Abstract
Imaging, surgical, and lesion studies suggest that the prefrontal cortex (orbitofrontal and anterior cingulate cortexes), basal ganglia, and thalamus are involved in the pathogenesis of obsessive-compulsive disorder (OCD). On the basis of these findings several models of OCD have been developed, but have had difficulty fully integrating the psychological and neuroanatomical findings of OCD. Recent research in the field of cognitive neuroscience on the normal function of these brain areas demonstrates the role of the orbitofrontal cortex in reward, the anterior cingulate cortex in error detection, the basal ganglia in affecting the threshold for activation of motor and behavioral programs, and the prefrontal cortex in storing memories of behavioral sequences (called “structured event complexes” or SECs). The authors propose that the initiation of these SECs can be accompanied by anxiety that is relieved with completion of the SEC, and that a deficit in this process could be responsible for many of the symptoms of OCD. Specifically, the anxiety can form the basis of an obsession, and a compulsion can be an attempt to receive relief from the anxiety by repeating parts of, or an entire, SEC. The authors discuss empiric support for, and specific experimental predictions of, this model. The authors believe that this model explains the specific symptoms, and integrates the psychology and neuroanatomy of OCD better than previous models.
“… Continually tormented by an inner sense of imperfection, connected with the perception that actions or intentions have been incompletely achieved.”
—Pierre Janet1
Obsessive-compulsive disorder (OCD) is a relatively common, and often disabling, psychiatric disorder.2,3 It is characterized by obsessions (unwanted, recurrent intrusive thoughts that cause anxiety) and compulsions (repetitive behaviors that the patient feels driven to perform, often in response to an obsession) which generally coexist.4 Imaging studies have consistently shown abnormalities in specific brain areas in patients with OCD. However, how the normal functioning of these brain areas is altered to produce the symptoms of OCD remains unknown. In this article, we assert that the completion of complex behaviors is normally accompanied by a reward signal, and that abnormalities in this process could account for some of the symptoms of OCD. We present evidence for this view and propose testable hypotheses.
This article is separated into five parts. The first part reviews the literature on the brain areas associated with OCD. A review of imaging studies on OCD was conducted by performing a MEDLINE search through 2006 on the term “obsessive-compulsive disorder” and one of the following terms: “imaging,” “CT,” “computed tomography,” “MRI,” “magnetic resonance imaging,” “PET,” “positron emission tomography.” The resulting abstracts were screened by one of the authors (EDH), and relevant studies were reviewed. In addition, we evaluated selected reviews.5–8 The second part of this article reviews recent findings from the field of cognitive neuroscience on the functions of these brain regions. We focus on the role of the orbitofrontal cortex (OFC) and reward structures in reinforcement, the basal ganglia in setting the threshold for activation of motor activity and complex behaviors, and the anterior cingulate cortex (ACC) for error detection (see Table 1). The third part discusses the role of the prefrontal cortex (PFC) in the execution and reinforcement of complex behaviors. The fourth part presents some previous models of OCD. Finally, the fifth part proposes a new model that integrates the findings presented in the first three parts of the paper.
TABLE 1.
Anatomic Areas of the Brain
| Brain area | Definition |
|---|---|
| Prefrontal cortex | Area anterior to the supplementary motor area and premotor cortex. |
| Orbitofrontal cortex | The ventral surface of the prefrontal cortex including parts of BA 10, 11, and 47 in the human, and these areas plus areas 12, 13, and 14 in the macaque [Kringelbach and Rolls 2004, Fuster 1997, Petrides and Pandya 1994]. See Figure 1. |
| Dorsolateral prefrontal cortex | Area of prefrontal cortex that extends over the superior and middle frontal gyri (core: BA 9, 46, and 9/46; anterior portion: BA 10; posterior portion: BA 8) [Petrides and Pandya 1999]. |
| Anterior cingulate cortex | BA 24. |
| Basal ganglia | A group of subcortical nuclei including the caudate, putamen, and globus pallidus (internal and external segments). The caudate and putamen are collectively termed the striatum. |
| Limbic and paralimbic structures | A group of structures involved in emotion, motivation, and the interaction of emotion and memory. Variably defined, but can include the amygdala, the hypothalamus, cingulate cortex, and memory structures such as the hippocampus. |
| Reward structures | Brain structures implicated in delivering a reward signal, including the ventral tegmental area, superior temporal sulcus, and the nucleus accumbens. |
| Supplementary motor area | Dorsal subregion of BA 6. |
| Premotor cortex | Ventral subregion of BA 6. |
BA = Brodmann areas
Kringelbach ML, Rolls ET: The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004; 72:341–372
Fuster JM: The Prefrontal Cortex. Philadelphia, Lippincott-Raven, 1997
Petrides M, Pandya DN: Comparative architonic analysis of the human and macaque frontal cortex, in Handbook of Neuropsychology. Edited by Boller F, Grafman J. Amsterdam, Elsevier, 1994, pp 17–58
Petrides M, Pandya DN: Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 1999; 11:1011–1036
The Brain Areas Involved in OCD
The majority of both structural and functional imaging studies have shown differences in the PFC, basal ganglia, ACC, and/or thalamus between patients with OCD and healthy comparison subjects (see Table 2).5 A recent meta-analysis reviewed functional imaging studies in OCD and found that the OFC (orbital gyrus) and head of the caudate were the only brain areas that significantly and consistently demonstrated increased tracer uptake in OCD patients relative to comparison subjects.8 We will discuss the OFC, basal ganglia, ACC, and thalamus in this review, but will focus on the OFC and basal ganglia because these brain areas are most consistently associated with OCD in imaging studies.8
TABLE 2.
A Review of Imaging Studies of OCD
| Structural Modality | Brain Area | Finding |
|---|---|---|
| CT | VBR, asymmetry, sulcal prominence | No significant differences between patients with OCD and healthy control subjects [113] |
| CT | Caudate, lenticular nuclei, third and lateral ventricles | Caudate volume lower in OCD patients compared to healthy control subjects [114] |
| CT | Ventricular-brain ratio | Patients with OCD had a higher ventricle-brain ratio [115] |
| MRI | Caudate | No structural difference in caudate between patients with OCD and healthy control subjects [116] |
| MRI | Caudate, cingulate gyrus, intracaudate/frontal horn ratio, corpus collosum | No significant differences between patients with OCD and healthy control subjects [117] |
| MRI | OFC, ACC, thalamus, caudate, putamen | Patients with OCD had a smaller left OFC volume compared to healthy control subjects [118] |
| MRI | Superior frontal gyrus, ACC, OFC, hippocampus, amygdala | Patients with OCD had decreased bilateral OFC and amygdala volume compared to healthy control subjects [119] |
| MRI | Grey matter | Increased gray matter regional density in multiple areas including left OFC and subcortical areas [120] |
| MRI | Frontal-striatal circuitry | Increase in volume of ventral PFC and striatum [121] |
| MRI | White matter | Spin-lattice relaxation time (T1) differences in right frontal white matter of OCD patients compared to control subjects [122] |
| MRI | Head of caudate | Increase in volume of right caudate head in OCD patients compared to healthy subjects [123] |
| MRI | Caudate, putamen, globus pallidus, ACC, superior frontal gyrus | OCD patients had smaller globus pallidus volumes and more total gray matter in the ACC compared to healthy control subjects [124] |
| MRI | PFC, caudate, lateral and third ventricles, and whole brain | Caudate volume lower in OCD patients [125] |
| MRI | PFC, striatum, lateral and third ventricles, and intracranial volume | Patients with OCD had smaller striatal, and larger third ventricle, volumes than control subjects [126] |
| MRI | Corpus callosum | Patients with OCD had increased size of the corpus callosum compared to healthy control subjects [127] |
| MRI | Whole brain volume | Decreased total white matter and greater total cortex and opercular volumes in patients with OCD compared to healthy control subjects [128] |
| MRI and 1H-MRS | Caudate and corpus striatum | No difference between volumes of caudate between patients with OCD and healthy control subjects. Decreased N-acetylaspartate levels in left corpus striatum [129] |
| MRI DTI | White matter | Lower fractional anisotropy in ACC white matter, partietal region, right posterior cingulate, and left occipital lobe compared to healthy control subjects [130] |
| MRI VBM | Regions defined a priori as likely to be involved in OCD | Increased grey matter in the OFC and parahippocampal regions, decreased grey matter in ACC in OCD patients compared to healthy control subjects [131] |
| Functional Modality | Finding | |
| 1H MRS | N-Acetylaspartate levels decreased in the left corpus striatum in patients with OCD compared to healthy control subjects [129] | |
| 1H MRS | N-Acetylaspartate levels decreased in the ACC and right striatum in patients with OCD compared to healthy control subjects [132] | |
| 1H MRS | N-Acetylaspartate levels decreased in the thalamus in patients with OCD compared to healthy control subjects [133] | |
| 1H MRS | Decreased thalamic choline in OCD patients compared to healthy control subjects and patients with major depressive disorder [134] | |
| 99mTc HMPAO SPECT | Hyperperfusion in right thalamus, left frontotemporal cortex, and bilateral OFC in patients with OCD compared to healthy control subjects [135] | |
| 99mTc HMPAO SPECT | Hyperperfusion in right superior and inferior frontal cortex and bilateral thalamus in OCD patients compared to healthy control subjects [136] | |
| 99mTc HMPAO SPECT | Hyperperfusion in OFC, dorsal parietal cortex, and left posterofrontal cortex [137] | |
| 99mTc HMPAO SPECT | Increase in metabolism of bilateral superior frontal cortices and right caudate in patients with OCD and PTSD compared to patients with panic disorder and healthy control subjects [138] | |
| 99mTc HMPAO SPECT | Higher ratio of medial/frontal to whole brain perfusion in patients with OCD compared to healthy control subjects [139] | |
| 99mTc HMPAO SPECT | Differences in regional brain perfusion between early-onset and late-onset OCD [140] | |
| 99mTc HMPAO SPECT | Decrease in right OFC perfusion in OCD patients without motor tics compared to healthy control subjects [141] | |
| FDG-PET | Metabolic rate increased in the left OFC and bilateral caudate nuclei compared to healthy control subjects and patients with unipolar depression [142] | |
| FDG-PET | Increased metabolic rate in left OFC, right sensorimotor, bilateral prefrontal and ACC regions in OCD patients compared to healthy control subjects [143] | |
| FDG-PET | Increased metabolic rate in cingulate cortex, thalamus, and pallidum/putamen complex. Successful SSRI treatment lowered metabolism in the cingulate [144] | |
| FDG-PET | Decreased metabolism in whole, and prefrontal lateral, cortex in patients with OCD compared to healthy control subjects [145] | |
| fMRI | Symptom induction was associated with activation of the OFC, superior frontal, and the DLPFC; the anterior, medial, and lateral temporal cortex; and the right anterior cingulate in patients with OCD [146] | |
| fMRI | Increased activation in OFC, lateral frontal, anterior temporal, ACC, insula, caudate, lenticulate, and amygdala in patients with OCD compared to healthy control subjects [147] | |
| fMRI | Patients with OCD showed greater error-related activation of the ACC than healthy control subjects [107] | |
| fMRI | Symptom improvement with successful SSRI treatment resulted in decreased symptom-provoked activation of OFC, dorsolateral prefrontal cortex, and ACC [148] | |
| O15 PET | Increased metabolism in OFC, premotor, and midfrontal cortex in patients with obsessional slowness compared to healthy control subjects [149] | |
| O15 PET | Symptom provocation resulted in increased blood flow to the right caudate, left ACC, and bilateral OFC in patients with OCD [150] | |
OCD = obsessive-compulsive disorder; VBR = ventricular brain ratio; OFC = orbitofrontal cortex; ACC = anterior cingulate cortex; PFC = prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; SSRI = selective serotonin reuptake inhibator; fMRI = functional magnetic resonance imaging; MRI DTI = magnetic resonance imaging diffusion tensor imaging; MRI VBM = magnetic resonance imaging voxel-based morphometry; 1H-MRS = proton magnetic resonance spectroscopy; HMPAO = Tc-Hexamethylpropyleneamine Oxime; SPECT = single photon emission computed tomography; FDG-PET = 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography
The imaging findings reviewed in Table 2 are corroborated by the finding that disrupting connections between the OFC, ACC, thalamus, and basal ganglia by means of a cingulotomy, anterior capsulotomy or subcaudate tractotomy results in a symptomatic improvement in most OCD patients.6,9–14
Some studies have examined the development of symptoms of OCD after brain injury.15 Damage to the basal ganglia (especially the caudate), the OFC, and the ACC16–22 are associated with the acquisition of OCD symptoms following brain injury.15 Dysfunction of the basal ganglia secondary to a streptococcal infection23 or encephalitis lethargica24 has also been associated with the development of OCD symptoms. One report showed an association between lesions in the mesial frontal region (including the ACC) and collecting behavior resembling OCD.25 Another demonstrated that repetitive motor activity in patients with dementia is uniquely associated with right ACC hypometabolism.26 We have observed that repetitive motor activity is associated with right caudate and OFC atrophy in patients with frontotemporal dementia (Huey, presentation, UCSF 5th International Conference on Frontotemporal Dementia, 2006).
