Abstract
Microglia are the resident innate immune cells of the brain. Although embryologically and functionally distinct, they are morphologically similar to peripheral monocyte-derived cells resulting in a poor ability to discriminate between the two cell types. The purpose of this study was to develop a rapid and reliable method to simultaneously characterize, quantify, and discriminate between whole populations of myeloid cells from the brain in a murine model of traumatic brain injury (TBI). Male C57BL/6 mice underwent TBI (n=16) or sham injury (n=14). Brains were harvested at 24 hours post injury. Multiparameter flow cytometry and sequential gating analysis was performed allowing for discrimination between microglia and infiltrating leukocytes as well as for the characterization and quantification of individual subtypes within the infiltrating population. The proportion of infiltrating leukocytes within the brain increased with the severity of injury and the predominate cell types within the infiltrating population were monocyte-derived (p=0.01). Additionally, the severity of injury altered the overall makeup of the infiltrating monocyte-derived cells. In conclusion, we describe a flow cytometry based technique for gross discrimination between infiltrating leukocytes and microglia as well as the ability to simultaneously characterize and quantify individual myeloid subtypes and their maturation states within these populations.
Introduction
Microglia, the resident innate immune cells of the central nervous system (CNS), are embryologically distinct from peripheral monocyte-derived macrophages. An inability to rapidly and reliably distinguish between these two morphologically and functionally similar cell types has limited our understanding of their complex interactions (1). Traditionally, the CNS is considered an immune privileged site barring entry of peripheral leukocytes via the blood brain barrier (2, 3). This leaves the resident microglia to maintain CNS homeostasis through immune surveillance as well as by providing neurotropic support and neural repair (4-6). However, under a variety of neuroinflammatory conditions, disruption of the blood brain barrier occurs allowing peripheral monocyte-derived macrophages to infiltrate into the CNS (7). Additionally, neuroinflammation rapidly activates microglia causing both morphological as well as functional changes that render them difficult to distinguish from infiltrating macrophages (8). This phenotypic similarity has lead to a potentially flawed perception that microglia and infiltrating macrophages perform functionally similar roles within the CNS. In fact, a number of recent studies have shown that infiltrating macrophages play critical roles that the resident microglia are incapable of (9-12). Microglia and infiltrating macrophages, therefore, represent two embryologically and functionally distinct cell populations with each relying on distinctive sets of transcription factors leading to separate patterns of gene expression (13, 14). Understanding the nature of their interaction raises the possibility of therapeutically altering phenotypes and activation states thereby altering the overall immune response to CNS injury.
The current standard for distinguishing between microglia and infiltrating macrophages is immunohistochemistry. Although immunohistochemistry is useful for assessing morphology, proliferation, and sites of activation it has a number of drawbacks limiting its use (15). It requires preparation of fixed tissue which is susceptible to artifact. Additionally, immunohistochemistry only permits semi quantitative analysis from localized regions of the CNS. Several investigative groups have focused on this problem. Gordon and colleagues described a column free magnetic separation protocol to isolated primary mouse microglia in culture (16). Similarly, Nikomedova et al. and Bedi et al. have described the isolation of live microglia based on CD11b immunomagnetic enrichment combined with flow cytometric analysis (15, 17). However, the need to distinguish microglia from infiltrating leukocytes while simultaneously characterizing their maturation status remains unsolved. Thus, current strategies to investigate the complex interplay between microglia and infiltrating leukocytes after CNS injury are inadequate. A method allowing for the simultaneous characterization and quantification of whole populations of myeloid cells from the CNS is needed. Here we describe a flow cytometry-based method for the gross differentiation between microglia and infiltrating leukocytes in a murine model of closed head traumatic brain injury. Most importantly,, this method allowed us to simultaneously characterize and quantify individual infiltrating myeloid subtypes and their maturation states within these populations.
Materials and Methods
Animals
C57BL/6 (B6) male mice (12-14 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, Maine). Mice were maintained in a pathogen-free barrier facility within Northwestern University's Center for Comparative Medicine. Animals were treated and cared for in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals. The Northwestern University Institutional Animal Care and Use Committee approved the experimental protocol.
