Summary
Immunodeficient mice engrafted with human immune systems provide an exciting model to study human immunobiology in an in vivo setting without placing patients at risk. The essential parameter for creation of these “humanized models” is engraftment of human hematopoietic stem cells (HSC) that will allow optimal development of human immune systems. However there are a number of strategies to generate humanized mice and specific protocols can vary significantly among different laboratories. Here we describe a protocol for the co-implantation of human HSC with autologous fetal liver and thymic tissues into immunodeficient mice to create a humanized model with optimal human T cell development. This model, often referred to as the Thy/Liv or BLT (bone marrow, liver, thymus) mouse, develops a functional human immune system, including HLA-restricted human T cells, B cells and innate immune cells.
Keywords: Hematopoietic stem cells, SCID, Thymus, HSC, humanized mice, BLT
1. Introduction
The study of human immunobiology is constrained by ethical and logistical concerns of working with patients (1, 2). Given these complications, our basic understanding of many fundamental biological processes in humans has been shaped by experimental studies in animal models, particularly in rodents. However, many characteristics and properties of mammalian biological systems, particularly mouse and human immune systems, are species-specific, and these species differences often limit the translation of experimental findings from rodent studies to the clinic (3–5). Moreover the use of non-human primates for basic research in Europe and United States is severely restricted (6). Immunodeficient mice that are engrafted with human hematopoietic stem cells (HSC) and that develop human immune systems or “humanized mice” are an attractive alternative to study immunobiology (7–9).
The ideal mouse strains for engraftment of human HSC are immunodeficient stocks of scid, Rag1null or Rag2null mice (9) that express targeted mutations within the IL-2 receptor common gamma chain (IL2rγ) gene. The IL2rγ chain is a critical component of the IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 receptors (10, 11). The absence of this IL2rγ chain leads to severe impairments in adaptive immune system development and function and completely prevents NK cell development (12, 13). Immunodeficient mice bearing a mutated IL2rγ gene support greatly enhanced engraftment of human HSC (13) and fetal thymic tissues (14) as compared with previous models.
Humanized mice can be generated using human HSC derived from a number of sources, including umbilical cord blood, G-CSF mobilized peripheral blood, bone marrow and fetal liver (15). NOD-PrkdcscidIL2rgTm1Wjl (NSG) mice engraft efficiently with human HSC and give rise to functional human immune systems (16, 17). However human T cell development in immunodeficient mice injected with HSC is hindered by the absence of human thymic epithelium that is required for T cell education (18). This problem can be overcome by co-implanting human fetal thymic tissues with autologous fetal liver derived HSC (19). This model, originally referred to as the SCID-hu mouse, has been modified over recent years and allows for optimal generation of functional human T cells that are HLA-restricted (20–23). This chapter describes a current technique to generate these Thy/Liv or “BLT” (bone marrow, liver thymus) mice as practiced by our lab.
2. Materials
2.1 Fetal tissue preparation
Wash Buffer: RPMI 1640 (Gibco, Life technologies, Grand Island, NY USA) supplemented with 1% Penicillin/Streptomycin (Gibco, Life Technologies, Grand Island, NY USA), 2.5 µg/mL Fungizone (Gibco, Life Technologies, Grand Island, NY USA) and 10 µg/mL Gentamicin (APP Pharmaceuticals, Lake Zurich, IL, USA). Keep washing buffer sterile and chilled on ice. The buffer can be stored at 4°C
Quenching Buffer: Wash Buffer supplemented with 3% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA, USA)
Human fetal liver and fetal thymus tissue (Advanced Bioscience Resources, Alameda, CA). (See NOTE 1)
100 mm plastic petri dishes (BD Falcon, Franklin Lakes, NJ, USA), sterile
No. 21 disposable scalpel (Feather Safety Razor Co., LTD, Kita-Ku, Osaka, Japan)
50mL centrifuge tubes (BD Falcon, Franklin Lakes, NJ, USA)
Liver digest buffer (Gibco, Life technologies, Grand Island, NY USA)
Water bath
Parafilm
Nutating mixer (VWR international, Randor, PA, USA)
Laboratory tape or alternatively rubber bands
85mm tissue grinder homogenizer cup with size 50 metal sieve (Sigma-Aldrich, St Louis, MO, USA)
10mm Syringe
Histopaque-1077 (Sigma-Aldrich, St Louis, MO, USA)
2.2 CD3 T cell Depletion
Human CD3 microbead kit (Miltenyi Biotech, Auburn, CA, USA) (See NOTE 2)
MidiMACS Separator (Miltenyi Biotech, Auburn, CA, USA)
MACS multistand (Miltenyi Biotech, Auburn, CA, USA)
LD columns (Miltenyi Biotech, Auburn, CA, USA)
MACS Buffer: PBS supplemented with 2% FBS, 1mM EDTA. Sterilize buffer through a vacuum flask with a 0.2µm filter.