The Functions of the Brain Areas Involved in OCD
The Orbitofrontal Cortex (OFC)
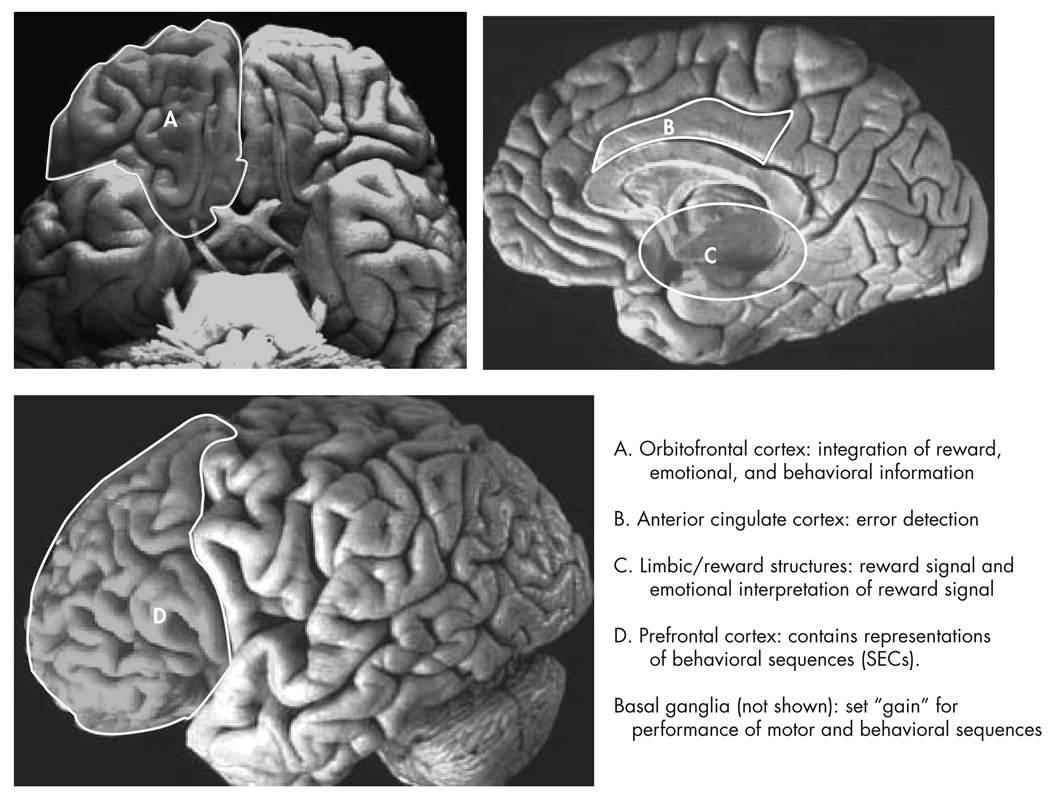
In this section we discuss the role of the OFC in reward learning, emotion, and social behaviors (Figure 1). In the final section of this article, we hypothesize how disruption of the normal functions of the OFC results in the symptoms of OCD.
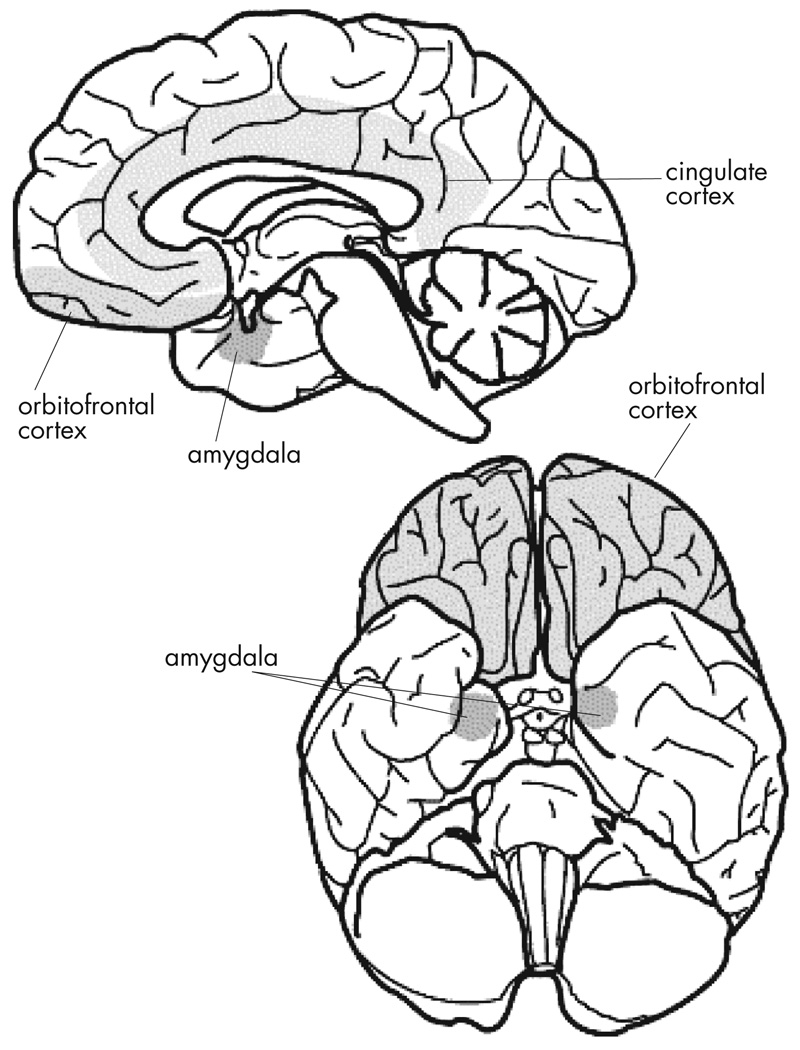
FIGURE 1. The Key Brain Structures Implicated in Reward and Emotion.
The position of the amygdala, orbitofrontal cortex and cingulate cortex are shown on a midsagittal view (top), and on a ventral view (bottom) of the human brain. Reproduced with permission from Luxenberg et al. 1988 (114)
The OFC appears to be involved with reward learning and emotional processes, and with the integration of these processes in social tasks.27,28 Rolls and colleagues28 have demonstrated that neurons in the OFC of the macaque represent the reward value of tastes. Taste neurons in the OFC, in contrast to neurons in the primary taste cortex,29,30 stop responding to the taste of a food if the monkey is fed to satiety with that food.31 Also, monkeys will work to receive electrical stimulation of the OFC if they are hungry, but not if they are satiated.32,33 A comparable role of the OFC in humans is supported by functional MRI (fMRI) studies that have demonstrated satiety-specific OFC activation to foods in humans.34,35 The human OFC is activated by sensory stimuli such as taste and olfaction,36–40 and by more abstract rewards such as money,41 attractive faces,42 cooperation,43 and altruistic donation.44 O’Doherty45 and Knutson and Cooper46 have reviewed imaging studies on reward in humans.
Macaques with OFC lesions have difficulty learning which stimuli are rewarding and which are not, and they have particular difficulty modifying behavior when reward contingencies change.47 For example, macaques with OFC damage continue to pick a response that was once rewarded, even if it is no longer rewarded.47–49 Humans with ventromedial PFC damage typically demonstrate disruption of social and emotional behaviors with relative preservation of memory, language, and tests of executive function.50,51
The Basal Ganglia
Imaging studies suggest that the basal ganglia can be involved in the pathogenesis of OCD. O’Reilly, Frank, and colleagues52–54 have proposed a model for the interaction of the basal ganglia and OFC in reward learning. Their model is based on previous models that had been developed to explain the role of the basal ganglia in motor control, “[s]pecifically in the motor domain, various authors suggest that the basal ganglia are specialized to selectively facilitate adaptive motor actions, while suppressing others.55 This same functionality may hold for more advanced tasks, in which the “action” to facilitate is the updating of prefrontal working memory representations.”52–54 In their model, the basal ganglia serve a gating function by biasing the activation of representations in the PFC (i.e., set the “gain” for activation of motor and action series in the frontal lobes). Graybiel and Rauch56 have also stressed the role of the basal ganglia in influencing motor pattern generators in the brainstem and spinal cord, and influencing “cognitive pattern generators” in the cerebral cortex.
