Abstract
In an inbred Iraqi Jewish family, we have studied three siblings with presenile cataract first noticed between the ages of 20 and 51 years and segregating in an autosomal recessive mode. Using microsatellite repeat markers in close proximity to 25 genes and loci previously associated with congenital cataracts in humans and mice, we identified five markers on chromosome 19q that cosegregated with the disease. Sequencing of LIM2, one of two candidate genes in this region, revealed a homozygous T→G change resulting in a phenylalanine-to-valine substitution at position 105 of the protein. To our knowledge, this constitutes the first report, in humans, of cataract formation associated with a mutation in LIM2. Studies of late-onset single-gene cataracts may provide insight into the pathogenesis of the more common age-related cataracts.
Cataract, defined as opacities within the clear lens of the eye, is a multifactorial disorder with an extreme variability in the age at onset and a strong genetic predisposition (McCarty and Taylor 2001). Nonsyndromic congenital cataracts have an estimated frequency of 1–6 per 10,000 live births (Lambert and Drack 1996). At least one-third of the cases are familial, most often transmitted in an autosomal dominant mode. To date, 20 human loci and genes have been associated with autosomal dominant congenital cataract (MIM 116600, 600897, 123680, 603212, 605749, 602669, 123590, 154050, 601885, 115650, 605728, 116800, 123740, 601202, 600881, 115660, 134790, 605387, 123580; and 601547).
Age-related cataract is a major health problem around the world, with >1.5 million cataract operations performed annually in the United States alone (Thylefors et al. 1995; Foster 1999). The etiology of age-related cataract is more complex. Factors such as age, female sex, smoking, diet, and ultraviolet light exposure, in addition to a strong genetic predisposition (McCarty and Taylor 2001), may contribute to its pathogenesis. Recent segregation and twin studies suggest that ∼50% of age-related cataracts could be accounted for by single-gene inheritance; many of these are transmitted as autosomal recessive traits (Heiba et al. 1993; Hammond et al. 2000, 2001). In contrast to the large number of genes and loci identified in human autosomal dominant cataract, only three such loci have been described in autosomal recessive cataract. Two of these loci, CRYAA (Pras et al. 2000) and a locus on chromosome 3p with an unidentified gene (Pras et al. 2001), cause congenital cataracts, whereas a recessive form of cataract that appears at a relatively late age was recently described in a Swiss family (Heon et al. 2001). Affected individuals from the Swiss family suffered from cortical pulverulent cataracts and required surgery at a mean age of 44 years (range 36–55 years). The disease has been mapped to chromosome 9q13-9q22, but the disease gene has not yet been cloned.
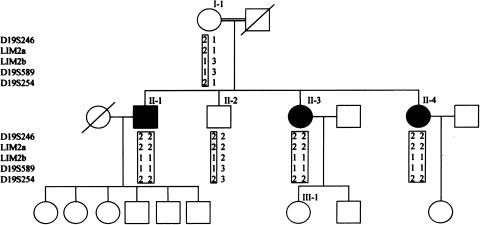
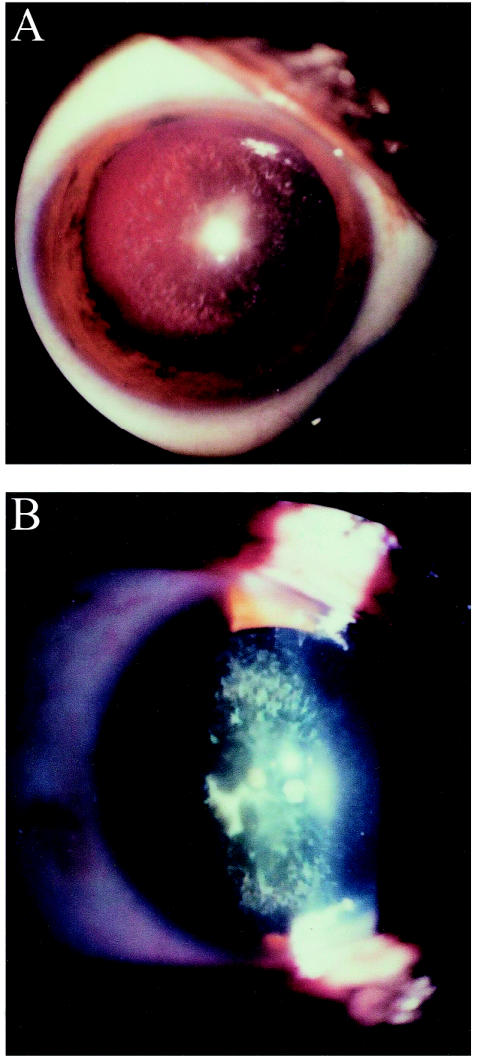
