Abstract
Keratitis-ichthyosis-deafness syndrome (KID) is a rare ectodermal dysplasia characterized by vascularizing keratitis, profound sensorineural hearing loss (SNHL), and progressive erythrokeratoderma, a clinical triad that indicates a failure in development and differentiation of multiple stratifying epithelia. Here, we provide compelling evidence that KID is caused by heterozygous missense mutations in the connexin-26 gene, GJB2. In each of 10 patients with KID, we identified a point mutation leading to substitution of conserved residues in the cytoplasmic amino terminus or first extracellular domain of Cx26. One of these mutations was detected in six unrelated sporadic case subjects and also segregated in one family with vertical transmission of KID. These results indicate the presence of a common, recurrent mutation and establish its autosomal dominant nature. Cx26 and the closely related Cx30 showed differential expression in epidermal, adnexal, and corneal epithelia but were not significantly altered in lesional skin. However, mutant Cx26 was incapable of inducing intercellular coupling in vitro, which indicates its functional impairment. Our data reveal striking genotype-phenotype correlations and demonstrate that dominant GJB2 mutations can disturb the gap junction system of one or several ectodermal epithelia, thereby producing multiple phenotypes: nonsyndromic SNHL, syndromic SNHL with palmoplantar keratoderma, and KID. Decreased host defense and increased carcinogenic potential in KID illustrate that gap junction communication plays not only a crucial role in epithelial homeostasis and differentiation but also in immune response and epidermal carcinogenesis.
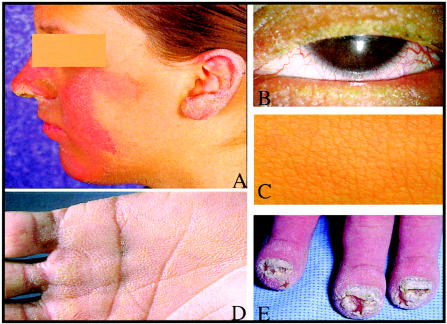
Keratitis-ichthyosis-deafness syndrome (KID [MIM 148210]) is a rare heritable ectodermal dysplasia with severe sensory impairment. Corneal epithelial defects, scarring, and neovascularization cause progressive decline of visual acuity and may eventually lead to blindness. Congenital sensorineural hearing loss (SNHL) is generally severe and bilateral, although unilateral or moderate hearing impairment has been observed (Szymko et al.2002). The skin is thickened and often has a coarse-grained appearance. Patients usually develop follicular hyperkeratoses and well-circumscribed, erythematous, hyperkeratotic plaques that are symmetrically distributed on the face and extremities. Palmoplantar keratoderma (PPK) with a grainy surface is invariably present (fig. 1). Other features include dystrophic hair and nail, dental anomalies, and heat intolerance. Increased susceptibility to mucocutaneous infections is common and sometimes fatal in the neonatal period. Squamous cell carcinoma of the skin and oral mucosa is a rare but serious complication that can shorten life expectancy. To date, ∼70 cases, the majority of which are sporadic, have been described in the world literature (Caceres-Rios et al. 1996). However, autosomal dominant and autosomal recessive inheritance has been reported in a small number of families (Legrand et al. 1982; Grob et al. 1987; Tuppurainen et al. 1988; Nazzaro et al. 1990; Kone-Paut et al. 1998).
Figure 1.
Clinical features of KID. A, Sharply demarcated, figurate outlined, red-brown hyperkeratotic plaques on the central face and outer rim of the ear (KID 05). B, Rarefied eyelashes and vascularizing keratitis (KID 05). C, Acanthosis of the skin with a heavy-grained leather appearance (KID 08). D, Diffuse palmar keratoderma with grainy surface (KID 03). E, Chronic Candida albicans onychia and paronychia with hypertrophy of distal digits, acanthosis, and hyperkeratosis of the skin (KID 09).