Traumatic brain injury (TBI)
Mice were anesthetized with 50mg/kg ketamine (Ketaset, Fort Dodge, IA) and 2.5mg/kg xylazine (Anased, Shenandoah, IA) via intraperitoneal injection. Once anesthetized, a 1-cm scalp incision was made allowing identification of the sagittal and coronal sutures of the skull and an impact area 2mm left of the sagittal suture and 2mm rostral to the coronal suture was marked. A closed-head traumatic brain injury was induced via a weight drop technique as previously described by our laboratory (18). In brief, a 333gm impacting rod with a 3-mm diameter tip was dropped from a height of 3cm imparting 0.06J to the closed skull. Sham-injured mice underwent anesthesia induction and scalp incision only. All groups were recovered in separate cages over a warming pad. The severity of injury was assessed at 2, 4, and 24 hours post-injury via administration of the Neurological Severity Score (NSS)1 as initially described by Flierl et al. and as used by our laboratory previously (18, 19). Sham-injured mice all scored 0 out of 10 (N= 14). Mild TBI was defined as a NSS from 1-5 (N=11) and severe TBI was defined as a NSS from 6-10 (N=5). Mice were euthanized at 24 hours post-injury and brains were harvested.
Cell isolation
Immediately after euthanasia, mice were perfused with 30mL cold 1xDPBS via cardiac injection. Whole brains were then removed, weighed, and split into hemispheres. Individual hemispheres were homogenized and processed via the Miltenyi neural dissociation kit according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). Subsequent to neural dissociation, myelin was removed with myelin removal beads via the Miltenyi autoMACS pro (Miltenyi Biotech). The myelin negative fraction was collected and centrifuged at 300g for 10 minutes at 4 degrees Celsius. Red blood cells were lysed with BD Pharm Lyse Buffer (BD Biosciences, Sparks, MD). The separated hemispheres were then recombined to allow for total brain cell counts obtained with a Countess automated cell counter (Invitrogen, Carlsbad, CA). Trypan blue staining (Life Technologies, Carlsbad, CA) excluded nonviable cells.
Flow cytometry
Approximately 3 million cells per brain were prepared for flow cytometric analysis. Viable cells were labeled with LIVE/DEAD Fixable Aqua Dead Cell Stain (Invitrogen). Samples were washed with PBS and treated with Fc Block to inhibit nonspecific binding (BD Biosciences, Sparks, MD). Cells were then labeled with FITC Ly6c (AL21), PE Cy7 CD11c (HL3), Alexa Fluor 700 Ly6G (1A8), and APC Cy7 CD45 (30-F11). A common channel for PE-CF594 CD3 (145-2C11), CD19 (1D3), Siglec F (E50-2440), and NK1.1 (PK136) was used (BD Biosciences). Additionally cells were labeled with PerCP Cy5.5 MHCII (M5/114.15.2), PE CD64 (x54-5/7.1) (Biolegend, San Diego, CA), Alexa Fluor 647 F4/80 (BMA) (Abdserotec, Raleigh, NC), and efluor450 CD11b (M1-70) (eBioscience, San Diego, CA). Following incubation, all cells were washed with PBS and fixed with 1.6% paraformaldehyde.
Flow cytometry data were acquired on a BD LSR II cytometer (BD Immunocytometry Systems, San Jose, CA) having 405-nm, 488-nm, 561-nm, and 640-nm excitation lasers as previously described by our laboratory (20). Data collection was performed using BD FACS Diva Software (BD Biosciences), and data analyses were performed using FlowJo Software (Treestar, Ashland, OR). Bioexponential transformation was adjusted when necessary.
Statistical analysis
The data are reported as the mean ± standard error of the mean using the statistical software program Prism (Graphpad Software Inc., La Jolla, CA). Data involving two groups were analyzed by a Student's t test and data involving more than two groups were analyzed using one-way analysis of variance with Tukey multiple comparison post-test. Significance was accepted at p<0.05. N = x mice in the sham-injury group, y mice in the mild TBI group, and z mice in the severe TBI group.
