Abstract
Background: Simvastatin is a widely used medication in cardiac care. Here we evaluate the role of ATP sensitive potassium (KATP) channels in simvastatin induced renal protection after renal ischemia/reperfusion (I/R) injury.
Methods: A total of 81 male Wistar rats, were treated with simvastatin (10 and 20mg/kg/day; gavage, one week). Some groups received glibenclamide (KATP channel inhibitor; 5mg/kg) before ischemia (45min) and reperfusion (24h). Finally the kidneys were processed for histological analysis and measurement of biochemical parameters including tissue malondialdehyde (MDA), blood urea nitrogen (BUN), fractional excretion of sodium (FENa), creatinine clearance rate (CCr) and Bcl2-associated X protein (Bax) expression.
Results: IR significantly increased serum Cr (p< 0.01) and BUN levels (p< 0.01), elevated FENa (p<0.01) and tissue MDA (p<0.01), and decreased CCr (p< 0.01) and induced histological damage. Bax pro-apoptotic protein was upregulated in renal tissue after I/R injury and downregulated in simvastatin pretreated group. Simvastatin at doses of 10 and 20mg/kg/day significantly reduced serum Cr and BUN levels (p< 0.05 vs. IR group), tissue MDA contents and FENa (p< 0.05 vs. I/R) and increased CCr (p< 0.05 vs. IR). Renal tissue injury was improved only in simvastatin 20mg/kg/day group (p< 0.05). Glibenclamide significantly abolished protective effects of simvastatin and increased serum Cr and BUN and FENa and decreased CCr (p< 0.05). It also abolished the effects of simvastatin on tissue injury and MDA contents and downregulated the Bax protein after IR injury (p< 0.05).
Conclusion: Opening of KATP channels is essential for simvastatin-induced renal protection against I/R injury.
Keywords: Creatinine Clearance, KATP channels, Renal ischemia/reperfusion, Simvastatin, Glibenclamide
Introduction
Ischemia/reperfusion (I/R) injury, a common clinical problem in different types of renal surgery and transplantation, is a major cause of acute kidney injury (AKI) (1,2). Pharmacological modulations to increase kidney tolerance against ischemic injury would help to reduce the incidence of early graft dysfunction. However, successful strategies to moderate renal I/R injury are inadequate and novel therapies are required (3). Several mechanisms are involved in renal cell damage after I/R injury. Oxidative stress is one of the pathogenic mechanisms known to be involved in renal tubular damage (4). A recent study by Fonseca et al. revealed that malondialdehyde (MDA) level one day after transplantation is inversely correlated with early graft function after renal transplantation and its level 7 days after transplantation is inversely correlated with 1-year graft survival (5). MDA is a marker of oxidative stress in tissue which has been shown to significantly increase early after I/R injury (5,6). Another mechanism which is believed to be involved in I/R injury is the mitochondrial membrane depolarization and translocation of cytochrome C from mitochondria to the cytosol which trigger the apoptotic death of renal cell (7, 8). Jones et al. has demonstrated that simvastatin attenuates membrane depolarization subsequent to oxidative stress by opening ATP-dependent (K+) (KATP) ion channels in the mitochondrial membrane.
It has been suggested that the prevention of I/R injury in transplantation settings should be started before organ recovery by donor pretreatment (9). Several pharmacological agents, including simvastatin, have been suggested for use as pretreatment to reduce I/R injury adverse outcomes. Simvastatin is a 3-Hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase inhibitor which has been widely used in clinical practice for reduction of serum cholesterol levels and subsequent risk for coronary events (10). Recent studies have demonstrated its protective effects against I/R injury when used as pretreatment in different organs including myocardium (11), kidney (12), and spinal cord (13). Studies have revealed that this effect of simvastatin in cardiac tissue is mediated, at least in part, by opening KATP ion channels (11, 14). There are also many reports on the antioxidant properties of the simvastatin (15-17). However, underlying mechanisms of renoprotection against renal I/R injury mediated by simvastatin has not been completely understood.
In the current study we investigated different aspects of renal protection against renal I/R injury by simvastatin. We studied changes in the MDA level, both as a new marker of graft survival and a marker of antioxidant capacity of the tissue, after renal I/R injury in rats pretreated with simvastatin. We also investigated the potential involvement of KATP ion channels in the protection and its effects on apoptotic cell death, as measured by the ratio of Bcl-2/Bax protein levels.
Methods
Animals and groups: Experiments were conducted on male Wistar rats weighing 210-250 g. The rats were housed in groups of 9 with food and water available, under a 12-hour light-dark cycle (light 7:00 a.m. to 7:00 p.m.) and controlled temperature (22 ± 2 ˚C). A total of 81 rats were used in the present study and each animal was used only once. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Ministry of Health and Medical Education and in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
The following drugs were administered: pentobarbital (45mg/kg, ip, Sigma, St. Louis, MO, USA), simvastatin (10 and 20 mg/kg, gavage: Sigma, St Louis, Mo, USA), and glibenclamide (5 mg/kg, ip: non selective KATP channels blocker, Sigma, St Louis, Mo, USA). Briefly, for activation of simvastatin 4 mg simvastatin was dissolved in 100μl ethanol then incubated at 50 Ċ for 2 hours. The pH was brought to 7.0 and the final concentration of stock solution was adjusted to 4 mg/ml and kept at 4 Ċ (18).