Recent advances in the molecular understanding of hearing loss, vision, and skin disorders have emphasized the pivotal role that gap junction cell-cell communication plays in development and homeostasis of ectodermally derived tissues. Gap junctions are tightly packed assemblies of intercellular channels that control and coordinate a variety of cellular activities through the exchange of small ions, metabolites, and signaling molecules. Each connexin (Cx) channel consists of two connexon hemichannels that are built by hexameric oligomerization of connexins (Cxs), a family of integral membrane proteins. Gap junctions can be composed of similar or different Cx proteins, forming homotypic or heterotypic channels with unique properties (Bevans et al. 1998). Dominant mutations in the genes encoding Cx26, Cx30, and Cx31, each of which is expressed in inner ear and skin, are detrimental to the function of these tissues, causing SNHL, skin disorders, or both (Kelsell et al. 2001; Richard 2001). Cutaneous disorders include those with Cx mutations affecting GJB2 (GenBank accession number XM_007169) in PPK/SNHL (MIM 148350) (Richard et al. 1998b) and Vohwinkel syndrome (MIM 124500) (Maestrini et al. 1999), GJB6 (GenBank accession numbers XM_007168 and NT_009917) in Clouston syndrome (MIM 129500) (Lamartine et al. 2000), and GJB3 (GenBank accession number AF_099730) and GJB4 in erythrokeratodermia variabilis (EKV [MIM 133200]) (Richard et al. 1998a; Macari et al. 2000). On the basis of the partially overlapping clinical features of these disorders and those of KID, we considered GJB2, GJB3, and GJB6 as strong candidate genes.
We ascertained, as part of clinical and genetic studies (by L.J.R., E.W.J., and G.R.) of KID, 8 unrelated subjects affected with sporadic disease and 15 unaffected first-degree relatives (fig. 2). Patients ranged in age from 2 to 47 years, and each demonstrated the cardinal features of KID: progressive, vascularizing keratitis, SNHL confirmed by audiometry, PPK with reticulate surface, hyperkeratotic plaques on face or extremities, and acanthosis of other skin areas (fig. 1). Frequency of mucocutaneous infections, degree of erythema, and involvement of hair and teeth were variable. At the time of examination, none of the patients we studied had evidence of squamous cell carcinoma. Two additional patients, an affected father and his son, were previously reported by Tuppurainen et al. (1988) (fig. 2). All families were of European descent; five originated in the United States, and one each originated in Australia, Finland, Hungary, and the United Kingdom. All participants gave informed consent, and DNA samples were collected from patients and family members by use of either buccal swabs or venous blood samples. We screened the coding and flanking sequences of GJB2, GJB3, and GJB6 of each patient by bidirectional direct DNA sequencing of PCR amplicons obtained from genomic DNA, as described elsewhere (Richard et al. 1998a, 1998b).
Figure 2.
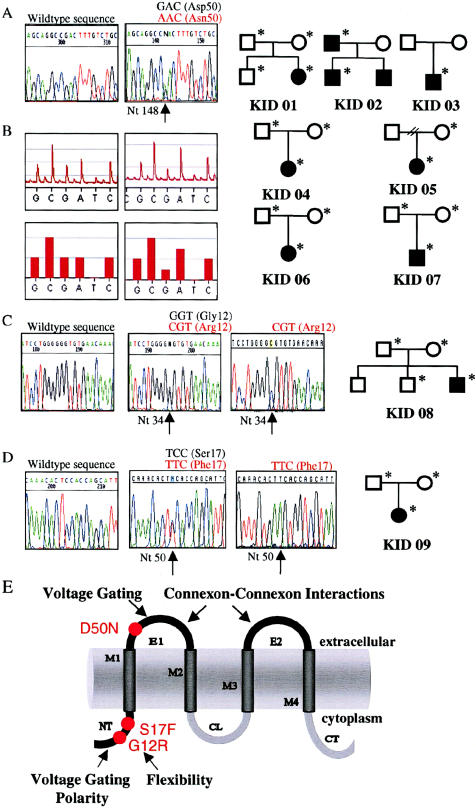
Mutation analysis of GJB2 in eight individuals affected with sporadic disease and one multiplex family with KID. A, Sequence chromatograms of GJB2from unaffected (left panel) and affected (right panel) family members depicting the heterozygous transition 148G→A at codon 50 encoding asparagine instead of aspartic acid (D50N) observed in seven unrelated families with KID. B, Genotyping by automated pyrosequencing of biotinylated single-stranded DNA templates (Pyrosequencing) confirmed the presence of D50N in the affected patients (top row,pyrogram; bottom row, outcome as bar graph; both generated with Pyrosequencing software), whereas it was excluded from unaffected parents and siblings and from 54 control subjects. C and D, Sequence chromatograms of GJB2 from unaffected (left panel) and affected (middle panel) family members and the mutant allele (right panel) subcloned in the pcDNA2.1 vector (Invitrogen), showing (C) the heterozygous G→C transversion in codon 12, replacing glycine with arginine (G12R) in patient KID 08, and (D) the transition 50C→T, leading to substitution of serine 17 with phenylalanine (S17F), in one allele of patient KID 09. Individuals from whom DNA was obtained are marked with an asterisk (*). E, Schematic of the Cx26 polypeptide that depicts structural motifs and location of mutations in KID. NT = amino-terminus; M1–M4 = transmembrane domains 1–4, respectively; E1 and E2 = extracellular domains 1 and 2, respectively; CL = cytoplasmic loop; and CT = carboxy-terminus.
