Abstract
Histone modifications affect chromatin dynamics on several levels by serving as binding sites for regulatory proteins. In many cell types, including embryonic stem cells (ESCs), a subset of genes is marked with histone modifications thought to be both activating and repressing: H3 lysine 4 trimethylation (H3K4me3) and lysine 27 trimethylation (H3K27me3), respectively. As a result, genes bearing this “bivalent” mark are transcribed at low levels, but are primed for activation, should the cell receive the appropriate cues during differentiation. Recently, we found that the Tip60-p400 acetyltransferase and histone exchange complex is necessary to maintain normal self-renewal in mouse ESCs. While Tip60-p400 has histone acetyltransferase activity, which is generally associated with transcriptional activation, it acts predominantly as a repressor of genes expressed during differentiation. Surprisingly, in ESCs Tip60-p400 localizes to the promoters of genes marked by H3K4me3, which include both highly expressed genes and “bivalent” genes expressed at low levels. Tip60-p400 acetylates histones at these targets, including the promoters for developmental regulators it helps to silence in ESCs. This suggests that the effect of chromatin modifications on transcription is not always simply positive or negative. Rather, we propose that the impact of specific modifications at each promoter is determined by the chromatin context in which they are found.
Keywords: Tip60, p400, Nanog, embryonic stem cells, self-renewal, chromatin, histone, methylation, acetylation
Histone Modifications are Binding Sites for Regulators of Chromatin and Transcription
In eukaryotes, the compaction of DNA into chromatin inhibits binding of proteins that require access to DNA, including those that mediate transcription, DNA replication, recombination and chromosome segregation. Accordingly, many of these processes are regulated in part through alteration of chromatin structure. Changes in chromatin structure can be used locally to regulate expression of individual genes. For example, in yeast, the ATP-dependent nucleosome remodeling complex Isw2 localizes to intergenic regions, where it represses transcription and suppresses the formation of aberrant intergenic transcripts.1,2 The Isw2 complex functions by sliding only a few nucleosomes at each target site into thermodynamically less favorable states.3,4 At the other extreme, large regions of the genome can be coordinately regulated by modulating chromatin structure. For example, dosage compensation in flies, mammals and worms results in accumulation of specific covalent histone modifications along the entire length of the sex chromosomes.5 These chromatin marks render most genes on the affected chromosome more (D. melanogaster) or less (C. elegans, mammals) permissive for expression.
One prominent mechanism for the generation of transcriptionally active or silent chromatin domains is through the placement of covalent modifications on histones at specific regions of the genome. These modifications come in a number of forms, including acetylation, methylation and ubiquitylation of lysines, methylation of arginines and phosphorylation of serines.6 As with covalent modifications on signal transduction proteins, histone modifications serve as binding sites for effector proteins that “read” the message encoded by each modification.7 These proteins not only contain one or more domains that bind specific histone modifications, but also typically contain domains that mediate protein-protein interactions, nucleosome remodeling or disruption, chromatin assembly, DNA binding, or other functions. In addition, many proteins with histone modification-binding domains reside within multi-subunit complexes with chromatin modifying activities.
Histone modifications are often classified as “activating” or “repressive” according to the effects that their downstream effectors have on gene expression. One of the first heterochromatic modifications identified, trimethylation of histone H3 on lysine 9 (H3K9me3),8 binds the chromodomain of the HP1 protein,9,10 and mediates both short range repressive effects on chromatin structure (such as the recruitment of histone modifying and DNA methyltransferase proteins11,12), as well as long-range chromosome looping.13 Another repressive histone modification is H3K27me3, which is catalyzed by the Polycomb Repressive Complex PRC2.14 H3K27me3 is bound by several chromodomain-containing proteins including Polycomb (Pc), a component of another Polycomb Repressive Complex, PRC1. PRC1 then catalyzes formation of an additional repressive chromatin mark, ubiquitylation of histone H2A on lysine 119.15 While the mechanism by which these marks inhibit transcription is largely unknown, the binding of PRC1 alone inhibits ATP-dependent nucleosome remodeling on chromatin templates in vitro.16 In this way, repression by the Polycomb complexes is self-reinforcing, since the presence of one repressive histone modification leads to the placement of another.