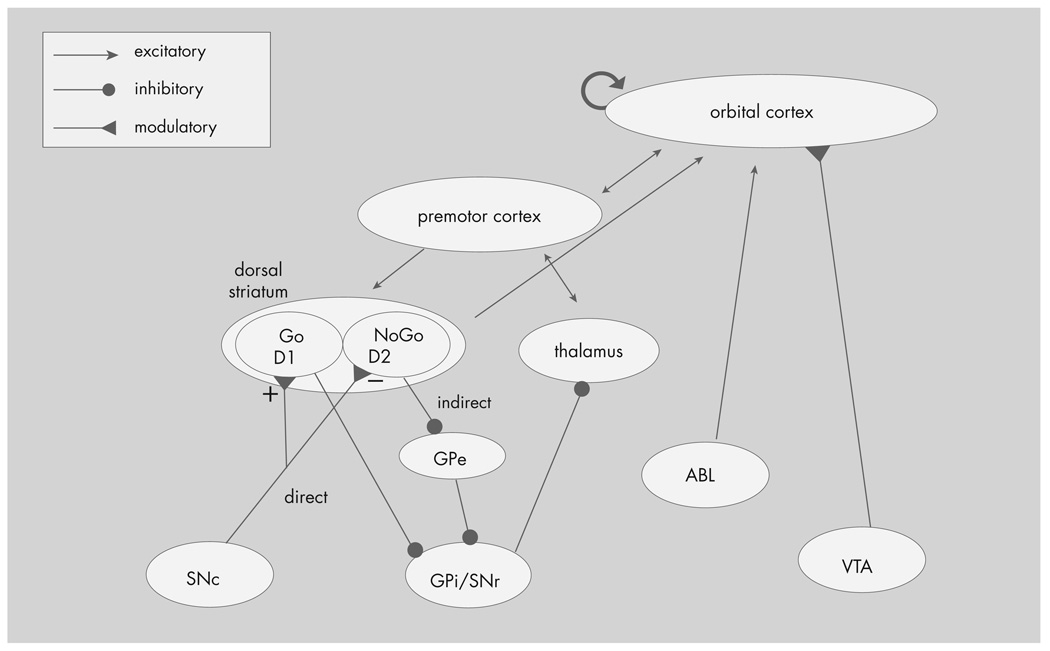
The basal ganglia have two opposing pathways: the direct “Go” pathway and the indirect “NoGo” pathway (see Figure 2). Cells in the direct pathway primarily express excitatory D1, and cells in the indirect pathway express inhibitory D2, dopaminergic receptors. Thus reinforcement, coded by an increase in dopamine, can bias the gating of the basal ganglia toward future activation of the rewarded behavior (i.e., facilitate learning). O’Reilly, Frank, and colleagues52–54 propose that the OFC exerts top-down control of the basal ganglia by representing reinforcement magnitudes to the basal ganglia. Thus, in their view, the basal ganglia and the OFC provide a dynamic system which both evaluates the reinforcement of current stimuli and reinforces rewarded behaviors.54 In their model, the amygdala codes for stimulus intensity, but not valence. Their model is supported by findings that dopamine-dependent reward mechanisms are activated in motor and habit learning in rats and can be disrupted by striatal lesions,57 findings with patients with Parkinson’s disease,58 healthy control subjects given medications that affect the dopamine system,59 and computational modeling.52,54
FIGURE 2. Model of Interaction of Basal Ganglia with Other Brain Structures.
GPi = internal segment of globus pallidus; GPe = external segment of globus pallidus; SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata; VTA = ventral tegmental area; ABL = basolateral amygdala. Reproduced with permission from Frank et al. 2006 (54)
Anterior Cingulate Cortex (ACC)
The ACC also appears to be involved in OCD based on the imaging findings discussed above. The ACC plays a role in decision making. It responds to the occurrence of conflicts in information processing,60 including errors61–65 and increased likelihood of errors.66 Errors appear to be detected as discrepancies between actual and intended events.67 There is evidence that the ACC is associated with negative emotional states; activation of the ACC is observed with anxiety (including in disorders other than OCD)68 and physical pain.69
Thalamus
The thalamus shows more activation in patients with OCD compared to healthy comparison subjects.5 This is likely related to the role of the thalamus as a relay and integrative site for other brain areas activated in OCD, such as the basal ganglia and the OFC. A large literature supports the existence of parallel circuits linking the basal ganglia, thalamus, and cortex with circuits communicating with separate areas of the frontal cortex.70,71 These circuits have been the basis of several of the neuroanatomical models of OCD.
The Prefrontal Cortex (PFC) in the Execution and Reinforcement of Complex Behaviors
Inherent in our discussion so far is the assumption of a conservation of mechanisms of reward between nonhuman primates and humans and between events rewarding to nonhuman primates and humans (e.g., receiving food) and events that are specifically rewarding to humans (e.g., receiving money). However, comparing human and nonhuman reward raises the following question: what are the boundaries of the rewarding event for complex behaviors? Rewarding events for complex behaviors are often associated with several superordinate and subordinate rewarding events. For example, the rewarding experience of enjoying a dinner in a restaurant with a friend lasts a few hours, but it is a component of the larger rewarding friendship (which could last for a lifetime) and is composed of shorter rewarded events (e.g., enjoying a story your friend tells during the dinner). How are the boundaries set?
Our laboratory has proposed that the PFC stores memories of behavioral sequences termed “structured event complexes” (SECs) that have beginnings and ends, but exist in nested hierarchies. For example, eating in a restaurant would be such an SEC, and it would exist as several different variants (e.g., eating at a fast-food restaurant, eating at a fancy French restaurant, etc.) which would come under the superordinate category of eating in a restaurant. We have proposed that these SECs are abstractly encoded in the PFC similar to the way in which memories of complex motor programs are encoded in more posterior cortexes. We hypothesize that the perceived boundaries of these SECs signal transitions for the purposes of reward, and that completing SECs can be inherently rewarding. In support of this hypothesis, prefrontal cortical neurons in macaques exhibit phasic peaks of spike activity at the beginning and endpoint of sequential tasks.72
In this theory, representations in the PFC differ from other types of memories that people are more familiar with, for example, semantic memory processes (Table 3). Semantic (knowing the capital of France) memory is usually explicit (associated with conscious awareness), but it can be implicitly primed. SECs, in contrast, are usually implicitly recalled and executed often over long periods of time in the absence of directly relevant stimuli. This mechanism is most similar to that of procedural memory in the premotor cortex and supplementary motor area; one is not consciously aware of the ability to swim or ride a bicycle, yet one can execute these motor memories with minimal conscious control. We hypothesize that behavioral programs in the human PFC evolved from simpler motor programs in more posterior cortex.73
TABLE 3.
Some Types of Memory
| Memory System | Major Anatomical Structures Involved | Length of Storage of Memory | Type of Awareness | Examples | Disorder that can Impair Memory |
|---|---|---|---|---|---|
| Semantic memory | Extrasylvian temporal lobes | Minutes to years | Explicit (associated with conscious awareness) or implicit | Knowing who was the first president of the United States, the color of a lion, and how a fork differs from a comb | Semantic dementia |
| Episodic memory | Medial temporal lobes, hippocampus, anterior thalamic nucleus, mammillary body, fornix, prefrontal cortex | Minutes to years | Usually explicit | Remembering what you had for dinner last night and what you did for your last birthday | Alzheimer’s disease |
| Procedural memory | Basal ganglia, cerebellum, parietal lobe, supplementary motor area, premotor cortex | Minutes to years | Usually implicit | Riding a bicycle, learning the sequence of numbers on a touchtone phone “by touch” | Ideational and ideomotor apraxia |
| SECs |
Social: ventral prefrontal cortex Non-social: dorsal prefrontal cortex |
Minutes to years | Usually implicit |
Social: how to go on a date Non-social: how to set the clock on your VCR |
Behavioral-variant frontotemporal dementia |
Adapted with permission from Budson AE, Price BH: Memory dysfunction. N Engl J Med 2005; 352:692–699
SEC = structured event complex.
The types of memory outlined in Table 3 work together in an integrated manner. For example, imagine you meet someone at a party and he or she gives you his or her telephone number. You will likely encode an episodic memory that this event occurred. You will keep the telephone number active in working memory until you can either write it down or encode the number in long-term memory through active rehearsal. If you dial the number enough, you may forget the actual digits and instead rely on the procedural memory of dialing the number on a touch-tone phone. Assuming that getting the other person’s number is a successful social outcome, you will encode a memory in the PFC of the behavioral sequence that led to this outcome, to be able to best repeat it in a similar situation.73–75
This theory asserts that related SECs are neuroanatomically localized together in specific areas of the PFC, an assertion that has obtained empiric support. The frequency with which healthy subjects had experienced an event determined how anterior or posterior fMRI activation was observed when the subjects determined if the events were correctly ordered,76 neurons in the lateral PFC of monkeys selectively exhibit activity for specific categories of behaviors77 and when the monkeys remember and perform particular action sequences.78 Patients with PFC lesions (and thus disruption of their SECs) should show deficits in ordering events into a coherent sequence. Patients with PFC damage have particular difficulty sequencing events,79 can generate a normal number of actions, but have difficulty ordering those actions into a coherent script,80,81 and appear to lose infrequently used SECs before frequently used (and thus overlearned) SECs.80,82 Patients with dementias affecting the frontal lobes typically demonstrate deficits in social behaviors with relative preservation of episodic memory, while patients with dementia initially affecting the medial temporal lobes (e.g., Alzheimer’s disease) typically demonstrate initial deficits in episodic memory with relative preservation of social behavior.83
The human brain can flexibly respond to events with an almost infinite variety of behaviors. How can such a large number of potential behaviors be encoded as memories? We hypothesize that humans (and other animals) can flexibly coactivate and combine SECs to form a large number of behaviors. This process could be analogous to language; a finite number of words and linguistic rules allow humans to form an almost infinite variety of expressions. In support of this, healthy adults are able to flexibly order the components of a plan while young children and patients with PFC damage tend to rigidly execute plans.84 Also, rather than performing an infinite variety of behaviors, healthy comparison subjects generally perform a relatively small number of high frequency behaviors in their daily lives.85
So far, we have explained the reinforcing properties of performing SECs. However, the interaction between behavior and reward is bidirectional and dynamic. If performing a certain SEC is rewarding, being prevented from performing that sequence would be punishing. Completion of a punishing SEC would result in reinforcement when the punishment is removed after completion of the behavior. An example of this is doing one’s taxes. Few people enjoy doing their taxes, but they do enjoy the feeling of relief when they have completed this onerous, but necessary, task.