Without exception, we identified a heterozygous point mutation in GJB2 in each of 10 patients with KID from nine unrelated families. Six of eight sporadic cases carried transition 148G→A resulting in substitution of aspartic acid with asparagine in codon 50 (D50N). The same heterozygous mutation was detected in an affected father and his son (fig. 2A, 2B, and 2E), confirming autosomal dominant inheritance of KID on a molecular level. The presence of this mutation in seven unrelated probands of varying ethnic origins but not in any of the unaffected parents and siblings strongly suggests that D50N has arisen de novo in each family and that it is a common mutation in KID. This amino acid replacement occurs in the highly conserved first extracellular loop of Cx26 (fig. 2E), which is crucial for voltage gating (Rubin et al. 1992) and connexon-connexon interactions (White et al. 1996). Therefore, D50N may seriously compromise these functions. Two sporadic cases of KID harbored different GJB2 mutations, including transversion 34G→C, which leads to replacement of glycine with arginine (G12R) and transition 50C→T, which substitutes serine with phenylalanine (S17F). Both nonconservative mutations (fig. 2C–E) affect residues in the cytoplasmic amino-terminus that are conserved among all β–type Cxs of different species, and both are predicted to alter charge and structure of this domain. These changes may interfere with conformation and flexibility of the amino-terminus, as well as regulation of Cx selectivity and gating polarity (Verselis et al. 1994; Falk et al. 1997; Purnick et al. 2000). The functional importance of these residues is underscored by the fact that missense mutations of corresponding or neighboring residues cause EKV (Cx31 [G12R and G12D], Cx30.3 [G12D]), Clouston syndrome (Cx30 [G11R]) and X-linked Charcot-Marie-Tooth disease (Cx32 [G12S]) (Richard 2001). No other sequence variants were identified in the patients’ coding sequences of GJB2, GJB3, and GJB6. Mutations S17F, G12R, and D50N were not present in at least 66 white control individuals nor in the hundreds of individuals worldwide, who were screened for recessive deafness mutations (see Connexin-Deafness Homepage), excluding the possibility that these sequence aberrations represent frequent polymorphisms. Therefore, we conclude that these dominant GJB2 mutations cause KID. The mutations are likely to disturb biogenesis of Cx26 or to dominantly inhibit the function of gap junctions, similar to mutations observed in other Cx disorders (Martin et al. 1999; VanSlyke et al. 2000; Rouan et al. 2001).
