Abstract
Background: The effectiveness of classic standard triple therapy regimen of helicobacter pylori (H. pylori) eradication has decreased to unacceptably low levels, largely related to development of resistance to metronidazole and clarithromycin. Thus successful eradication of H. pylori infections remains challenging. Therefore alternative treatments with superior effectiveness and safety should be designed and appropriately tested in all areas depending on the native resistance patterns. Furazolidone has been used successfully in eradication regimens previously and regimens containing furazolidone may be an ideal regimen.
Methods: H. pylori infected patients with proven gastric or duodenal ulcers and /or gastric or duodenal erosions at Imam Khomeini Hospital in Sari/Northern Iran, were randomly allocated into three groups: group A (OABF) with furazolidone (F) (200 mg bid.), group B (OABM-F) metronidazole (M) (500 mg bid.) for the first five days, followed by furazolidone (F) (200 mg bid.) for the second five days and group C (OAF) with furazolidone (F) (200 mg tid.). Omeprazole (O) (20 mg bid.) and amoxicillin (A) (1000 mg bid.) were given in all groups; bismuth (B) (240 mg bid.) was prescribed in groups A & B. Duration of all eradication regimens were ten days. Eight weeks after treatment, a 14C-urea breath test was performed for evaluation of H. pylori eradication.
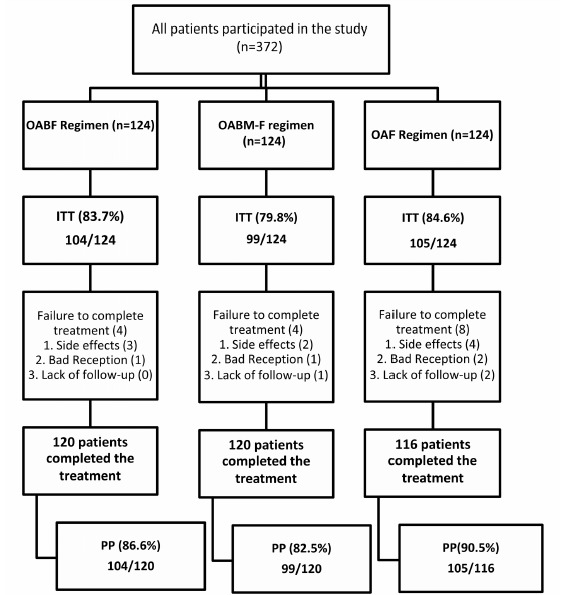
Results: A total of 372 patients were enrolled in three groups randomly (124 patients in each group); 120 (97%) patients in group A (OABF), 120 (97%) in group B (OABM-F) and 116 (93%) in group C (OAF) completed the study. The intention-to-treat eradication rates were 83.7% (95% CI= 77.3–90.4), 79.8% (95% CI= 72.6–87), and 84.6% (95% CI= 78.2–91.1) and per-protocol eradication rates were 86.6% (95% CI= 80.5–92.8), 82.5% (95% CI= 75.6–89.4), and 90.5% (95% CI= 85.1–95.9) for groups OABF, OABM-F, and OAF, respectively. No statistical significant differences were found in case of severe drug adverse effects between the above mentioned three groups (p> 0.05). The most common side effects, namely nausea and fever, occurred in all groups, but more frequently in group C (OAF) (p< 0.05).
Conclusion: In developing countries such as Iran, furazolidone-based regimens can substitute clarithromycinbased regimens for H. pylori eradication because of a very low level of resistance, low cost and high effectiveness. Considering per-protocol eradication rate of ten days OAF regimen, and the acceptable limit of ninety percent, we recommend this regimen in developing countries such as Iran to be substituted of classic standard triple therapy. In order to minimize rare serious adverse effects, one week high dose OAF regimen should be taken into consideration in other studies.
Keywords: Helicobacter pylori, Furazolidone, Bismuth, Treatment effectiveness
Introduction
Helicobacter pylori (H. pylori) is one of the most prevalent infectious agent in the world and approximately half of the world's population infected with this organism, predominantly live in Asia. It has a major role in the peptic ulcer disease, gastric adenocarcinoma, maltoma, chronic gastritis and atrophic gastritis.Its prevalence in patients over 25 years of age has been reported more than 90% in Iran. The ideal treatment for H. pylori infection is a regimen that is well tolerated, with minor side effects(<5%), eradication rate by “per-protocol” and “intention–to-treat” method is more than 90-95% and 80%, respectively (1-3).A more successful regimen should be studied in other regions (4).Although various regimens for treatment of H. pylori has been introduced; but the optimal regimen has not been defined yet (5). The classic standard triple drug regimen in American and European countries led to increased resistance to antibiotics such as metronidazole and clarithromycin, and reduced eradication rate. Therefore we needed a regimen being able to overcome these antibiotic resistance. Then, a sequential regimen (10 days) was used and became the preferred regimen as the first-line therapy previously (3,6,7). Sequential regimen is still a good treatment but not ideal, because eradication rate decreased in some areas due to dual drug resistanceto metronidazole and clarithromycin. It is probably that the concomitant quadruple regimen (bismuth or non-bismuth containing) or hybrid regimen are more effective, due to overcoming dual drug resistance. Recently sequential regimen is conducted and approved in Northern Iran (7,8).