2.3 Flow cytometry analysis
FACS buffer: phosphate buffered saline (PBS) supplemented with 1% FBS, and 0.1% Sodium azide. (See NOTE 3)
Human CD29 FITC, Clone K20 (Beckman Coulter, Marseille, France,)
HLA-A2 FITC, Clone BB7.2 (BD Biosciences, San Jose, CA, USA)
Human CD29 PE, Clone MAR4 (BD Biosciences, San Jose, CA, USA)
Human CD34 PE, Clone 581 (BD Biosciences, San Jose, CA, USA)
Human CD3 PE, Clone UCHT1 (BD Biosciences, San Jose, CA, USA)
Human CD45 APC, Clone HI30 (BD Biosciences, San Jose, CA, USA)
Human CD45 APC-H7, Clone 2D1 (BD Biosciences, San Jose, CA, USA)
Human CD3 FITC, Clone UCHT1 (BD Biosciences, San Jose, CA, USA)
Human CD20 APC, Clone 2H7 (BD Biosciences, San Jose, CA, USA)
Human CD4 Pacific Blue, Clone RPA-T4 (BD Biosciences, San Jose, CA, USA)
Human CD8 PE, PTA-T8 (BD Biosciences, San Jose, CA, USA)
Mouse Ly5 PerCP, Clone 30-F11 (BD Biosciences, San Jose, CA, USA)
2.4 Reagents and materials for tissue implant
Immunodeficient mice: Our laboratory primarily uses NOD-Prkdcscid IL2rgTm1Wjl (NSG) mice between 8 to 12 weeks of age. (See NOTE 4)
Ketamine/Xylene solution (store at 4°C), Working concentration is 15mg/mL of Ketamine and 2mg/mL of xylene, (Hospira, Lake Forest, IL, USA) diluted in PBS. Dosage per mouse is 150mg/kg and 10mg/kg of body weight, respectively.
Buprinex SR (slow release), 1mg/ml (Zoopharm, Laramie, WY, USA). Dosage per mouse is 2.4mg/kg. Store at 4°C.
Cefazolin/Gentamicin (Sandoz, Princeton, NJ, USA / APP Pharmaceuticals, Lake Zurich, IL, USA): Working concentration is 8.3mg/mL of cefazolin and 2mg/mL gentamicin. Dosage per mouse is 0.83mg and 0.2mg, respectively. Store working stock in 1 ml aliquots of at −20°C.
Sterile surgical draping
Betadine surgical scrub: 10% Povidone-iodine (Purdue Pharma L.P., Stamford, CT, USA)
70% ethanol: Mix 70 mL of absolute ethanol with 30 mL of deionized water
Sterile physiological (o.85%) saline: 10 mL bottle
100-mm plastic petri dishes (BD Falcon, Franklin Lakes, NJ, USA), sterile
30-mm plastic petri dishes (BD Falcon, Franklin Lakes, NJ, USA), sterile
Ohaus portable balance (Fisher Scientific, Pittsburgh, PA, USA)
Sterile Curity gauze sponges 4” × 4” (Covidien, Mansfield, MA, USA)
No. 21 disposable scalpel
Electric clipper with size 40 blade
10 mL syringe with 20-G needle
1 mL syringe with 25-G needle
2.4 Surgical instruments: sterilized by placing in an instant sealing sanitization pouch (Fisher Scientific, Pittsburgh, PA, USA) and autoclaving before use
Micro-dissecting forceps curved (small)
Dressing forceps (large)
Operating scissors: straight, sharp (Large)
Micro dissecting scissors: straight, sharp (small)
16-G Trocar
Olsen-Hegar needle holder
Surgical suture
Autoclip wound clip applicator and 9-mm autoclips
3. Method
This protocol involves the extensive manipulation of human tissues and cells. Extreme care should be taken when handling the specimens and all work should be done in a standard laminar flow hood. All unused tissues and waste should be disposed of using protocols approved by an Institutional Biosafety Committee. All mouse surgeries should be done using protocols approved by an Institutional Animal Care and Use Committee.