Nine experimental groups included: 1) Sham operated control; 2) SIMV10 (Simvastatin 10mg/kg/day; gavage for 7 days before sham operation) + sham operation; 3) SIMV20 (Simvastatin 20mg/kg/day for 7 days before sham operation) + sham operation; 4) Untreated left kidney ischemia (45 min) and reperfusion (24 h) (I/R); 5) SIMV10 (Simvastatin 10mg/kg/day for 7 days before I/R) + I/R; 6) SIMV20 (Simvastatin 20 mg/kg/day for 7 days before I/R) + I/R; 7) glibenclamide (5mg/kg i.p. 45 minutes before ischemia) + I/R; 8) SIMV10 + glibenclamide + I/R; 9) SIMV20 + glibenclamide + I/R.
Experimental protocol for I/R and renal function measurements: Experimental protocol for induction of renal I/R injury has been discussed previously (19). Briefly, 3 weeks before ischemia, the right kidney was removed through a small flank incision under general anesthesia with Pentobarbital sodium (50 mg/kg, i.p.). For induction of I/R injury, under general anesthesia, the left renal artery was exposed and occluded with a non-traumatic arterial clamp for 45 minutes, then blood flow was re-established by releasing the clamp and each rat was placed in a metabolic cage for 24 hours of reperfusion period. Urine volume was collected during reperfusion period for measurement of creatinine and sodium concentrations. After 24 hours of reperfusion animals were killed humanely and blood samples were prepared and serum was separated by centrifugation and used for measurement of serum creatinine (SCr) and blood urea nitrogen (BUN) and renal functional parameters.
Creatinine and BUN levels were determined in serum and urine by a Hitachi multi-analyzer and sodium concentration was determined using a flame photometer (Hitachi, 205D; Hitachinaka, Japan). The data were applied for calculation of creatinine clearance rate (CCr) and fractional excretion of sodium (FENa).
MDA measurement: Renal tissue malondialdehyde (MDA) levels after I/R or sham operation was determined by thiobarbituric acid method in which MDA, as a thiobarbituric acid reactive substance (TBARS), reacts with thiobarbituric acid (TBA Sigma, St. Louis, MO, USA) to produce a red colored complex that has maximum absorbance at 532 nm(20). The amount of MDA in tissue is used as an index of lipid peroxidation. MDA concentration was calculated from the intensity of pink color of the final product at 532 nm (Malondialdehyde Assay kit, NWLSS, WA, USA). Results were expressed as nmol MDA per gram of wet tissue.
Morphological examination of the kidney: The abdominal cavity was opened and after minimal dissection both kidneys were removed. The kidneys were divided longitudinally and were processed for light microscopic observation. The kidneys were fixed in phosphate-buffered 10% formalin and then embedded in paraffin, cut at (4-5μm) and stained with Hematoxylin and Eosin (H&E). Prepared sections were graded for histopathological damage by a histologist in a double blind manner. Twenty fields were read in two consecutive rows. All proximal tubular profiles presenting each field were counted. The severity of the histological lesions was graded from 1 to 4 according to the following criteria (19):
0: No signs of necrosis (no damage)
1+: Necrosis of individual cells.
2+: Necrosis of all cells in adjacent proximal convoluted tubules, with survival of surrounding tubules.
3+: Necrosis confined to the distal third of the proximal convoluted tubule with a band of necrosis extending across the inner cortex.
4+: Necrosis affecting all the three segments of the proximal convoluted tubule.
Western blotting
The kidney sections for western blotting were immersed in a lysis buffer (including sodium dodecyl sulfate 2.5%, glycerol 10%, Tris-HCl 62.5mM and pH 6.8) and then placed in a thermomixer at 95° for eight minutes. Then samples were transferred into freezer at -80 °C for storage until the time of Western blot analysis. Before Western blotting, bicinchoninic acid protein assay was performed to determine protein concentration (absorbance= 560 nm, Pierce). Equal amount of protein from each sample (45 µg) was applied on a 12% sodium dodecyl sulfate polyacrylamide gel. Electrophoretic separation of proteins was performed using electrophoresis in denaturing running buffer system (25 mM Tris, 190mM glycine, 0.1% SDS, pH 8.5). 50 V electric field was applied for 5 minutes and then voltage increased to 150 for 60 minutes. Transfer was at 100 V for 120 minutes at room temperature in wet transfer system (buffer containing 25mM Tris, 190mM glycine and 20% methanol). Proteins were transferred onto PVDF membranes, and then membranes were incubated at 4°C in 0.1 M sodium phosphate buffer (pH= 7.4) and incubated for 2 h with a 1:3500 dilution of rabbit polyclonal anti-rat Bcl-2 and Bax antibodies (Abcam: catalog nos. ab7973 and ab53154, respectively) in 5% non-fat skimmed milk. HRP-conjugated Rabbit IgG secondary antibody–H&L was used for detection (Abcam: catalog no. ab7090). ECL chemiluminescence system (Amersham Pharmacia Biotech, Braunschweig, Germany) was used for visualization of the blots, and the photosensitive film was developed and used for measurement of optical density.