Several histone modifications activate transcription by increasing the rate of transcription initiation or elongation. For example, acetylation of multiple lysines within the N-terminal tails of all four core histones plays a role in transcriptional activation.17 TAF1 (also known as TAFII250), a component of TFIID, contains two bromodomains, which directly bind acetylated histone tails as well as acetylated transcription factors.18 Therefore, acetylated histones can directly recruit the transcriptional machinery. In addition, the Swi2/Snf2 bromodomain of SWI/SNF complex also binds acetylated histones, enriching SWI/SNF binding at acetylated promoters.19 The SWI/SNF complex disrupts histone-DNA contacts within nucleosomes, causing nucleosome sliding or eviction of histones, which leads to greater access of transcriptional machinery to DNA.20 Another mark associated with transcriptional activation is H3K4me3, catalyzed by Set1-family methyltransferase complexes.21–23 As with histone acetylation, H3K4me3 can directly recruit the general transcription machinery through the TAF3 subunit of TFIID, which binds H3K4me3 through its PHD (plant homeodomain) finger.24 Furthermore, PHD fingers within nucleosome remodeling complexes such as NURF can also bind this mark.25 In sum, proteins that bind histone modifications at promoters and enhancers can activate or repress gene expression. In addition, the numerous possible combinations of histone modifications at each gene may allow transcriptional outputs to be fine-tuned by a variety of inputs.26
A “Bivalent” Pattern of Histone Modifications Marks many Developmental Genes in ESCs
Stem cells are broadly defined by two properties, the ability to divide as stem cells indefinitely (self-renewal) and the ability to differentiate into some (adult stem cells) or all (ESCs) cell types of the adult organism. Stem cells have a unique problem: they must stably maintain their normal gene expression pattern through self-renewing cell divisions, but must also be flexible enough to alter their gene expression pattern if they receive appropriate differentiation signals. ESCs are capable of differentiating into many different cell types in vitro, meaning that their choice is not merely between the ESC and one type of “differentiated cell” gene expression pattern; rather they must choose among gene expression patterns specific for ESCs, neuroblasts, primitive endoderm, mesenchymal cells, and other cell types.
One mechanism by which mammalian cells deal with the conflicting requirements of maintaining stability and flexibility of their transcriptome is by superimposing both activating and repressive histone modifications at key developmental regulators. Many developmental regulators and other differentiation-induced genes are silent in ESCs, and are induced when cells are directed down a particular lineage. When induced during differentiation, these factors contribute to lineage specification by activating or repressing downstream lineage-specific gene expression. In ESCs, the promoters for genes encoding many developmental regulators are marked with both activating (H3K4me3) and repressive (H3K27me3) histone modifications and are termed “bivalent”.27,28 Although these genes are silenced in ESCs, they are thought to be primed for activation should the cell commit to a lineage in which their expression is necessary. This transcriptional priming model is consistent with the known roles of Polycomb Group (PcG) and Trithorax Group (trxG) mutants in developmental gene regulation.29 Members of the PcG family (to which PRC2 belongs) were initially identified in D. melanogaster as repressors of homeotic gene expression, and this function is conserved in mammals. Conversely, members of the trxG family (to which Set1-family genes belong) were identified as suppressors of PcG genes and activate homeotic gene expression.
Consistent with the hypothesis that bivalent genes are primed for expression, genes encoding a number of developmental regulators in human ESCs (hESCs) initiate transcription, but fail to elongate full length transcripts.30,31 During differentiation, the repressive H3K27me3 mark is lost in cells of the appropriate lineage, and full-length transcripts can accumulate. On the other hand, in differentiated cells in which a particular developmental regulator is not expressed, the activating H3K4me3 mark is lost, and the repressive H3K27me3 mark accumulates.28
Tip60-p400 is Required for Normal ESC Self-renewal
In an RNAi screen for chromatin regulators important for mouse ESC self-renewal we identified several genes encoding components of the Tip60-p400 complex.32 Tip60-p400 is a 17 subunit chromatin remodeling complex33 with a lysine acetyltransferase (KAT) subunit (Tip60) and a Swi2/Snf2 family ATPase (p400) that exchanges canonical H2A for H2A variants in nucleosomes.