In Iran, during the years 2003-2011, clarithromycin and metronidazole resistance increased markedly from 1.2% to 30-34% and from 32% to 78%, respectively, while there was only a slight increase in furazolidone resistance (0% to 4.5%) (9-14).In developing countries such as Iran, furazolidone, due to low cost and drug resistance and high effectiveness, may be a better alternative to clarithromycin or metronidazole in the first-line regimen of eradication (15,16).Meanwhile, it is recommended to evaluate more regimens containing furazolidone to find the proper dose of furazolidone (15). It seems that regimens with less than 14 days duration of treatment are not very effective in Iran (9,17) . However, the arrangement of a regimen is not fixed and the eradication rate of that regimen can be increased through changing the type of the drugs, dose of the drugs, interval between doses and duration of treatment (4). Resistance of H. pylori to clarithromycin and metronidazole in recent years is reported in the world and Iran. The study originality underlies the reduced effectiveness of some regimens containing clarithromycin, the high cost of some antibiotics such as clarithromycin and ofloxacin in developing countries, recommendation of some researchers to do more regional studies on regimens containing furazolidone and the results obtained especially in developing countries (3,6,7,16). We designed this study to compare the effectiveness and safety of quadruple and triple 10-day regimens containing furazolidone for H. pylori eradication in all patients with H. pylori infected gastric or duodenal ulcer or erosions referred to the clinic/office of gastroenterologists or endoscopy unit of Imam Khomeini Hospital in Sari, Iran.
Methods
Data
This clinical trial was performed on 372 patients at Imam Khomeini Hospital in Sari/Northern Iran. Participants were patients’ whose duodenal ulcer, gastric ulcer and/or erosions was diagnosed by endoscopy and H. pylori infection was detected by rapid urease test (RUT, Shim-anzym,Tehran, Iran) or histological Geimsa staining on biopsied specimens from antrum and body during endoscopy. We considered the difference of about16% in eradication rate of these three regimens (according to alpha= 0.05 and beta= 0.2) and based on pilot study and statistical calculations, at least a total number of 372 patients were chosen to participate in this study (at least 124 patients in each group). Exclusion criteria were; age less than 18 years, pregnancy or lactation, history of previous H. pylori eradication, taking antibiotics and/or nonsteroid anti-inflammatory drugs (NSAID) or bismuth during the previous 4 weeks, concomitant use of alcohol, anticoagulant, monoamine oxidase inhibitors (MAOIs), sympathomimetics and/or corticosteroid, history of gastric surgery, known case of neoplasms and severe underlying disease such as; liver, renal, pulmonary or cardiac disease, G6PD deficiency and history of drug allergy to any mentioned medication in this study .
The aim and nature of the study was fully explained for patients and informed consent was obtained. This study was accepted in the Ethics Committee of Mazandaran University of Medical Sciences (Code number: 91-139), and the proposal was also registered in Iranian Registry of Clinical Trials (IRCT code: 2013120715701N1).We used permuted (computer-generated) randomized blocks for randomization and each group was treated with one of the three following regimens:
Group A (OABF): Omeprazole (20mg), Amoxicillin (1000mg), Bismuth (240mg), Furazolidone (200mg); all prescribed twice a day for ten days.
Group B (OABM-F): Omeprazole (20mg), Amoxicillin (1000mg) and Bismuth (240 mg), all prescribed twice a day for the whole ten days. Metronidazole (500mg twice a day) during the first 5 days and Furazolidone (200mg twice a day) during the second remaining 5 days were prescribed.
Group C (OAF): Omeprazole (20 mg), Amoxicillin (1000mg), twice a day and Furazolidone (200mg) three times a day, all prescribed for ten days.
Ulcer characteristics (location, diameter in mm, number and depth) and the presence or absence of bulb deformity were recorded in each patient. In addition to demographic data, history of gastrointestinal bleeding and current smoking were recorded in each patient. All patients received one of three above treatments randomly. In addition, all patients were advised to avoid consumption of thyramine-containing foods and MAOI medications during the ten days of treatment (List of thyramine-containing foods and MAOI medications delivered to patients by patient’s informative sheet). The patients were given both oral and written instructions about the significance of taking the medications regularly and were suggested not to stop medication in the event of mild to moderate side effects. Patients recorded adverse effects of drugs on a daily basis in their informative sheets and were advised to call the physician or senior physician in charge of study in case of any problematic or severe adverse effects. Patients referred to the clinic 2, 6 and 12 weeks after starting the treatment.