We have studied an inbred Iraqi Jewish family in which three sibs had a relatively late-onset form of cataract (fig. 1). The parents were first cousins, and the three affected children in the second generation noted blurred vision and glare at a mean age of 40 years (range 20–51 years). Figure 2 discloses results of a slit lamp examination performed on patient II-3, revealing cortical bluish and white opacifications in concentric layers, with nuclear opacities emerging from prominent sutures. Similar findings were seen in patients II-1 and II-4. Examination of the mother (I-1), who is 83 years old, showed a mild nuclear sclerosing cataract without cortical opacifications, which could clearly be differentiated from those of her offspring by its morphology, severity, and the age at which it appeared and most likely represents a mild form of age-related cataract. The father, who died at the age of 67 years, was reported to have good functional vision in his last years. Individuals II-2 and III-1 were examined and had no evidence of cataract formation. Additional clinical details are provided in table 1. The Helsinki Committee at the Sheba Medical Center approved the study, and participants gave informed consent. Twenty milliliters of blood were drawn from each family member, and DNA was extracted by use of a commercial kit (Gentra Systems).
Figure 1.
Family pedigree and typings for five chromosome 19q markers. Carrier chromosomes are presented in boxes.
Figure 2.
Slit lamp photographs of patient II-3. Retroilluminative (a) and tangential (b) views disclose concentric bluish and white cortical opacities, with prominent nuclear sutures.
Table 1.
Clinical Characteristics of the Family Members
|
Age (years) at |
|||||
| Individual | Preoperative Visual Acuitya | Present | First VisualSymptoms | Lens Morphology | Other Systemic Findings |
| I-1 | RE 20/100; LE 20/100 | 83 | 70 | Nuclear sclerosing cataract | Hypertension; diabetes mellitus |
| II-1 | RE 20/200; LE 20/40 | 61 | 50 | Pulverulent cortical cataract | … |
| II-2 | Noncooperative | 57 | None | Clear lens | Mental retardation |
| II-3 | RE 20/100; LE 20/200 | 54 | 51 | Pulverulent cortical cataract | Hypertension |
| II-4 | RE 20/40; LE 20/40 | 51 | 20 | Pulverulent cortical cataract | … |
| III-1 | RE 20/20; LE 20/20 | 26 | None | Clear lens | … |
RE indicates right eye, and LE indicates left eye.
In an attempt to identify the gene that causes cataract in this family, we used polymorphic microsatellite markers in close proximity to 25 candidate genes or loci previously involved in cataract formation in humans or in mice (MIM 116600, 600897, 123680, 603212, 605749, 602669, 123590, 154050, 601885, 115650, 605728, 116800, 123740, 601202, 600881, 115660, 134790, 605387, 123580, 601547, 603307, 154045, 123730, 601094, and Pras et al. 2001), using PCR conditions described elsewhere (Pras et al. 2001). Twenty-three of these genes and loci were excluded because of lack of segregation between the markers and the disease (data not shown).