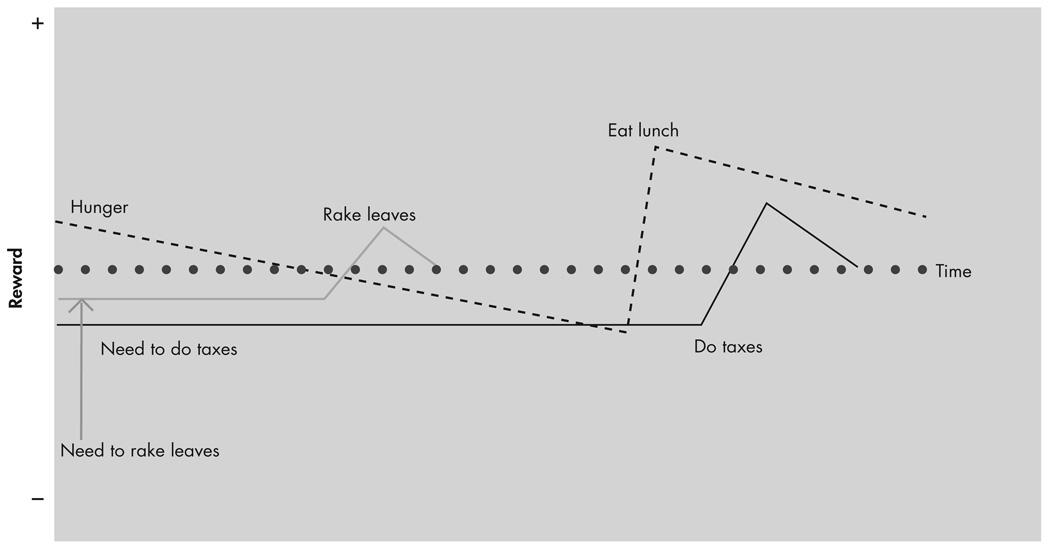
Expectation of outcome can affect the reward value of an event. Schultz 86 has demonstrated the importance of “prediction error” in reward and learning. Prediction error refers to the difference (positive or negative) between the expected and received reward. Certain dopamine neurons in the pars compacta of the substantia nigra and the medially adjoining ventral tegmental area (groups A8, A9, and A10) and the OFC of macaques respond most to a stimulus that is paired with an unpredicted reward.86,87 Thus, the same stimulus could be rewarding or punishing depending on expectation. For example, you could receive punishment by learning that one-half of your lottery winnings will go to taxes after learning that you have won the lottery, even though it is a large net financial gain. The dynamic nature of reward over time and the role of expectation make the concept of a “baseline” of reward state for an animal difficult to define. We believe that the reward state of an animal at any given time is, in part, a summation of the reward values associated with the many different SECs active and at different stages of completion at that moment (see Figure 3).
FIGURE 3. Reward Values Associated with Active SECs at a Given Time.
This figure shows a hypothesized schematic representation of the changes in a few active motivational/reward states. The overall reward state at a given time of an animal will be the summation of the component reward states, and the emotional “flavor” of the reward state is provided through interactions with limbic structures.
SECs = structured event complexes
Previous Models of OCD
So far, we have presented research from Rolls47 showing that the OFC is central for reward mechanisms. The ACC plays a central role in error detection for complex behaviors. Frank, O’Reilly, and colleagues52–54 have proposed a model with the basal ganglia setting the “gain” for activation of representations in the PFC similar to the way in which the basal ganglia sets the “gain” for activation of motor programs in the supplemental motor area and premotor cortex. Our laboratory has asserted that SECs exist in the PFC similarly to motor programs contained in the supplementary motor area and premotor cortex, and that performance of those SECs is rewarded. Schultz and colleagues86,87 have demonstrated that areas involved in reward, including the OFC, are activated by a difference between the expected and observed outcomes of events. In this part, we discuss some current models of OCD (see Table 4 for a comparison). In the next section we propose a new model that suggests that the symptoms of OCD arise from abnormalities in the reward mechanisms of complex behaviors.
TABLE 4.
A Comparison Between Some Models of OCD
| Model | Type | Strengths | Limitations |
|---|---|---|---|
| Standard | Neuroanatomic | Consistent with imaging, surgical, and lesion findings | Does not explain psychological symptoms of OCD |
| Direct/indirect striatal pathway | Neuroanatomic | Refines the standard model | Does not explain psychological symptoms of OCD |
| Executive dysfunction | Integrative | Patients with prefrontal cortex damage and executive dysfunction can demonstrate perseverative behaviors | The extent of executive dysfunction in idiopathic OCD is unclear |
| Failure of inhibition | Integrative | OCD patients demonstrate deficits of response inhibition | Connection of finding to symptoms of OCD not yet clear |
| Release | Integrative | Utilizes findings on the basal ganglia from animal research | Focuses on basal ganglia, difficulty applying simple behaviors in lizards to the symptoms of OCD in humans |
| Feeling of knowing | Psychological | Provides a richer explanation of the symptoms of OCD | Does not specify nature or anatomical location of representations responsible for symptoms |
| SEC/OCD model | Integrative | Integrates neuroanatomical and psychological explanations of the symptoms of OCD | Many hypotheses, such as the rewarding properties of behavioral sequences and the behavioral effects of brain lesions, are untested |
SEC = structured event complex; OCD = obsessive-compulsive disorder
The Standard Model
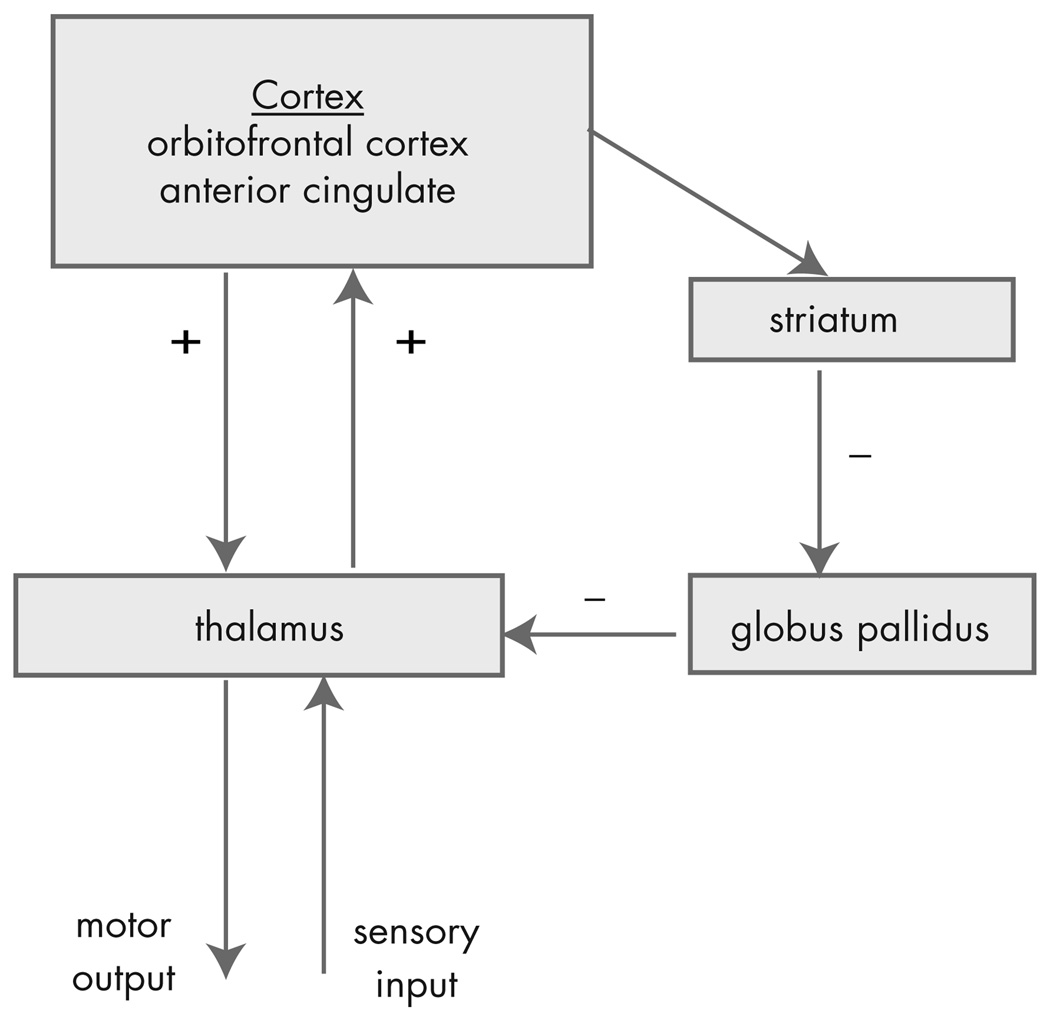
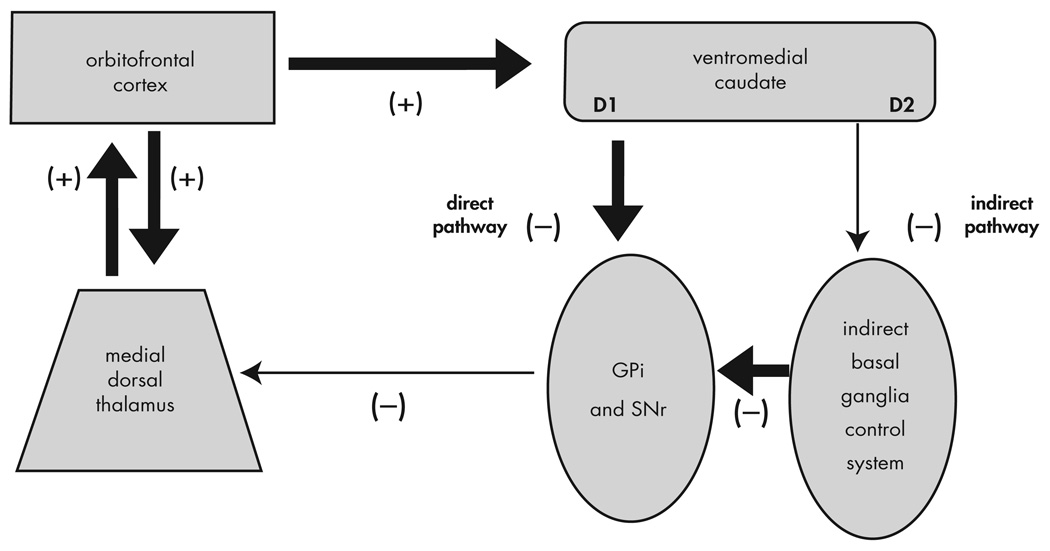
The most accepted neuroanatomic model of OCD is based on the finding that there are separate cortico-basal ganglia-thalamic-cortical loops,70,71 (although recent evidence suggests that these loops are not as separate as previously thought).88 The standard anatomic model of OCD proposes that the symptoms of OCD are caused by dysfunction of elements of a PFC-basal ganglia-thalamic-PFC loop89–93 (Figure 4). The imaging findings presented above support that these structures are involved in OCD. In addition, surgical interruption6,9–14 or deep brain stimulation94 of the anterior internal capsule can reduce the symptoms of OCD. Overactivation of this loop is suggested by the hypermetabolism of these structures observed in the imaging studies presented in the first part of this article.
