Abstract
Renin-angiotensin system (RAS) blockers have potential protective effects against atrial fibrillation (AF). The purpose of this study was to determine if patient characteristics and underlying co-morbidities could predict the efficacy of RAS blockers in AF prevention. Patients aged ≥ 45 years with hypertension were identified from the Taiwan National Health Insurance Research Database. After propensity-score matching, a total of 22,324 patients were included in this study. Risk of new-onset AF in RAS blockers users and non-users was estimated. During up to 10 years of follow-up, 1,475 patients experienced new-onset AF. Overall, RAS blockers reduced the risk of AF by 36% (adjusted HR 0.64; 95% CI 0.58 to 0.71; p < 0.001). Subgroup analysis showed that RAS blockers use was beneficial for AF prevention in patients aged ≥ 55 years or with a CHADS2 score of 1, 2, or 3. The therapy provided no obvious beneficial effect for AF prevention in those aged less than 55 years or with a CHADS2 score ≥ 4. In conclusion, RAS blockers reduced the risk of new-onset AF in patients aged ≥ 55 years or with a CHADS2 score of 1, 2, or 3, but not in patients aged less than 55 years or with a CHADS2 score ≥ 4.
Atrial fibrillation (AF) is the most common arrhythmia and is associated with an increased risk of stroke, mortality, and health care costs1,2. Old age, male gender, hypertension, heart failure, diabetes mellitus, vascular disease, pulmonary disease, thyroid disease, chronic renal disease, and valvular heart disease are risk factors for AF occurrence2,3,4,5. Among these risk factors, hypertension is the most common condition and is associated with a 40–50% increased risk of developing new-onset AF3. As the elderly population increased in recent years, AF has become more prevalent. Therefore, a major focus of disease management is to effectively prevent new-onset AF in hypertensive patients1.
The focus of AF primary prevention in patients with hypertension has recently shifted to renin-angiotensin system (RAS) blockers; such agents include angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs)6,7. Studies have suggested that RAS blockers have favorable potency because of their effect against atrial remodeling8,9. The key targets of these therapies are electrical and structural changes in the atria, such as inflammation, hypertrophy, and oxidative stress6. Although some large randomized trials10,11 and nationwide retrospective studies7,12 have shown that RAS blockers can reduce the risk of new-onset AF in patients with significant structural heart disease, the evidence is less robust in hypertensive patients with mild-to-moderate heart disease6,13.
Since trials investigating the effect of RAS blockers on AF prevention in hypertensive patients without significant heart disease reported conflicting results,7 whether these therapies can prevent AF in these subgroups of patients remains a subject of debate. Our recent studies suggest that CHADS2 scores could be used for predicting the AF preventive effect of statin, another upstream therapy for AF prevention14,15,16. However, it is still unclear whether this co-morbidity score can be used to predict RAS blockers effects on AF prevention. The purpose of the present study was to determine if patient characteristics or cardiovascular co-morbidity scoring systems could predict the effectiveness of RAS blockers in primary AF prevention in a nationwide population-based cohort.
Methods
Study database
The National Health Insurance program, which covers about 99% of population and all forms of health care services in Taiwan, was implemented in 1995. The National Health Research Institute (NHRI) of Taiwan has established a National Health Insurance Research Database. In the present study, we used the Longitudinal Health Insurance Database 2000, a systemic sampling of patient data released by the NHRI, which includes a total of 1,000,000 subjects. The NHRI has confirmed these random samples to be representative of the general Taiwanese population; i.e., there were no statistically significant differences in age and gender between overall population and the sample.
Patients’ demographic characteristics were included in the database. These data also contain information about prescriptions, including the drug use duration and prescribed dosage. The information about diagnoses and prescriptions is of high quality, and has previously been used for several epidemiological studies17,18. The NHRI safeguards the privacy of individuals and provides the data to researchers after ethical approval has been obtained. The NHRI made data at the individual level available to us in an anonymous format, in which specific individuals cannot be identified. This study was approved by the Institutional Review Board of Taichung Veterans General Hospital (CE14148).