The patients were asked about their compliance to treatment, number of remaining pills (if any), and presence and severity of side effects verbally and/or written in patient’s informative sheet, 2 weeks after starting treatment. The gathered data transformed to data gathering sheets for analysis. The severity of side effects was classified as 0= no side effect; 1= mild side effects (no limitation in daily activities); 2= moderate (partial limitation in daily activities); and 3= severe (profound limitation in daily activities). Medications were discontinued if any intolerable adverse event occurred. The compliance to treatment was considered to be excellent if the patient had consumed more than 80% of prescribed medications, acceptable or good for 60–80% medication consumption, and poor for < 60%. Omeprazole was continued 4 weeks after staring treatment. Next, no antibiotics or omeprazole was taken for 8 weeks. Twelve weeks after starting the treatment, H. pylori eradication was assessed by 14 C-urea breath test (UBT). To perform UBT, patients swallowed 37kBq (lCi) encapsulated 14 C-labeled urea composition (Helicap Institute of Isotopes, Budapest, Hungary) with water. After 10 minutes, patients exhaled into a cartridge (Heliprobe breath card, Kibion Uppsala, Sweden) until the indicator of the card changed from orange to yellow. The cards were inserted into a Geiger–Muller counter (Heliprobe Analyser, Kibion AB), and radioactivity of samples was automatically measured after 250 seconds. Based on radioactivity, as count per minute (cpm), counts more than 50cpm were considered infected with H. pylori (Positive UBT) and count less than 25cpm were considered H. pylori eradication. The borderline UBT values were considered as eradicated.
Statistical Analysis
Data were analyzed using SPSS 16. Discriptive statistics are reported as mean ± SD. We used ANOVA for comparing means between three groups and Chi square or Fisher exact tests for comparing proportion between groups and p< 0.05 was considered statistically significant.
The statistician analyzer of ultimate results was unsighted to the assignment of patients.
To calculate the intention-to-treat (ITT) eradication rates, we included everyone who entered the study was considered, and to calculate per-protocol (PP) eradication rates, only those who completed the entire protocol with more than 90% compliance to treatment and returned for final test of eradication.
Results
Three hundred and seventy two patients were enrolled in this study; 47.3% (176) were women and 52.7% (196) were men. The mean (±SD) age of the patients was 42.28 (±.29) years (range: 18-83 years). The mean (±SD) age of patients in three groups were 43.7 (±13.91), 40.8 (±14.60) and 42.3 (± 14.31), respectively.
There were not statistically significant difference between the three groups according to age, sex, history of gastrointestinal bleeding, current smoking (p> 0.05) and endoscopic findings (Table 1).
Table 1 . Frequency distribution of the endoscopic findings in the three groups .
|
A(OABF) N=124 n (%) |
B(OABM-F) N=124 n (%) |
C(OAF) N=124 n (%) |
p | ||
| Gender | Male | 64 (53) | 65 (54) | 67 (58) | 0.927 |
| Female | 60 (47) | 59 (46) | 57 (42) | ||
| Current Smoking | 14(11.3) | 15(12.1) | 22(17.1) | 0.274 | |
| History of GIB | 13(10.5) | 14(11.3) | 15(12.1) | 0.923 | |
| Endoscopic findings | DU | 73(58.9) | 76(61.3) | 77(62.1) | 0.926 |
| GU | 14(11.3) | 14(11.3) | 12(9.7) | 0.926 | |
| GU +DU | 3(2.4) | 3(2.4) | 2(1.6) | 0.926 | |
| DE | 7(5.6) | 9(7.3) | 5 (4) | 0.926 | |
| GE | 24(19.4) | 19(15.3) | 24(19.4) | 0.926 | |
| GE+DE | 3(2.4) | 3(2.4) | 4(3.2) | 0.926 | |
| Ulcer size >7 mm | 59(34.7) | 63(37.1) | 63(37.1) | 0.233 | |
| Deformity of bulb | 22(17.7) | 23(18.6) | 23(18.6) | 0.974 |
GIB= Gasterointestinal bleeding, DU= Duodenal Ulcer, GU= Gasreric Ulcer, DE= Duodenal Erosions, GE= Gasteric Erosions
The number and types of side effects in the three treatment groups are described in Table 2. The most common complication was fever and nausea which were significantly higher in group C (OAF) than two other groups.
Table 2 . The number and types of drug side effects in three treatment groups .
