Abstract
Background: Infection with parvovirus B19 may cause fetal losses including spontaneous abortion, intrauterine fetal death and non-immune hydrops fetalis. The aim of this study is to determine the frequency of parvovirus B19 in formalin fixed placental tissues in lost fetuses using real-time PCR method.
Methods: In this cross-sectional study, 100 formalin fixed placental tissues with unknown cause of fetal death were determined using real-time PCR method after DNA extraction.
Results: Six out of 100 cases (6%) were positive for parvovirus B19 using real-time PCR. Gestational age of all positive cases was less than 20 weeks with a mean of 12.3 weeks. Three cases have a history of abortion and all of positive cases were collected in spring. Mean age of positive cases were 28 years.
Conclusion: Parvovirus B19 during pregnancy can infect red precursor cells and induces apoptosis or lyses these cells that resulting in anemia and congestive heart failure leading to fetal death. Management of parvovirus B19 infection in pregnant women is important because immediate diagnosis and transfusion in hydropsic fetuses can decrease the risk of fetal death.
Keywords: Human Parvovirus B19, Placenta, Fetal death
Introduction
Parvoviruses are small DNA-containing viruses, single-stranded and none enveloped. Parvo B19V (Parvovirus B19) belongs to Erythro-viruses genus that is pathogenic for humans. B19V has three major viral proteins, one non-structural protein NS1 (77 kDa), two structural proteins VP1 (83 kDa) and VP2 (53 kDa and two minor proteins (1,2). NS1 has important role in activation of viral gene tr anscription, viral replication and induces apoptosis in target cells. This protein cause cell death by apoptosis by the pathway caspase 3 and is inhibited by Bcl-2 (3).
P antigen is the most important cellular receptor for B19V (4), which is a glycol sphingo lipid globoside that exists on the surfaces of progenitors erythrocyte cells, endothelium, synovium, placental trophoblast, myocardial cells and fetal hepatic cells (4,5). B19V have no polymerase of its own: this virus needs active cell division for replication. Like other Parvo viruses, B19V induces cells into S phase for own replication. Having a large number of cells in mitosis makes fetuses vulnerable to B19V. The most important of these precursors cells are CFU-E (erythroid colony forming units) and BFU-E (erythroid burst-forming units); B19V simply infect and replicate in this cells (2,6,7). In addition to cytolytic activity, B19V has an apoptosis inducing factor, and an ability to arrest cells at either G1 or G2 cell cycle phases. So, B19V can destruct many of erythroid progenitor cells that leads to anemia (8). Moreover, B19V may infect platelets, lymphocytes and granulocytes. Infection with B19V mostly occurs through respiratory droplets; other ways of transmission are blood and blood-derived products and vertical transmission from mother to fetus (9). Rate of vertical transmission in maternal infection is 33–51% of cases, and the rate of adverse fetal effect is approximately 10% (10,11). B19V is the most important infectious cause of fetal anemia. Fetuses during the hepatic stage of hematopoiesis are severely affected by B19V infection and they are noticeably vulnerable (12). The rate of incidence of fetal mortality and morbidity related to B19V infection depend on gestational age (9,13). Decreasing the rate of fetal losses after 25 weeks is related to passive transfer of maternal antibodies and decreasing expression of P antigen within the villous trophoblast layer (14).
Fetal infection with B19V is related to non-immune hydrops fetalis (NIHF), intrauterine fetal death (IUFD), myocarditis, thrombocytopenia, and neurological manifestations. Fetal infection may also remain clinically unrecognized (15).
B19V infection-associated fetal death and hydrops fetalis occur mostly during the second trimester (16). The aim of the current study was to determine the relation between parvovirus B19 infection and fetal mortality and spontaneous abortion in Iranian women.
Mehtods
Clinical specimens
In this cross sectional study, 100 formalin fixed placental tissues from autopsies done following fetal-loss cases including spontaneous abortion and non-immune hydrops fetalis and IUFD. Autopsies were done in 2012 at obstetrics and gynecology medical center hospital, affiliated to Arak University of medical sciences. Patients were admitted from all regions of markazi state, representing a wide spectrum of socioeconomic levels.
Detailed clinical, autopsy and placental examination reports were reviewed on all 100 tissue samples. The time from the last menstruation was used for calculation of gestational age.
DNA Extraction
For nucleic acid isolation specimens were frozen and thawed for three times; then DNA was extracted using Nucleic Acid isolation kit (Roche Applied Science) as described by the manufacturer. Genome was re-suspended in a final volume of 100μl elution buffer and stored at -80 °C.