Study population
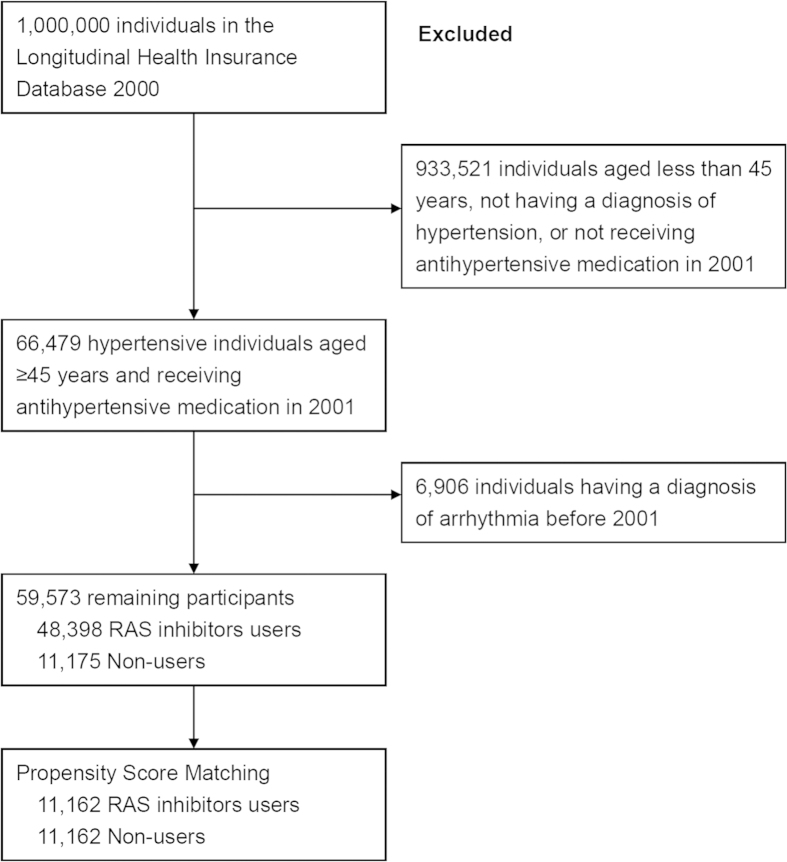
We identified individuals with a hypertension diagnosis and receiving anti-hypertensive therapy in 2000 and 2001. We excluded all individuals suffering from AF or other arrhythmia. We matched patients on RAS blockers 1:1 with individuals using other antihypertensives. Each matched cohort was followed from January 1, 2002 until a diagnosis of AF, or end of follow-up on December 31, 2011, whichever came first. The propensity-score matching was performed in an attempt to address differences in medical history between users as previously described19. Factors used in the propensity-score matching were age, gender, co-morbidities, and Charlson co-morbidity index. Inclusion and exclusion criteria of study participants are shown in Fig. 1.
Figure 1. Inclusion and exclusion criteria of study participants.
Study endpoint
The study endpoint was defined as new-onset AF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 427.31) during the 10-year follow-up period (2002-2011). All occurrences of AF were confirmed by the claims data. To ensure the diagnostic validity, only patients with an inpatient AF diagnosis or at least 3 consensus AF diagnoses at outpatient departments (to avoid misclassification by including patients with tentative diagnosis for exams and those retrieving exam reports) were included. The date of the end-point event (AF) was defined as the index date.
Medications and co-morbidities
RAS blockers (ACEIs and ARBs) use records were retrieved from outpatient and inpatient claims database. The prescription dates and the number of pills per prescription were collected. Patients were divided into RAS blockers users group and non-users group according to their ACEIs and ARBs use between January 1, 2001, and the index date (if AF occurred), or December 31, 2011. Patients who used ACEIs or ARBs for more than 28 days were defined as RAS blockers users. Other medications were identified between January 1, 2000, and December 31, 2001.
We identified many cardiovascular co-morbidities as a potential confounders by using ICD-9-CM code between January 1, 2000, and December, 31, 2001. The CHADS2 score was calculated for each patient by assigning 1 point each for the presence of hypertension, heart failure, age ≥ 75 years, and diabetes, and 2 points for a history of stroke or transient ischemic attack (TIA)20. The CHA2DS2VASc score was calculated for each patient by assigning 1 point each for the presence of hypertension, heart failure, age 65–74 years, diabetes, vascular disease, and female gender, and 2 points for a history of stroke or TIA, and age ≥ 75 years21.