3.1 Fetal tissue preparation
Place fetal liver and thymus tissues into 100mm plastic petri dishes with 10mL of Wash Buffer. Reserve medium that tissues were shipped in to combine with liver preparation (see below). Using a scalpel cut one piece of liver and one piece of thymus of sufficient size to prepare the quantity of 1mm3 pieces needed to implant all recipient mice. Place pieces in separate 50 mL conical tubes in 30 mL of Wash Buffer (See Step 3.3.3 below) and keep on ice until transplant (See NOTE 5). In addition, dissect 1mm3 pieces of liver and thymus for optional flow cytometric analysis to evaluate HLA-A2 status (See 3.4.1). Dissect the remaining fetal liver tissue into 4 to 5 mm3 pieces that are of sufficient size to pass through the bore of a 25 mL pipette. Combine liver pieces and Wash Buffer with reserved liver shipping medium into two 50 mL tubes and centrifuge (400 × g for 10 min at 4°C).
Thaw Liver Digest Buffer at room temperature. Resuspend liver tissue pellets from both tubes in a total of 25 mL of Liver Digest Buffer and combine into one 50 ml conical tube. Seal tube lid tightly with parafilm. Place the 50ml conical tube with digesting liver tissues on a Nutator in a 37°C incubator for 20 to 25 minutes. Secure tube to platform with laboratory tape or rubber bands.
Decant contents into a size 50 mesh in the 85 mm tissue grinder homogenizer cup that is sitting in a fresh 100 mm petri dish. Using a 10 mL syringe plunger, homogenize tissue through the sieve. Rinse the sieve well with 25 mL of Quenching Buffer to stop digestion reaction and split volume equally in two 50 mL conical tubes (approximately 60 mL total volume) (See NOTE 6).
Allow cell suspension to settle for 3 min to preferentially pellet the fetal liver cells Each tube should have a large pellet that is brown in color and that contains primarily nucleated cells and some RBCs. Carefully remove supernatant (leukocyte rich) without disturbing the hepatocyte pellet and spit volume equally into four fresh 50 mL conical tubes. Bring total volume to 30 mL per tube using Quenching Buffer.
Underlay with 12 mL of histopaque in each tube by slowly pipetting the histopaque into bottom of tube. Ensure a clear interface is maintained between the histopaque and the cell-containing medium. Centrifuge the layered tubes at 400 × g for 30 minutes at 18 to 20°C.
Carefully harvest cells along the interface, collecting all visible cells. Transfer interface cells to fresh 50 mL conical tubes and add at least 4 volumes of Quenching Buffer. Centrifuge the tubes at 100 × g for 10 minutes at 18 to 20°C. Resuspend the pellets in a total volume of 10 mL Quenching Buffer and determine viable cell number. Keep recovered cells on ice. Reserve 200 µL of cells for flow cytometric evaluation (See 3.4).
3.2 Depletion of CD3+ T cells from fetal liver hematopoietic cells. For the depletion step we use reagents from Miltenyi Biotech and following the manufactures recommendations (See NOTE 3)
Resuspend recovered cells in 1mL RPMI and perform viable cell counts.
Reserve 100,000 cells for flow cytometric analysis to validate depletion of CD3 T cells and to quantify the CD34+ cells. (See Section 3.4.2 and 3.4.3)
Based on results of CD34+ cell analysis, resuspend recovered liver cells at 4.0 × 105 CD34+ cells per mL in Wash Buffer. Keep cells on ice until injection.
3.3 Preparation of mice for surgery and tissue implant
Irradiate recipient mice by whole body gamma irradiation (a 137Cs radioactive source is most commonly used). For the NSG mouse strain, our laboratory use a conditioning dose of 200 cGy, which is normally well tolerated by adult NSG mice. Tissues implant can be performed immediately after irradiation. However HSC injection must be performed at four hour to 24 hours after irradiation(See NOTE 7).
Using fetal thymic and liver pieces from step 3.1.1 above, place the tissues and medium into a 100 mm petri dish. Dissect fetal tissues into 1mm3 pieces using a scalpel.
Determine mouse weight using balance and use ear punch to give each mouse a unique identifier.
Inject each mouse with 0.1 ml of cefazolin/gentamicin antibiotic mixture subcutaneously (See NOTE 8).
Anesthetize mice by intraperitoneal injection of ketamine/xylene solution at a dose of 150mg/kg and 10mg/kg, respectively. When mice are fully anesthetized, proceed to the next step. This generally takes between 10 to 15 minutes.