FIGURE 4. The Standard Model of OCD.
OCD = obsessive-compulsive disorder.
Excitatory connections are labeled +; inhibitory connections are labeled −. Reproduced with Permission from Rauch et al. 2006 (94)
The advantage of this model is that it is consistent with the evidence collected to date on OCD. This model forms the neuroanatomic basis of most subsequent models. The limitation of the standard model is that while it specifies the brain structures involved, it does not provide a psychological explanation for the specific symptoms of OCD.
Direct/Indirect Striatal Pathways
The standard anatomic model has been refined by specifying that overactivation of the direct pathway in the basal ganglia relative to the indirect pathway results in an orbitofrontal-subcortical hyperactivity (Figure 5). According to this model, “[p]atients with OCD, however, may have a low threshold for system ‘capture’ by socioterritorial stimuli, possibly caused by excess ‘tone’ in the direct relative to the indirect orbitofrontal-subcortical pathway, allowing for concerns about danger, violence, hygiene, order, and sex to rivet attention to themselves.”95
FIGURE 5. A Neuroanatomical Model That Incorporates Direct and Indirect Striatal Pathways.
GPi = globus pallidus interna; SNr = substantia nigra pars reticulata
In OCD, the direct pathway is strongly activated in relation to the indirect pathway resulting in OFC-subcortical hyperactivity. Large arrows represent inputs that are strengthened in patients with OCD. Reproduced with permission from Stein 2006 (96)
This model adds explanatory power to the standard model by proposing a specific mechanism within the striatum that results in overactivation of the neuroanatomical loop of the standard model. In support of this model, patients with excessive nigrostriatal dopaminergic input (such as patients with Huntington’s disease) have excessive motor output.95 This model is supported by the imaging findings presented in the first part of this article and because damage to specific basal ganglia structures (e.g., caudate) is associated with the development of symptoms of OCD. The limitation of this model, similar to the standard neuroanatomical model, is that it does not specify or explain the psychological mechanisms of OCD. For example, how do concerns “rivet attention to themselves?” It also focuses on dysfunction in the basal ganglia, but does not specify the role of the OFC or explain how patients with OFC lesions can develop aspects of the OCD syndrome.
Models Based on Other Brain Areas
Other theorists have focused more on the orbitofrontal cortex (OFC) in modeling OCD. Chamberlain et al.7 discuss the failure of inhibition in patients with OFC lesions and propose that a similar failure to inhibit contributes to symptoms of OCD. Some have implicated the anterior cingulate cortex (ACC) by proposing that faulty error detection may be central to the pathogenesis of OCD.91,96 Others have suggested that dysfunction of reward mechanisms may contribute to the symptoms of OCD.6
“Release” Models
Baxter92 demonstrated the role of the basal ganglia in “releasing” territorial display programs in lizards and proposed that the basal ganglia in patients with OCD may inappropriately “release” territorial behaviors. Stein and Lochner97 have conceptualized OCD as a “dysfunction in the control of procedural strategies with inappropriate release of symptoms ranging from simple motoric stereotypies to more complex behavioral programs.” In the same paper, they note that many of the structures involved in OCD are also involved in learning and reward. They also observe that dopaminergic agonists can increase the symptoms of OCD and putative OCD spectrum disorders, including Tourette’s syndrome. Where in the brain and how these “behavioral programs” are represented, or why they are inappropriately released in OCD, is not specified. Graybeil and Rauch56 hypothesized that anxiety suffered by OCD patients may indicate a “lack of loop closure between expected outcomes and the chunks of behavior that should generate them.”
“Feeling of Knowing” Models
Szechtman and Woody98 have proposed a model of OCD based on the hypothesis that the symptoms of OCD arise from an inability to generate a normal “feeling of knowing” that would otherwise signal task completion. This deficit results in an overactivation of neural systems designed to respond to danger in the environment (which they term the “security motivation system”). They base their work on earlier cognitive theories of OCD including those of Janet,1 Pitman,99 and Reed.100 Their theory is supported by interviews that revealed that the majority of OCD patients describe their symptoms as being “unable to stop” the behavior rather than being forced to continue.100,101 Also, patients with OCD often engage in few but extended episodes of compulsive behavior during the day rather than excessively frequent episodes but normal duration, which is consistent with an inability to stop the compulsive behavior.102
The “Structured Event Complex” Model of OCD
The Structured Event Complex (SEC)/OCD model builds upon those proposed by Stein and Lochner97 and Szechtman and Woody.98 However, in the SEC/OCD model we specify how abnormal interactions of representations of complex behaviors in the PFC, reward information in the OFC, error detection in the ACC, and reward and limbic structures can result in the symptoms of OCD. To our knowledge, this is the first model of OCD to fully integrate the neuroanatomy and psychological experience of OCD.
We propose that the initiation of an SEC is accompanied by a motivational signal, likely determined through interaction between reward structures (including the OFC) and limbic structures, experienced as motivational anxiety. This anxiety likely exists to motivate animals to complete necessary SECs. Completion of the SEC is accompanied by a reward signal, experienced as relief from anxiety. People with OCD have a deficiency in this process. They may receive only a fraction of the full relief from anxiety that most healthy people receive upon completing the SEC. Even after completion, the patient is left with the unpleasant sensation that the SEC is not done. In this way, our model resembles the “feeling of knowing” model proposed by Szechtman and Woody.98 A difference is that we specify the nature and location of the task that is perceived as not completed (SECs contained in the PFC), and the relative contribution of other brain systems involved (see Figure 6).
FIGURE 6. Schematic Representation of the SEC/OCD Model.
Brain areas are listed with summary of function. In healthy people, initiation of an SEC can generate motivational anxiety. Completion of such an SEC results in a reward signal and a reduction in anxiety. People with OCD do not receive the full reward signal and reduction of anxiety upon completion of an SEC, giving them the sensation of leaving a task undone, which they attempt to remove by repeatedly performing an SEC or segments of an SEC. Symptoms of OCD can be acquired by damage to the basal ganglia, OFC, or ACC.
OCD = obsessive-compulsive disorder; SEC = structured event complex; OFC = orbitofrontal cortex; ACC = anterior cingulate cortex;
In this review, we have focused on the separable roles of the brain structures found to be involved in OCD. However, all of these brain areas communicate extensively, and are frequently coactivated in imaging studies of OCD. Our laboratory has previously proposed that the subjective experience of a particular mental state is biologically represented by synchronous activity of a system of brain areas, each of which contributes a component to the experience.51,103 In our model of OCD, the experience of OCD symptoms comes from binding together SECs in the prefrontal cortex (PFC), the reward signal in reward structures and the OFC, the threshold for activation of SECs in the basal ganglia, emotional relevance by limbic structures, and the error signal by the ACC. For example, an active contamination obsession in a patient with OCD could involve representations of the following:
an error signal of an incomplete task generated in the ACC;
punishment represented in the OFC and reward structures;
the emotional experience of anxiety arising from limbic structures;
a lowering of the threshold for a compensatory SEC in the basal ganglia;
activation of the compensatory SEC contained in the PFC.
The specific degree and nature of these coactivations could correspond to the particular cognitive and emotional state of the patient with OCD. In this way, we argue against the commonly held view that the PFC opposes emotional input from the limbic system.104
In the SEC/OCD model, the OCD patient’s cognitive interpretation of the feeling of leaving an SEC incomplete forms the basis of an obsession. The feeling is unconscious, but the patient explicitly attempts to assign it a cause and reduce it through conscious action. The explicit interpretation of the feeling is the “obsession” and the conscious attempt to reduce it is the “compulsion.” This interpretation is influenced by individual and societal attributes, but is often based on common themes (e.g., contamination or pathologic doubt). The societal influence on this interpretation can be observed in the change that has occurred in obsessional themes over time (e.g., no one had germ contamination obsessions prior to the proposal of the germ theory of infection). Patients with OCD may receive a fraction of the full reward signal with each performance of the SEC (or its elements). Thus each performance of the SEC (or its elements) can be partially successful at alleviating the anxiety reinforcing a maladaptive learning mechanism. For example, most of us, if our hands are dirty, feel a motivational anxiety to perform the SEC of washing our hands. Upon completion of this SEC we receive relief from the motivational anxiety. Patients with OCD do not receive full relief from the anxiety upon completion of the SEC, but may receive partial relief. They may, understandably, cognitively appraise the implicit continued motivational anxiety to mean that their hands are still dirty, despite having washed them. Thus there is dissociation in patients with OCD between the conscious awareness that one’s hands are clean, and the “feeling” that they are not. Consequently, the patient may repeat the hand-washing SEC, receiving partial relief with each repetition, until the motivational anxiety is resolved.