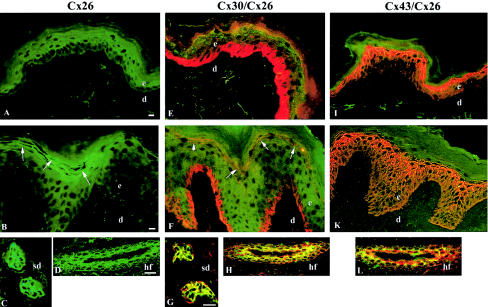
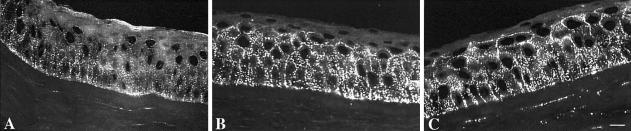
Gap junctions formed by Cx26 are found in many epithelial organs, including cochlea, palmoplantar epidermis, hair follicles, and sweat glands and ducts. To assess the effect that KID mutations have on the cutaneous Cx network, we studied Cx expression in lesional skin from the back of proband KID 08, who is heterozygous for G12R (fig. 3). Cx26 (green) was predominantly expressed in a punctate pattern at cell membranes of eccrine sweat ducts and the outer root sheet of hair follicles, similar to control skin. These observations demonstrate that G12R does not interfere with the synthesis and intracellular distribution of Cx26 in vivo. In these adnexal structures, Cx26 colocalized (yellow) extensively with Cx30 (red), indicating that wild-type and/or mutant Cx26 may interact with Cx30 at junctional plaques. In contrast to the absence of Cx26 staining in normal truncal epidermis, we observed weak, focal, ectopic staining of Cx26 in the granular layers of lesional epidermis, which also expressed Cx30 and Cx43. Since distribution and intensity of Cx43 and Cx30 were not significantly altered, we hypothesize that expression of mutant Cx26 in KID may be detrimental to the function of gap junctions by changing the normal level of Cx signaling in skin. In normal human cornea (fig. 4A–C), Cx43 and Cx30 were abundant, with widespread and overlapping yet distinct distribution patterns that differed from the distribution reported for Cx50 (Matic et al. 1997). In contrast, immunostaining revealed weak expression of Cx26, limited to basal epithelial cells. This distribution parallels that in rabbit cornea, in which Cx26 was shown to be transiently upregulated during wound regeneration (Ratkay-Traub et al. 2001). These findings suggest that the progressive keratitis in KID may result from perpetually induced expression of mutant Cx26.
Figure 3.
Immunofluorescence (confocal microscopy) of normal (A, E, and I) and lesional (B–D, F–H, K, and L) skin in KID (KID 08) with Cx26, Cx43, and Cx30 antibodies. Immunohistochemical analyses were performed on 5-μm–thick sections of snap-frozen skin biopsies of normal control skin and lesional skin, as described elsewhere (Rouan et al. 2001). The primary monoclonal anti-Cx26 antibody (mouse [Zymed Laboratories; catalog number 33-5800]), which does not cross-react with Cx30, was detected with fluorescein-conjugated anti-mouse immunoglobin (green). The polyclonal anti-Cx43 and anti-Cx30 antibodies (rabbit [Zymed Laboratories]) were visualized with Texas Red–conjugated anti-rabbit immunoglobin (red). Areas of focal colocalization of Cx26 with either Cx30 or Cx43 are visualized in yellow. A, Normal epidermis, Cx26 (green). B–D, KID syndrome, Cx26 (green). Note the weak immunostaining of Cx26 in the granular cell layers (B, arrows) but strong expression in epithelial cells of sweat ducts (C) and outer root sheath of hair follicles (oblique cut) (D). E, Normal epidermis, Cx30 (red). F–H, KID syndrome, Cx30 (red) and Cx26 (green). Cx30 staining appears strictly compartmentalized with membranous and cytoplasmic staining in basal keratinocytes and punctate membrane staining of the upper differentiated cell layers. Note the focal colocalization of Cx26 and Cx30 in the granular epidermal layers (F, arrows), as well as in the sweat ducts (G) and hair follicles (H). I, Normal epidermis, Cx43 (red). K and L, KID syndrome, Cx43 (red) and Cx26 (green). Comparable expression of Cx43 throughout the epidermis in normal (I) and lesional skin (K) and colocalization of Cx26 with Cx43 in hair follicles (L). d = dermis; e = epidermis; sd = sweat duct; and hf = hair follicle. Scale bars = 10 μm.
Figure 4.
Immunofluorescence of normal human corneal epithelium with Cx26 (A), Cx30 (B), and Cx43 (C) antibodies. All connexin proteins showed, as expected, punctate staining at the cell membranes. A, Cx26 staining was relatively weak and was limited to the basal layer of the epithelium. B, Comparatively strong Cx30 expression throughout the basal and lower wing layers, sparing the superficial wing and squamous layers. C, Similar distribution of Cx43, with highest protein levels in the basal layer but staining extended throughout the wing layers. Scale bar = 10 μm.