Place anesthetized mouse on sterile draping with left side facing up. Shave the left side of the mouse from the level of the shoulder joint to that of the hip joint with an electric clipper. Scrub the left side of the mouse 6 times alternating between betadine and 70% alcohol (Figure 1A).
Use a pair of operating scissors and dressing forceps to make a left-side skin incision (1.5 cm long) ventral to the spine and between the last rib and the hip joint. Loosen connective tissue under the skin using the blunt side of the operating scissors. Make a 0.5-cm incision in a longitudinal direction in the abdominal wall (through muscle wall) (Figure 1B).
Using a pair of straight micro-dissecting forceps, grasp the fatty tissue at the base of the kidney and gently expose the kidney through the incision. Do not apply direct pressure to the kidney to prevent damaging the organ. Secure the kidney by grasping the renal vessels and ureter just below kidney between the flaps of skin with a second pair of straight micro dissecting forceps. Throughout the surgical procedure, keep the exposed kidney moist, by frequently applying sterile PBS to tissue surface with a 10-mL syringe with an 18-G needle (Figure 1C).
Place the trocar plunger inside the trocar barrel. Using a pair of curved dissecting forceps, place a 1mm3 piece of fetal thymus on the tip of the trocar, and aspirate it inside by drawing back the plunger piece of the trocar. Place a 1mm3 piece of fetal liver and aspirate it inside with same technique.
Make a small (1 to 2 mm) incision in kidney capsule at the posterior lateral side with a scalpel. Insert the trocar loaded with human tissue as far as possible through the incision in the kidney capsule and staying as superficial as possible. The kidney capsule is delicate and caution should be taken to not rupture. Push the plunger piece of the trocar to expel the loaded tissues. Remove trocar from kidney. To prevent tissues from sliding out with the trocar, light pressure can be applied to the kidney and against the tissues using a pair of straight micro dissecting forceps as the trocar is removed (Figure 1D and 1E).
Gently pull apart the two cut edges of the muscular wall using two pairs of micro dissecting forceps. The kidney will retract into the peritoneal cavity. Close the abdominal wall with two separate sutures, using the Olsen-Hegar needle holder and surgical suture. Cut the ends of the suture and close the knots. Close the skin incision with three autoclips, using an autoclip wound-clip applicator (Figure 1F).
Once mice recover from the anesthesia and are active, dose each mouse with 2.4mg/kg buprenex SR (1 mg/mL) by intraperitoneal injection. Check mice daily for surgical would healing and remove autoclips, 5 to 7 days after surgery.
Inject 2×105 CD34+ fetal liver hematopoietic cells isolated earlier (See 3.2.3) through the lateral tail vein at least 4 hours after irradiation (see NOTE 9 – 10).
At 12 weeks after tissue transplants run a FACS analysis on the peripheral blood of the mice to validate human immune cell engraftment.
Figure 1. Surgical procedure for implantation of human fetal thymus and liver.
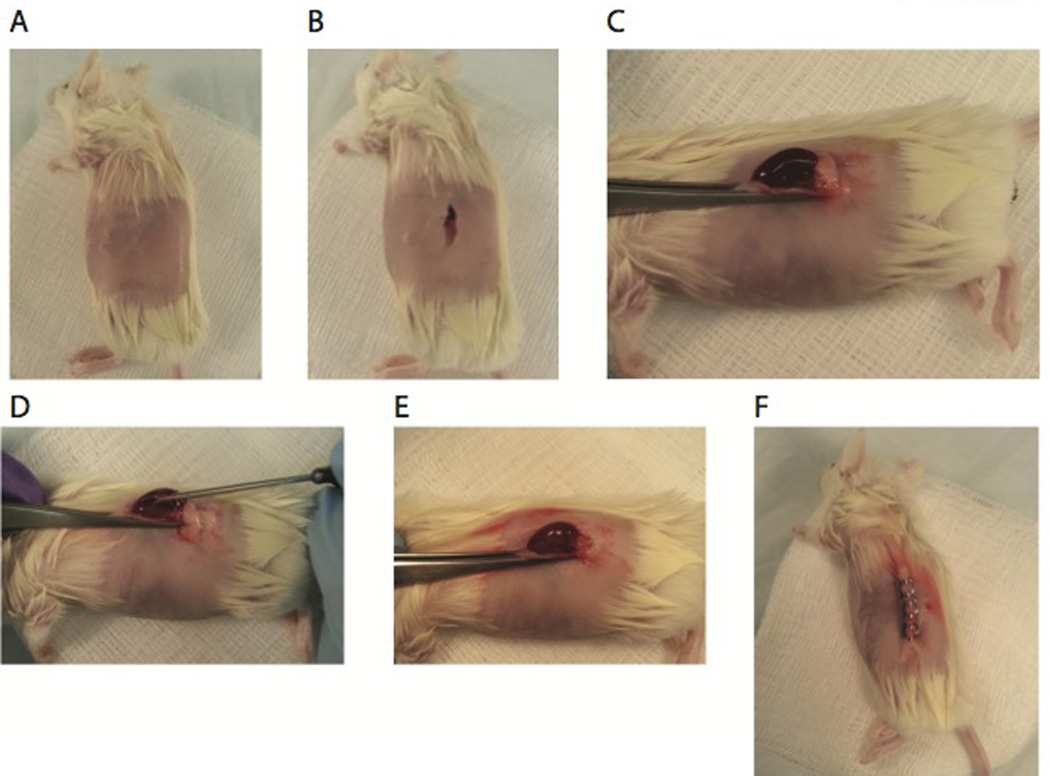