Findings presented above suggest that the basal ganglia can set the threshold for activation of motor programs in the premotor cortex and supplemental motor area by facilitating some motor programs and inhibiting others through reinforcement learning.53,54,105 In the SEC/OCD model, the basal ganglia perform a similar role for the activation of SECs in the PFC. We propose that the basal ganglia set the threshold for activation of SECs, and if this threshold is lowered, SECs can be overactivated, resulting in excessive motor activity (e.g., tics) and/or the excessive activation of SECs. Thus damage to the basal ganglia that results in a reduction of its net inhibitory output to the OFC (such as occurs with caudate damage) can result in symptoms of OCD, as observed in cases of autoimmune basal ganglia damage.106 The role of the basal ganglia in our model has some of the properties proposed in the “release” models of OCD.92,97
Evidence for the SEC/OCD Model
The Structured Event Complex (SEC)/OCD model fits well with the research presented above about the roles of the OFC, the basal ganglia, and the ACC. The OFC is central for receiving and interpreting reward signals in a social and behavioral context, and can be especially activated when there is a large difference between the expected and received reward signal, as would be the case for patients with OCD in the SEC/OCD model. The basal ganglia set the threshold for activation of SECs. If this threshold were lowered, these SECs could be overactivated, resulting in excessive motor activity (e.g., tics) and/or the excessive activation of SECs. The ACC is involved in error detection (especially a discrepancy between expected and observed outcome), and thus in the SEC/OCD model we would expect it to be overactive in OCD where the OFC is receiving a neural message that the SEC was not successfully completed (as is observed in imaging studies). Patients with OCD demonstrate greater ACC activation than healthy subjects when the patients make errors that do not elicit OCD symptoms.107
Most of the evidence in favor of the “feeling of knowing” model supports the SEC/OCD model as well: patients with OCD usually report that they experience their symptoms as a feeling of being unable to stop an action with a lack of a sense of completion of an action,100,101 and engage in few but extended episodes of compulsive behavior during the day rather than episodes of excessive frequency but normal duration, suggestive of an inability to stop the compulsive behavior.102
The SEC/OCD model asserts that obsessions are primary in OCD and that compulsions are a secondary response to the obsession. In support of this, obsessions and compulsions most frequently co-occur in idiopathic OCD in adults,4 obsessions and compulsions are usually thematically related,108 and the majority of OCD patients report the obsession as the primary motivation for the compulsion.100,101 Specific compulsions are associated with particular brain areas,109 consistent with the theory from our laboratory that specific SECs are regionally separable.73 There is some evidence that the performance of SECs is inherently rewarding. Direct stimulation of reward pathways in rats resulted in repetitive stereotyped complex behaviors.99,110 Humans performing SECs111 or responding to novel stimuli112 show activation of reward structures on fMRI.
Experimental Predictions
The SEC/OCD model provides several testable hypotheses. Most previous anatomical models have proposed a primary source of the psychopathology of OCD (usually either the basal ganglia or OFC). A limitation of that approach is that it fails to explain how damage to several different structures can lead to acquired symptoms of OCD in lesion studies. In the SEC/OCD model, we hypothesize that damage to different brain structures, or damage to the communication between structures, will result in different and separable aspects of the OCD syndrome (Table 5). Because the PFC contains memories of SECs, patients with acquired OCD from PFC damage should show impaired performance of SECs. This is in contrast to patients with idiopathic OCD and patients with OCD acquired from basal ganglia damage who should have relatively preserved performance of SECs. We also hypothesize that, because of the role of the OFC in perceiving and interpreting reward and anxiety signals, patients with acquired symptoms of OCD from OFC damage will have fewer obsessions and less anxiety compared to patients with idiopathic OCD who have comparable levels of compulsive behavior. This has been reported,17 and we have clinically observed this in our laboratory in patients with frontotemporal dementia (Huey, presentation, UCSF 5th International Conference on Frontotemporal Dementia, 2006). However the literature on this topic is limited because studies have been performed retrospectively on patients identified and defined by having the entire OCD syndrome. We also hypothesize that patients with symptoms of OCD acquired from brain injury will show hypometabolism of injured structures and areas of the brain that are closely connected, in contrast to the hyperactivation of these brain areas observed in idiopathic OCD. Prospective studies on patients with brain damage should be performed to determine the relative contributions of different brain areas to the OCD syndrome (Table 5). Newer imaging techniques such as diffusion tensor imaging may be useful for exploring abnormalities in white matter tracts between brain areas involved in idiopathic OCD.
TABLE 5.
Predictions of the SEC/OCD Model for Idiopathic OCD and Symptoms of OCD Acquired from Brain Lesions
| Idiopathic OCD | OCD Symptoms Secondary to PFC Damage | OCD Symptoms Secondary to Basal Ganglia Damage | |
|---|---|---|---|
| Type of repetitive behavior | Complex | Simple and complex | Simple and complex |
| Executive dysfunction | Less | Yes | Less |
| Performance of SECs | Intact | Impaired | Intact |
| Anxiety | Yes | No | Less |
| Obsessions | Yes | No | Less |
| Associated with motor tics | Yes | No | Yes |
| Functional imaging | ↑ tracer uptake in OFC, caudate, ACC | ↓ tracer uptake in OFC, caudate, ACC | ↓ tracer uptake in basal ganglia (esp. caudate), ↑ in OFC |
SEC = structured event complexes; OCD = obsessive-compulsive disorder; OFC = orbitofrontal cortex; ACC = anterior cingulate cortex; PFC = prefrontal cortex
The potential rewarding properties of the completion of structured event complexes (SECs) in healthy subjects have been minimally examined. Functional MRI studies of symptom provocation with careful clinical correlation in patients with idiopathic OCD could be performed with the hypothesis that the ACC will be initially activated (and correspond to the initial detection of the provoking stimulus), followed by OFC and limbic activation (corresponding to the anxiety provocation), then the basal ganglia and PFC (corresponding to activation of the compensatory compulsion). The SEC model of OCD is amenable to computer modeling, similar to that performed by Frank, O’Reilly, and colleagues52,54 to model basal ganglia-OFC interactions. Finally, the SEC/OCD model suggests that lesioning certain brain areas in animals can result in behaviors similar to aspects of the idiopathic OCD syndrome (Table 5).
CONCLUSION
Imaging, surgical, and lesion studies suggest that the OFC, basal ganglia, and ACC are involved in the pathogenesis of OCD. Recent research on the normal function of these brain areas demonstrates the role of the OFC in reward, the basal ganglia in affecting the threshold for activation of motor and behavioral programs, and the ACC in error detection. We discussed theories that the PFC stores memories of behavioral sequences (called SECs) and that initiation of an SEC results in motivational anxiety that is relieved upon completion. We discussed previous models of OCD and proposed a new model of OCD (the SEC/OCD model), which hypothesizes that a deficit in the relief of anxiety that usually accompanies the completion of an SEC is responsible for the symptoms of OCD. Specifically, this anxiety forms the basis of an obsession, and a compulsion is an attempt to receive relief from the anxiety by repeating parts of, or an entire, SEC. We discussed empiric support for the SEC/OCD model and specific experimental predictions of the model. We believe that the SEC/OCD model explains the specific symptoms of OCD and integrates the neuroanatomy and psychology of this disorder better than previous models.
Addendum
Studies identified in a non-systematic review of papers published between the completion and publication of this article are mostly supportive of the SEC/OCD theory. Frontotemporal dementia affecting the OFC is associated with stereotypic behaviors as predicted in Table 5.151 Deficits in reversal learning linked to the OFC were demonstrated in patients with OCD152,153 and their unaffected relatives,153 supporting the assertion of the SEC/OCD theory that the inability to stop an action is associated with risk for OCD. Similar results were found for task-switching in patients with OCD.154 Damage to the medial striatum (including the head of the caudate) in monkeys also resulted in impairments in reversal learning, as would be predicted from Table 5.155 One study showed specific sites of grey and white matter volume decreases associated with specific symptom clusters of OCD, which is supportive of the SEC/OCD theory, which proposes that the PFC contains separable representations.156 However, some of the associations occurred in brain areas other than the PFC, which is not supportive of the theory (although the association seen in these areas, such as caudate, could be related to their action on the PFC).
Acknowledgments
We thank Ben Greenberg and Anthony Pinto of Brown University for their very helpful comments and Nicole Armstrong for her help with manuscript preparation. This research was supported by the Intramural Research Program of NIH, NINDS, and NIMH.
Contributor Information
Edward D. Huey, Litwin-Zucker Research Center for the Study of Alzheimer’s Disease and Memory Disorders in Great Neck, N.Y.
Roland Zahn, Cognitive Neuroscience Section, National Institute of Neurological Disorders and Stroke, at NIH in Bethesda, M.D.
Frank Krueger, Cognitive Neuroscience Section, National Institute of Neurological Disorders and Stroke, at NIH in Bethesda, M.D.
Jorge Moll, Cognitive and Behavioral Neuroscience Unit at LABS–D’Or Hospital Network in Rio de Janeiro, Brazil
Dimitrios Kapogiannis, Cognitive Neuroscience Section, National Institute of Neurological Disorders and Stroke, at NIH in Bethesda, M.D.
Eric M. Wassermann, Brain Stimulation Unit at the National Institute of Neurological Disorders and Stroke, NIH, in Bethesda.
Jordan Grafman, Cognitive Neuroscience Section, National Institute of Neurological Disorders and Stroke, at NIH in Bethesda, M.D.