| Side effects / treatment group |
A (OABF) (n)% |
B (OABM-F) (n)% |
C(OAF) (n)% |
p |
|
Dyspepsia Weakness |
(4)3.2 (8)6.5 |
(5)4.0 (4)3.2 |
(5)4.0 (9)7.3 |
.929 .348 |
|
* Nausea Vomiting |
(13)10.5 (2)1.6 |
(8)6.5 (1)0.8 |
(21)16.9 (4)3.2 |
.031 .363 |
|
Abdominal cramp Urine orange discoloration |
(1)0.8 (10)8.1 |
(0)0 (8)6.5 |
(1)0.8 (17) 13.7 |
.607 .121 |
|
Diarrhea Constipation |
(3)2.4 (1)0.8 |
(1)0.8 (2)1.6 |
(4)3.2 (0)0 |
.411 .367 |
|
Glossitis Anorexia |
(2)1.6 (3)2.4 |
(0)0 (1)0.8 |
(0)0 (2)1.6 |
.135 .604 |
|
Bad taste Heartburn |
(0)0 (1)0.8 |
(0)0 (1)0.8 |
(2)1.6 (0)0 |
.135 .607 |
|
Skin rash Urticaria |
(2)1.6 (1)0.8 |
(0)0 (1)0.8 |
(1)0.8 (2)1.6 |
.367 .778 |
|
Drug Fever* Arthralgia |
(2)1.6 (1)0.8 |
(0)0 (1)0.8 |
(8)6.5 (2)1.6 |
.005 .778 |
| Bloating | (1)0.8 | (0)0 | (0)0 | .369 |
|
Dizziness Orthostatic changes |
(10)8.1 (5)4.0 |
(6)4.8 (3)2.4 |
(14)11.3 (8)6.5 |
.176 .291 |
|
Itching Chest pain |
(0)0 (0)0 |
(2)1.6 (1)0.8 |
(0)0 (1)0.8 |
.135 .607 |
| Headache | (6)4.8 | (1)0.8 | (6)4.8 | .137 |
* p<0.05
The majority of serious side effects in each treatment group were observed in the second half of the treatment period: 4 of 5 patients in the regimen A (OABF); and 4 of 4 patients in the regimen B (OABM-F) and 5 of 6 patients in the regimen C (OAF). Prevalence of mild, moderate and severe drug adverse effects in three studied regimens is described in Table 3. Also, the number of drug withdrawal due to severe side effects for three groups were 3, 2 and 4, respectively.
Table 3 . Prevalence of mild, moderate and severe drug adverse effects of three treatment groups .
|
Severity of side effects |
Group A (OABF) n (%) |
Group B (OABM-F) n (%) |
Group C (OAF) n (%) |
p |
| Mild | 41 (33.1) | 30 (24.2) | 37 (29.8) | |
| Moderate side effects | 2 (1.6) | 2(1.6) | 18(14.5) | |
| Severe* | 5(4) | 4(3.2) | 6(4.8) | 0.812 |
*P value has been calculated for the incidence of severe side effects among three groups versus others
In group A (OABF) a total of 4 patients did not complete their treatment (3 patients due to side effects such as rash, vomiting, diarrhea and severe orthostatic changes and 1 patient with poor compliance). In group B (OABM-F) a total of 4 patients did not complete their treatment (2 patients due to side effects such as vomiting, nausea, sever dizziness and 1 patient with poor compliance and one lost follow-up due to appendectomy). In group C (OAF) a total of 8 patients did not complete their treatment (4 patients due to side effects such as severe headache, vomiting, urticaria, fever, and severe orthostatic changes and 2 patients with poor compliance and 2 patients lost follow-up due to MI in one 74 years old patient and car accident in another).
Among the participants in 3 treatment groups, 353 patients (95%) had excellent treatment compliance (Table 4). ITT and PP eradication rates are shown in Table 5. The algorithm of patients presented in study is shown in Fig. 1 .
Table 4 . Compliance in three treatment regimens .
|
Compliance rate/ medical group |
Group A(OABF) n(%) |
Group B (OABM-F) n(%) |
Group C(OAF) n (%) |
p |
| High (> 80% of drugs) | 120 (96.7) | 120(96.7) | 116(93.5) | 0.089 |
| Acceptable (60-80% of drugs ) | 3(2.4) | 2(1.6) | 5(4.0) | |
| Poor (< 60% of drugs) | 1(0.8) | 2(1.6) | 3(2.4) |
*P value has been calculated for the high compliance in three groups versus others
Table 5 . Intention-to-treat and per-protocol eradication rates in three treatment regimens .
|
Total enrolled patients(n=372) |
Group A(OABF) (n=124) | Group B(OABM-F) (n=124) |
Group C(OAF) (n=124) |
p |
| Number of patients who have consumed >80% of drugs | 120 | 120 | 116 | |
| ITT* eradication rate with 95% CI (n=372) | 83.7% (104/124) (95%CI=77.3-90.4) | 79.8% (99/124) (95%CI=72.6-87.0) | 84.6%(105/124) (95%CI=78.2-91.1) | 0.557 |
| PP* eradication rate with 95% CI(n=356) | 86.6% (104/120)- (95%CI=80.5-92.8) | 82.5% (99/120) (95%CI=75.6-89.4) | 90.5%(105/116)-(95%CI=85.1-95.5) | 0.197 |
The cure rates was higher in treatment regimen C(OAF), but in general, the differences were not statistically significant (p=0.197, p=0.557, respectively for PP and ITT) and therefore, as far as helicobacter pylori eradication level is considered, the treatment regimen C(OAF) is in Grade B (good), and treatment regimen B(OABM-F) is in Grade D (poor) and treatment regimen A(OABF) is in Grade C (moderate or acceptable).
Fig. 1 .
Eradication rate based on intention-to-treat and per-portocol analyzes
Discussion
Although various treatment regimens have been introduced to treat H. pylori, but an ideal treatment regimen has not yet been defined. The most common regimen introduced during the last two decades, is a two weeks triple clarithromycin-based treatment with eradication rates of 75% -90% in developed countries; currently, these eradication rates reached to 75-80.5% (3,5,18). However, eradication rate with this regimen in developing countries is much lower than in developed countries. Reasons for the difference in eradication rate in the geographical area, covers a wide range of difference in pathogenic strains of H. pylori, antibiotic resistance pattern, type and duration of treatment and pharmacological drug metabolism (19). In Iran, eradication rate of triple therapy is less than 60% (16).