(A) Fur is removed from anesthetized NSG mice and (B) a 0.5-cm incision in a longitudinal direction in the abdominal wall (through muscle wall). (C) Using a pair of straight micro-dissecting forceps, expose kidney through the incision. (D) Secure the kidney by grasping the renal vessels and ureter just below kidney between the flaps of skin with a pair second pair of straight micro dissecting forceps. Make a small (1 to 2 mm) incision in kidney capsule at the posterior lateral side with a scalpel. Insert the trocar loaded with human tissue as far as possible through the incision in the kidney capsule and staying as superficial as possible. Push the plunger piece of the trocar to expel the loaded tissue. (E) Remove trocar from kidney. Gently pull apart the two cut edges of the muscular wall using two pairs of micro dissecting forceps. The kidney will retract into the peritoneal cavity. (E) Close the abdominal wall with two separate sutures, using the Olsen-Hegar needle holder and surgical suture. Cut the ends of the suture and close the knots. Close the skin incision with three autoclips, using an autoclip wound-clip applicator. (F) Mice were then placed on a warming tray and observed until they awoke from the anesthesia.
3.4. Flow cytometry analysis
For the optional HLA-A2 FACS, mechanically dissociate the saved liver and thymus tissue pieces to make a suspension, keeping tissues separate. Stain with anti-CD29 FITC (1:50) as a FITC control, and stain test sample with anti-HLA-A2 FITC (2:50).
To validate the CD3+ T cell depletion, stain the saved cells from section 3.2.3 with anti-CD45 PE (1:50) as PE control, anti-CD45 APC (1:50) as an APC control and stain the test sample with anti-CD3 PE (1:50) and anti-CD45 APC (1:50).
To determine the CD34+ cell levels, stain the saved cells with anti-CD45 PE (1:50) as PE control, anti-CD45 APC (1:50) as APC control and stain the test sample with anti-CD34 PE (1:50) and anti-CD45 APC (1:50).
To determine the engraftment of human lymphocytes, stain 120 µL of blood with the following antibody mixture:CD45 APC-H7 (1:100), CD3 FITC (1:50), CD20 APC (1:25), CD8 PE (1:50), CD4 Pacific Blue (1:50) and mLy5 PerCP (1:100) (Figure 2A–2D).
Figure 2. Evaluation of human cell chimerism.

At 12 weeks post-implant human immune cell chimerism levels can be evaluated in the peripheral blood. Our standard panel includes antibodies specific for mouse CD45, human CD45, human CD3, human CD20, human CD4 and human CD8. (A) The gating strategy for this panel includes first evaluating percentages of human CD45+ and mouse CD45+ cells. (B) Human CD45+ cells are evaluated for expression of human CD3 to enumerate T cells and human CD20 to enumerate B cells and (C) then the T cells populations are further defined by CD4 and CD8 expression. (D) In addition the human thymocytes recovered from the thymic organoid can be evaluated by expression of human CD4 and CD8.
Acknowledgements
We thank Jamie Kady, Meghan Dolan, Pamela St Louis, Linda Paquin, Michael Bates, Bruce Gott, and Allison Ingalls for excellent technical assistance. This work was supported by National Institutes of Health research grants AI046629 and grants from the Juvenile Diabetes Research Foundation, International and the Helmsley Charitable Trust. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
The gestational ages of the human tissues are between 16 and 20 weeks
Injection of mature human T cells into immunodeficient mice such as the NSG mice results in the development of a severe xenogeneic graft-versus-host disease (GVHD). For this reason we use a magnetic-bead based approach to deplete T cells from the liver cell preparation. The frequency of human T cells within the recovered cells generally falls between 0.3 and 0.5% of total cells. Alternatively CD34+ HSC can be isolated by a positive selection approach, which will exclude T cells and other cell types from the final cell preparation (21). We routinely use the CD3 depletion approach as accessory cells (CD34-negative and CD3 negative) present within the liver cell preparation have been shown to facilitate engraftment of human HSC in immunodeficient mice (19).
