Abstract
Early life stress exposure (ELS) yields risk for psychiatric disorders that might occur though a population-specific mechanism that impacts prefrontal cortical development. Sex differences in ELS effects are largely unknown and are also essential to understand social and cognitive development. ELS can cause dysfunction within parvalbumin (PVB)-containing inhibitory interneurons in the prefrontal cortex and in several prefrontal cortex-mediated behaviors including social interaction. Social behavior deficits are often the earliest observed changes in psychiatric disorders, therefore the time-course and causation of social interaction deficits after ELS are important to determine. PVB interneuron dysfunction can disrupt social behavior, and has been correlated in males with elevated markers of oxidative stress and inflammation, such as cyclooxygenase-2 after ELS. Here, we measured the effects of maternal separation ELS on social interaction behaviors in males and females. Prefrontal cortex PVB and cyclooxygenase-2 were also measured in juveniles and adolescents using Western blots. ELS led to social interaction alterations earlier in females than males. Sexually dimorphic behavioral changes were consistent with prefrontal cortex PVB loss after ELS. PVB levels were decreased in ELS-exposed juvenile females, while males exposed to ELS do not display parvalbumin decreases until adolescence. Early behavioral and PVB changes in females did not appear to be mediated through cyclooxygenase-2, since levels were not affected in ELS females. Therefore, these data suggest that ELS affects males and females differently and with distinct developmental profiles.
Individuals that have exposure to early life stress (ELS) are vulnerable to psychiatric disorders such as schizophrenia, anxiety and depression, which sustain throughout adulthood [29, 33]. The mechanisms through which ELS can perturb development are therefore of interest. While some consequences of ELS arise in childhood, they often manifest during adolescence or young adulthood [36], making the timing of assessment critical for understanding neuronal and behavioral effects over the lifespan. Recent evidence has linked ELS with prefrontal cortex (PFC) changes [14, 15, 35]. The PFC is a late-maturing region that subserves all higher order emotional and cognitive functions [1]. The maternal separation model of ELS in rodents leads to later, peri-pubertal deficits in PFC-mediated behaviors such as learned helplessness and working memory [6, 10, 21]. These behaviors are largely mediated by GABAergic interneurons within the PFC that express the calcium-binding protein parvalbumin (PVB) [23, 40]. Maternal separation and other early life insults lead to a loss of PVB in the PFC [6, 7]. Therefore, PFC PVB loss is a likely mechanistic substrate for behavioral effects of ELS.
While the cause of PVB loss is not yet understood, PVB neurons have been proposed to be vulnerable to oxidative stress [7]. One downstream molecule of oxidative stress is cyclooxygenase-2 (COX-2), which is produced in the brain in response to stress signals, glutamatergic activity and presence of inflammatory cytokines [13, 24]. We recently reported that in adolescent ELS-treated male rats, COX-2 upregulation was correlated with PFC PVB loss, suggesting a role for oxidative stress or neuroinflammation in PVB loss after ELS [6]. However, there is very little existing knowledge regarding sex differences in physiological and behavioral effects of ELS.
ELS has been shown to induce changes in social behaviors, including avoidance, fear, and decreased social interaction [11, 37]. In humans, social dysfunction is also highly comorbid with psychiatric disorders such as depression and anxiety, and generally appears before these disorders, e.g. prodromal phase of schizophrenia. Therefore studying dysfunctional social interaction is important for understanding derailed development in response to stress [25] A large body of evidence indicates that males and females adapt to and are affected by stress differently [27]. However, the relationship between sex differences and ELS-related changes has been scarcely investigated and results are inconsistent [5, 18, 19]. Taken together, investigating sex differences in the effects of ELS on social behavior and brain development helps explain how animals respond differently to their early environment.
In this study we used an open-field social interaction paradigm to assess sex differences and developmental effects after a maternal separation ELS paradigm. Differential expression of PVB and COX-2 in the PFC over development was also examined. Sexually dimorphic effects of ELS would indicate a distinct pattern of development in response to postnatal stress between males and females.
2. Materials and methods
2.1 Animals and tissue collection
Pregnant female multiparous Sprague-Dawley rats (250–275g) were obtained from Charles River Laboratories (Wilmington, MA) on day 15 of gestation. Rats were housed with food and water available ad libitum in constant temperature and humidity conditions on a 12-h light/dark cycle (light period 0700-1900). This experiment was conducted in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (NIH) and was approved by the Institutional Animal Care and Use Committee at Northeastern University.