Statistical analyses
Results for measurement of Cr, BUN, MDA, CCr and FENa are presented as means ± S.E.M. Means of groups were compared by one-way analysis of variances (ANOVA) then post-hoc analysis (Tukey test) was performed for assessing specific group comparisons. Mann-Whitney U test was used for comparison of histological data. The level of statistical significance was set as p <0.05. Calculations were performed using the SPSS 14.
Results
Serum creatinine (SCr) and BUN: SCr and BUN levels were normal in sham-operated groups receiving 10 and 20 mg/kg/day of simvastatin (0.68 ± 0.12 and 0.71 ± 0.1 mg/dl and 10.88 ± 3.82 and 14.35 ± 3.22 mg/dl, respectively). I/R significantly increased SCr (1.93 ± 0.24mg/dl, p<0.01; Fig. 1a) and BUN levels (53.3 ± 7.44mg/dl, p<0.01; Fig. 1b) in comparison with sham group. Pretreatment with simvastatin 10 and 20 mg/kg/day significantly reduced SCr and BUN levels (1.37 ± 0.18 and 1.09 ± 0.13 mg/dl: p<0.05 vs. I/R and 35.71 ± 6.5 and 30.62 ± 4.11: p<0.05 vs. I/R; Figure 1).
Fig. 1 .
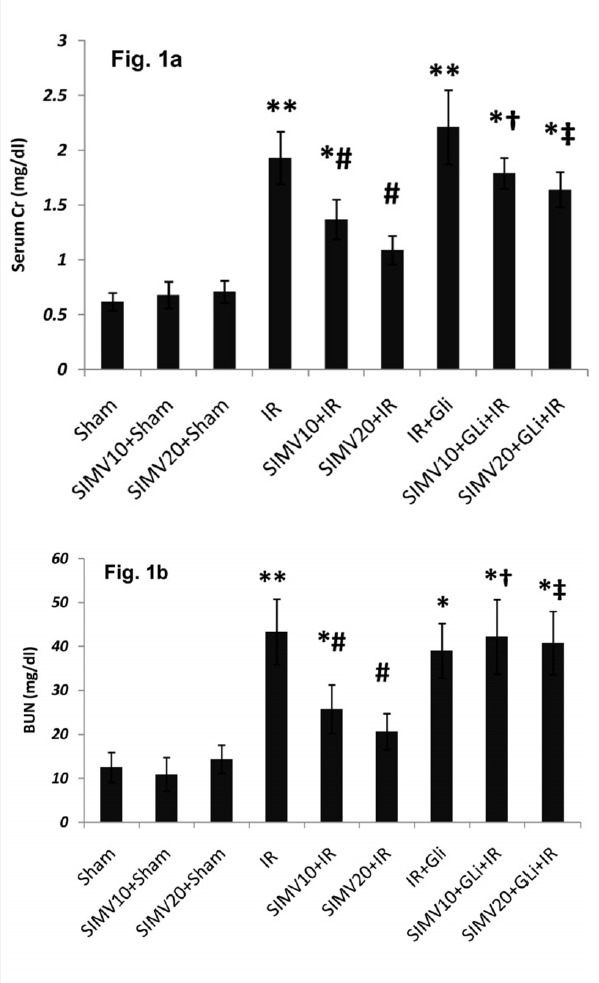
SCr and BUN levels in different groups (a) SCr and (b) serum BUN was determined in samples which were collected during the reperfusion period. All data were shown as mean±SEM. I/R: Ischemia (45 min) and reperfusion (24 h), SIMV (Simvastatin 10 or 20 mg/kg/day for 7 days), GLi: Glibenclamide (5mg/kg 45 min before ischemia I.P.). * p<0.05 and ** p<0.01 vs. sham, # p<0.05 vs. I/R, †p<0.05 vs. SIMV10+I/R and ‡ p<0.05 vs. SIMV20+I/R.
Although glibenclamide by itself had no significant effect on SCr and BUN levels, it abolished the effect of simvastatin 10 and 20 mg/kg/day on decreasing the SCr and BUN levels (p<0.05).
Simvastatin 10 and 20 mg/kg/day treatment increased CCr from 0.36 ± 0.1 ml/min in I/R group to 0.48±0.13 and 0.84±0.21 ml/min, respectively (p<0.05) and decreased FENa from 27±5.62% to 20±3.16% and 12±2.73%, respectively (p<0.05). However, in groups which received glibenclamide, simvastatin could not produce significant change in CCr or FENa in comparison to the I/R group (Fig. 2a and 2b).
Fig. 2 .