Tip60-p400 has well established roles in response to DNA damage, apoptosis and cell cycle regulation.34 Upon DNA damage, Tip60-p400 acetylates histones near the site of DNA damage and has been shown to exchange modified H2A variants for unmodified versions.35 Tip60-p400 also plays a role in activation of the DNA damage signaling protein ATM.36 In addition, Tip60-p400 regulates expression of the cell cycle inhibitors p14ARF and p21, acting primarily through the p53 pathway.37–41 Tip60 also acts at a second step of the p53 pathway, interfering with p53 turnover by Mdm2.37 Finally, Tip60 has been shown to be a haplo-insufficient tumor suppressor, due to its requirement for the oncogene-induced DNA damage response.42
Recently, we reported that ESCs knocked down for members of Tip60-p400 complex exhibit several prominent phenotypes reminiscent of differentiated cells. Tip60-p400 knockdown ESCs have a flattened and elongated cellular morphology, altered cell-cell adhesion, defects in teratoma formation, weak alkaline phosphatase activity, and a reduction in proliferation rate.32 Together, these phenotypes indicate that Tip60-p400 is necessary for normal ESC self-renewal.
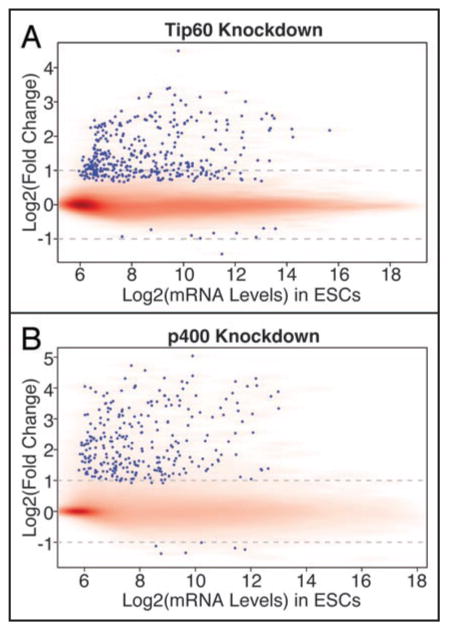
The Tip60 KAT has been shown to function as a coactivator of transcription in conjunction with sequence-specific transcriptional activators.34 In addition, Tip60-catalyzed acetylation modulates the function of non-histone proteins including p53, Rb and others.39,43,44 To our surprise, we found that Tip60-p400 complex appears to function primarily as a transcriptional repressor in ESCs.32 In Tip60 or p400 knockdown ESCs, a large number of genes are upregulated. Closer inspection of upregulated genes reveals a bias toward genes that are normally expressed at low levels in ESCs (Fig. 1), including a significant proportion of developmental regulators. Therefore, Tip60-p400 may play a role in the silencing of genes primed for expression during differentiation.
Figure 1.
Knockdown of Tip60 or p400 results mainly in derepression of normally silent genes. Density scatterplots of the transcriptional changes (Log2 scale) upon knockdown of Tip60 (A) or p400 (B) as a function of gene expression level in mouse ESCs (Log2 of mRNA hybridization signal by microarrray). Blue spots mark genes with high significance (adjusted p < 0.05). Dotted lines mark two-fold changes in gene expression in both directions.