Statistical analysis
All analyses were performed on the propensity score 1:1 matched cohorts. We matched RAS blockers users and non-users for age, gender and co-morbidities; paired matching was included in the model as a stratification variable. The data are presented as the mean values and standard deviations (SD) for continuous variables, and proportions for categorical variables. The t test was used to analyze differences between continuous variables, and chi-square test was used to analyze differences between categorical variables. Multivariate Cox proportional hazard regression was used to estimate the hazard ratio (HR) and 95% confidence interval (CI), which was used to determine the association between the use of RAS blockers and the occurrence of AF. The AF-free survival curves were plotted via the Kaplan–Meier method with statistical significance examined by the log-rank test. All statistical analyses were performed by SAS software version 9.2 (SAS Institute, Inc., Cary, NC, USA). A p value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the RAS blockers users and non-users are shown in Table 1. A total of 22,324 patients aged ≥ 45 years were enrolled in this study after matching, of whom 11,162 (50%) had used RAS blockers. The mean age of the study population was 65.5 ± 11.3 years, with 30.4% of them aged 65–74 years, and 22.3% of them aged ≥ 75 years. Females accounted for 51.5% of the population. Among the study subjects, 4.8% had heart failure, 10.8% had diabetes mellitus, 10.5% had stroke or TIA, 1.9% had vascular disease, 1.3% had thyroid disease, 1.1% had valvular heart disease, 10.6% had chronic lung disease, and 2.5% had chronic renal disease. There was no significant difference in the distribution of age, gender and co-morbidities between groups. The rate of statin, aspirin, alpha-blocker, beta-blocker, calcium channel blocker, diuretics, and digoxin usage was higher among RAS blockers users than non-users (p = 0.022 for digoxin; p < 0.001 for other agents). The average CHADS2 score was 1.6 ± 0.9 and the average CHA2DS2VASc score was 2.6 ± 1.3.
Table 1. Baseline characteristics.
| Variables | All patients | RAS blockers users | Non-users | P value |
|---|---|---|---|---|
| (n = 22,324) | (n = 11,162) | (n = 11,162) | ||
| No.(%) | No.(%) | No.(%) | ||
| Age at entry, years | ||||
| mean ± SD | 65.5 ± 11.3 | 65.5 ± 11.1 | 65.5 ± 11.4 | 0.954 |
| 45-54 | 5,012 (22.5) | 2,463 (22.1) | 2,549 (22.8) | 0.059 |
| 55-64 | 5,540 (24.8) | 2,796 (25.1) | 2,744 (24.6) | |
| 65-74 | 6,792 (30.4) | 3,468 (31.1) | 3,324 (29.8) | |
| ≥75 | 4,980 (22.3) | 2,435 (21.8) | 2,545 (22.8) | |
| Female | 11,505 (51.5) | 5,742 (51.4) | 5,763 (51.6) | 0.779 |
| Medical diseases | ||||
| Heart failure | 1,076 (4.8) | 555 (5.0) | 521 (4.7) | 0.288 |
| Diabetes mellitus | 2,412 (10.8) | 1,182 (10.6) | 1,230 (11.0) | 0.301 |