Results
Flow cytometry grossly distinguishes infiltrating leukocytes from microglia
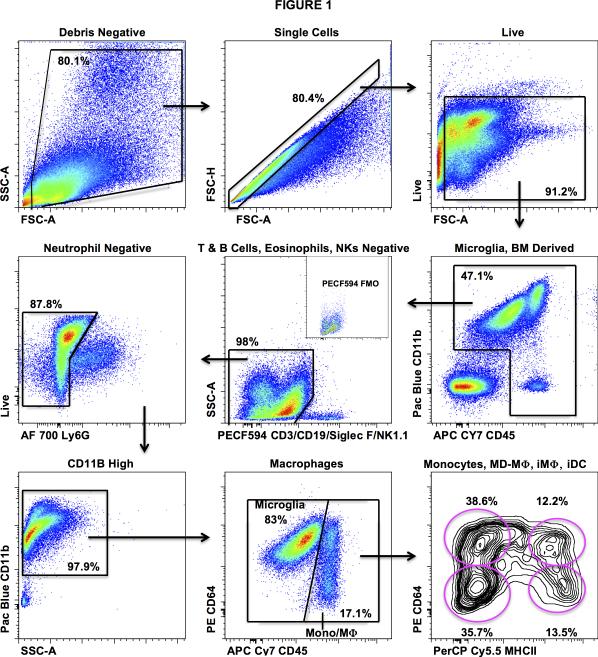
Prior studies have reported efficient isolation of myeloid cells from the brain using immunomagnetic separation based on expression of CD11b (15, 17). However, the flow cytometric analysis of these immunomagnetically enriched myeloid populations demonstrated poor resolution of peripheral monocyte-derived myeloid cells from microglia. To identify myeloid populations in the brain we performed 10-color flow cytometry and sequential gating analysis (Fig. 1). Using this technique we were able to fully characterize the infiltrating leukocyte population into its principle components via flow cytometry (lymphocytes, NK cells, eosinophils, neutrophils, monocytes, monocyte-derived macrophages, and monocyte-derived dendritic cells). In addition, our antibody panel and sequential gating strategy also allowed for a detailed examination of monocyte-derived populations. First, the infiltrating myeloid population (CD3−CD19−NK1.1−SiglecF−Ly6G−CD11b+CD45Hi) was grossly distinguished from microglia (CD3−CD19−NK1.1−SiglecF−Ly6G−CD11b+CD45Lo) via differential expression of CD45 and confirmed via backgating based on the differential forward scatter characteristics and CD11b expression of the two populations. Following the differentiation of monocyte-derived cells from microglia, we were able to delineate between monocytes (CD64−MHCII−), early monocyte-derived (CD64+MHCII−) macrophages (MD-Mϕ), inflammatory (CD64+MHCII+) macrophages (iMϕ), and inflammatory (CD64−MHCII+) dendritic cells (iDC) (Fig. 3, bottom panels) (20, 21). While our particular panel focused on identifying the various maturation states of monocyte-derived cells, one could easily alter the panel to focus on the lymphoid population or other granulocyte populations in greater detail.
Figure 1. Gating strategy for the differentiation of microglia from monocytic cells.
Cells were isolated from enzymatically digested, myelin depleted, mouse brains. After the exclusions of doublets and debris, immune cells were identified by CD45 staining. Live cells were separated from dead cells using an amine reactive dye. Lymphocytes, eosinophils, and NK cells were excluded using a common channel for CD3/CD19, Siglec F, and NK1.1 respectively. Neutrophils were identified and gated out using surface staining for Ly6G. Microglia (CD3-CD19−NK1.1−SiglecF−Ly6G−CD11b+CD45Lo) were then differentiated from infiltrating monocytes and macrophages (CD3−CD19−NK1.1−SiglecF−Ly6G−CD11b+CD45Hi) by differential expression of CD64 and CD45. Backgating based on the differential forward scatter characteristics and CD11b expression aided identification of these populations. Monocytes (CD64−MHCII−), early monocyte-derived (CD64+MHCII−) macrophages (MD-Mϕ), inflammatory (CD64+MHCII+) macrophages (iMϕ), and inflammatory (CD64−MHCII+) dendritic cells (iDC) were then identified via overlapping expression patterns of CD64 and MHCII.