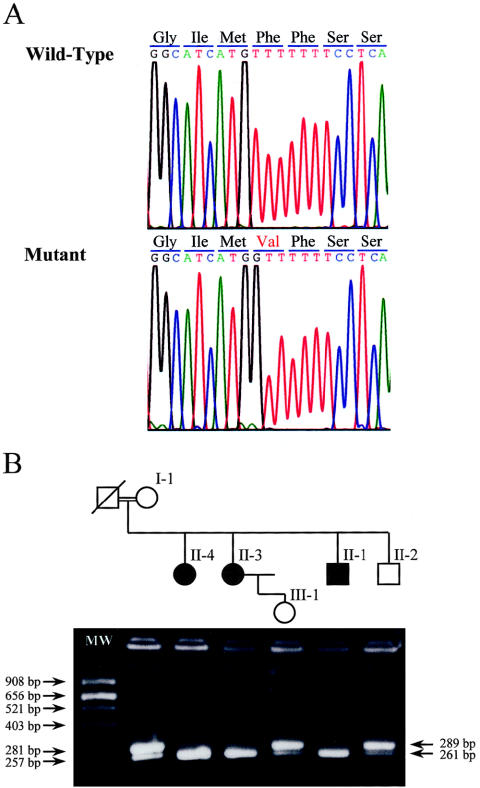
Cosegregation of the cataract trait was noticed initially with alleles of three microsatellite markers, D19S246, D19S589, and D19S254. These markers map close to two candidate genes, FTL and LIM2, located 6 Mb from each other on the long arm of chromosome 19. From a genomic clone that spans LIM2, AC008750, we have identified two additional microsatellite CA repeats that we named LIM2a and LIM2b. These were amplified with the primers 5′-GGC AGT GCT CAG CTC ATA CA-3′ and 5′-AGT ACC CAG AAT GCC CAA TG-3′, 5′-ACA AAT GCT GTG CTC AGA GTG-3′ and 5′-CAC GCA CAT ACC CAG AAA CA-3′, respectively. Haplotypes for the five markers are presented in figure 1. Sequencing of the entire FTL coding region failed to disclose any alterations, but in LIM2, a homozygous T→G change was detected at position 313 of the cDNA (fig. 3a). This resulted in a valine replacing phenylalanine at position 105 of the protein (F105V). The sequence variation was confirmed and extended to all family members by restriction digestions performed on PCR-amplified DNA (fig. 3b), using the primers 5′-GCA TCT CCC GGC CCT TCT CTG GCA CCA TG -3′ and 5′-GGA GTC AAA CAC AAA TTT TAG GTG AT-3′. The former primer was modified at its 3′ end (underlined base) so that, in the patients, an NcoI restriction site is formed. Two hundred control chromosomes were screened by means of the restriction digest, but only the wild-type variant was found (data not shown). A database search revealed that LIM2 has been sequenced in four different species, all of which show conservation of the phenylalanine at this position (fig. 4).
Figure 3.
a, Sequence chromatograms from a normal control subject and a patient. A T→G change in the patient results in valine replacing phenylalanine at codon 105. b, NcoI restriction digest of the mutation. In the carrier chromosome, the 289-bp PCR product is cleaved to yield 261- and 28-bp fragments.
Figure 4.
A multiple alignment of a segment of the human LIM2 protein with the corresponding segments in some of its homologues. The sequences were selected using a BLASTP (Altschul et al. 1997; NCBI BLAST home page) search of the human LIM2 against the nonredundant protein database. Multiple alignment of the entire protein length was performed with the ClustalW program, using the default parameters (Higgins et al. 1996). The top sequence is for the mutated human LIM2, and the arrow indicates the F105V mutated position. The GenBank accession numbers of the aligned proteins are listed in the Electronic-Database Information section.
This report constitutes the first description of a mutation in LIM2 associated with cataract formation in humans, and the fourth gene/locus associated with the autosomal recessive form of the disease. Of interest, there is a mouse model for the LIM2 gene. In the To-3 mouse, a valine-to-glycine substitution at position 15 (V15G) was shown to cause congenital cataract, inherited in a semidominant fashion, with both heterozygous and homozygous mice exhibiting dense opacity of the lens (Steele et al. 1997). Histological sections from the eyes of the homozygote mice revealed the existence of vacuoles, gross disorganization of the fibers, leakage of lens material into the vitreous chamber, and microphthalmia. Transgenic mice harboring the V15G mutation showed very similar findings (Steele at al. 2000).