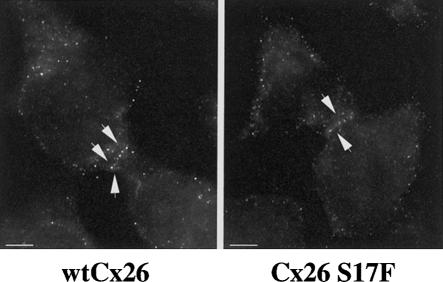
To test the ability of mutant Cx26 to form functional gap junction channels, we stably expressed wild-type and mutant (S17F) Cx26 in coupling-incompetent HeLa cells (fig. 5A and 5B). Then we injected the cytoplasm of individual cells expressing either wild-type Cx26 or mutant Cx26 with carboxyfluorescein (Mw 376 Da), a fluorescent tracer that can freely pass through gap junction channels, and we scored the number of surrounding cells receiving dye through gap junction coupling assessed by means of fluorescence microscopy. In cells expressing wild-type Cx26, we observed a dye spread to neighboring cells, with an average dye coupling of 3.94 ± 0.32 cells (18 experiments). In contrast, cells expressing comparable amounts of Cx26 S17F were completely uncoupled (0.0 ± 0.00; 25 experiments), indicating that S17F abolishes the function of mutant Cx26 in vitro (table 1). Our results are consistent with the functional impairment determined for other dominant GJB2 mutations, which were also shown to dominantly inhibit the function of wild-type Cx26 (Richard et al. 1998b; Martin et al. 1999; Rouan et al. 2001). A possible mechanism could be the integration of mutant proteins into homo- or heteromeric connexons, which might block their interactions with connexons in apposing cells, produce structurally incomplete channels, or alter selective properties of gap junction channels.
Figure 5.
Immunolocalization of wild-type Cx26 (A) and Cx26 S17F (B). HeLa cells expressing a tTA vector were stably transfected with Cx26-MYC-cDNA/pCEP-TetP vectors, grown in selective medium to >80% confluency, fixed, and stained with a monoclonal Cx26 antibody. A, WtCx26. B, Cx26 S17F. Arrows indicate plasma membrane staining at cell-cell contacts. Scale bar = 10 μm.
Table 1.
Functionality of Wild-Type and Mutant Cx26
| Connexin | No. of CellsInjected | No. of NeighboringCells ObtainingCarboxyfluorescein |
| Wt Cx26 | 18 | 3.94 ± .32 |
| Cx26 S17F | 25 | 0.00 ± .00a |
P<.000001.
KID is the third distinct disorder attributable to dominantly inherited GJB2 mutations and highlights the remarkable relationship that exists between GJB2 genotype and phenotype. Specific amino acid substitutions clustered within the extracellular domain E1 of Cx26 (fig. 2E) can affect only the nonsensory epithelium of the cochlea (W44C and W44S), the inner ear and epidermis (ΔE42, G59A, D66H, and R75W), or, in addition, the corneal epithelium, hair follicles, and nails (D50N). Although the determinants of this extraordinary phenotypic variability are not understood, character and location of mutations likely influence structural and functional parameters of the gap junction channels containing mutant Cx26. In addition, faulty Cx26 may, directly or indirectly, hinder the function of one or more partners in the Cx network. For example, certain GJB2 mutations causing PPK/SNHL exert a selective, trans-dominant inhibitory effect on the function of Cx43 in vitro (Rouan et al. 2001). We suspect that the GJB2 mutations described here cause KID via a similar mechanism. For example, a dominant interference with Cx30—which is expressed in epidermis, skin appendages, and cornea—could lead to the involvement of these tissues in KID. This hypothesis is supported by the fact that dominant germline mutations in the Cx30 gene (GJB6) cause the ectodermal dysplasia Clouston syndrome, which also shows adnexal involvement, and by the focal colocalization of Cx26 and Cx30 observed in lesional skin. Corneal involvement could also stem from additional interactions with the abundantly expressed Cx43 or Cx50 (Matic et al. 1997).
The development of squamous cell carcinoma reported in several patients with KID highlights the importance of intact intercellular communication for growth control of epithelial cells. Epithelial neoplasias, including squamous cell carcinoma, show a significant loss of Cx26 expression and downregulation of intercellular communication (Kamibayashi et al. 1995; Sawey et al. 1996). Transfection of Cx26 into coupling-deficient HeLa cells restores their ability to communicate and significantly decreases their proliferative potential. That dominant negative Cx26 mutants can reverse such a tumor-suppressive effect supports the concept that Cx26 has a specific growth-regulatory function (Mesnil et al. 1995).