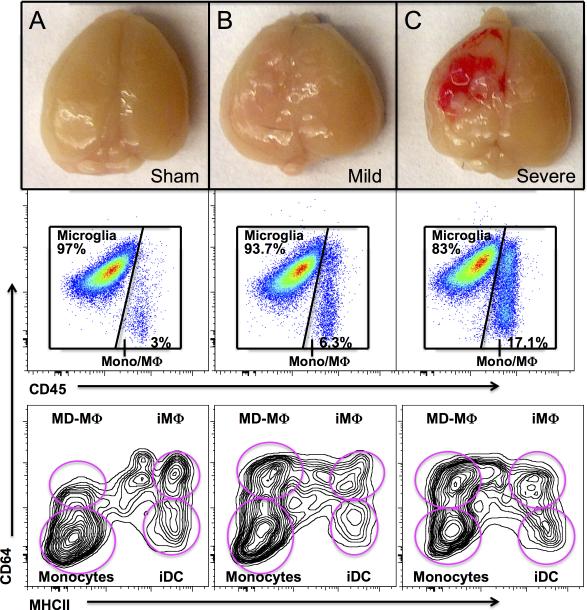
Figure 3. Degree of brain injury alters the maturation state of infiltrating monocyte-derived cells at 24 hours post injury.
A, The majority of peripheral leukocytes within the sham-injured brain are monocytes. B, After mild TBI, peripheral leukocytes infiltrate into the brain tissue with a significant increase in the number of early monocyte-derived (CD64+MHCII−) macrophages (MD-Mϕ) (p<0.02) as compared to sham injury. C, Severe TBI results in a marked increase in infiltrating leukocytes within the injury brain. Although monocytes and MD-Mϕ are still present in large numbers, the proportion of inflammatory (CD64−MHCII+) dendritic cells (iDC) increased substantially after severe TBI as compared to both sham injury (p<0.001) as well as mild TBI (p<0.002).
Infiltrating leukocytes counts increase in proportion to the severity of traumatic brain injury
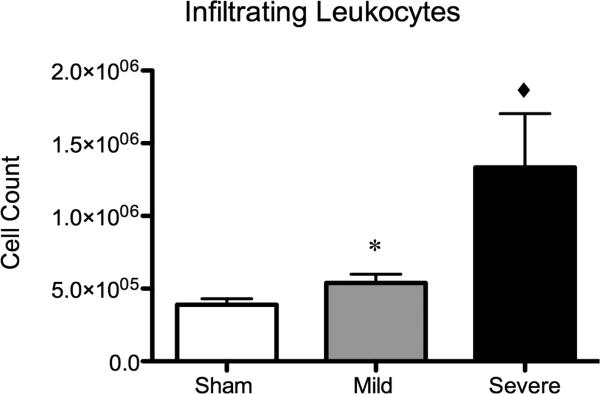
Our analysis revealed a significant difference in the frequency of leukocyte infiltration into the injured brains dependent on the severity of injury (Fig. 2). At 24 hours post TBI, brains from animals sustaining a mild TBI demonstrated a 1.4 fold increase in infiltrating leukocytes as compared to sham injury (p<0.05) whereas mice sustaining a severe traumatic brain injury demonstrated a 3.4-fold increase in infiltrating leukocytes over sham-injured mice (p<0.001). This correlated with gross pathological examination of the injured brain (Fig. 3). Interestingly, the frequency of microglia remains unchanged after TBI (data not shown).
Figure 2. Severity of brain injury determines degree of peripheral leukocyte infiltration.
Mild TBI without any gross evidence of hemorrhage resulted in a significant increase in overall cell counts of peripheral leukocytes within the injured brain (*=p<0.05) as compared to sham injury. Severe injury, with grossly evident hemorrhage within the brain, resulted in a 3.4-fold increase in peripheral leukocytes within the injured brain (◆=p<0.001) as compared to sham injury.
Monocyte-derived cells constitute the majority of infiltrating leukocytes after TBI
Prior studies have suggested that neutrophils comprise the majority of infiltrating leukocytes after CNS injury (22, 23). Our analysis revealed that monocyte-derived cells comprise the majority of the infiltrating population. Monocyte-derived cells constituted 42.5% ± 2.0% of all peripheral bone marrow-derived leukocytes within brains of sham-injured mice. The remainder of the infiltrating population is split amongst neutrophils, lymphocytes, NK cells, and eosinophils. The proportion of monocyte-derived cells within the infiltrating leukocyte population increased as the severity of brain injury increased with mild TBI resulting in 46.5% ± 2.6% monocyte-derived cells and severe injury resulting in 52.9% ± 2.3% monocyte derived cells (p=0.01). The proportion of neutrophils within the infiltrating population did not change substantially after TBI—34.7% ± 1.6% in sham-injury, 35.1% ± 2.9% in mild TBI, and 31.6% ± 3.3% in severe TBI (p=NS).