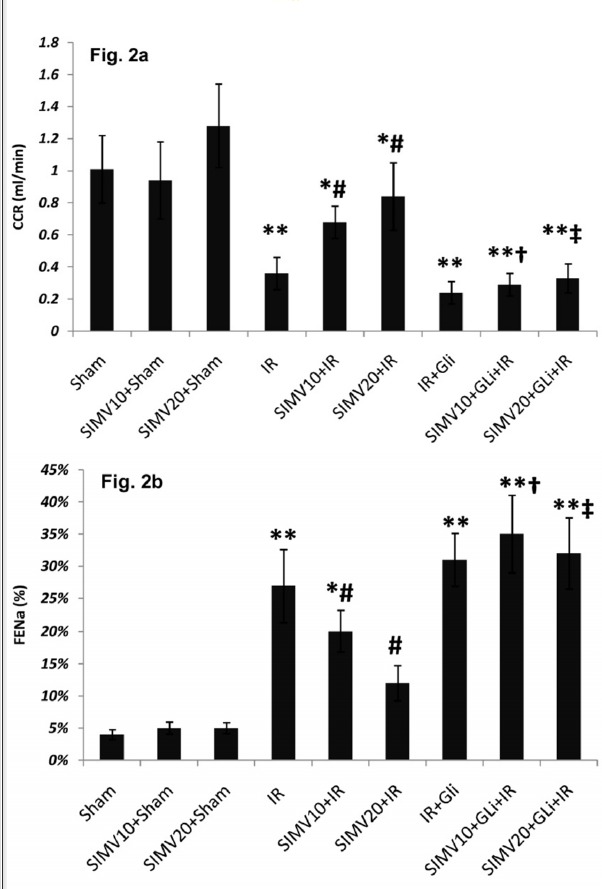
Creatinine clearance rate (CCR: ml/min) and fractional excretion of sodium (FENa) in different groups. (a) CCR was calculated from Cr concentrations in urine and serum and urine volume during 24 hours after ischemia. (b) FENa was calculated from CCr and sodium concentrations in urine and serum during 24 hours after ischemia. All data was shown as mean±SEM. I/R: Ischemia (45 min) and reperfusion (24 h), SIMV (Simvastatin 10 or 20 mg/kg/day for 7 days), GLi: Glibenclamide (5mg/kg 45 min before ischemia I.P.). * p<0.05 and ** p<0.01 vs. sham, # p<0.05 vs. I/R, †p<0.05 vs. SIMV10+I/R and ‡ p<0.05 vs. SIMV20+I/R.
MDA levels and lipid peroxidation: Renal tissue MDA levels increased after I/R and reached to 60.44±7.1nmol/gr tissue (p<0.01 vs. sham; Figure 3). Pretreatment with both doses of simvastatin attenuated MDA level (49.5±5.29 and 28.01±4.31 nmol/mg tissue p<0.01) which was abolished by glibenclamide treatment 45 min before ischemia (58.11±6.51 and 53.7±7.02nmol\mg tissue, p<0.05 vs. simvastatin+ I/R treated groups).
Fig. 3 .
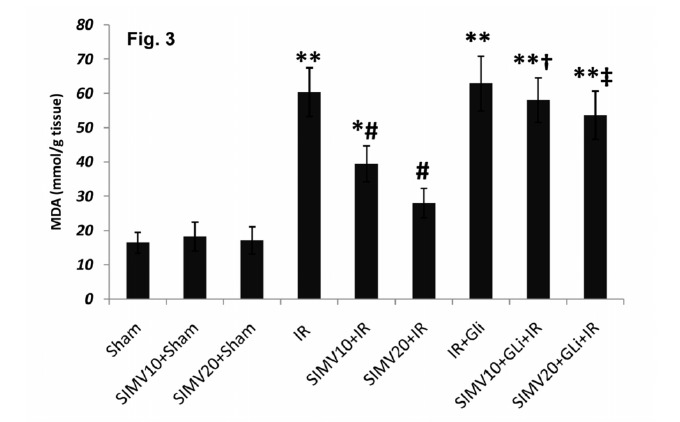
MDA contents of renal tissue 24h after ischemia in different groups. All data was shown as mean±SEM. I/R: Ischemia (45 min) and reperfusion (24 h), SIMV (Simvastatin 10 or 20 mg/kg/day for 7 days), GLi: Glibenclamide (5mg/kg 45 min before ischemia I.P.). * p<0.05 and ** p<0.01 vs. sham, # p<0.05 vs. I/R, †p<0.05 vs. SIMV10+I/R and ‡ p<0.05 vs. SIMV20+I/R.
Histological assessment: Results of histological examinations are shown in Table 1 and Figures 4 and 5. In sham-operated groups saline or simvastatin 10 and 20mg/kg/day had no effect on histological profiles (Figs. 4a, 4b and 4c). I/R induced moderate to severe histological damage (p<0.001 vs. sham, Table 1) with lose of brush border in the main parts of proximal tubules, a large number of cell debris and detached epithelial structures which obstructed tubules and led to swelling of the tubules (Fig. 4d).
Table 1 . Histological scores obtained from each group and comparison of the severity of tissue damage between groups .