Our data also constitute the first report linking a disorder with immune dysfunction to mutations in a Cx gene. Various immune-competent cells show a differential expression of Cx proteins that seems tightly regulated by TNF-α, IL-2β, and other cytokines (Hu et al. 1997; Saez et al. 2000). There is mounting evidence that gap junction–mediated intercellular signaling coordinates and synchronizes cellular actions to produce a timely and effective immune response (Hu and Cotgreave 1997; Saez et al. 2000). The exact mechanisms by which dominant GJB2 mutations in KID reduce host defense and influence susceptibility to mucocutaneous infections, particularly with Candida albicans (Caceres-Rios et al. 1996), remain to be determined.
Acknowledgments
We are grateful to the families who participated in our studies and acknowledge the support of the Foundation for Ichthyosis and Related Skin Types and the National Foundation for Ectodermal Dysplasias. We thank the referring physicians, in particular A. Fryer and S. Kaye, for providing clinical information. We are grateful to C. Lo, for invaluable technical assistance with dye transfer studies; R. Mountford, for DNA banking; and F. Ringpfeil and C. Lo, for stimulating discussion. This study was supported by NIH/NIAMS grants K08-AR02141 and P01-AR38923 (G.R.), the Dermatology Foundation Fellowship (F.R.), the National Foundation for Ectodermal Dysplasias (L.R. and E.W.J.), and the British Medical Association and Royal Liverpool University Hospital Research and Development Fund (C.W.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Connexin-Deafness Homepage, http://www.IRO.es/deafness/
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for GJB2 [accession number XM_007169], GJB3 [accession number AF_099730], GJB6 [accession numbers XM_007168 and NT_009917])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for KID [MIM 148210], PPK/SNHL [MIM 148350], Vohwinkel syndrome [MIM 124500], Clouston syndrome [MIM 129500], and EKV [MIM 133200])
References
- Bevans CG, Kordel M, Rhee SK, Harris AL (1998) Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem 273:2808–2816 [DOI] [PubMed] [Google Scholar]
- Caceres-Rios H, Tamayo-Sanchez L, Duran-Mckinster C, de la Luz Orozco M, Ruiz-Maldonado R (1996) Keratitis, ichthyosis, and deafness (KID syndrome): review of the literature and proposal of a new terminology. Pediatr Dermatol 13:105–113 [DOI] [PubMed] [Google Scholar]
- Falk MM, Buehler LK, Kumar NM, Gilula NB (1997) Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J 16:2703–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob JJ, Breton A, Bonafe J-L, Sauvan-Ferdani M, Bonerandi J-J (1987) Keratitis, ichthyosis, and deafness (KID) syndrome. Arch Dermatol 123:777–782 [PubMed] [Google Scholar]
- Hu J, Cotgreave IA (1997) Differential regulation of gap junctions by proinflammatory mediators in vitro. J Clin Invest 99:2312–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamibayashi Y, Oyamada Y, Mori M, Oyamada M (1995) Aberrant expression of gap junction proteins (connexins) is associated with tumor progression during multistage mouse skin carcinogenesis in vivo. Carcinogenesis 16:1287–1297 [DOI] [PubMed] [Google Scholar]
- Kelsell DP, Di WL, Houseman MJ (2001) Connexin mutations in skin disease and hearing loss. Am J Hum Genet 68:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kone-Paut I, Hesse S, Palix C, Rey R, Remediani K, Garnier JM, Berbis P (1998) Keratitis, ichthyosis, and deafness (KID) syndrome in half sibs. Pediatr Dermatol 15:219–221 [DOI] [PubMed] [Google Scholar]
- Lamartine J, Munhoz Essenfelder G, Kibar Z, Lanneluc I, Callouet E, Laoudj D, Lemaitre G, Hand C, Hayflick SJ, Zonana J, Antonarakis S, Radhakrishna U, Kelsell DP, Christianson AL, Pitaval A, Der Kaloustian V, Fraser C, Blanchet-Bardon C, Rouleau GA, Waksman G (2000) Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet 26:142–144 [DOI] [PubMed] [Google Scholar]
- Legrand J, Litoux P, Quere M, Stalder JF, Ertus M (1982) A rare oculo-auriculo-cutaneous syndrome (Burns' syndrome). J Fr Ophtalmol 5:441–445 [PubMed] [Google Scholar]