Also, the rate of metronidazole resistance has been increased in many countries. However, this high resistance was in vitro, and the drug has been relatively effective in clinical trials (20,21). North American and European institutions recommended clarithromycin as a good substitute for metronidazole. However, this drug may not be an appropriate option in developing countries, such as Iran, due to the increase of drug-resistant strains and its high cost (17).Evaluation of eradication regimens in Iran showed more toxic and special species that do not have a good response to the usual regimens in developed countries (22).
Sequential clarithromycin-based regimen as an effective alternative therapy for triple therapy was introduced. Several randomized studies have failed to demonstrate the superiority of this method (22,23)and the results of this method have not been promising in Asia as well (24)which could be due to increased resistance to clarithromycin (25-27). In Iran, based on the last reports, 78.6% of H. pylori are resistant to metronidazole, 34% to clarithromycin, 10% to amoxicillin and 4.5% to furazolidone (11,14). Furazolidone is one of nitroimidazole antibiotics with a good intestinal absorption and tissue distribution (28,29). In the last decade, this drug was effective as the first, second and even third -line therapy (20,30-33).
Many researchers believe that this drug can be an appropriate and effective alternative for clarithromycin (17).Ten to 14-day bismuth quadruple regimen containing furazolidone (100 mg Tid) were effective as the first and second-line regimen in eradicating H. pylori. Meanwhile, in areas where this drug is available, carrying out more studies to find the proper dose of furazolidone has been recommended (15).
In Iran, furazolidone, combined with amoxicillin, bismuth, clarithromycin and a PPI in the form of quadruple and triple therapy, is used as the first-line therapy with the different results based on the dose and duration of treatment (4,17,30,34-38). Fakheri et al in different studies on furazolidone- containing regimens in the north of Iran, have shown that there was no significant difference between bismuth and clarithromycin-containing quadruple regimen and bismuth and furazolidone-containing quadruple regimen considering eradication rate (34). In a study, they showed that low doses of furazolidone (100mg bid) is not an effective treatment, but the 14-day quadruple therapy containing bismuth and furazolidone is associated with eradication rates of 92% (35). In another study, two 14-day modified quadruple regimens containing first week furazolidone while one regimen included metronidazole and the other bismuth, were compared. Eradication rates were 86.36% and 90.27%, respectively. In addition, the side effects in the bismuth- containing group were lower compared to the metronidazole-containing group (32,39).
In a recent study that compared the sequential and modified furazolidone-based quadruple regimen, both of them showed the same efficacy in eradicating H. pylori (88.7%, 89.1%, respectively); however, due to the high price of clarithromycin in Iran, modified quadruple regimen is more cost-effective (38).
Also in a study, Khatibian et al. reported that the modified furazolidone–based quadruple therapy had good effectiveness and low cost in H. pylori eradication (36). Meanwhile, the incidence of intolerable side effects in furazolidone-containing regimen is more than other regimens and similar to our study, adverse effects are mostly appeared in the second half of the treatment (32,39).Rare but serious side effects of this drug have limited its common use. It seems that these side effects are due to monoamine oxidase inhibitory of this drug which is occurrence is preventable by avoiding certain drugs (such as sympatomimtic and monoamine oxidase inhibitors) and thyramine containing foods such as old kinds of cheeses (17).
However, dose and duration of furazolidone are effective on the incidence of side effects (35).Shortening the duration of treatment and adding another drug such as bismuth in combination with amoxicillin and PPI can be a reasonable approach to reduce the side effects and to increase the effectiveness of furazolidone (40).
In a study in Brazil, a high dose of furazolidone (600mg/day) plus tetracycline, and a PPI was used for one week. The incidence of serious side effects, patients’ compliance and the eradication rate were 7.6%, 96% and 91.8%, respectively (41). However, other studies in Latin America were conducted with a high dose of furazolidone for 4 and 10 days which had no serious side effects. Increasing the duration of treatment to 10 days had no effect on the eradication rate (42). On the other hand, in a study in Peru, a high dose of furazolidone (600 mg a day) plus tetracycline, and a PPI were used for one week, the severe side effect was 15% and eradication rate was 75% (32). Dual therapy with low dose of furazolidone and omeprazole was used for one week in a Latin American country which was stopped in the middle course of treatment due to low eradication rate (50%), though the side effect was mild (28).
In this study, we aimed at: shortening treatment duration -although it seems to reduce regimen efficacy-and examining the rate of eradication, patient compliance and drug side effects in regimens containing high dose of furazolidone for the first time in Iran. The eradication rate in high-dose OAF regimen was higher than the other two quadruple regimens (PP= 90.5%, ITT= 84.6% compared with the PP= 82.5% and ITT= 79.8% in OABM-F regimen and PP= 86.6% and ITT=83.7% in OABF regimen). However, the difference was not statistically significant. It seems that increasing the dose of drug is more effective than adding another drug such as bismuth to the regimen. On the other hand, 10-days OABM-F regimen had an eradication rate of 91.3% in a recent study conducted in Iran (30), whereas in our study the eradication rate was 82.5%. It seems that the difference in eradication rate is related to the factors such as differences in pathogenic strains of H. pylori, antibiotic resistance pattern and pharmacological differences in drug metabolism in our area.