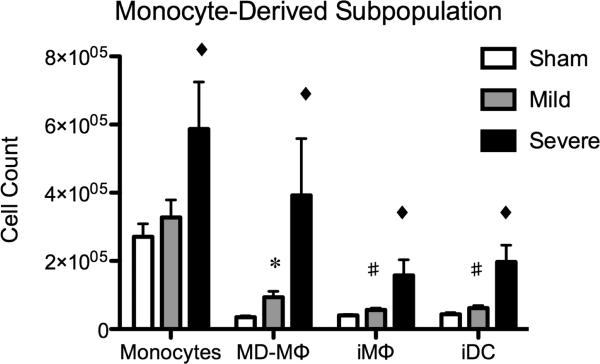
The severity of traumatic brain injury alters the maturation state of infiltrating monocyte-derived cells at 24 hours post injury
To our knowledge, no study to date has characterized the maturation state of infiltrating monocyte-derived cells after TBI. We observed significant alterations in the makeup of the monocyte-derived population at 24 hours post TBI (Fig. 4). First, monocytes traffic to the injured brain. They then differentiate into early MHCII− monocyte-derived macrophages (MDMϕ) 2 and progress to MHCII+ inflammatory tissue macrophages (iMϕ) 3. Alternatively, monocytes can also give rise to monocyte-derived dendritic cells (iDC)4 (20, 21). Our analysis revealed that mild TBI did not produce a significant change in the proportion of monocytes within the injured brain. However, there was a 1.4 fold increase in MD-Mϕ's (p<0.02). There was also a modest, albeit statistically significant, increase in iMϕ and iDC's. The most dramatic changes in the maturation state of infiltrating monocyte-derived cells occurred in mice sustaining a severe TBI. Monocytes increased 2.2 fold (p<0.01), MD-Mϕ increased 11.3 fold (p<0.002), iMϕ increased 3.9 fold (p<0.001), and the proportion of iDC's increased 4.5 fold as compared to sham (p<0.001).
Figure 4. The severity of traumatic brain injury alters the maturation state of infiltrating monocyte-derived cells at 24 hours post injury.
Mild TBI did not produce a significant change in the proportion of monocytes within the injured brain. Mild TBI did result in a 1.4 fold increase in monocyte-derived (MD-Mϕ) macrophages (*=p<0.002). There was also a modest, albeit statistically significant, increase in inflammatory tissue macrophages and inflammatory (iDC) dendritic cells (#=p<0.04). Severe TBI resulted in a 2.2 fold increase in infiltrating monocytes, an 11.3 fold increase in MD-Mϕ, a 3.9 fold increase in iMϕ, and a 4.5 fold increase in iDC as compared to sham (◆=p<0.001).
Discussion
In this study, we described the use of multiparameter flow cytometry with sequential gating to grossly differentiate infiltrating leukocytes from microglia, characterize the makeup of infiltrating leukocytes, and determine the maturation state of infiltrating monocyte-derived macrophages after the induction of a closed-head traumatic brain injury. The value of our system lies in its ability to simultaneously characterize and quantify entire populations of cells from whole brains or hemispheres in a rapid and reliable fashion. Furthermore, by incorporating ten-color antibody panels we are able to concurrently delineate leukocyte subpopulations in a manner not possible with the three-color limitation of traditional immunohistochemical techniques.