Real time PCR
The primers and TaqMan probe for quantitative real-time PCR were selected from the most conserved sequences encoding the non-structural protein (NS1) of parvovirus B19 viruses using the “Beacon Designer” software program (PREMIER Biosoft International, USA) (Table1).
Table1 . Sequence primer and probe .
| Name | Sequence | Position |
| PB forward | 5´-AATGCAGATGCCCTCCACG-3´ | 1468–1485 |
| PB Reverse | 5´-ATGATTCTCCTGAACTGGTCCA-3´ | 1740–1760 |
| PB Probe | 5´-FAM-AACCCCGCGCTCTAGTACCGA-TAMARA-3´ | 1711–1728 |
Reactions were performed using Universal TaqMan Master Mix (Applied Bio systems) in a final volume of 25µL with primers and probe at a concentration of 30 and 100 nM, respectively. Following initial incubations at 50°C for 2 minutes and 95°C for 15 minutes, 40 cycles of thermal cycling at 95°C for 15 seconds and 60°C for 40 seconds were performed in a CFX96 BioRAD real-time system. Data were collected in FAM channel.
PVB19- infected bone marrow tissue from a patient suffering from acute aplastic anemia served as a positive control that was tested with a commercial real-time PCR kit (Inter Lab Service, Russia). All samples were analyzed in duplicate.
Statistical analysis
The data were analyzed using SPSS 19. The relation between parvovirus B19 positivity and the time of sample collection was analyzed using chi-square test. A P-value of <0.05 was considered statistically significant.
Result
Samples were collected during 2012 from idiopathic abortions. Six cases infected with parvovirus B19 (6%) occurred in 10, 11, 11, 13, 13 and 16 weeks gestational age. Mean (±SD) gestational age of infected cases is12.6 (±3.4) weeks. All of those fetal losses infected by B19 V were less than 20 weeks of gestational age. All positive cases were found in spring; the seasonal prevalence of fetal death related to parvovirus B19 was statistically meaningful (P value<0.001) ( Fig. 1).
Fig. 1 .
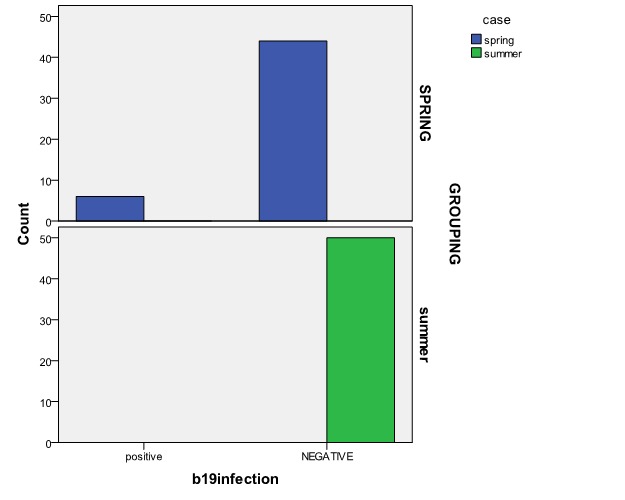
comparative prevalence of parvovirus B19 infection in different season
Moreover, 3 cases out of 6 cases had a history of abortion or intrauterine fetal death (IUFD). All of infected cases declared a history of contact with children and these cases were anemic. Number of pregnancies of positive cases was in a range of 1 to 5. One of the infected cases was aborted in first pregnancy (Table 2).
Table 2 . Findings and their relation with seasonal incidence, gestational age, number of pregnancies and contact with children .
| Result | Having history of abortion |
Gestational age (age of fetus) |
The number of pregnancy |
Having contact with children |
|
Positive (6%) Case 1 Case 2 Case 3 Case 4 Case 5 Case 6 |
No Yes No Yes Yes no |
Less down 20 weeks 10 weeks 11 weeks 13 weeks 13 weeks 16 weeks 11 weeks |
different 2 5 2 4 4 1 |
Yes Yes Yes Yes Yes yes |
| Negative (94%) | different | different | different | different |
Discussion
Parvovirus B19 is an infectious agent results in fetal losses during pregnancy. B19V during pregnancy can kill red cell precursors resulting in anemia and congestive heart failure and finally fetal death can occur. Within late gestation no signs of fetal involvement might happen (17,19). So, the rate of parvovirus B19 is higher than we conceive. Determination of etiology of the anemia and hydrops is very important. If the cause of hydrops is B19V, the intrauterine blood transfusion is very critical and chance of fetal survival can improve 60% to 80%; in other hand, in untreated cases this rate is 15% to 30% (20,21-23). In our cross-sectional study, with the aim of determination of the frequency of parvovirus B19 infection in fetal losses from 100 samples, 6 samples (6%) of dead fetuses were infected with B19V. Other studies conducted in Tehran (10%), Tunisia (17.24%), Brazil (5.9%) and Greece showed similar results (24-27).