Reducing the duration of therapy in OABF regimen from 14 to 10 days causes the eradication rate to be decreased from 92% to 86.6%, still within acceptable eradication rate, and the rate of severe side effects decreased from 20.4% to 4% (35).
In our study severe drug side effects was less than 5% in each group, and the overall compliance rate was 94.9%, the lowest compliance rate was associated with high-dose OAF regimen (91.1%). Therefore, it shows that there is no significant difference in severe adverse drug effect and compliance between three groups. It seems that it can be justified by the time we spent to inform patients about the importance of eradication of H. Pylori and to avoid thyramine containing foods and monoamine oxidase inhibitor drugs during furazolidone consumption. Interestingly, in high dose furazolidone-containing regimen for the first time in Iran, the results of eradication (PP= 90.5% and ITT= 84.6%), the incidence of severe side effects (4.8%) and drug compliance (91.1%) were in the good level.
It seems that high doses of furazolidone has the same effect observed with high doses of metronidazole capable to eradicate the resistant strains of H. pylori (40).
Conclusion
According to the findings of this study, high price of clarithromycin and high level of single and dual resistance to clarithromycin and metronidazole in developing countries, the 10-days high dose OAF regimen is acceptable with a good effectiveness and safety profile as the first-line treatment regimen for H. pylori eradication. In order to minimize rare serious adverse effects, we recommended one week high dose OAF regimen should be taken into consideration in other studies.
Cite this article as: Mokhtare M, Hosseini V, Tirgar Fakheri H, Maleki I, Taghvaei T, Valizadeh S.M, Sardarian H, Agah Sh, Khalilian A. Comparison of quadruple and triple Furazolidone containing regimens on eradication of helicobacter pylori. Med J Islam Repub Iran 2015 (6 April). Vol. 29:195.
References
- 1.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007 Aug;12(4):275–8. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 2.Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World Journal of Gastroenterology. 2011 Sep 21;17(35):3971–5. doi: 10.3748/wjg.v17.i35.3971. Pubmed Central PMCID: 3199554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D. et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut . 2007 Jun;56(6):772–81. doi: 10.1136/gut.2006.101634. Pubmed Central PMCID: 1954853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakheri H, Fakhri M, Shahmohamadi S. Efficacy of short-term Furazolidone in two different quadruple regimens of H pylori eradication. Pak J Med Sci. 2011;27(4):887–91. [Google Scholar]
- 5.Chey WD, Wong BC. ractice Parameters Committee of the American College of G. . American College of Gastroenterology guideline on the management of Helicobacter pylori infection. American Journal of Gastroenterology. 2007 Aug;102(8):1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 6.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Annals of Internal Medicine. 2008 Jun 17;148(12):923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 7.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F. et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Annals of Internal Medicine. 2007 Apr 17;146(8):556–63. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 8.Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013 Apr;18(2):129–34. doi: 10.1111/hel.12017. [DOI] [PubMed] [Google Scholar]
- 9.Malekzadeh R, Merat S, Derakhshan MH, Siavoshi F, Yazdanbod A, Mikaeli J. et al. Low Helicobacter pylori eradication rates with 4- and 7-day regimens in an Iranian population. Journal of Gastroenterology And Hepatology. 2003 Jan;18(1):13–7. doi: 10.1046/j.1440-1746.2003.02897.x. [DOI] [PubMed] [Google Scholar]
- 10.Kohanteb J, Bazargani A, Saberi-Firoozi M, Mobasser A. Antimicrobial susceptibility testing of Helicobacter pylori to selected agents by agar dilution method in Shiraz-Iran. Indian Journal of Medical Microbiology. 2007 Oct;25(4):374–7. doi: 10.4103/0255-0857.37342. [DOI] [PubMed] [Google Scholar]
- 11.Siavoshi F, Saniee P, Latifi-Navid S, Massarrat S, Sheykholeslami A. Increase in resistance rates of H pylori isolates to metronidazole and tetracycline--comparison of three 3-year studies. Archives of Iranian Medicine. 2010 May;13(3):177–87. [PubMed] [Google Scholar]