| Groups | Number (%) of each grade observations | Total (%) | |||||
| 0 | 1 | 2 | 3 | 4 | |||
| 1 | Sham | 82 (91.1) | 7 (7.8) | 1 (1.1) | - | - | 90 (100%) |
| 2 | SIMV10+Sham | 85 (94.4) | 5 (5.6) | 0 | 0 | 0 | 90 (100%) |
| 3 | SIMV20+Sham | 79 (87.8) | 9 (10) | 2 (2.2) | 0 | 0 | 90 (100%) |
| 4 | IR *** | 6 (6.6) | 17 (18.8) | 31 (34.4) | 37 (41.0) | 2 (2.2) | 90 (100%) |
| 5 | SIMV10+IR ** | 9 (10) | 22 (24.5) | 27 (30.0) | 30 (33.3) | 2 (2.2) | 90 (100%) |
| 6 | SIMV20+IR * # † | 18 (20.0) | 44 (48.9) | 19 (21.1) | 8 (8.9) | 1 (1.1) | 90 (100%) |
| 7 | GLi+IR *** | 5 (6.25) | 8 (10.0) | 32 (40.0) | 32 (40.0) | 3 (3.75) | 80 (100%) |
| 8 | SIMV10+GLi+IR *** † | 6 (6.7) | 18 (20.0) | 31 (34.4) | 33 (36.7) | 2 (2.2) | 90 (100%) |
| 9 | SIMV20+GLi+IR ***‡ | 10 (11.1) | 24 (26.7) | 28 (31.1) | 28 (31.1) | 0 | 90 (100%) |
Each row shows the number (percent) of total observation of that grade in each group. From any of left kidneys 5 slices were prepared and graded by two specialist who were blinded about experimental design and groups; Total observation = N of rats in each group × N of slices from each kidney × 2 pathologist. I/R: Ischemia (45 min) and reperfusion (24 h), SIMV (Simvastatin 10 or 20 mg/kg/day for 7 days), GLi: Glibenclamide (5mg/kg 45 min before ischemia I.P.). Mann-Whitney U test results: * p<0.05, ** p<0.01 and *** p<0.01 vs. sham, # p<0.05 and ##p<0.01 vs. IR, †p<0.05 vs. SIMV10+I/R and ‡ p<0.05 vs. SIMV20+IR. Number of rats in all groups was 9 except Gli+I/R with 8 rats (One of rats died at the end of reperfusion period).
Fig. 4 .
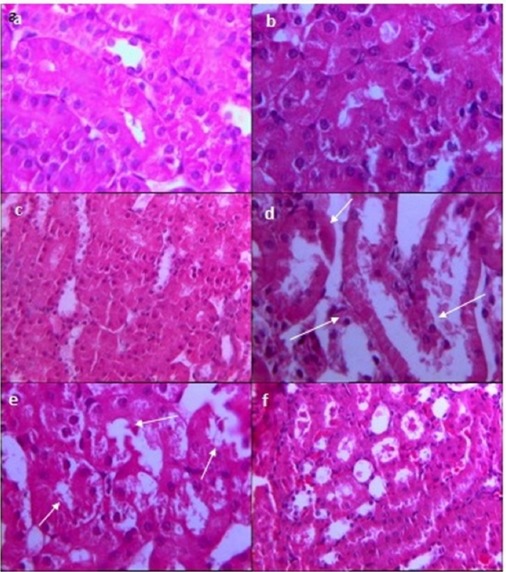
The effect of simvastatin on renal histology after I/R injury. (a) Sham, (b) simvastatin 10 mg/kg/day and (c) simvastatin 20 mg/kg/day showed normal appearance in most of the slices and marked as grade 0 or 1 in pathological scoring system. (d) I/R induced moderate to severe histological damage in most slices which is shown by the loss of brush border in the main parts of proximal tubules, large numbers of cell debris and detached epithelial structures which obstructed tubules and tubular swelling (white arrows). (e) Simvastatin (10mg/kg/day) could not change I/R induced renal injury; cellular debris exists in most parts of the studied field with distended tubular spaces (white arrows). (f) Simvastatin (20mg/kg) restricted cellular debris and tubular swelling to some parts of the studied fields; grade 1 was the frequent feature of slices prepared from this group.
Fig. 5 .
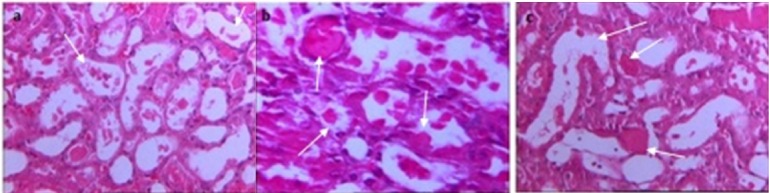
Renal histology in groups which were treated with (a) glibenclamide alone or in combination with (b) Simvastatin 10 or (c) Simvastatin 20 mg/kg/day. Severe tubular damage was apparent in prepared slices from all of these groups and all types of tubular damage were apparent in most of the slices (white arrows show tubular swelling, cellular debris and detachment of epithelium).
Treatment with simvastatin (10mg/kg) could not change renal histology, and microscopic field similar to the I/R group was reported by histologists (Fig. 4e). In contrast simvastatin (20mg/kg) significantly reduced tubular damage (p<0.05 vs. I/R group and p<0.05 vs. simvastatin 10mg/kg; Table 1 and Fig. 4f) and restricted cellular debris and tubular swelling to some parts of studied fields. When glibenclamide was administered alone or in combination with simvastatin 10 or 20 mg/kg group, severe tubular damage were apparent in prepared slices (p<0.05; Table 1 and Figs. 5a, 5b and 5c).
Fig. 6 .