To determine which genes are directly targeted by Tip60-p400 we performed promoter location analysis of the p400 subunit using ChIP-chip. We found that Tip60-p400 localizes to over half of promoters in ESCs, including those of both strongly and weakly expressed genes, but with the strongest enrichment at those that are highly expressed.32 This result was unexpected since the largest effect of Tip60/p400 knockdown is to increase expression of genes that are normally weakly expressed, suggesting that Tip60/p400 acts as a repressor. This apparent contradiction may be explained by the observation that p400 binding closely mirrors the H3K4me3 mark, which is found at both highly expressed genes as well as “bivalent” genes that also have the PRC2-catalyzed mark, H3K27me3. When H3K4me3 is reduced by knocking down Ash2l, a subunit of Set1-family methyltransferase complexes, p400 binding to its target genes is also reduced. Furthermore, it is likely that Tip60-p400 complex binds H3K4me3 directly, since one of its subunits, Ing3, contains a PHD finger that binds peptides with this mark in vitro.45 Ing3 is a member of the ING family of tumor suppressor proteins implicated in a number of different cancers.46 The PHD domains of ING family members bind methylated H3K4, with the strongest binding to H3K4me3.45 Interestingly, the H3K4me3-binding Ing2 protein has also been implicated in transcriptional repression, via recruitment of the Sin3a-HDAC1 complex.45 In sum, Tip60-p400 binds the promoters of active genes and silent genes that are primed for expression, but its role in transcriptional regulation in ESCs appears to be mainly in silencing.
Tip60-p400 Acetylates the Same Target Genes it Represses in ESCs
The Tip60 KAT is involved in transcriptional activation at a number of loci. Furthermore, histone acetylation is an important step in transcriptional activation of many genes. Why, then, is the primary response of Tip60-p400 knockdown in ESCs upregulation of genes that are normally silent? One possibility is that Tip60-p400 does not acetylate histones at these genes and instead acts through independent mechanisms to repress transcription. However, acetylation of the H4 N-terminal tail decreases in Tip60 or p400 knockdown ESCs at targets repressed by Tip60-p400.32 Therefore, Tip60-p400 appears to acetylate histones at all of its target genes, both expressed and silent. What, if any, role histone acetylation by Tip60-p400 plays in gene silencing will be discussed below.
In addition to its KAT function, Tip60-p400 has activity to exchange H2A for H2AZ or H2AX via the p400 subunit.35,47–49 It is therefore possible that the primary function of Tip60-p400 in ESC self-renewal is to mediate H2A/H2A-variant exchange at its target genes. However, H2AX−/− ESCs exhibit normal self-renewal and do not have any of the phenotypes observed upon Tip60-p400 knockdown.32,50 Furthermore, H2AZ knockdown does not phenocopy that of Tip60-p400 subunits,32 suggesting that histone exchange may not be the most important function of this complex in ESC self-renewal.
Models for Binding and Function of Tip60-p400 Complex in ESCs
Although H3K4me3 is required for optimal binding of Tip60-p400 to its chromatin targets in ESCs, it is not the only binding determinant. We also found that knockdown of the ESC transcription factor Nanog decreases p400 binding at its targets. While Tip60 has been shown to be recruited to a number of target genes through direct interactions with transcription factors, Nanog does not appear to directly recruit Tip60-p400.32 Despite significant overlap between genes regulated by Nanog and Tip60-p400, there are many genes regulated by only one or the other. Furthermore, Nanog knockdown also reduces p400 binding at genes not directly bound by Nanog. Therefore, Nanog appears to act indirectly to promote binding of Tip60-p400 to chromatin. We found that Nanog knockdown did not affect H3K4me3 and vice versa, suggesting that Nanog and H3K4me3 are independent determinants of Tip60-p400 binding (Fig. 2). Nanog-dependent binding of Tip60-p400 could occur indirectly through expression of another transcription factor that directly binds Tip60-p400 (Fig. 2A), of a chromatin-modifying enzyme that catalyzes a mark that binds Tip60-p400 in addition to Set1-complex catalyzed H3K4me3 (Fig. 2B), or of an enzyme which directly modifies the Tip60-p400 complex, allowing chromatin interaction (Fig. 2C).
Figure 2.

Models of Nanog- and H3K4me3-dependent binding of Tip60-p400 to its chromatin targets. (A) Transcription factor model. In this model, Nanog induces expression of a transcription factor (TF) that, along with H3K4me3 (deposited by a Set1-family histone methyltransferase, or HMTase), recruits Tip60-p400 to its target genes. (B) Dual-histone modification model. In this model, the requirement for Nanog is due to its role in expression of a histone modifying enzyme that places a mark bound by Tip60-p400 complex. Both this mark and H3K4me3 are required for Tip60-p400 binding. (C) Tip60-p400 post-translational modification model. Here, Nanog induces expression of a signaling protein (SP) that adds a post-translational modification to one or more subunits of Tip60-p400, which (along with H3K4me3) allows the complex to bind its chromatin targets.