The primary immune cell component of the myelin depleted brain suspensions consisted of CD3-CD19−NK1.1−SiglecF−Ly6G−CD11b+CD45Lo microglia. Only a small fraction of cells, corresponding to peripheral leukocytes, were found in the CD3−CD19−NK1.1−SiglecF−Ly6GCD11b+CD45Hi gate. However, after induction of traumatic brain injury there was a significant stepwise increase in the cell counts of the infiltrating peripheral leukocyte population corresponding to the severity of brain injury. Early experiments defined this population as resident brain macrophages. More recent data has argued that neutrophils are the predominant cell type within the infiltrating leukocyte population (22, 23). These studies, however, utilized anti-Gr-1 antibodies to identify neutrophils and data from own laboratory and others has shown that the anti-Gr-1 antibody recognizes both Ly6C and Ly6G antigens (20). This leads one to question whether prior studies have misidentified infiltrating monocyte-derived cells as neutrophils. This hypothesis is consistent with the data reported herein as monocytes and monocyte-derived macrophages are the predominant cell type within the infiltrating leukocyte population after TBI. The importance of this observation cannot be overstated as there is growing evidence that infiltrating macrophages play a critical role in neurologic repair that resident microglia are incapable of. For example, infiltrating macrophages have been shown to restrict amyloid-β plaque in Alzheimer's disease, facilitate functional recovery in spinal cord injury, and aid in cell renewal in the injured retina (9-12). Additionally, there is early evidence to suggest that infiltrating monocyte-derived macrophages orchestrate the polarization of microglia towards inflammatory (M1) or anti-inflammatory (M2) phenotypes (9, 11, 24, 25). Understanding the nature of the interaction between infiltrating monocyte-derived macrophages and microglia raises the possibility of therapeutically altering phenotypes and activation states thereby altering the overall immune response to CNS injury. An important limitation of the current study is that microglia up-regulate CD45 after injury whereas highly activated monocyte-derived macrophages down-regulate CD45 expression. This most certainly results in some degree of crossover between the two populations and it must be acknowledged that some inflammatory microglia may be included within the initially identified monocyte-derived population. Likewise, some highly activated monocytes may be included within the initially identified microglia population. Although backgating based on the differential forward scatter characteristics and CD11b expression of microglia and infiltrating monocytes/macrophages aids in more definitive identification of these populations, these differences may be too subtle and allow for too much subjectivity for investigators without long term experimental experience. Another limitation is that flow cytometry does not allow for definitive discrimination between M1 and M2 polarization states. While several studies have attempted to use flow cytometry as a tool for M1/M2 discrimination, it is has become clear that M1 and M2 polarization states are not binary. Rather, they represent a spectrum of phenotypes controlled by a number of different genes (17, 26-28). Although flow cytometry may be useful as a screening tool in this regard, a method allowing for the simultaneous detection of genes covering the entire M1/M2 monocyte and macrophage activation spectrum will be required.
In addition to identifying monocytes and monocyte-derived macrophages as the predominant cell type in the infiltrating leukocyte population during brain injury, we further characterized these cells according to their state of maturation. This, in turn, corresponded to the degree of brain injury. The maturation state of the monocyte-derived population was stratified based on expression of CD64 (Fc-Gamma receptor 1) and MHC II as described previously by our laboratory (20, 21). Monocytes were defined as CD3−CD19−NK1.1−SiglecF−Ly6GCD11b+CD45HiCD64−MHCII−. As the monocytes matured into monocyte-derived macrophages they began to express CD64 and were identified as CD3−CD19−NK1.1−SiglecF−Ly6GCD11b+CD45HiCD64+MHCII−. As monocyte-derived macrophage matured into inflammatory macrophages they began to express MHCII and were identified as CD3−CD19−NK1.1−SiglecFLy6G−CD11b+CD45HiCD64+MHCII+. Lastly, monocyte-derived dendritic cells, or inflammatory dendritic cells, do not express CD64 and are defined as CD3−CD19−NK1.1−SiglecF−Ly6GCD11b+CD45HiCD64−MHCII+. The overall maturation state of the infiltrating monocyte-derived population, as determined by the multiparameter flow cytometry technique described above, changed dramatically as the severity of injury increased. Infiltrating monocytes were the predominant cell type in both sham injury and mild TBI. However, as the injury became more severe, the infiltrating monocytes rapidly matured along the spectrum of early monocyte-derived macrophages, inflammatory macrophages, and inflammatory dendritic cells (Fig. 4).
In conclusion, we describe a flow cytometry based technique for the gross discrimination between infiltrating leukocytes and microglia in a murine model of closed-head traumatic brain injury. Most notably, we were able to simultaneously characterize and quantify individual infiltrating myeloid subtypes and their maturation states within these populations. We observed that the predominant infiltrating cell types within the injured brain are monocyte-derived and that this correlated with the severity of injury. The importance of this observation is highlighted by the growing evidence that infiltrating macrophages play a critical role in neurologic repair that resident microglia are incapable of. By gaining a deeper understanding of their progression and maturation after TBI, infiltrating monocytes and macrophages may soon represent a novel therapeutic target for the treatment of traumatic brain injury.