In another study conducted by Thomas Tolfeveston et al, from 47 cases of fetal death, 7 cases were infected by parvovirus B19 (28). Our study have similar result and the small differences might be related to specimen pupulation.
The outcome of intrauterine parvovirus B19 infection is not completely known and is related to factors such as variations of viral spread or gestational age of subjects and other causes also may be responsible for fetal death such as chromosomal abnormalities (29). Women with confirmed infection show a significantly higher rate of second-trimester fetal loss (11.8%) compared with the control group (30,31).
This study that evaluated the relation between fetal losses and the infection caused by B19 virus, have several important and noticeable results: fetal losses were affected by this virus occurred in different ages from 10 to 16 weeks, but all 6 positive cases occurred in less than 20 week of gestational age. In similar studies conducted by Hamkar et al (27) (on 31 placental tissues) and Enders at al. (on 1018 cases) (32), all of fetal deaths had occurred less than 20 weeks.
The yearly peak prevalence of infection happens in spring; in addition, larger epidemics happen every 4 years (33). In our study all positive cases of this study were detected in spring. Our findings also showed that 3 positive cases had a history of abortion or intrauterine fetal losses. So, more studies are needed to study the exact role of this virus in continual abortion. The limitation of this study was lack of access to patients’ serum to study the level of antibody. Although we screened all samples, other causes of fetal losses such as taking drugs for abortion, trauma, genetic factors and etc should be evaluated. Therefore, it cannot certainly be proved that virus was the main cause of fetal losses. It is also advised to do serologic test before pregnancy, because 34 to 65% women are sensitive to parvovirus B19 in different parts of the world (34).
The most important risk factors for exposure to B19V are having contact with young children and also the number of children at home (35, 36).
This virus is easily transferred by small children at school. We suggest another study with control group at the time of the prevalence of this virus. It is also recommended that high-risk women, for example teachers and daycare workers, be monitored for parvovirus. If hydrops fetalis is suspected in these groups, reviewing prenatal findings can help in finding the etiology and management can be started sooner. Evaluation of the role of this virus in continual and repeated abortions is also suggested.
Conclusion
This study showed that fetal mortality is probably associated with parvovirus B19 infection in its peak prevalence time. Immediate diagnosis and management of parvovirus B19 infection in pregnant women is important and transfusion in hydropic fetuses can decrease the risk of fetal death.
Acknowledgments
This investigation has been funded by Tehran University of Medical Sciences.
Cite this article as: Shabani Z, Esghaei M, Keyvani H, Shabani F, Sarmadi F, Mollaie H, Monavari S.H. Relation between parvovirus B19 infection and fetal mortality and spontaneous abortion. Med J Islam Repub Iran 2015 (7 April). Vol. 29:197.