How does Tip60-p400 repress transcription of its target genes in ESCs when its KAT activity would seem to act counter to this purpose? At least two possibilities might explain this apparent contradiction (Fig. 3). First, in addition to histone tails, Tip60 might acetylate one or more unknown transcriptional repressors, and this modification might be necessary for their binding to chromatin and/or repressor activity. In this model, histone acetylation is either ineffective in contributing to transcriptional activation, or the effect of histone acetylation is overcome by the hypothetical repressor(s) (Fig. 3A). A second possibility is that the transcriptional read-out of acetylated histone tails—in the presence of additional histone modifications—may be repression rather than activation. This model predicts the presence of a transcriptional repressor or co-repressor complex that contains one or more bromodomains in combination with a binding module for specific DNA sequences or additional chromatin marks (Fig. 3B). The repressor would bind specifically to chromatin with both histone acetylation and another chromatin mark, to ensure selectivity.26
Figure 3.

Possible mechanisms for Tip60-p400-mediated repression. (A) Acetylation-mediated activation of a repressor. Tip60-p400, upon recruitment to a target gene, acetylates (green triangle) and activates a transcriptional repressor (TR), which directly represses the target gene. Tip60-p400 also acetylates nearby histone tails, but these marks are ineffective in activation of transcription, or their effects are simply overcome by the repressor protein. (B) Dual-mode repressor binding. This model posits a transcriptional repressor with domains allowing it to bind both acetylated histone tails and a second chromatin modification (or alternatively, a specific DNA sequence). Both marks are predicted to be necessary for repressor binding, imparting specificity, and allowing a normally activating chromatin mark (acetylation) to serve as a repressive mark in this limited context. HMC: histone modifying complex.
Tip60-p400 adds to the growing list of chromatin and transcriptional regulators important for ESC self-renewal and/or pluripotency. In addition, the surprising role of Tip60-p400 in repression of differentiation-induced genes raises a number of questions. What is the role of H2A variant exchange in ESCs? H2AZ in yeast plays a role in “transcriptional memory,”51 suggesting that H2AZ knockdown in ESCs might exhibit a stronger phenotype upon a change in growth conditions, perhaps during differentiation. In addition, it will be important to test whether the KAT activity of Tip60-p400 is essential for its repression activity by testing the phenotype of a catalytically inactive Tip60 subunit. Furthermore, how is Tip60-p400 mediated repression reversed during differentiation? Since Tip60-p400 indirectly requires Nanog for chromatin binding in ESCs, down-regulation of Nanog during differentiation is one obvious possibility. In addition, changes in histone modifications during differentiation may also alter the array of Tip60-p400 targets. Finally, is Tip60-p400 required for transcription factor-mediated reprogramming of differentiated cells into induced pluripotent stem (iPS) cells? In hESCs, NANOG has been shown to facilitate iPS cell formation, in conjunction with other transcription factors.52 Since mouse Nanog is required for Tip60-p400 to bind chromatin, Tip60-p400 could play a role in re-establishment of the ESC-specific gene expression pattern during cellular reprogramming.
Acknowledgments
We thank J. Benanti for comments on the manuscript. We also thank J.M. Bishop for advice and assistance. T.G.F. was a fellow of the Jane Coffin Childs Memorial Fund for Medical Research and is funded by an NCI training grant (T32CA09043) to UCSF. J.T.H. is funded by a UCSF NIH Genetics Training Grant.