Acknowledgments
Sources of Support: This study was supported by the Shock Society Research Fellowship for Early Career Investigators and a grant from the American Association for the Surgery of Trauma Research and Education Foundation to S.J.S. Further support from NIH grants (AR050250, AR054796, AI092490, and HL108795) and Funds provided by Solovy/Arthritis Research Society Professor to H.P.
Footnotes
NSS—neurological severity score
MD-Mϕ—early MHCII− monocyte-derived macrophage
iMϕ—inflammatory tissue macrophage
iDC—monocyte-derived dendritic cell
Contributor Information
Diane M. Trahanas, Northwestern University, Department of Surgery, Division of Trauma and Critical Care diane.trahanas@northwestern.edu
Carla M. Cuda, Northwestern University, Department of Medicine, Division of Rheumatology c-cuda@northwestern.edu
Harris Perlman, Northwestern University, Department of Medicine, Division of Rheumatology h-perlman@northwestern.edu
Steven J. Schwulst, Northwestern University, Department of Surgery, Division of Trauma and Critical Care
References
- 1.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature neuroscience. 2007;10(12):1538–43. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 3.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339(6116):156–61. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 5.Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55(3):233–8. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 6.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(13):3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. The Journal of comparative neurology. 2007;500(2):267–85. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 8.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–9. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 9.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS medicine. 2009;6(7):e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. The Journal of experimental medicine. 2011;208(1):23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London A, Cohen M, Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Frontiers in cellular neuroscience. 2013;7:34. doi: 10.3389/fncel.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49(4):489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 14.Gomez Perdiguero E, Schulz C, Geissmann F. Development and homeostasis of “resident” myeloid cells: the case of the microglia. Glia. 2013;61(1):112–20. doi: 10.1002/glia.22393. [DOI] [PubMed] [Google Scholar]
- 15.Nikodemova M, Watters JJ. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. Journal of neuroinflammation. 2012;9:147. doi: 10.1186/1742-2094-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon R, Hogan CE, Neal ML, Anantharam V, Kanthasamy AG, Kanthasamy A. A simple magnetic separation method for high-yield isolation of pure primary microglia. Journal of neuroscience methods. 2011;194(2):287–96. doi: 10.1016/j.jneumeth.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedi SS, Smith P, Hetz RA, Xue H, Cox CS. Immunomagnetic enrichment and flow cytometric characterization of mouse microglia. Journal of neuroscience methods. 2013;219(1):176–82. doi: 10.1016/j.jneumeth.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwulst SJ, Trahanas DM, Saber R, Perlman H. Traumatic brain injury-induced alterations in peripheral immunity. The journal of trauma and acute care surgery. 2013;75(5):780–8. doi: 10.1097/TA.0b013e318299616a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nature protocols. 2009;4(9):1328–37. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- 20.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2012;81(4):343–50. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. American journal of respiratory cell and molecular biology. 2013;49(4):503–10. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. Journal of immunology. 1995;154(9):4309–21. [PubMed] [Google Scholar]
- 23.Stirling DP, Yong VW. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. Journal of neuroscience research. 2008;86(9):1944–58. doi: 10.1002/jnr.21659. [DOI] [PubMed] [Google Scholar]
- 24.Jung S, Schwartz M. Non-identical twins - microglia and monocyte-derived macrophages in acute injury and autoimmune inflammation. Frontiers in immunology. 2012;3:89. doi: 10.3389/fimmu.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain : a journal of neurology. 2009;132(Pt 9):2487–500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 26.Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. Journal of neuroinflammation. 2014;11:82. doi: 10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW, Knawy BA. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem cell reviews. 2013;9(5):620–41. doi: 10.1007/s12015-013-9455-2. [DOI] [PubMed] [Google Scholar]
- 28.Jin X, Ishii H, Bai Z, Itokazu T, Yamashita T. Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PloS one. 2012;7(7):e41892. doi: 10.1371/journal.pone.0041892. [DOI] [PMC free article] [PubMed] [Google Scholar]