- 12.Shokrzadeh L, Jafari F, Dabiri H, Baghaei K, Zojaji H, Alizadeh AH. et al. Antibiotic susceptibility profile of Helicobacter pylori isolated from the dyspepsia patients in Tehran, Iran. Saudi Journal of Gastroenterology : Official Journal of The Saudi Gastroenterology Association. 2011 Jul-Aug;17(4):261–4. doi: 10.4103/1319-3767.82581. Pubmed Central PMCID: 3133984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abadi AT, Taghvaei T, Mobarez AM, Carpenter BM, Merrell DS. Frequency of antibiotic resistance in Helicobacter pylori strains isolated from the northern population of Iran. Journal of Microbiology. 2011 Dec;49(6):987–93. doi: 10.1007/s12275-011-1170-6. Pubmed Central PMCID: 3275342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talebi Bezmin Abadi A, Ghasemzadeh A, Taghvaei T, Mobarez AM. Primary resistance of Helicobacter pylori to levofloxacin and moxifloxacine in Iran. Internal And Emergency Medicine. 2012 Oct;7(5):447–52. doi: 10.1007/s11739-011-0563-1. [DOI] [PubMed] [Google Scholar]
- 15.Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi Journal of Gastroenterology : Official Journal of The Saudi Gastroenterology Association. 2012 Jan-feb;18(1):1–2. doi: 10.4103/1319-3767.91724. Pubmed Central PMCID: 3271686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daghaghzadeh H, Emami MH, Karimi S, Raeisi M. One-week versus two-week furazolidone-based quadruple therapy as the first-line treatment for Helicobacter pylori infection in Iran. Journal of Gastroenterology and Hepatology. 2007 Sep;22(9):1399–403. doi: 10.1111/j.1440-1746.2007.05029.x. [DOI] [PubMed] [Google Scholar]
- 17.Hasan SR, Vahid V, Reza PM, Roham SR. Short-duration furazolidone therapy in combination with amoxicillin, bismuth subcitrate, and omeprazole for eradication of Helicobacter pylori. Saudi Journal of Gastroenterology: Official Journal of the Saudi Gastroenterology Association. 2010 Jan-Mar;16(1):14–8. doi: 10.4103/1319-3767.58762. Pubmed Central PMCID: 3023095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ. et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. Journal of Gastroenterology and Hepatology. 2009 Oct;24(10):1587–600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 19.Vakil N. Are there geographical and regional differences in Helicobacter pylori eradication? Canadian Journal of Gastroenterology. 2003 Jun;17 Suppl B:30B–2B. doi: 10.1155/2003/903494. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H, Hu FL. Furazolidone, amoxicillin, bismuth and rabeprazole quadruple rescue therapy for the eradication of Helicobacter pylori. World Journal of Gastroenterology. 2009 Feb 21;15(7):860–4. doi: 10.3748/wjg.15.860. Pubmed Central PMCID: 2653387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Group CHpR, Gastroenterology. CSo. Prevalence of Helicobacter pylori resistance to antibiotics and its influence on the treatment outcome in China: A multicenter clinical study. Weichang Bingxue. 2007;12:525-30. Transplantation proceedings; 2002. Elsevier.
- 22.Choi WH, Park DI, Oh SJ, Baek YH, Hong CH, Hong EJ. et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. The Korean Journal of Gastroenterology . 2008 May;51(5):280–4. [PubMed] [Google Scholar]
- 23.Park S, Chun HJ, Kim EJ, Seo YS, Jeen YT, Um S. et al. The 10-Day Sequential Therapy for Helicobacter pylori Eradication in Korea: Less Effective than Expected. Gastroenterology. 2009 May;136(5):339–40. [Google Scholar]
- 24.Gisbert JP, Calvet X, O'Connor A, Megraud F, O'Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. Journal of Clinical Gastroenterology. 2010 May-Jun;44(5):313–25. doi: 10.1097/MCG.0b013e3181c8a1a3. [DOI] [PubMed] [Google Scholar]
- 25.Paoluzi OA, Visconti E, Andrei F, Tosti C, Lionetti R, Grasso E. et al. Ten and eight-day sequential therapy in comparison to standard triple therapy for eradicating Helicobacter pylori infection: a randomized controlled study on efficacy and tolerability. Journal of Clinical Gastroenterology. 2010 Apr;44(4):261–6. doi: 10.1097/MCG.0b013e3181acebef. [DOI] [PubMed] [Google Scholar]
- 26.Romano M, Cuomo A, Gravina AG, Miranda A, Iovene MR, Tiso A. et al. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut. 2010 Nov;59(11):1465–70. doi: 10.1136/gut.2010.215350. [DOI] [PubMed] [Google Scholar]
- 27.Tokunaga K, Tanaka A, Sugano H, Takahashi S. The present status and problems of Helicobacter pylori first-line eradication therapy. Japanese Journal of Clinical Medicine. 2009 Dec;67(12):2291–6. [PubMed] [Google Scholar]
- 28.Van Zwet AA, Thijs JC, van der Wouden EJ, Kooy A. Low cure rate of Helicobacter pylori infection with omeprazole and furazolidone dual therapy for one week. Alimentary Pharmacology and Therapeutics . 1997 Jun;11(3):533–5. doi: 10.1046/j.1365-2036.1997.00166.x. [DOI] [PubMed] [Google Scholar]