- Macari F, Landau M, Cousin P, Mevorah B, Brenner S, Panizzon R, Schorderet DF, Hohl D, Huber M (2000) Mutation in the gene for connexin 30.3 in a family with erythrokeratodermia variabilis. Am J Hum Genet 67:1296–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestrini E, Korge BP, Ocana-Sierra J, Calzolari E, Cambiaghi S, Scudder PM, Hovnanian A, Monaco AP, Munro CS (1999) A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel's syndrome) in three unrelated families. Hum Mol Genet 8:1237–1243 [DOI] [PubMed] [Google Scholar]
- Martin PE, Coleman LS, Casalotti SO, Forge A, Evans WH (1999) Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal heriditary deafness. Hum Mol Genet 8:2369–2376 [DOI] [PubMed] [Google Scholar]
- Matic M, Petrov IN, Chen S, Wang C, Dimitrijevich SD, Wolosin JM (1997) Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation 61:251–260 [DOI] [PubMed] [Google Scholar]
- Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, Yamasaki H (1995) Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer Res 55:629–639 [PubMed] [Google Scholar]
- Nazzaro V, Blanchet-Bardon C, Lorette G, Civatte J (1990) Familial occurrence of KID (keratitis, ichthyosis, deafness) syndrome: case reports of a mother and daughter. J Am Acad Dermatol 23:385–388 [DOI] [PubMed] [Google Scholar]
- Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL (2000) Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys 381:181–190 [DOI] [PubMed] [Google Scholar]
- Ratkay-Traub I, Hopp B, Bor Z, Dux L, Becker DL, Krenacs T (2001) Regeneration of rabbit cornea following excimer laser photorefractive keratectomy: a study on gap junctions, epithelial junctions and epidermal growth factor receptor expression in correlation with cell proliferation. Exp Eye Res 73:291–302 [DOI] [PubMed] [Google Scholar]
- Richard G (2001) Connexin disorders of the skin. Adv Dermatol 17:243–277 [PubMed] [Google Scholar]
- Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EH Jr, DiGiovanna JJ, Compton JG, Bale SJ (1998a) Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet 20:366–369 [DOI] [PubMed] [Google Scholar]
- Richard G, White TW, Smith LE, Bailey RA, Compton JG, Paul DL, Bale SJ (1998b) Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum Genet 103:393–399 [DOI] [PubMed] [Google Scholar]
- Rouan F, White TW, Brown N, Taylor AM, Lucke TW, Paul DL, Munro CS, Uitto J, Hodgins MB, Richard G (2001) trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J Cell Sci 114:2105–2113 [DOI] [PubMed] [Google Scholar]
- Rubin JB, Verselis VK, Bennett MV, Bargiello TA (1992) Molecular analysis of voltage dependence of heterotypic gap junctions formed by connexins 26 and 32. Biophys J 62:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Branes MC, Corvalan LA, Eugenin EA, Gonzalez H, Martinez AD, Palisson F (2000) Gap junctions in cells of the immune system: structure, regulation and possible functional roles. Braz J Med Biol Res 33:447–455 [DOI] [PubMed] [Google Scholar]
- Sawey MJ, Goldschmidt MH, Risek B, Gilula NB, Lo CW (1996) Perturbation in connexin 43 and connexin 26 gap-junction expression in mouse skin hyperplasia and neoplasia. Mol Carcinog 17:49–61 [DOI] [PubMed] [Google Scholar]
- Szymko YM, Russell LJ, Bale SJ, Griffith AJ (2002) Auditory manifestations of keratitis ichthyosis-deafness (KID) syndrome. Laryngoscope 112:272–280 [DOI] [PubMed] [Google Scholar]
- Tuppurainen K, Fraki J, Karjalainen S, Paljarvi L, Suhonen R, Ryynanen M (1988) The KID-syndrome in Finland: a report of four cases. Acta Ophthalmol (Copenh) 66:692–698 [DOI] [PubMed] [Google Scholar]
- VanSlyke JK, Deschenes SM, Musil LS (2000) Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol Biol Cell 11:1933–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis VK, Ginter CS, Bargiello TA (1994) Opposite voltage gating polarities of two closely related connexins. Nature 368:348–351 [DOI] [PubMed] [Google Scholar]
- White TW, Bruzzone R (1996) Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J Bioenerg Biomembr 28:339–350 [DOI] [PubMed] [Google Scholar]