| Stroke or TIA | 2,341 (10.5) | 1,192 (10.7) | 1,149 (10.3) | 0.348 |
| Vascular disease | 416 (1.9) | 212 (1.9) | 204 (1.8) | 0.692 |
| Thyroid disease | 300 (1.3) | 150 (1.3) | 150 (1.3) | 1.000 |
| Valvular heart disease | 247 (1.1) | 132 (1.2) | 115 (1.0) | 0.277 |
| Chronic lung disease | 2,371 (10.6) | 1,181 (10.6) | 1,190 (10.7) | 0.845 |
| Chronic renal disease | 551 (2.5) | 279 (2.5) | 272 (2.4) | 0.763 |
| Charlson comorbidity index | ||||
| mean ± SD | 1.1 ± 1.4 | 1.1 ± 1.3 | 1.1 ± 1.5 | 0.794 |
| Medications | ||||
| Statin | 952 (4.3) | 586 (5.3) | 366 (3.3) | <0.001 |
| Aspirin | 3,710 (16.6) | 2,150 (19.3) | 1,560 (14.0) | <0.001 |
| Warfarin | 84 (0.4) | 49 (0.4) | 35 (0.3) | 0.126 |
| Alpha-blocker | 1,534 (6.9) | 885 (7.9) | 649 (5.8) | <0.001 |
| Beta-blocker | 7,351 (32.9) | 3,905 (35.0) | 3,446 (30.9) | <0.001 |
| Calcium channel blocker | 10,164 (45.5) | 5,570 (49.9) | 4,594 (41.2) | <0.001 |
| Diuretics | 5,251 (23.5) | 3,009 (27.0) | 2,242 (20.1) | <0.001 |
| Antiarrhythmic agent | 81 (0.4) | 39 (0.4) | 42 (0.4) | 0.738 |
| Digoxin | 278 (1.3) | 158 (1.4) | 120 (1.1) | 0.022 |
| CHADS2 score | ||||
| mean ± SD | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 0.9 | 0.778 |
| score =1 | 13,855 (62.1) | 6,909 (61.9) | 6,946 (62.2) | 0.597 |
| score =2 | 5,239 (23.5) | 2,659 (23.8) | 2,580 (23.1) | |
| score =3 | 2,010 (9.0) | 989 (8.9) | 1,021 (9.2) | |
| score ≥4 | 1,220 (5.5) | 605 (5.4) | 615 (5.5) | |
| CHA2DS2VASc score | ||||
| mean ± SD | 2.6 ± 1.3 | 2.6 ± 1.3 | 2.6 ± 1.3 | 0.988 |
| score =1 | 3,989 (17.9) | 2,050 (18.4) | 1,939 (17.4) | 0.007 |
| score =2 | 7,817 (35.0) | 3,790 (34.0) | 4,027 (36.1) | |
| score =3 | 5,327 (23.9) | 2,711 (24.3) | 2,616 (23.4) | |
| score ≥4 | 5,191 (23.3) | 2,611 (23.4) | 2,580 (23.1) | |
Treatment outcome
During up to 10 years of follow-up (a total of 177,798 person-years), 1,475 patients (6.6% of the study population) developed new-onset AF, and the overall incidence rate was 8.3 per 1,000 person-years. Table 2 shows the HRs for the development of new-onset AF in the cohort. AF occurred less frequently among RAS blockers users compared with non-users before and after an adjustment for variables between the 2 cohorts (adjusted HR 0.64; 95% CI 0.58 to 0.71). The incidence rate of AF decreased from 9.6 to 7.2 per 1,000 person-years after RAS blockers use. The adjusted HRs were 0.80, 0.64, and 0.77 for pure ACEIs users, pure ARBs users, and mixed users, respectively. ACEIs and ARBs therapy were both associated with lower incidences of new-onset AF compared with other agents (p = 0.007 for pure ACEIs users, p < 0.001 for pure ARBs users). Table 2 also shows the associations between the duration of RAS blockers use and the risk of AF. Among the RAS blockers users, 23.5% used RAS blockers for 28-365 days, and 76.5% of them used RAS