MP-20, the protein product of LIM2, is a 20-kDa membrane protein, with four intramembrane domains (Arneson and Louis 1998), expressed mainly in the cortical region of the lens (Tenbroek et al. 1992). The function of this protein remains unknown. MP-20 has been shown to be absent from proliferating epithelial cells in the lens, with expression becoming prominent in differentiating as well as in mature lens fiber cells. Taylor et al. (1995) suggested that it has an important role in the switch from proliferation to differentiation and in the maintenance of the cells in the differentiated state. Zhou et al. (2001) performed a hydropathic profile search and identified several proteins similar to MP-20. These included the Chlamydia pneumonia lipoprotein signal peptidase (LSPA Q9Z817), a protein that specifically catalyzes the removal of signal peptides from lipoproteins, and GMP-17, a granule membrane protein that moves to the plasma membrane of natural killer cells after target cell recognition. Several much larger proteins of diverse origin and function showed the MP-20 hydropathic profile as part of their C-terminal or N-terminal structure. These findings have led Zhou et al. to suggest that MP-20 is most likely to have a transport or an enzymatic function, although they could not rule out the possibility that it is purely a structural protein.
Although the mouse mutation is associated with a severe dominant congenital cataract, the human mutation is associated with a relatively mild, autosomal recessive, and late-onset phenotype. These differences could be accounted for by the distinct nature and location of the two mutations and by the influence that they may have on the MP-20 protein structure or function. The V15G mutation is located in the first cytoplasmic N-terminal domain (Arneson and Louis 1998). Steele et al. (2000) have shown that in the To-3 transgenic mice, a very small percentage of the total MP-20 population needs to be altered to have a severe consequence on normal lens development, findings that are likely to be explained by a negative dominant effect. Cross-linking studies on both purified MP-20 protein and native lens membranes indicated that MP-20 could form oligomers as large as hexamers (Jarvis and Louis 1995). In such a case, a small number of abnormal monomers could severely alter the functioning MP-20 units. In contrast, the F105V mutation is located in the third intermembrane domain and results in a small amino acid being replaced by a larger yet neutral one. An alteration in enzymatic or transport activity may best explain its recessive nature. In such cases one functional allele is usually enough to maintain function, and therefore heterozygotes remain asymptomatic. Such a functional alteration may also explain its late onset, since the deleterious results of a mild reduction in an enzymatic or transport function may take years to appear. The formation of cataract in this case may be due to a gradual accumulation of an unidentified substance, to a deficiency in one, and/or to a concomitant loss of function of other housekeeping genes that occurs with normal aging.
Two genes, CRYAA and LIM2, and two loci with as yet uncloned genes have been implicated in autosomal recessive cataract. Mutations in CRYAA and LIM2 have been associated with both dominant and recessive cataract, whereas the two loci with as yet uncloned genes have been associated only with autosomal recessive cataract. These findings indicate that, in some cases, autosomal recessive cataract can be caused by mild mutations in genes that cause autosomal dominant disease and, in others, by unique genes that have so far been implicated only with recessive disease.
The recessive inheritance and the late onset of cataract in this family support the possibility that at least some cases of age-related cataract represent single-gene disorders that are caused by mild mutations in genes that have already been implicated in congenital cataract.
Acknowledgments
This study was supported by the Vladimir Schreiber Fund for Medical Research and by the Claire and Amedee Martier Institute for the Study of Blindness and Visual Disorders, Sackler Faculty of Medicine, Tel Aviv University.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/GenbankOverview.html (for human LIM2, accession number gi/14754795; rat LIM2, accession number gi/477539; bovine LIM2, accession number gi/482519; and Mus musculus LIM2, accession number gi/12802692).