- 29.Xiao SD, Liu WZ, Xia DH, Jiang SJ, Wang RN, Zhang ZH. et al. The efficacy of furazolidone and metronidazole in the treatment of chronic gastritis associated with Helicobacter (Campylobacter) pylori--a randomized double-blind placebo-controlled clinical trial. Hepato-Gastroenterology. 1990 Oct;37(5):503–6. [PubMed] [Google Scholar]
- 30.Riahizadeh S, Malekzadeh R, Agah S, Zendehdel N, Sotoudehmanesh R, Ebrahimi-Dariani N. et al. Sequential metronidazole-furazolidone or clarithromycin-furazolidone compared to clarithromycin-based quadruple regimens for the eradication of Helicobacter pylori in peptic ulcer disease: a double-blind randomized controlled trial. Helicobacter. 2010 Dec;15(6):497–504. doi: 10.1111/j.1523-5378.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 31.Qasim A, Sebastian S, Thornton O, Dobson M, McLoughlin R, Buckley M. et al. Rifabutin- and furazolidone-based Helicobacter pylori eradication therapies after failure of standard first- and second-line eradication attempts in dyspepsia patients. Alimentary Pharmacology and Therapeutics. 2005 Jan 1;21(1):91–6. doi: 10.1111/j.1365-2036.2004.02210.x. [DOI] [PubMed] [Google Scholar]
- 32.Silva FM, Eisig JN, Chehter EZ, Silva JJ, Laudanna AA. Omeprazole, furazolidone, and tetracycline: an eradication treatment for resistant H pylori in Brazilian patients with peptic ulcer disease. Revista Do Hospital Das Clinicas . 2002 Sep-Oct;57(5):205–8. doi: 10.1590/s0041-87812002000500003. [DOI] [PubMed] [Google Scholar]
- 33.Eisig JN, Silva FM, Barbuti RC, Rodriguez TN, Malfertheiner P, Moraes Filho JP. et al. Efficacy of a 7-day course of furazolidone, levofloxacin, and lansoprazole after failed Helicobacter pylori eradication. BMC Gastroenterology. 2009;9:38. doi: 10.1186/1471-230X-9-38. Pubmed Central PMCID: 2695477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fakheri H, Malekzadeh R, Merat S, Khatibian M, Fazel A, Alizadeh BZ. et al. Clarithromycin vs furazolidone in quadruple therapy regimens for the treatment of Helicobacter pylori in a population with a high metronidazole resistance rate. Alimentary Pharmacology And Therapeutics. 2001 Mar;15(3):411–6. doi: 10.1046/j.1365-2036.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 35.Fakheri H, Merat S, Hosseini V, Malekzadeh R. Low-dose furazolidone in triple and quadruple regimens for Helicobacter pylori eradication. Alimentary Pharmacology and Therapeutics . 2004 Jan 1;19(1):89–93. doi: 10.1046/j.1365-2036.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 36.Khatibian M, Ajvadi Y, Nasseri-Moghaddam S, Ebrahimi-Dariani N, Vahedi H, Zendehdel N. et al. Furazolidone-based, metronidazole-based, or a combination regimen for eradication of Helicobacter pylori in peptic ulcer disease. Archives of Iranian Medicine. 2007 Apr;10(2):161–7. [PubMed] [Google Scholar]
- 37.Roghani HS, Massarrat S, Shirekhoda M, Butorab Z. Effect of different doses of furazolidone with amoxicillin and omeprazole on eradication of Helicobacter pylori. Journal of Gastroenterology and Hepatology. 2003 Jul;18(7):778–82. doi: 10.1046/j.1440-1746.2003.03058.x. [DOI] [PubMed] [Google Scholar]
- 38.Fakheri H, Taghvaei T, Hosseini V, Bari Z. A comparison between sequential therapy and a modified bismuth-based quadruple therapy for Helicobacter pylori eradication in Iran: a randomized clinical trial. Helicobacter. 2012 Feb;17(1):43–8. doi: 10.1111/j.1523-5378.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- 39.Malekzadeh R, Mohamadnejad M, Siavoshi F, Massarrat S. Treatment of Helicobacter pylori infection in Iran: low efficacy of recommended Western regimens. Archives of Iranian Medicine. 2004;7(1):1–8. [Google Scholar]
- 40.de Boer WA. Bismuth triple therapy: Still a very important drug regimen for curing Helicobacter pylori infection. European Journal of Gastroenterology and Hepatology. 1991;11:697–700. [PubMed] [Google Scholar]
- 41.Frota LC, Cunha MPSS, Luz CRL, Araujo-Filho AH, Frota LAS, Braga LLBC. Helicobacter pylori eradication using tetracycline and furazolidone versus amoxicillin and azithromycin in lanzoperazole based triple therapy: an open randomized clinical trial. Arq Gastroenterol. 2005 June;42(2):111–5. doi: 10.1590/s0004-28032005000200009. [DOI] [PubMed] [Google Scholar]
- 42.Xiao SD, Liu WZ, Hu PJ, Ouyang Q, Wang JL, Zhou LY. et al. A multicentre study on eradication of Helicobacter pylori using four 1-week triple therapies in China. Alimentary Pharmacology and Therapeutics. 2001 Jan;15(1):81–6. doi: 10.1046/j.1365-2036.2001.00895.x. [DOI] [PubMed] [Google Scholar]