References
- 1.Anderson MJ, Higgins PG, Davis LR, Willman JS, Jones SE, Kidd IM. et al. Experimental parvovirus infection in humans. The Journal of infectious diseases. 1985;152(2):257–65. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- 2.Ozawa K, Ayub J, Hao YS, Kurtzman G, Shimada T, Young N. Novel transcription map for the B19 (human) pathogenic parvovirus. Journal of virology. 1987;61(8):2395–406. doi: 10.1128/jvi.61.8.2395-2406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moffatt S, Yaegashi N, Tada K, Tanaka N, Sugamura K. Human parvovirus B19 nonstructural (NS1) protein induces apoptosis in erythroid lineage cells. Journal of virology. 1998;72(4):3018–28. doi: 10.1128/jvi.72.4.3018-3028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262(5130):114–7. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 5.Cooling LL, Koerner TA, Naides SJ. Multiple glycosphingolipids determine the tissue tropism of parvovirus B19. The Journal of infectious diseases. 1995;172(5):1198–205. doi: 10.1093/infdis/172.5.1198. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava A, Lu L. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. Journal of virology. 1988;62(8):3059–63. doi: 10.1128/jvi.62.8.3059-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown KE, Young NS. Parvovirus B19 infection and hematopoiesis. Blood reviews. 1995;9(3):176–82. doi: 10.1016/0268-960x(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 8.Chisaka H, Morita E, Yaegashi N, Sugamura K. Parvovirus B19 and the pathogenesis of anemia. Reviews in medical virology. 2003;13(6):347–59. doi: 10.1002/rmv.395. [DOI] [PubMed] [Google Scholar]
- 9.Enders M, Weidner A, Zoellner I, Searle K, Enders G. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenatal diagnosis. 2004;24(7):513–8. doi: 10.1002/pd.940. [DOI] [PubMed] [Google Scholar]
- 10.Norbeck O, Papadogiannakis N, Petersson K, Hirbod T, Broliden K, Tolfvenstam T. Revised clinical presentation of parvovirus B19-associated intrauterine fetal death. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;35(9):1032–8. doi: 10.1086/342575. [DOI] [PubMed] [Google Scholar]
- 11.Chisaka H, Morita E, Yaegashi N, Sugamura K. Parvovirus B19 and the pathogenesis of anaemia. Reviews in medical virology. 2003;13(6):347–59. doi: 10.1002/rmv.395. Epub 2003/11/20. [DOI] [PubMed] [Google Scholar]
- 12.Jordan JA, DeLoia JA. Globoside expression within the human placenta. Placenta. 1999;20(1):103–8. doi: 10.1053/plac.1998.0353. [DOI] [PubMed] [Google Scholar]
- 13.Nunoue T, Kusuhara K, Hara T. Human fetal infection with parvovirus B19: maternal infection time in gestation, viral persistence and fetal prognosis. The Pediatric infectious disease journal. 2002;21(12):1133–6. doi: 10.1097/00006454-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Jordan JA, DeLoia JA. Globoside expression within the human placenta. Placenta. 1999;20(1):103–8. doi: 10.1053/plac.1998.0353. [DOI] [PubMed] [Google Scholar]
- 15.De Jong E, Walther F, Kroes A, Oepkes D. Parvovirus B19 infection in pregnancy: new insights and management. Prenatal diagnosis. 2011;31(5):419–25. doi: 10.1002/pd.2714. [DOI] [PubMed] [Google Scholar]
- 16.Enders M, Klingel K, Weidner A, Baisch C, Kandolf R, Schalasta G. et al. Risk of fetal hydrops and non-hydropic late intrauterine fetal death after gestational parvovirus B19 infection. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2010;49(3):163–8. doi: 10.1016/j.jcv.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Enders M, Klingel K, Weidner A, Baisch C, Kandolf R, Schalasta G. et al. Risk of fetal hydrops and non-hydropic late intrauterine fetal death after gestational parvovirus B19 infection. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2010;49(3):163–8. doi: 10.1016/j.jcv.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Saito S. Cytokine cross-talk between mother and the embryo/placenta. Journal of reproductive immunology. 2001;52(1-2):15–33. doi: 10.1016/s0165-0378(01)00112-7. [DOI] [PubMed] [Google Scholar]
- 19.Norbeck O, Papadogiannakis N, Petersson K, Hirbod T, Broliden K, Tolfvenstam T. Revised clinical presentation of parvovirus B19-associated intrauterine fetal death. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;35(9):1032–8. doi: 10.1086/342575. [DOI] [PubMed] [Google Scholar]