blockers for >365 days. The incidence rates of AF were 9.6, 8.8, and 6.8 per 1,000 person-years among patients who used RAS blockers less than 28, 28 to 365, and more than 365 days, respectively. The occurrence of new-onset AF was significantly different between non-users and those who used RAS blockers for 28-365 days (adjusted HR 0.77; 95% CI 0.65 to 0.90) as well as those who used RAS blockers for >365 days (adjusted HR 0.60; 95% CI 0.54 to 0.67).
Table 2. Dose relation analysis for new-onset AF.
| Variables | No. of patients | No. of patients with AF | Incidence rate (per 1000 person-yrs) | Adjusted HR | (95% CI) | P value |
|---|---|---|---|---|---|---|
| Non-users (<28 days) | 11,162 | 782 | 9.6 | |||
| RAS blockers users | 11,162 | 693 | 7.2 | 0.64 | (0.58-0.71) | <0.001 |
| 28-365 days | 2,626 | 175 | 8.8 | 0.77 | (0.65-0.90) | 0.002 |
| >365 days | 8,536 | 518 | 6.8 | 0.60 | (0.54-0.67) | <0.001 |
| pure ACEIs users | 2,744 | 155 | 7.5 | 0.80 | (0.67-0.94) | 0.007 |
| pure ARBs users | 1,747 | 84 | 5.4 | 0.64 | (0.51-0.80) | <0.001 |
| mixed users | 6,671 | 454 | 7.6 | 0.77 | (0.69-0.86) | <0.001 |
Age, CHADS2 score, CHA2DS2VASc score and treatment outcome
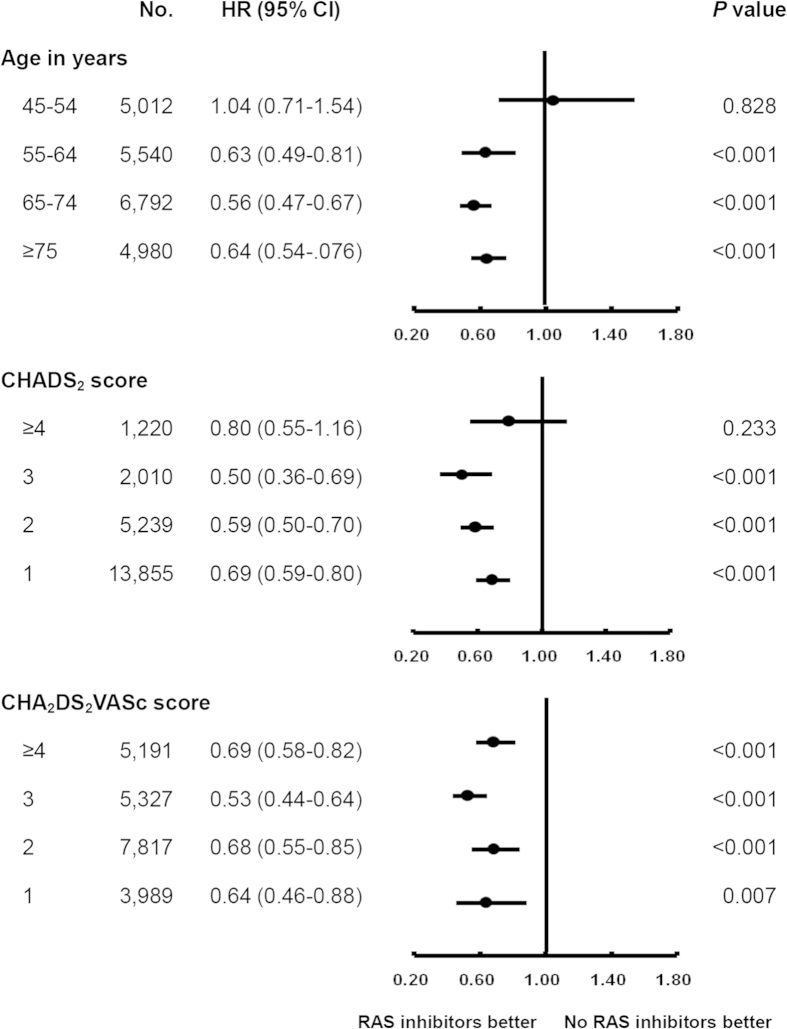
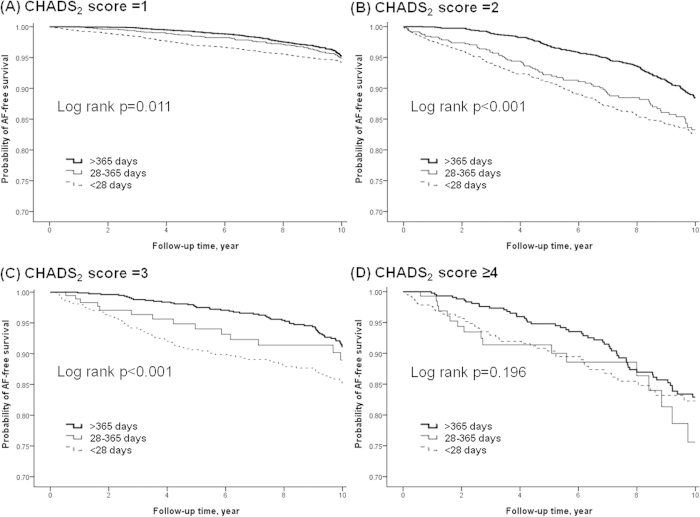
In subgroup analyses, there was a universal RAS blockers protective effect across gender categories (For female: adjusted HR 0.73; 95% CI 0.63–0.84. For male: adjusted HR 0.56; 95% CI 0.48–0.65). Figure 2 displays the hazard ratio plot of the protective effect of RAS blockers against AF according to age, CHADS2 score, and CHA2DS2VASc score. RAS blockers use was beneficial for AF prevention in patients aged ≥55 years (For age 55–64: adjusted HR 0.63; 95% CI 0.49–0.81. For age 65–74: adjusted HR 0.56; 95% CI 0.47–0.67. For age ≥ 75: adjusted HR 0.64; 95% CI 0.54–0.76), but provided no obvious beneficial effect in those aged less than 55 years (adjusted HR 1.04; 95% CI 0.71–1.54). By using established cardiovascular co-morbidity scoring systems, RAS blockers use was beneficial in patients with a CHADS2 score of 1, 2, or 3 (For a score of 1: adjusted HR 0.69; 95% CI 0.59–0.80. For a score of 2: adjusted HR 0.59; 95% CI 0.50–0.70. For a score of 3: adjusted HR 0.50; 95% CI 0.36–0.69). No significant benefit was found in patients with a CHADS2 score ≥ 4 (adjusted HR 0.80; 95% CI 0.55 to 1.16). RAS blockers therapy shows similar efficacy across every CHA2DS2VASc score category. The Kaplan-Meier survival plot presented in Figure 3 shows the protective effect of RAS blockers against AF according to RAS blockers use and CHADS2 score. The survival curves began to diverge early and continued to diverge throughout the course of the study in patients with a CHADS2 score of 1, 2, and 3. In patients with a CHADS2 score ≥ 4, the treatment showed no significant effect (log rank p = 0.196).