References
- 1.Janet P. Les Obsessions et la Psychasthenie [Obsessions and Psychasthenia] 2nd ed. Paris: Alcan; 1903. [Google Scholar]
- 2.Karno M, Golding JM, Sorenson SB, et al. The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry. 1988;45:1094–1099. doi: 10.1001/archpsyc.1988.01800360042006. [DOI] [PubMed] [Google Scholar]
- 3.Stein DJ. Obsessive-compulsive disorder. Lancet. 2002;360:397–405. doi: 10.1016/S0140-6736(02)09620-4. [DOI] [PubMed] [Google Scholar]
- 4.Foa EB, Kozak MJ, Goodman WK, et al. DSM-IV field trial: obsessive-compulsive disorder. Am J Psychiatry. 1995;152:90–96. doi: 10.1176/ajp.152.1.90. [DOI] [PubMed] [Google Scholar]
- 5.Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clin Psychol Rev. 2006;26:32–49. doi: 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Aouizerate B, Guehl D, Cuny E, et al. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery, and physiology. Prog Neurobiol. 2004;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain SR, Blackwell AD, Fineberg NA, et al. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Koran LM. Obsessive-compulsive and related disorders in adults. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 10.Mindus, Jenike MA. Neurosurgical treatment of malignant obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15:921–938. [PubMed] [Google Scholar]
- 11.Jenike MA. Neurosurgical treatment of obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;35:79–90. [PubMed] [Google Scholar]
- 12.Binder DK, Iskandar BJ. Modern neurosurgery for psychiatric disorders. Neurosurgery. 2000;47:9–23. doi: 10.1097/00006123-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg BD, Murphy DL, Rasmussen SA. Neuroanatomically based approaches to obsessive-compulsive disorder: neurosurgery and transcranial magnetic stimulation. Psychiatr Clin North Am. 2000;23:671–686. doi: 10.1016/s0193-953x(05)70188-x. xii. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg BD, Price LH, Rauch SL, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin N Am. 2003;14:199–212. doi: 10.1016/s1042-3680(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 15.Coetzer BR. Obsessive-compulsive disorder following brain injury: a review. Int J Psychiatry Med. 2004;34:363–377. doi: 10.2190/XENN-NNWT-7N2K-R26A. [DOI] [PubMed] [Google Scholar]
- 16.Laplane D, Levasseur M, Pillon B, et al. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study, part 3. Brain. 1989;112:699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- 17.Berthier ML, Kulisevsky J, Gironell A, et al. Obsessive-compulsive disorder associated with brain lesions: clinical phenomenology, cognitive function, and anatomic correlates. Neurology. 1996;47:353–361. doi: 10.1212/wnl.47.2.353. [DOI] [PubMed] [Google Scholar]
- 18.Berthier ML, Kulisevsky JJ, Gironell A, et al. Obsessive-compulsive disorder and traumatic brain injury: behavioral, cognitive, and neuroimaging findings. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:23–31. [PubMed] [Google Scholar]
- 19.Carmin CN, Wiegartz PS, Yunus U, et al. Treatment of late-onset OCD following basal ganglia infarct. Depress Anxiety. 2002;15:87–90. doi: 10.1002/da.10024. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigo Escalona P, Adair JC, Roberts BB, et al. Obsessive-compulsive disorder following bilateral globus pallidus infarction. Biol Psychiatry. 1997;42:410–412. doi: 10.1016/s0006-3223(97)00262-x. [DOI] [PubMed] [Google Scholar]
- 21.Cummings JL, Cunningham K. Obsessive-compulsive disorder in Huntington’s disease. Biol Psychiatry. 1992;31:263–270. doi: 10.1016/0006-3223(92)90049-6. [DOI] [PubMed] [Google Scholar]
- 22.Chacko RC, Corbin MA, Harper RG. Acquired obsessive-compulsive disorder associated with basal ganglia lesions. J Neuropsychiatry Clin Neurosci. 2000;12:269–272. doi: 10.1176/jnp.12.2.269. [DOI] [PubMed] [Google Scholar]
- 23.Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 24.Caparros-Lefebvre D, Cabaret M, Godefroy O, et al. PET study and neuropsychological assessment of a long-lasting post-encephalitic parkinsonism. J Neural Transm. 1998;105:489–495. doi: 10.1007/s007020050072. [DOI] [PubMed] [Google Scholar]
- 25.Anderson SW, Damasio H, Damasio AR. A neural basis for collecting behaviour in humans. Brain. 2005;128:201–212. doi: 10.1093/brain/awh329. [DOI] [PubMed] [Google Scholar]
- 26.Rosen HJ, Allison SC, Schauer GF, et al. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 28.Rolls ET. The functions of the orbitofrontal cortex. In: Stuss D, Knight R, editors. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. pp. 354–375. [Google Scholar]
- 29.Rolls ET, Scott TR, Sienkiewicz ZJ, et al. The responsiveness of neurones in the frontal opercular gustatory cortex of the macaque monkey is independent of hunger. J Physiol. 1988;397:1–12. doi: 10.1113/jphysiol.1988.sp016984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaxley S, Rolls ET, Sienkiewicz ZJ. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- 31.Rolls ET. Information processing in the taste system of primates. J Exp Biol. 1989;146:141–164. doi: 10.1242/jeb.146.1.141. [DOI] [PubMed] [Google Scholar]
- 32.Rolls ET. Neural processing related to feeding in primates. In: Legg CR, Booth DA, editors. Appetite: Neural and Behavioral Bases. Oxford: Oxford University Press; 1994. pp. 11–53. [Google Scholar]
- 33.Mora F, Avrith DB, Phillips AG, et al. Effects of satiety on self-stimulation of the orbitofrontal cortex in the rhesus monkey. Neurosci Lett. 1979;13:141–145. doi: 10.1016/0304-3940(79)90031-4. [DOI] [PubMed] [Google Scholar]
- 34.O’Doherty J, Rolls ET, Francis S, et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- 35.Kringelbach ML, O’Doherty J, Rolls ET, et al. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 36.O’Doherty J, Rolls ET, Francis S, et al. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 37.Small DM, Gregory MD, Mak YE, et al. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 38.Gottfried JA, Deichmann R, Winston JS, et al. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci. 2003;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- 40.Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 41.Elliott R, Newman JL, Longe OA, et al. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Doherty J, Winston J, Critchley H, et al. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- 43.Decety J, Jackson PL, Sommerville JA, et al. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23:744–751. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moll J, Krueger F, Zahn R, et al. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci U S A. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 47.Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1212–1225. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- 48.McEnaney KW, Butter CM. Perseveration of responding and nonresponding in monkeys with orbital frontal ablations. J Comp Physiol Psychol. 1969;68:558–561. doi: 10.1037/h0027639. [DOI] [PubMed] [Google Scholar]
- 49.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 50.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 51.Moll J, Zahn R, de Oliveira-Souza R, et al. Opinion: the neural basis of human moral cognition. Nat Rev Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- 52.O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- 53.Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 54.Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 55.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 56.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 57.Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 59.Frank MJ, O’Reilly RC. A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- 60.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Falkenstein M, Hoormann J, Christ S, et al. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 62.Carter CS, Braver TS, Barch DM, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 63.Braver TS, Barch DM, Gray JR, et al. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 64.Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- 65.Menon V, Adleman NE, White CD, et al. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 67.Ito S, Stuphorn V, Brown JW, et al. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 68.Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13:372–378. doi: 10.1177/026988119901300418. [DOI] [PubMed] [Google Scholar]
- 69.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: a review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 70.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 71.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 72.Fujii N, Graybiel AM. Representation of action sequence boundaries by macaque prefrontal cortical neurons. Science. 2003;301:1246–1249. doi: 10.1126/science.1086872. [DOI] [PubMed] [Google Scholar]
- 73.Huey ED, Krueger F, Grafman J. Representations in the human prefrontal cortex. Current Directions in Psychological Science. 2006;15:167–171. [Google Scholar]
- 74.Grafman J. Similarities and distinctions among current models of prefrontal cortical functions. Ann N Y Acad Sci. 1995;769:337–368. doi: 10.1111/j.1749-6632.1995.tb38149.x. [DOI] [PubMed] [Google Scholar]
- 75.Grafman J. The structured event complex and the human prefrontal cortex. In: Stuss D, Knight RT, editors. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. pp. 292–310. [Google Scholar]
- 76.Zahn R, Moll J, Krueger F, et al. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:6430–6645. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shima K, Isoda M, Mushiake H, et al. Categorization of behavioural sequences in the prefrontal cortex. Nature. 2007;445:315–318. doi: 10.1038/nature05470. [DOI] [PubMed] [Google Scholar]
- 78.Averbeck BB, Lee D. Prefrontal neural correlates of memory for sequences. J Neurosci. 2007;27:2204–2211. doi: 10.1523/JNEUROSCI.4483-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sirigu A, Cohen L, Zalla T, et al. Distinct frontal regions for processing sentence syntax and story grammar. Cortex. 1998;34:771–778. doi: 10.1016/s0010-9452(08)70780-9. [DOI] [PubMed] [Google Scholar]
- 80.Sirigu A, Zalla T, Pillon B, et al. Selective impairments in managerial knowledge following pre-frontal cortex damage. Cortex. 1995;31:301–316. doi: 10.1016/s0010-9452(13)80364-4. [DOI] [PubMed] [Google Scholar]
- 81.Sirigu A, Zalla T, Pillon B, et al. Encoding of sequence and boundaries of scripts following prefrontal lesions. Cortex. 1996;32:297–310. doi: 10.1016/s0010-9452(96)80052-9. [DOI] [PubMed] [Google Scholar]
- 82.Goel V, Grafman J, Tajik J, et al. A study of the performance of patients with frontal lobe lesions in a financial planning task. Brain. 1997;120:1805–1822. doi: 10.1093/brain/120.10.1805. [DOI] [PubMed] [Google Scholar]
- 83.Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103–123. doi: 10.1007/978-3-7091-6892-9_6. [DOI] [PubMed] [Google Scholar]
- 84.Rattermann M, Spector L, Grafman J, et al. Partial and total-order planning: evidence from normal and prefrontally damaged populations. Cognitive Science. 2001;25:941–975. [Google Scholar]
- 85.Rosen VM, Caplan L, Sheesley L, et al. An examination of daily activities and their scripts across the adult lifespan. Behav Res Methods Instrum Comput. 2003;35:32–48. doi: 10.3758/bf03195495. [DOI] [PubMed] [Google Scholar]
- 86.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 87.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 88.McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Insel TR. Toward a neuroanatomy of obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49:739–744. doi: 10.1001/archpsyc.1992.01820090067011. [DOI] [PubMed] [Google Scholar]
- 90.Modell JG, Mountz JM, Curtis GC, et al. Neurophysiologic dysfunction in basal ganglia/limbic striatal and thalamocortical circuits as a pathogenetic mechanism of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 1989;1:27–36. doi: 10.1176/jnp.1.1.27. [DOI] [PubMed] [Google Scholar]
- 91.Schwartz JM. A role of volition and attention in the generation of new brain circuitry: toward a neurobiology of mental force. J Consciousness Stud. 1999;6:115–142. [Google Scholar]