- NCBI BLAST home page, http://www.ncbi.nlm.nih.gov/BLAST/ (for LIM2 homologues)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for GJA8 [MIM 600897], crystalline gamma complex [MIM 123680], BFSP2 [MIM 603212], CRYGS [MIM 123730], PITX3 [MIM 602669], CRYAB [MIM 123590], LIM1/ AQP0 [MIM 154050], GJA3 [MIM 601885], CRYM [MIM 123740], CRYBA [MIM 600881], GLK1 [MIM 115660], LIM2 [MIM 154045], FTL [MIM 134790], CRYAA [MIM 123580], CRYBB [MIM 601547], BFSP1 [MIM 603307], FOXE3 [MIM 601094] and for known loci with unspecified genes [MIM 116600, 116800, 601202, 605728, 605749, 605387, and 115650])
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneson M, Louis C (1998) Structural arrangement of lens fiber cell plasma membrane protein MP20. Exp Eye Res 66:495–509 [DOI] [PubMed] [Google Scholar]
- Foster A (1999) Cataract-A global perspective: output, outcome and outlay. Eye 13:449–453 [DOI] [PubMed] [Google Scholar]
- Hammond CJ, Duncan DD, Snieder H, deLange ME, West SK, Spector TD, Gilbert CE (2001) The heritability of age related cortical cataract: the twin eye study. Invest Ophthalmol Vis Sci 42:601–605 [PubMed] [Google Scholar]
- Hammond CJ, Snieder H, Spector TD, Gilbert CE (2000) Genetic and environmental factors in age related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med 342:1786–1790 [DOI] [PubMed] [Google Scholar]
- Heiba IM, Elston RC, Klein BEK, Klein R (1993) Genetic etiology of nuclear cataract: evidence for a major gene. Am J Med Genet 47:1208–1214 [DOI] [PubMed] [Google Scholar]
- Heon E, Paterson AD, Fraser M, Billingsley G, Pritson M, Balmer A, Schorderet DF, Verner A, Hudson TJ, Munier FL (2001) A progressive autosomal recessive cataract locus maps to chromosome 9q13-q22. Am J Hum Genet 68:772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DG, Thompson JD, Gibson TJ (1996) Using CLUSTAL for multiple sequence alignments. Methods Enzymol 266:383–402 [DOI] [PubMed] [Google Scholar]
- Jarvis LJ, Louis CF (1995) Purification and oligomeric state of the major lens fiber cell membrane proteins. Curr Eye Res 14:799–808 [DOI] [PubMed] [Google Scholar]
- Lambert SL, Drack AV (1996) Infantile cataracts. Surv Ophthalmol 40:427–458 [DOI] [PubMed] [Google Scholar]
- McCarty A, Taylor HR (2001) The genetics of cataract. Invest Ophthalmol Vis Sci 42:1677–1678 [PubMed] [Google Scholar]
- Pras E, Frydman M, Levy-Nissenbaum E, Bakhan T, Raz J, Assia EI, Goldman B, Pras E (2000) A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Ophthalmol Vis Sci 41:3511–3515 [PubMed] [Google Scholar]
- Pras E, Pras E, Bakhan T, Levy-Nissenbaum E, Lahat H, Assia EI, Garzozi HJ, Kastner DL, Goldman B, Frydman M (2001) A gene causing autosomal recessive cataract maps to the short arm of chromosome 3. Isr Med Assoc J 3:559–562 [PubMed] [Google Scholar]
- Steele EC, Kerscher S, Lyon MF, Glenister PH, Favor J, Wang J, Church RL (1997) Identification of a mutation in the MP19 gene, LIM2, in the cataractous mouse mutant TO3. Mol Vis 3:5 [PubMed] [Google Scholar]
- Steele EC, Wang J, Lo W, Saperstein DA, Li X , Church RL (2000) Lim2 (To3) transgenic mice establish a causative relationship between the mutation identified in the Lim2 gene and cataractogenesis in the To3 mouse mutant. Mol Vis 6:85–94 [PubMed] [Google Scholar]
- Taylor V, Welcher AA, Program AE, Suter U (1995) Epithelial membrane protein-1, Peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J Biol Chem 270:28824–28833 [DOI] [PubMed] [Google Scholar]
- Tenbroek E, Arneson M, Jaris L, Louis C (1992) The distribution of the fiber cell intrinsic membrane proteins MP20 and connexin 46 in the bovine lens. J Cell Science 103:245–257 [DOI] [PubMed] [Google Scholar]
- Thylefors B, Negrel A, Pararajasegaram R, Dadzie K (1995) Global data on blindness. Bull World Health Organ 73:115–121 [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li XL, Church RL (2001) The mouse lens fiber-cell intrinsic membrane protein MP19 gene (LIM2) and granule membrane protein GMP-17 gene (NK-17): isolation and sequence analysis of two neighboring genes. Mol Vis 7:79–88 [PubMed] [Google Scholar]