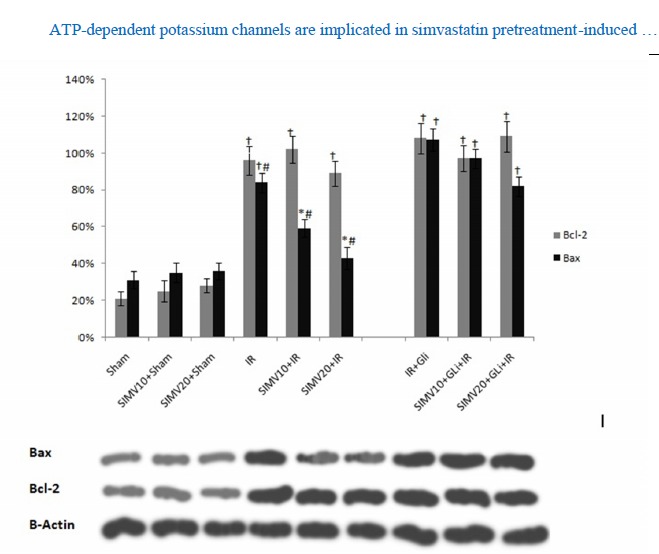
level of Bax proteins and Bcl-2 in rat kidney tissues. * p<0.01 vs sham, †p<0.01 vs sham, #p<0.05 vs. I/R, I/R+Gli, SIMV10+Gli+IR and SIMV20+Gli+I/R I/R: ischemia reperfusion, SIMV10: simvastatin 10 mg/kg, SIMV20: simvastatin 20 mg/kg, Gli: glibenclamide
Western blot
Western blot analysis revealed that simvastatin in all applied doses did not alter the expression of Bcl-2 and Bax proteins. While I/R injury significantly altered the expression of these proteins compared to sham operated group, simvastatin treatment with doses of 10 and 20mg/kg significantly reduced the expression of Bax protein while having no significant effect on Bcl-2 protein expression. Glibenclamide did not affect the Bcl-2 and Bax proteins level when compared to I/R group. In I/R group receiving simvastatin and glibenclamide, Bax protein level was increased compared to groups receiving simvastatin with doses 10 and 20 mg/kg (p<0.05; Fig. 6). Simvastatin’s effect on Bax protein level appeared to be dose dependent.
Discussion
In the current study we investigated the protective effects of the simvastatin on renal tissue after renal I/R injury. The results revealed that simvastatin at doses of 10 and 20mg/kg administered one week before I/R injury, protected the kidney against injury and improved renal function, reduced MDA level and decreased the expression of pro-apoptotic Bax protein in I/R-injured tissue. It also decreased renal tubular tissue damage. Glibenclamide prevented these effects of simvastatin and worsened renal function, decreased tissue antioxidant capacity, increased renal tubular damage, and reversed the effects of simvastatin on Bax protein level in I/R-injured kidney tissue of animals treated with simvastatin at doses 10 and 20mg/kg and 5mg/kg glibenclamide.
The HMG-CoA reductase inhibitors (statins), known for their cholesterol lowering effects, have been tested for application in the treatment of I/R injuries in various kinds of tissues such as kidney (12, 21), liver (22-24), heart (11) and spinal cord (13). In the current study our data demonstrated that simvastatin significantly improves outcomes after renal I/R injury in male Wistar rats. I/R injury is regularly associated with reduced antioxidant capacity of the tissue (1, 20). In the current study, we observed reduced antioxidant capacity of the tissue after I/R injury which was improved in groups pretreated with simvastatin. This is consistent with other studies presenting the data on simvastatin protection of kidney against oxidative stress induced injury (25, 26). Tissue MDA level was significantly increased after I/R injury in non-treated groups, while this metabolite was significantly less in simvastatin treated groups. There was an inverse correlation between MDA levels and renal function after I/R injury. This is consistent with the observations by Fonseca et al. (5), which demonstrated the inverse correlation between MDA levels on day 1 after transplantation and early graft function. Their study also demonstrated that MDA level 7 days after transplantation is an independent predictor of 1-year graft function. In the current study we were only able to observe the renal function early after I/R injury. Other studies are required for investigating the effects of simvastatin pretreatment on graft function for longer times after I/R injury.
Jones et al. reported that simvastatin protective effects against I/R injury in isolated neonatal rat cardiac myocytes are mediated by induction of nitric oxide synthase (NOS) and KATP channels (14). In the current study we observed that administration of 5 mg/kg of glibenclamide, a non-selective inhibitor of KATP ion channels, abolished the beneficial effects of simvastatin on I/R-injured kidneys. This implies to the role of KATP ion channels by simvastatin in the renal protection after I/R injury. Zhao et al. achieved similar results with simvastatin pretreatment on cardiac I/R injury (11). Their study revealed that simvastatin improved the outcomes after cardiac I/R injury by opening mitochondrial KATP ion channels. Inhibition of KATP ion channels with simvastatin is associated with the membrane depolarization and influx of Ca2+ ions and decrease mitochondrial membrane potential (14). Coalescence of Ca2+ ions in the cytosol triggers apoptotic cell death. This is potentially mediated by calpains, an intracellular calcium-dependent protease. This protease is activated in the presence of Ca2+ ions and mediates a series of events which result in Bax-mediated apoptotic cell death (27). As it has been demonstrated by Ma et al. that statins suppress calpain activation (28), we speculated that reduced apoptotic cell death and renal tissue preservation is possibly mediated, at least in part, by this activity of statins. As treatment with glibenclamide prevent this activity of statins, it appears that KATP ion channels are having a role in this signaling cascade.
