson's disease and multiple system atrophy? Acta Neuropathol. 2013 May;125(5):753–69. doi: 10.1007/s00401-013-1096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesage S, Anheim M, Letournel F, Bousset L, Honoré A, Rozas N, et al. G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. 2013 Apr;73(4):459–71. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 7.Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Mov Disord. 2013 Jun;28(6):811–3. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 8.Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, et al. A novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease-type pathology. Neurobiol Aging. 2014 Sep;35(9):2180, e1–5. doi: 10.1016/j.neurobiolaging.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999 Oct 1;58(1):120–9. [PubMed] [Google Scholar]
- 10.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998 Apr 17;273(16):9443–9. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 11.El-Agnaf OM, Jakes R, Curran MD, Middleton D, Ingenito R, Bianchi E, et al. Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Lett. 1998 Nov 27;440(1-2):71–5. doi: 10.1016/s0014-5793(98)01418-5. [DOI] [PubMed] [Google Scholar]
- 12.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha- synuclein linked to early-onset Parkinson disease. Nat Med. 1998 Nov;4(11):1318–20. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 13.Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999 Mar 19;274(12):7619–22. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 14.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, et al. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005 Mar 4;280(9):7800–7. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh D, Mondal M, Mohite GM, Singh PK, Ranjan P, Anoop A, et al. The Parkinson's disease-associated H50Q mutation accelerates α-Synuclein aggregation in vitro. Biochemistry. 2013 Oct 8;52(40):6925–7. doi: 10.1021/bi400999d. [DOI] [PubMed] [Google Scholar]
- 16.Rutherford NJ, Moore BD, Golde TE, Giasson BI. Divergent effects of the H50Q and G51D SNCA mutations on the aggregation of α-synuclein. J Neurochem. 2014 Dec;131(6):859–67. doi: 10.1111/jnc.12806. [DOI] [PubMed] [Google Scholar]
- 17.Fares M- B, Ait-Bouziad N, Dikiy I, Mbefo MK, Jovičić A, Kiely A, et al. The novel Parkinson's disease linked mutation G51D attenuates in vitro aggregation and membrane binding of α-synuclein, and enhances its secretion and nuclear localization in cells. Hum Mol Genet. 2014 Sep 1;23(17):4491–509. doi: 10.1093/hmg/ddu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh D, Sahay S, Ranjan P, Salot S, Mohite GM, Singh PK, et al. The Newly Discovered Parkinson's Disease Associated Finnish Mutation (A53E) Attenuates α-Synuclein Aggregation and Membrane Binding. Biochemistry. 2014 Oct 21;53(41):6419–21. doi: 10.1021/bi5010365. [DOI] [PubMed] [Google Scholar]
- 19.Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001 Jan 26;276(4):2380–6. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 20.Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, et al. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001 Oct 15;21(20):8053–61. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, et al. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson's disease. J Neurosci Res. 2000 Feb 15;59(4):528–33. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Waxman EA, Giasson BI. Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J Neuropathol Exp Neurol. 2008 May;67(5):402–16. doi: 10.1097/NEN.0b013e3186fc995. [DOI] [PMC free article] [PubMed] [Google Scholar]