Figure 2. The effectiveness of RAS blockers against new-onset AF according to age, CHADS2 score, and CHA2DS2VASc score.
Figure 3. AF-free survival rate according to RAS blockers use and CHADS2 score.
Discussion
The main finding of this nationwide cohort study is that the risk of AF was lower in RAS blockers users than non-users among patients aged ≥55 years, but not among patients aged less than 55 years. This study also showed that RAS blockers therapy reduces the risk of new-onset AF for those with a CHADS2 score of 1, 2, or 3. To the best of our knowledge, this is the first study to analyze the relationship between CHADS2 score and the protective effects of RAS blockers against AF. The results suggest that the CHADS2 score can help identify the patients who will benefit most from RAS blockers use for the prevention of AF. These findings support the inference that RAS blockers have anti-arrhythmic effects and explain the heterogeneity among previous trials.
Our results showed that RAS blockers reduce the risk of new-onset AF in patients aged ≥ 55 years. Of note, this therapy provided no obvious beneficial effect in those aged less than 55 years. AF is a result of electrical and structural remodeling of the atria, which involves progression of underlying heart disease, genetic factors, and ageing process. RAS blockers target both the formation and evolution of the substrate for AF, and thus prevent the occurrence of new-onset AF. Young patients have a low incidence of developing AF and fewer processes of atrial remodeling than elderly patients22. Therefore, our results may imply that RAS blockers do not play a major role in AF prevention in young patients. These findings may also explain why previous studies reached different conclusions23– heterogeneity of patient characteristics may confound effectiveness of RAS blockers against AF.
Our study also showed that RAS blockers are more beneficial in hypertensive patients with a CHADS2 score of 1, 2, or 3. Patients with scores ≥ 4 get no obvious AF preventive benefits from RAS blockers use. Meanwhile, grouping patients by using CHA2DS2VASc score showed a universal protective effect of this therapy. Therefore, the CHADS2 score is a more useful scoring system than the CHA2DS2VASc score for predicting the effectiveness of RAS blockers in AF prevention. The CHADS2 score is a convenient scoring system used for evaluating the complexity of patient’s co-morbidities. Previous study demonstrates that high CHADS2 scores are associated with atrial remodeling and a larger left atrium size24,25. Furthermore, patients with higher CHADS2 scores had a significantly larger amount of atrial fibrosis and advanced atrial remodeling when compared to those with lower CHADS2 scores26,27. We propose that atrial fibrosis and fixed atrial remodeling may therefore reduce the potency of RAS blockers in patient with high CHADS2 scores.
Although studies focusing on the protective effects of RAS blockers against AF have yielded consistently significant results in people with heart failure, these effects are less clear in patients with multiple risk factors, such as hypertension, diabetes mellitus, hypercholesterolemia, and coronary artery disease6. A recent meta-analysis, which included patients at high cardiovascular risk, showed a 19% risk reduction in the occurrence of AF with RAS blockers28. However, heterogeneity among studies was noted, especially in subjects with mild-to-moderate underlying heart disease. Hence, whether a specific population of hypertensive patients may benefit from RAS blockers in preventing AF is the question addressed in the present study. Our results suggest that RAS blockers reduce the risk of new-onset AF in patients with cardiovascular diseases, and the effect was confound by age and CHADS2 score. This finding provides an explanation for the heterogeneity among studies.
Our previous studies revealed that statin was more beneficial in patients with higher CHADS2 scores than those with lower scores for AF prevention15. In the present study, RAS blockers seemed to have a different pattern of efficacy for AF prevention in regard to this co-morbidity score: RAS blockers were less effective in patients with high CHADS2 score. The result of our study implies that the main mechanisms of AF prevention by statin and RAS blockers may be different. RAS blockers can reduce the occurrence of new-onset AF via counteracting the profibrotic process, but not fixed atrial fibrosis6. On the other hand, anti-inflammatory effect of statin may be more obvious in regarding to AF prevention16. Patients with higher CHADS2 scores may have a more severe inflammation status and a more advanced fixed atrial fibrosis than those with lower scores. This difference may therefore result in the different efficacy pattern of stain and RAS blockers.