Another potential mechanism of increased apoptotic cell death after I/R injury is upregulation of Bax which has been demonstrated by Jin et al. to be mediated by p53 transcription factor activation. According to their report, p53 activation is mediated by adiponectin signaling (29). Adiponectin level increase after renal I/R injury (29) and its level is directly correlated with several adverse outcomes after renal I/R injury (30,31). Also, observations by Rajtík et al., demonstrated the upregulation of anti-apoptotic Bcl-2 and downregulation of pro-apoptotic markers to be induced by simvastatin after myocardial I/R injury in isolated rat hearts (32). In the current study we observed upregulation of Bax after renal I/R-injury which was downregulated by pretreatment with simvastatin. Treatment with glibenclamide abolished the effects of simvastatin on Bax protein level which indicates the potential involvement of KATP ion channels in this effect of simvastatin. Further experiments are required to uncover the relationship between KATP channel opening by simvastatin and adiponectin expression.
Conclusion
Up to present study, protective effect of simvastatin against renal I/R injury has a common feature with its cardioprotective effect, and both are mediated by KATP ion channels.
Acknowledgments
We appreciate Islamic Azad University, Najafabad Branch, Isfahan, Iran, for funding all aspects of the present work.
Cite this article as: Dowlatshahi K, Ajami M, Pazoki-Toroudi H, Hajimiresmaiel S.J. ATP-dependent potassium channels are implicated in simvastatin pretreatment-induced inhibition of apoptotic cell death after renal ischemia/reperfusion injury. Med J Islam Repub Iran 2015 (14 March). Vol. 29:191
References
- 1.Abela CB, Homer-Vanniasinkham S. Clinical implications of ischaemia-reperfusion injury. Pathophysiology. 2003;9:229–40. doi: 10.1016/s0928-4680(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 2.Kellum JA. Acute kidney injury. Critical Care Medicine. 2008;36:S141–5. doi: 10.1097/CCM.0b013e318168c4a4. [DOI] [PubMed] [Google Scholar]
- 3.Wever KE, Wagener FA, Frielink C, Boerman OC, Scheffer GJ, Allison A. et al. Diannexin protects against renal ischemia reperfusion injury and targets phosphatidylserines in ischemic tissue. PloS One. 2011;6:e24276. doi: 10.1371/journal.pone.0024276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himmelfarb J, McMonagle E, Freedman S, Klenzak J, McMenamin E, Le P. et al. Oxidative stress is increased in critically ill patients with acute renal failure. Journal of the American Society of Nephrology. 2004;15:2449–56. doi: 10.1097/01.ASN.0000138232.68452.3B. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca I, Reguengo H, Almeida M, Dias L, Martins LS, Pedroso S. et al. Oxidative stress in kidney transplantation: malondialdehyde is an early predictive marker of graft dysfunction. Transplantation. 2014;97:1058–65. doi: 10.1097/01.TP.0000438626.91095.50. [DOI] [PubMed] [Google Scholar]
- 6.Ajami M, Davoodi SH, Habibey R, Namazi N, Soleimani M, Pazoki-Toroudi H. Effect of DHA+EPA on oxidative stress and apoptosis induced by ischemia-reperfusion in rat kidneys. Fundamental & Clinical Pharmacology. 2013;27:593–602. doi: 10.1111/j.1472-8206.2012.01066.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirakawa A, Takeyama N, Nakatani T, Tanaka T. Mitochondrial permeability transition and cytochrome c release in ischemia-reperfusion injury of the rat liver. The Journal of Surgical Research. 2003;111:240–7. doi: 10.1016/s0022-4804(03)00091-x. [DOI] [PubMed] [Google Scholar]
- 8.DeSantiago J, Bare DJ, Banach K. Ischemia/ Reperfusion injury protection by mesenchymal stem cell derived antioxidant capacity. Stem Cells and Development. 2013;22:2497–507. doi: 10.1089/scd.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosieradzki M, Rowinski W. Ischemia/ reperfusion injury in kidney transplantation: mechanisms and prevention. Transplantation Proceedings. 2008;40:3279–88. doi: 10.1016/j.transproceed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 10. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9. [PubMed]
- 11.Zhao JL, Yang YJ, Cui CJ, You SJ, Gao RL. Pretreatment with simvastatin reduces myocardial no-reflow by opening mitochondrial K(ATP) channel. British Journal of Pharmacology. 2006;149:243–9. doi: 10.1038/sj.bjp.0706862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuuminen R, Nykanen AI, Saharinen P, Gautam P, Keranen MA, Arnaudova R. et al. Donor simvastatin treatment prevents ischemia-reperfusion and acute kidney injury by preserving microvascular barrier function. American Journal of Transplantation. 2013;13:2019–34. doi: 10.1111/ajt.12315. [DOI] [PubMed] [Google Scholar]
- 13.Saito T, Tsuchida M, Umehara S, Kohno T, Yamamoto H, Hayashi J. Reduction of spinal cord ischemia/reperfusion injury with simvastatin in rats. Anesthesia and Analgesia. 2011;113:565–71. doi: 10.1213/ANE.0b013e318224ac35. [DOI] [PubMed] [Google Scholar]