- 92.Baxter LR., Jr Basal ganglia systems in ritualistic social displays: reptiles and humans; function and illness. Physiol Behav. 2003;79:451–460. doi: 10.1016/s0031-9384(03)00164-1. [DOI] [PubMed] [Google Scholar]
- 93.Deckersbach T, Dougherty DD, Rauch SL. Functional imaging of mood and anxiety disorders. J Neuroimaging. 2006;16:1–10. doi: 10.1177/1051228405001474. [DOI] [PubMed] [Google Scholar]
- 94.Rauch SL, Dougherty DD, Malone D, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 95.Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 96.Stein DJ. Advances in understanding the anxiety disorders: the cognitive-affective neuroscience of “false alarms.”. Ann Clin Psychiatry. 2006;18:173–182. doi: 10.1080/10401230600801192. [DOI] [PubMed] [Google Scholar]
- 97.Stein DJ, Lochner C. Obsessive-compulsive spectrum disorders: a multidimensional approach. Psychiatr Clin North Am. 2006;29:343–351. doi: 10.1016/j.psc.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 98.Szechtman H, Woody E. Obsessive-compulsive disorder as a disturbance of security motivation. Psychological Review. 2004;111:111–127. doi: 10.1037/0033-295X.111.1.111. [DOI] [PubMed] [Google Scholar]
- 99.Pitman RK. Animal models of compulsive behavior. Biol Psychiatry. 1989;26:189–198. doi: 10.1016/0006-3223(89)90022-x. [DOI] [PubMed] [Google Scholar]
- 100.Reed GF. Obsessional Experience and Compulsive Behavior. Orlando, Fla: Academic Press; 1985. [Google Scholar]
- 101.Reed GF. The obsessional-compulsive experience: a phenomenological reemphasis. Philosophy and Phenomenological Research. 1977;37:381–384. [Google Scholar]
- 102.Neziroglu F, Yaryura-Tobias JA. Over and Over Again: Understanding Obsessive-Compulsive Disorder. Toronto, Ontario: Lexington Books; 1991. [Google Scholar]
- 103.Weingartner H, Grafman J, Boutelle W, et al. Forms of memory failure. Science. 1983;221:380–382. doi: 10.1126/science.6867715. [DOI] [PubMed] [Google Scholar]
- 104.Blumer D, Benson DF, editors. Psychiatric Aspects of Neurologic Disease. New York: Grune & Stratton; 1975. Personality changes with frontal and temporal lobe lesions. [Google Scholar]
- 105.O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- 106.Swedo SE, Rapoport JL, Cheslow DL, et al. High prevalence of obsessive-compulsive symptoms in patients with Sydenham’s chorea. Am J Psychiatry. 1989;146:246–249. doi: 10.1176/ajp.146.2.246. [DOI] [PubMed] [Google Scholar]
- 107.Fitzgerald KD, Welsh RC, Gehring WJ, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 108.Baer L. Factor analysis of symptom subtypes of obsessive compulsive disorder and their relation to personality and tic disorders. J Clin Psychiatry. 1994;55:18–23. [PubMed] [Google Scholar]
- 109.Mataix-Cols D, Wooderson S, Lawrence N, et al. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 110.Valenstein ES. Stereotypy and sensory-motor changes evoked by hypothalamic stimulation: possible relation to schizophrenic behavior patterns. In: Routtenberg A, editor. Biology of Reinforcement: Facets of Brain-Stimulation Reward. New York: Academic Press; 1980. pp. 39–52. [Google Scholar]
- 111.Mathiak K, Weber R. Toward brain correlates of natural behavior: fMRI during violent video games. Hum Brain Mapp. 2006;27:948–956. doi: 10.1002/hbm.20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 113.Insel TR, Donnelly EF, Lalakea ML, et al. Neurological and neuropsychological studies of patients with obsessive-compulsive disorder. Biol Psychiatry. 1983;18:741–751. [PubMed] [Google Scholar]
- 114.Luxenberg JS, Swedo SE, Flament MF, et al. Neuroanatomical abnormalities in obsessive-compulsive disorder detected with quantitative X-ray computed tomography. Am J Psychiatry. 1988;145:1089–1093. doi: 10.1176/ajp.145.9.1089. [DOI] [PubMed] [Google Scholar]
- 115.Behar D, Rapoport JL, Berg CJ, et al. Computerized tomography and neuropsychological test measures in adolescents with obsessive-compulsive disorder. Am J Psychiatry. 1984;141:363–369. doi: 10.1176/ajp.141.3.363. [DOI] [PubMed] [Google Scholar]
- 116.Aylward EH, Harris GJ, Hoehn-Saric R, et al. Normal caudate nucleus in obsessive-compulsive disorder assessed by quantitative neuroimaging. Arch Gen Psychiatry. 1996;53:577–584. doi: 10.1001/archpsyc.1996.01830070021006. [DOI] [PubMed] [Google Scholar]
- 117.Kellner CH, Jolley RR, Holgate RC, et al. Brain MRI in obsessive-compulsive disorder. Psychiatry Res. 1991;36:45–49. doi: 10.1016/0165-1781(91)90116-7. [DOI] [PubMed] [Google Scholar]
- 118.Kang DH, Kim JJ, Choi JS, et al. Volumetric investigation of the frontal-subcortical circuitry in patients with obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2004;16:342–349. doi: 10.1176/jnp.16.3.342. [DOI] [PubMed] [Google Scholar]
- 119.Szeszko PR, Robinson D, Alvir JM, et al. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- 120.Kim JJ, Lee MC, Kim J, et al. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- 121.Rosenberg DR, Keshavan MS. A.E. Bennett Research Award: toward a neurodevelopmental model of of obsessive-compulsive disorder. Biol Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- 122.Garber HJ, Ananth JV, Chiu LC, et al. Nuclear magnetic resonance study of obsessive-compulsive disorder. Am J Psychiatry. 1989;146:1001–1005. doi: 10.1176/ajp.146.8.1001. [DOI] [PubMed] [Google Scholar]
- 123.Scarone S, Colombo C, Livian S, et al. Increased right caudate nucleus size in obsessive-compulsive disorder: detection with magnetic resonance imaging. Psychiatry Res. 1992;45:115–121. doi: 10.1016/0925-4927(92)90005-o. [DOI] [PubMed] [Google Scholar]
- 124.Szeszko PR, MacMillan S, McMeniman M, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. 2004;161:1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- 125.Robinson D, Wu H, Munne RA, et al. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52:393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- 126.Rosenberg DR, Keshavan MS, O’Hearn KM, et al. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Arch Gen Psychiatry. 1997;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- 127.Rosenberg DR, Keshavan MS, Dick EL, et al. Corpus callosal morphology in treatment-naive pediatric obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1269–1283. doi: 10.1016/s0278-5846(97)00163-2. [DOI] [PubMed] [Google Scholar]
- 128.Jenike MA, Breiter HC, Baer L, et al. Cerebral structural abnormalities in obsessive-compulsive disorder: a quantitative morphometric magnetic resonance imaging study. Arch Gen Psychiatry. 1996;53:625–632. doi: 10.1001/archpsyc.1996.01830070073011. [DOI] [PubMed] [Google Scholar]
- 129.Bartha R, Stein MB, Williamson PC, et al. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am J Psychiatry. 1998;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- 130.Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- 131.Valente AA, Jr, Miguel EC, Castro CC, et al. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biol Psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 132.Ebert D, Speck O, König A, et al. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatry Res. 1997;74:173–176. doi: 10.1016/s0925-4927(97)00016-4. [DOI] [PubMed] [Google Scholar]
- 133.Fitzgerald KD, Moore GJ, Paulson LA, et al. Proton spectroscopic imaging of the thalamus in treatment-naive pediatric obsessive-compulsive disorder. Biol Psychiatry. 2000;47:174–182. doi: 10.1016/s0006-3223(99)00286-3. [DOI] [PubMed] [Google Scholar]
- 134.Smith EA, Russell A, Lorch E, et al. Increased medial thalamic choline found in pediatric patients with obsessive-compulsive disorder versus major depression or healthy control subjects: a magnetic resonance spectroscopy study. Biol Psychiatry. 2003;54:1399–1405. doi: 10.1016/s0006-3223(03)00474-8. [DOI] [PubMed] [Google Scholar]
- 135.Alptekin K, Degirmenci B, Kivircik B, et al. Tc-99m HMPAO brain perfusion SPECT in drug-free obsessive-compulsive patients without depression. Psychiatry Res. 2001;107:51–56. doi: 10.1016/s0925-4927(01)00086-5. [DOI] [PubMed] [Google Scholar]
- 136.Lacerda AL, Dalgalarrondo P, Caetano D, et al. Elevated thalamic and prefrontal regional cerebral blood flow in obsessive-compulsive disorder: a SPECT study. Psychiatry Res. 2003;123:125–134. doi: 10.1016/s0925-4927(03)00061-1. [DOI] [PubMed] [Google Scholar]
- 137.Rubin RT, Villanueva-Meyer J, Ananth J, et al. Regional xenon 133 cerebral blood flow and cerebral technetium 99m HMPAO uptake in unmedicated patients with obsessive-compulsive disorder and matched normal control subjects. Determination by high-resolution single-photon emission computed tomography. Arch Gen Psychiatry. 1992;49:695–702. doi: 10.1001/archpsyc.1992.01820090023004. [DOI] [PubMed] [Google Scholar]
- 138.Lucey JV, Costa DC, Adshead G, et al. Brain blood flow in anxiety disorders: OCD, panic disorder with agoraphobia, and posttraumatic stress disorder on 99mTc HMPAO single photon emission tomography (SPET) Br J Psychiatry. 1997;171:346–350. doi: 10.1192/bjp.171.4.346. [DOI] [PubMed] [Google Scholar]
- 139.Machlin SR, Harris GJ, Pearlson GD, et al. Elevated medial-frontal cerebral blood flow in obsessive-compulsive patients: a SPECT study. Am J Psychiatry. 1991;148:1240–1242. doi: 10.1176/ajp.148.9.1240. [DOI] [PubMed] [Google Scholar]
- 140.Busatto GF, Buchpiguel CA, Zamignani DR, et al. Regional cerebral blood flow abnormalities in early-onset obsessive-compulsive disorder: an exploratory SPECT study. J Am Acad Child Adolesc Psychiatry. 2001;40:347–354. doi: 10.1097/00004583-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 141.Crespo-Facorro B, Cabranes JA, López-Ibor Alcocer MI, et al. Regional cerebral blood flow in obsessive-compulsive patients with and without a chronic tic disorder: a SPECT study. Eur Arch Psychiatry Clin Neurosci. 1999;249:156–161. doi: 10.1007/s004060050081. [DOI] [PubMed] [Google Scholar]
- 142.Baxter LR, Jr, Phelps ME, Mazziotta JC, et al. Local cerebral glucose metabolic rates in obsessive-compulsive disorder: a comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- 143.Swedo SE, Schapiro MB, Grady CL, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 1989;46:518–523. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- 144.Perani D, Colombo C, Bressi S, et al. [18F]FDG PET study in obsessive-compulsive disorder: a clinical/metabolic correlation study after treatment. Br J Psychiatry. 1995;166:244–250. doi: 10.1192/bjp.166.2.244. [DOI] [PubMed] [Google Scholar]
- 145.Martinot JL, Allilaire JF, Mazoyer BM, et al. Obsessive-compulsive disorder: a clinical, neuropsychological and positron emission tomography study. Acta Psychiatr Scand. 1990;82:233–242. doi: 10.1111/j.1600-0447.1990.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 146.Adler CM, McDonough-Ryan P, Sax KW, et al. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 147.Breiter HC, Rauch SL, Kwong KK, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 148.Nakao T, Nakagawa A, Yoshiura T, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 149.Sawle GV, Hymas NF, Lees AJ, et al. Obsessional slowness. Functional studies with positron emission tomography. Brain. 1991;114:2191–2202. doi: 10.1093/brain/114.5.2191. [DOI] [PubMed] [Google Scholar]
- 150.Rauch SL, Jenike MA, Alpert NM, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 151.Nyatsanza S, Shetty T, Gregory C, et al. A study of stereotypic behaviours in Alzheimer’s disease and frontal and temporal variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2003;74:1398–1402. doi: 10.1136/jnnp.74.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Remijnse PL, Nielen MM, van Balkom AJ, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 153.Chamberlain SR, Menzies L, Hampshire A, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 154.Gu BM, Park JY, Kang DH, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–164. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- 155.Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesion of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.van den Heuvel OA, Remijnse PL, Mataix-Cols D, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. doi: 10.1093/brain/awn267. (Epub ahead of print, Oct 24, 2008) [DOI] [PubMed] [Google Scholar]