Our study has a number of strengths. The data on this study cohort was retrieved from a computerized population-based database, allowing evaluation of a large cohort in a 10-year follow-up period. In addition, because the data on RAS blockers use includes all prescription information before the diagnosis of AF, recall bias can be avoided. Furthermore, we conducted multivariate analysis to eliminate the misclassifications, and the results revealed no significant changes in the HRs before and after the analyses. Finally, we matched individuals on a propensity score to address differences in medical history between groups. This method further reduces the likelihood of bias by indication.
There were some limitations in the present study. First, the study population mainly included Asian subjects. Second, types of AF and duration of each AF episode were not included in our research database. Third, the AF event definition in our study may be under-reported. We did not capture silent AF. Fourth, several confounding factors, including smoking, body mass index, and alcohol intake, were not included in our database. We also had no information on actual blood pressure control or whether the administered dose resulted in the target blood pressure. Finally, we assumed that patients took all prescribed medications. This may overestimate the actual dosage used.
Conclusions
RAS blockers reduce the risk of new-onset AF, and are beneficial in patients aged ≥ 55 years or with a CHADS2 score of 1, 2, or 3. However, these therapies showed no obvious AF preventive effect in patients aged less than 55 years or with a CHADS2 score ≥ 4. Further studies focusing on possible biological mechanisms are needed.
Additional Information
How to cite this article: Hung, C.-Y. et al. Age and CHADS2 Score Predict the Effectiveness of Renin-Angiotensin System Blockers on Primary Prevention of Atrial Fibrillation. Sci. Rep. 5, 11442; doi: 10.1038/srep11442 (2015).
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes (Registered number 101095, 102148, 103122, 103274). The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes.This study was supported in part by grants from Taichung Veterans General Hospital, Taiwan (TCVGH-NHRI10405, TCVGH-1047324D, TCVGH-1047312C, TCVGH-104G211, TCVGH-1033103C) and the National Science Council, Taiwan (MOST 103-2314-B-075A-006). The authors would like to thank the Healthcare Service Research Center (HSRC) of Taichung Veterans General Hospital for statistical support.
Footnotes
Author Contributions Conceived and designed the experiments: C.Y.H., Y.C.H., C.H.L., T.J.W. Performed the experiments: C.Y.H., T.J.W. Analyzed the data: C.H.L. Prepare Tables and Figure: C.Y.H., Y.C.H., C.H.L., J.L.H. Wrote the paper: C.Y.H., Y.C.H. All authors reviewed the manuscript.
References
- Wolf P. A., Mitchell J. B., Baker C. S., Kannel W. B. & D’Agostino R. B. Impact of atrial fibrillation on mortality, stroke, and medical costs. Archives of internal medicine 158, 229–234 (1998). [DOI] [PubMed] [Google Scholar]
- Krahn A. D., Manfreda J., Tate R. B., Mathewson F. A. & Cuddy T. E. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. The American journal of medicine 98, 476–484, doi:10.1016/S0002-9343(99)80348-9 (1995). [DOI] [PubMed] [Google Scholar]
- Benjamin E. J. et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. Jama 271, 840–844 (1994). [PubMed] [Google Scholar]
- Macfarlane P. W. et al. The incidence and risk factors for new onset atrial fibrillation in the PROSPER study. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 13, 634–639, doi:10.1093/europace/eur016 (2011). [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Wolf P. A., Benjamin E. J. & Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. The American journal of cardiology 82, 2N–9N (1998). [DOI] [PubMed] [Google Scholar]
- Savelieva I., Kakouros N., Kourliouros A. & Camm A. J. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 13, 308–328, doi:10.1093/europace/eur002 (2011). [DOI] [PubMed] [Google Scholar]
- Marott S. C., Nielsen S. F., Benn M. & Nordestgaard B. G. Antihypertensive treatment and risk of atrial fibrillation: a nationwide study. European heart journal 35, 1205–1214, doi:10.1093/eurheartj/eht507 (2014). [DOI] [PubMed] [Google Scholar]
- Boldt A. et al. Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease. Journal of the American College of Cardiology 42, 1785–1792 (2003). [DOI] [PubMed] [Google Scholar]