Abbreviations
- ESC
embryonic stem cell
- Pc
polycomb
- PRC2
polycomb repressive complex 2
- PRC1
polycomb repressive complex 1
- KAT
lysine acetyltransferase
- TF
transcription factor
- H3K4me3
histone H3 trimethylated on lysine 4
- H3K27me3
histone H3 trimethylated on lysine 27
- H3K9me3
histone H3 trimethylated on lysine 9
- PHD
plant homeodomain
References
- 1.Gelbart ME, Bachman N, Delrow J, Boeke JD, Tsukiyama T. Genome-wide identification of Isw2 chromatin-remodeling targets by localization of a catalytically inactive mutant. Genes Dev. 2005;19:942–54. doi: 10.1101/gad.1298905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehouse I, Rando OJ, Delrow J, Tsukiyama T. Chromatin remodelling at promoters suppresses antisense transcription. Nature. 2007;450:1031–5. doi: 10.1038/nature06391. [DOI] [PubMed] [Google Scholar]
- 3.Whitehouse I, Tsukiyama T. Antagonistic forces that position nucleosomes in vivo. Nat Struct Mol Biol. 2006;13:633–40. doi: 10.1038/nsmb1111. [DOI] [PubMed] [Google Scholar]
- 4.Fazzio TG, Tsukiyama T. Chromatin remodeling in vivo: evidence for a nucleosome sliding mechanism. Mol Cell. 2003;12:1333–40. doi: 10.1016/s1097-2765(03)00436-2. [DOI] [PubMed] [Google Scholar]
- 5.Lucchesi JC, Kelly WG, Panning B. Chromatin remodeling in dosage compensation. Annu Rev Genet. 2005;39:615–51. doi: 10.1146/annurev.genet.39.073003.094210. [DOI] [PubMed] [Google Scholar]
- 6.Nightingale KP, O’Neill LP, Turner BM. Histone modifications: signalling receptors and potential elements of a heritable epigenetic code. Curr Opin Genet Dev. 2006;16:125–36. doi: 10.1016/j.gde.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14:1025–40. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–9. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 9.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–20. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 10.Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–4. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 11.Zhang CL, McKinsey TA, Olson EN. Association of class II histone deacetylases with heterochromatin protein 1: potential role for histone methylation in control of muscle differentiation. Mol Cell Biol. 2002;22:7302–12. doi: 10.1128/MCB.22.20.7302-7312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson JP, Lindroth AM, Cao X, Jacobsen SE. Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature. 2002;416:556–60. doi: 10.1038/nature731. [DOI] [PubMed] [Google Scholar]
- 13.Seum C, Delattre M, Spierer A, Spierer P. Ectopic HP1 promotes chromosome loops and variegated silencing in Drosophila. Embo J. 2001;20:812–8. doi: 10.1093/emboj/20.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–8. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 16.Shao Z, Raible F, Mollaaghababa R, Guyon JR, Wu CT, Bender W, et al. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell. 1999;98:37–46. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- 17.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson RH, Ladurner AG, King DS, Tjian R. Structure and function of a human TAFII250 double bromodomain module. Science. 2000;288:1422–5. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- 19.Hassan AH, Prochasson P, Neely KE, Galasinski SC, Chandy M, Carrozza MJ, et al. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell. 2002;111:369–79. doi: 10.1016/s0092-8674(02)01005-x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson CN, Adkins NL, Georgel P. Chromatin remodeling complexes: ATP-dependent machines in action. Biochem Cell Biol. 2005;83:405–17. doi: 10.1139/o05-115. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P, et al. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol Cell. 2001;8:1207–17. doi: 10.1016/s1097-2765(01)00405-1. [DOI] [PubMed] [Google Scholar]
- 22.Roguev A, Schaft D, Shevchenko A, Pijnappel WW, Wilm M, Aasland R, et al. The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. Embo J. 2001;20:7137–48. doi: 10.1093/emboj/20.24.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briggs SD, Bryk M, Strahl BD, Cheung WL, Davie JK, Dent SY, et al. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 2001;15:3286–95. doi: 10.1101/gad.940201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeulen M, Mulder KW, Denissov S, Pijnappel WW, van Schaik FM, Varier RA, et al. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell. 2007;131:58–69. doi: 10.1016/j.cell.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Soliman MA, Riabowol K. After a decade of study-ING, a PHD for a versatile family of proteins. Trends Biochem Sci. 2007;32:509–19. doi: 10.1016/j.tibs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–94. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–8. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 29.Francis NJ, Kingston RE. Mechanisms of transcriptional memory. 2001;2:409–21. doi: 10.1038/35073039. [DOI] [PubMed] [Google Scholar]