- 14.Jones SP, Teshima Y, Akao M, Marban E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circulation Research. 2003;93:697–9. doi: 10.1161/01.RES.0000097262.21507.DF. [DOI] [PubMed] [Google Scholar]
- 15.Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL. et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–31. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 16.Tavridou A, Efthimiadis A, Efthimiadis I, Paschalidou H. Antioxidant effects of simvastatin in primary and secondary prevention of coronary heart disease. European Journal of Clinical Pharmacology. 2006;62:485–9. doi: 10.1007/s00228-006-0097-z. [DOI] [PubMed] [Google Scholar]
- 17.Ungureanu D, Filip C, Artenie A, Artenie R. Evaluation of simvastatin antioxidant effects. Revista Medico-Chirurgicala. 2003;107:66–71. [PubMed] [Google Scholar]
- 18.Sadeghi MM, Collinge M, Pardi R, Bender JR. Simvastatin modulates cytokine-mediated endothelial cell adhesion molecule induction: involvement of an inhibitory G protein. Journal of Immunology. 2000;165:2712–8. doi: 10.4049/jimmunol.165.5.2712. [DOI] [PubMed] [Google Scholar]
- 19.Pazoki-Toroudi HR, Ajami M, Habibey R. Pre-medication and renal pre-conditioning: a role for alprazolam, atropine, morphine and promethazine. Fundamental & Clinical Pharmacology. 2010;24:189–98. doi: 10.1111/j.1472-8206.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- 20.Habibey R, Ajami M, Ebrahimi SA, Hesami A, Babakoohi S, Pazoki-Toroudi H. Nitric oxide and renal protection in morphine-dependent rats. Free Radical Biology & Medicine. 2010;49:1109–18. doi: 10.1016/j.freeradbiomed.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Inman SR, Davis NA, Olson KM, Lukaszek VA. Simvastatin attenuates renal ischemia/reperfusion injury in rats administered cyclosporine A. The American Journal of the Medical Sciences. 2003;326:117–21. doi: 10.1097/00000441-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Lai IR, Chang KJ, Tsai HW, Chen CF. Pharmacological preconditioning with simvastatin protects liver from ischemia-reperfusion injury by heme oxygenase-1 induction. Transplantation. 2008;85:732–8. doi: 10.1097/TP.0b013e3181664e70. [DOI] [PubMed] [Google Scholar]
- 23.Dibazar F, Hajipour B, Hosseinian MM, Hemmati MR, Ghandiha A. Simvastatin decreases hepatic ischaemia/reperfusion-induced liver and lung injury in rats. Folia Morphologica. 2008;67:231–5. [PubMed] [Google Scholar]
- 24.Gracia-Sancho J, Garcia-Caldero H, Hide D, Marrone G, Guixe-Muntet S, Peralta C. et al. Simvastatin maintains function and viability of steatotic rat livers procured for transplantation. Journal of Hepatology. 2013;58:1140–6. doi: 10.1016/j.jhep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Maheshwari RA, Sailor GU, Patel L, Balaraman R. Amelioration of cisplatin-induced nephrotoxicity by statins. Indian Journal of Pharmacology. 2013;45:354–8. doi: 10.4103/0253-7613.115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Otaibi KE, Al Elaiwi AM, Tariq M, Al-Asmari AK. Simvastatin attenuates contrast-induced nephropathy through modulation of oxidative stress, proinflammatory myeloperoxidase, and nitric oxide. Oxidative Medicine and Cellular Longevity. 2012;2012:831748. doi: 10.1155/2012/831748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobhan PK, Seervi M, Deb L, Varghese S, Soman A, Joseph J. et al. Calpain and reactive oxygen species targets Bax for mitochondrial permeabilisation and caspase activation in zerumbone induced apoptosis. PloS One. 2013;8:e59350. doi: 10.1371/journal.pone.0059350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma T, Zhao Y, Kwak YD, Yang Z, Thompson R, Luo Z. et al. Statin's excitoprotection is mediated by sAPP and the subsequent attenuation of calpain-induced truncation events, likely via rho-ROCK signaling. The Journal of Neuroscience. 2009;29:11226–36. doi: 10.1523/JNEUROSCI.6150-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin X, Chen J, Hu Z, Chan L, Wang Y. Genetic deficiency of adiponectin protects against acute kidney injury. Kidney International. 2013;83:604–14. doi: 10.1038/ki.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E. et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. Journal of the American Society of Nephrology. 2005;16:1091–8. doi: 10.1681/ASN.2004090742. [DOI] [PubMed] [Google Scholar]
- 31.Jorsal A, Tarnow L, Frystyk J, Lajer M, Flyvbjerg A, Parving HH. et al. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney International. 2008;74:649–54. doi: 10.1038/ki.2008.201. [DOI] [PubMed] [Google Scholar]
- 32.Rajtik T, Carnicka S, Szobi A, Mesarosova L, Matus M, Svec P. et al. Pleiotropic effects of simvastatin are associated with mitigation of apoptotic component of cell death upon lethal myocardial reperfusion-induced injury. Physiological Research. 2012;61 Suppl 2:S33–41. doi: 10.33549/physiolres.932420. [DOI] [PubMed] [Google Scholar]