- Ehrlich J. R., Hohnloser S. H. & Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. European heart journal 27, 512–518, doi:10.1093/eurheartj/ehi668 (2006). [DOI] [PubMed] [Google Scholar]
- Schmieder R. E. et al. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. Journal of hypertension 26, 403–411, doi:10.1097/HJH.0b013e3282f35c67 (2008). [DOI] [PubMed] [Google Scholar]
- Wachtell K. et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. Journal of the American College of Cardiology 45, 712–719, doi:10.1016/j.jacc.2004.10.068 (2005). [DOI] [PubMed] [Google Scholar]
- Schaer B. A. et al. Risk for incident atrial fibrillation in patients who receive antihypertensive drugs: a nested case-control study. Annals of internal medicine 152, 78–84, doi:10.7326/0003-4819-152-2-201001190-00005 (2010). [DOI] [PubMed] [Google Scholar]
- Goette A. et al. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circulation. Arrhythmia and electrophysiology 5, 43–51, doi:10.1161/CIRCEP.111.965178 (2012). [DOI] [PubMed] [Google Scholar]
- Hung C. Y., Hsieh Y. C., Huang J. L., Lin C. H. & Wu T. J. Statin Therapy for Primary Prevention of Atrial Fibrillation: Guided by CHADS2/CHA2DS2VASc Score. Korean circulation journal 44, 205–209, doi:10.4070/kcj.2014.44.4.205 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C. Y., Lin C. H., Loh el W., Ting C. T. & Wu T. J. CHADS(2) score, statin therapy, and risks of atrial fibrillation. The American journal of medicine 126, 133–140, doi:10.1016/j.amjmed.2012.06.027 (2013). [DOI] [PubMed] [Google Scholar]
- Hung C. Y. et al. Dosage of statin, cardiovascular comorbidities, and risk of atrial fibrillation: a nationwide population-based cohort study. International journal of cardiology 168, 1131–1136, doi:10.1016/j.ijcard.2012.11.087 (2013). [DOI] [PubMed] [Google Scholar]
- Wu C. Y. et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. Jama 308, 1906–1914 (2012). [DOI] [PubMed] [Google Scholar]
- Shih C. J. et al. Long-term clinical outcome of major adverse cardiac events in survivors of infective endocarditis: a nationwide population-based study. Circulation 130, 1684–1691, doi:10.1161/CIRCULATIONAHA.114.012717 (2014). [DOI] [PubMed] [Google Scholar]
- Nielsen S. F., Nordestgaard B. G. & Bojesen S. E. Statin use and reduced cancer-related mortality. The New England journal of medicine 367, 1792–1802, doi:10.1056/NEJMoa1201735 (2012). [DOI] [PubMed] [Google Scholar]
- Gage B. F. et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 110, 2287–2292, doi:10.1161/01.CIR.0000145172.55640.93 (2004). [DOI] [PubMed] [Google Scholar]
- European Heart Rhythm A. et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 12, 1360–1420, doi:10.1093/europace/euq350 (2010). [DOI] [PubMed] [Google Scholar]
- Teh A. W. et al. Electroanatomic remodelling of the pulmonary veins associated with age. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 14, 46–51, doi:10.1093/europace/eur275 (2012). [DOI] [PubMed] [Google Scholar]
- Hansson L. et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 353, 611–616 (1999). [DOI] [PubMed] [Google Scholar]
- Hrynkiewicz-Szymanska A. et al. Association of the CHADS and CHA DS -VASc scores with left atrial enlargement: a prospective cohort study of unselected atrial fibrillation patients. Journal of thrombosis and thrombolysis, doi:10.1007/s11239-014-1154-6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T. F. et al. Associations among the CHADS(2) score, atrial substrate properties, and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society 8, 1155–1159, doi:10.1016/j.hrthm.2011.03.016 (2011). [DOI] [PubMed] [Google Scholar]
- Daccarett M. et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. Journal of the American College of Cardiology 57, 831-838, doi:10.1016/j.jacc.2010.09.049 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H. et al. The electroanatomical remodelling of the left atrium is related to CHADS2/CHA2DS2VASc score and events of stroke in patients with atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 13, 1541–1549, doi:10.1093/europace/eur135 (2011). [DOI] [PubMed] [Google Scholar]
- Schneider M. P. et al. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. Journal of the American College of Cardiology 55, 2299–2307, doi:10.1016/j.jacc.2010.01.043 (2010). [DOI] [PubMed] [Google Scholar]