- 30.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stock JK, Giadrossi S, Casanova M, Brookes E, Vidal M, Koseki H, et al. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–35. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- 32.Fazzio TG, Huff JT, Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134:162–74. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Y, Jin J, Florens L, Swanson SK, Kusch T, Li B, et al. The mammalian YL1 protein is a shared subunit of the TRRAP/TIP60 histone acetyltransferase and SRCAP complexes. J Biol Chem. 2005;280:13665–70. doi: 10.1074/jbc.M500001200. [DOI] [PubMed] [Google Scholar]
- 34.Squatrito M, Gorrini C, Amati B. Tip60 in DNA damage response and growth control: many tricks in one HAT. Trends Cell Biol. 2006;16:433–42. doi: 10.1016/j.tcb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates JR, 3rd, et al. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science. 2004;306:2084–7. doi: 10.1126/science.1103455. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci USA. 2005;102:13182–7. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legube G, Linares LK, Tyteca S, Caron C, Scheffner M, Chevillard-Briet M, et al. Role of the histone acetyl transferase Tip60 in the p53 pathway. J Biol Chem. 2004;279:44825–33. doi: 10.1074/jbc.M407478200. [DOI] [PubMed] [Google Scholar]
- 38.Park JH, Roeder RG. GAS41 is required for repression of the p53 tumor suppressor pathway during normal cellular proliferation. Mol Cell Biol. 2006;26:4006–16. doi: 10.1128/MCB.02185-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyteca S, Vandromme M, Legube G, Chevillard-Briet M, Trouche D. Tip60 and p400 are both required for UV-induced apoptosis but play antagonistic roles in cell cycle progression. Embo J. 2006;25:1680–9. doi: 10.1038/sj.emboj.7601066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell cycle arrest and apoptosis. Mol Cell. 2006;24:827–39. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Gevry N, Chan HM, Laflamme L, Livingston DM, Gaudreau L. p21 transcription is regulated by differential localization of histone H2A. Z Genes Dev. 2007;21:1869–81. doi: 10.1101/gad.1545707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorrini C, Squatrito M, Luise C, Syed N, Perna D, Wark L, et al. Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature. 2007;448:1063–7. doi: 10.1038/nature06055. [DOI] [PubMed] [Google Scholar]
- 43.Sykes SM, Mellert HS, Holbert MA, Li K, Marmorstein R, Lane WS, McMahon SB. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–51. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leduc C, Claverie P, Eymin B, Col E, Khochbin S, Brambilla E, et al. p14ARF promotes RB accumulation through inhibition of its Tip60-dependent acetylation. Oncogene. 2006;25:4147–54. doi: 10.1038/sj.onc.1209446. [DOI] [PubMed] [Google Scholar]
- 45.Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature. 2006;442:96–9. doi: 10.1038/nature04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker LA, Allis CD, Wang GG. PHD fingers in human diseases: Disorders arising from misinterpreting epigenetic marks. Mutat Res. 2008 doi: 10.1016/j.mrfmmm.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303:343–8. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 48.Krogan NJ, Keogh MC, Datta N, Sawa C, Ryan OW, Ding H, et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol Cell. 2003;12:1565–76. doi: 10.1016/s1097-2765(03)00497-0. [DOI] [PubMed] [Google Scholar]
- 49.Kobor MS, Venkatasubrahmanyam S, Meneghini MD, Gin JW, Jennings JL, Link AJ, et al. A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A. Z into euchromatin PLoS Biol. 2004;2:131. doi: 10.1371/journal.pbio.0020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bassing CH, Chua KF, Sekiguchi J, Suh H, Whitlow SR, Fleming JC, et al. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc Natl Acad Sci USA. 2002;99:8173–8. doi: 10.1073/pnas.122228699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brickner DG, Cajigas I, Fondufe-Mittendorf Y, Ahmed S, Lee PC, Widom J, et al. H2A. Z-Mediated Localization of Genes at the Nuclear Periphery Confers Epigenetic Memory of Previous Transcriptional State. PLoS Biol. 2007;5:81. doi: 10.1371/journal.pbio.0050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]