- 20.Cosmi E, Mari G, Delle Chiaie L, Detti L, Akiyama M, Murphy J. et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia resulting from parvovirus infection. American journal of obstetrics and gynecology. 2002;187(5):1290–3. doi: 10.1067/mob.2002.128024. [DOI] [PubMed] [Google Scholar]
- 21.Nyman M, Tolfvenstam T, Petersson K, Krassny C, Skjöldebrand-Sparre L, Broliden K. Detection of Human Parvovirus B19 Infection in First‐Trimester Fetal Loss. Obstetrics & Gynecology. 2002;99(5, Part 1):795–8. doi: 10.1016/s0029-7844(02)01937-3. [DOI] [PubMed] [Google Scholar]
- 22.Skjoldebrand-Sparre L, Nyman M, Broliden K, Wahren B. All cases of intrauterine fetal death should be evaluated for parvovirus B19 viral deoxyribonucleic acid. American journal of obstetrics and gynecology. 1999;180(6 Pt 1):1595–6. doi: 10.1016/s0002-9378(99)70059-1. [DOI] [PubMed] [Google Scholar]
- 23.Aberham C, Pendl C, Gross P, Zerlauth G, Gessner M. A quantitative, internally controlled real-time PCR Assay for the detection of parvovirus B19 DNA. Journal of virological methods. 2001;92(2):183–91. doi: 10.1016/s0166-0934(00)00292-5. [DOI] [PubMed] [Google Scholar]
- 24.Landolsi H, Yacoubi M, Bouslama L, Lahmar A, Trabelsi A, Hmissa S. et al. Detection of the human Parvovirus B19 in nonimmune hydrops fetalis using immunohistochemistry and nested-PCR in formalin-fixed and paraffin-embedded placenta and fetal tissues. Pathologie Biologie. 2009;57(3):e1–e7. doi: 10.1016/j.patbio.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Quemelo PR, Lima DM, da Fonseca BA, Peres LC. Detection of parvovirus B19 infection in formalin-fixed and paraffin-embedded placenta and fetal tissues. Revista do Instituto de Medicina Tropical de Sao Paulo. 2007;49(2):103–7. doi: 10.1590/s0036-46652007000200007. [DOI] [PubMed] [Google Scholar]
- 26.Syridou G, Spanakis N, Konstantinidou A, Piperaki ET, Kafetzis D, Patsouris E. et al. Detection of cytomegalovirus, parvovirus B19 and herpes simplex viruses in cases of intrauterine fetal death: association with pathological findings. Journal of medical virology. 2008;80(10):1776–82. doi: 10.1002/jmv.21293. [DOI] [PubMed] [Google Scholar]
- 27.Shahsiah R, Monajemzadeh M, Hoseinzadeh H, Alamooti AA, Mahjoub F. Parvovirus B19 Infection Frequency in Placenta of Fetal Loss Cases in Children Medical Center, Tehran, Iran. Iranian Journal of Pathology. 2011;6(4):202–7. [Google Scholar]
- 28.Tolfvenstam T, Papadogiannakis N, Norbeck O, Petersson K, Broliden K. Frequency of human parvovirus B19 infection in intrauterine fetal death. Lancet. 2001;357(9267):1494–7. doi: 10.1016/S0140-6736(00)04647-X. [DOI] [PubMed] [Google Scholar]
- 29.Al-Buhtori M, Moore L, Benbow EW, Cooper RJ. Viral detection in hydrops fetalis, spontaneous abortion, and unexplained fetal death in utero. Journal of medical virology. 2011;83(4):679–84. doi: 10.1002/jmv.22007. [DOI] [PubMed] [Google Scholar]
- 30.Nyman M, Tolfvenstam T, Petersson K, Krassny C, Skjöldebrand-Sparre L, Broliden K. Detection of Human Parvovirus B19 Infection in First‐Trimester Fetal Loss. Obstetrics & Gynecology. 2002;99(5, Part 1):795–8. doi: 10.1016/s0029-7844(02)01937-3. [DOI] [PubMed] [Google Scholar]
- 31.Miller E, Fairley CK, Cohen BJ, Seng C. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. British journal of obstetrics and gynaecology. 1998;105(2):174–8. doi: 10.1111/j.1471-0528.1998.tb10048.x. [DOI] [PubMed] [Google Scholar]
- 32.Yaegashi N. Pathogenesis of nonimmune hydrops fetalis caused by intrauterine B19 infection. The Tohoku journal of experimental medicine. 2000;190(2):65–82. doi: 10.1620/tjem.190.65. [DOI] [PubMed] [Google Scholar]
- 33.Bosman A, Wallinga J, Kroes A. Elke vier jaar de vijfde ziekte. Parvovirus B19 Infectieziekten Bull. 2002;13:215–9. [Google Scholar]
- 34.Harger JH, Adler SP, Koch WC, Harger GF. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstetrics & Gynecology. 1998;91(3):413–20. doi: 10.1016/s0029-7844(97)00701-1. [DOI] [PubMed] [Google Scholar]
- 35.Rohrer C, Gartner B, Sauerbrei A, Bohm S, Hottentrager B, Raab U. et al. Seroprevalence of parvovirus B19 in the German population. Epidemiology and infection. 2008;136(11):1564–75. doi: 10.1017/S0950268807009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Rijckevorsel GG, Sonder GJ, van der Loeff MF, van den Hoek JA. Population-based study on the seroprevalence of parvovirus B19 in Amsterdam. Journal of medical virology. 2009;81(7):1305–9. doi: 10.1002/jmv.21528. [DOI] [PubMed] [Google Scholar]