We substituted BSA for FBS.
NSG mice are available from the Jackson Laboratory. The mice are maintained on medicated (sulfamethoxazole/trimethoptrim) water to prevent infections. An alternative strain for engraftment is NOD-scid mice.
In our experience we generally receive tissues that are sufficient to implant 30 to 40 mice but the size of the tissues can be variable. It is critical to have sufficient liver tissue for HSC recovery. The recovery of HSC can vary with the gestational age of the tissues.
For optimal cell recovery from the liver tissues, ensure that liver has been completely disaggregated and that sieve has been extensively rinsed.
The specific dose of irradiation used will be dependent on the specific strain being used as a recipient. Mice homozygous for the scid mutation are radiosensitive, while Rag1null or Rag2null mice are more radioresistant and require higher irradiation doses for optimal conditioning. Each laboratory will need to determine the optimal dose based on the mouse strain and the specific irradiation source.
The use of antibiotics is at the discretion of the laboratory. However, procurement of the fetal tissues is not done under sterile conditions and in our experience, pre-treatment with antibiotics reduces the risk of infection and pre-mature death of engrafted mice.
Cryopreserving/thawing the fetal thymus before transplantation into the mice has been reported to support human thymopoiesis and T-cell reconstitution while clearing preexisting thymocytes from the thymic graft (24).
Another modification for generating BLT mice involves transplantation of the autologous fetal thymic and liver tissue under the renal capsules of the mice. After 3 weeks, the engrafted mice are sub-lethally irradiated and injected through their tail veil with the autologous cryopreserved/thawed CD34+ cells isolated from the fetal liver (23).
References
- 1.Davis MM. A prescription for human immunology. Immunity. 2008;29:835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis MM. Immunology taught by humans. Science translational medicine. 2012;4:117fs112. doi: 10.1126/scitranslmed.3003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 4.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol. 2011;33:67–87. doi: 10.1007/s00281-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 6.Wadman M. Time called on chimp work. Nature. 2013;495:289–290. doi: 10.1038/495289a. [DOI] [PubMed] [Google Scholar]
- 7.Brehm MA, Shultz LD, Greiner DL. Humanized mouse models to study human diseases. Current opinion in endocrinology, diabetes, and obesity. 2010;17:120–125. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brehm MA, Powers AC, Shultz LD, et al. Advancing animal models of human type 1 diabetes by engraftment of functional human tissues in immunodeficient mice. Cold Spring Harbor perspectives in medicine. 2012;2:a007757. doi: 10.1101/cshperspect.a007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 10.Sugamura K, Asao H, Kondo M, et al. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annual review of immunology. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 13.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 14.Stoddart CA, Maidji E, Galkina SA, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rgamma(−/−) (NSG) BLT mice. Virology. 2011;417:154–160. doi: 10.1016/j.virol.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shultz LD, Brehm MA, Garcia-Martinez JV, et al. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brehm MA, Cuthbert A, Yang C, et al. Parameters for establishing humanized mouse models to study human immunity: Analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rγnull mutation. Clinical Immunology. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brehm MA, Bortell R, Diiorio P, et al. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD-Rag1null IL2rgammanull Ins2Akita mice. Diabetes. 2010;59:2265–2270. doi: 10.2337/db10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shultz LD, Saito Y, Najima Y, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covassin L, Jangalwe S, Jouvet N, et al. Human immune system development and survival of NOD-scid IL2rgamma (NSG) mice engrafted with human thymus and autologous hematopoietic stem cells. Clinical and experimental immunology. 2013 doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. Journal of virology. 2009;83:7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan P, Tonomura N, Shimizu A, et al. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 22.McCune JM, Namikawa R, Kaneshima H, et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 23.Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nature medicine. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 24.Kalscheuer H, Danzl N, Onoe T, et al. A model for personalized in vivo analysis of human immune responsiveness. Science translational medicine. 2012;4:125ra130. doi: 10.1126/scitranslmed.3003481. [DOI] [PMC free article] [PubMed] [Google Scholar]