A timeline illustrating experimental design is presented in Supplemental Figure 1. The day of birth was designated as postnatal day 0 (P0). At P1, litters were culled to 10 pups (5 males and 5 females), and litters were randomly assigned to either a maternal separation group (ELS group) or control group (CON group). Pups in the ELS group were isolated for 4 h per day between P2- 20, and kept in a thermoneutral environment of 36°C with a circulating water bath until they could regulate their own temperatures. The maternal separation procedure is identical to procedures used previously by this laboratory [3, 4] and similar to others [32]. Pups in the CON group were not disturbed after P2, except for weekly changes in cage bedding and when weighed. All rat pups were weighed on P9, P11, P15, and P20 and no significant difference in weight was observed between all groups at the separate time points. Rats were weaned on P21, and group-housed with same-sex littermates with 2–4 rats/cage until experimentation. Only one rat per litter was assigned to each experimental group to avoid litter effects. Rats were tested for social interaction during the juvenile stage (P25) or in adolescence (P40), ages that are based on achievement of milestones, including brain development and pubertal status [2]. A separate cohort of rats were rapidly decapitated at either P25-27 or P42-45. The prefrontal cortex was dissected, flash frozen on dry ice, and stored in a -80OC freezer until Western blot analysis.
2.2 Social Interaction Test
Separate cohorts of rats were tested for social interaction at either P25 or at P40 (n = 13–14; See Supplemental Figure 1). ELS or CON subjects were marked and placed individually into a Plexiglas open field arena (100cm × 100cm) for 10 minutes to habituate to the environment. A naïve conspecific of same sex and equal age was then placed into the arena for a separate 10 minute habituation period. The ELS or CON animal was then reintroduced to the arena facing away from the conspecific, at opposite sides of the arena. Both rats were monitored for 30 minutes by a CCTV camera (Panasonic WV- CP500, Secaucus NJ) suspended directly above the arena. The camera was interfaced with EthoVision (v9.0; Noldus Information Technology, Leesburn VA), which digitally analyzed nose-to-nose contacts, nose-to-tail contacts, locomotion, distance between subjects, approach (moving towards conspecific), and avoidance (moving away from conspecific). Cumulative duration, frequency, and latency to first of nose-to-nose and nose-to-tail contacts were measured using a minimum distance of 5cm set as the threshold for contacts [30, 34]. The cumulative duration of time subjects exhibited locomotion was recorded with a minimum velocity of 2cm/second set as the threshold. The mean distance between subjects for the social interaction trial was also measured. The frequency and cumulative duration of approach and avoidance was measured with a threshold of 50cm set as the minimum distance required for approach or avoidance behavior. The arena was cleaned with 30% ethanol between each test.
2.3 Gel electrophoresis and Western blot
At P25-27 (juvenile; n=5–7/group) or P42-45 (adolescent; n=5–7/group), a separate cohort of males and females were sacrificed by rapid decapitation and PFC was dissected and placed on dry ice until analysis. Tissue was processed for SDS-PAGE as described previously [8] Blots were then incubated in primary antibody (1:500 rabbit polyclonal anti-PVB [PA1-933, Thermo Scientific] or 1:500 rabbit polyclonal anti-COX-2 [236002, Millipore]). Subsequently blots were incubated in a secondary antibody (1:5000 Peroxidase goat anti-rabbit IgG antibody [PI-1000 Vector Laboratories]). Molecular weights for the target proteins used were: PVB, 12kDa; COX-2, 72kDa. After probing blots for PVB and COX-2, antibodies were stripped by incubation with stripping buffer (62.5 mM Tris, 2% SDS, 100 mM β-mercaptoethanol, pH 6.8) for 15 minutes at 50°C. Blots were then re-blocked and probed with anti-tubulin (1:30,000 mouse monoclonal anti-β-tubulin [T4026, Sigma-aldrich]) and an anti-mouse secondary (1:2,000 Peroxidase horse anti-mouse IgG antibody [PI-2000 Vector Laboratories]). SeeBlue Plus 2 (LC5925, Life Technologies) pre-stained standards were run for molecular weight estimation.
Protein immunoblots were analyzed using Carestream Molecular Imaging Software 5.0. Net intensity (the sum of the pixels within the band of interest minus the sum of the background pixels) was determined for each band.
2.4 Data Analysis
Latencies, durations, frequencies and distances collected during the social interaction assessment were compared between groups using three-way analysis of variance (ANOVA) with Sex, Group (ELS/CON), and Age as interacting factors. Two- and three-way interactions were followed up with Bonferroni post-hoc tests, or with Sidak-Bonferroni post-hoc when homogeneity of variances could not be assumed. Optical densities of each Western blot band were normalized with tubulin, and two-way ANOVAs followed by post-hoc tests compared Group and Age effects on male and female PVB and COX-2 levels in the PFC.
3. Results
3.1 Social interaction Behavior
Duration and latency of nose-to-nose contact
Three-way ANOVAs revealed an Age × Sex × Group interaction on the duration of nose-to-nose contact (F1,80=4.707; p=0.033) and on the latency for nose-to-nose contact with a conspecific (F1,80=8.441; p=0.005). As seen in Figure 1A, juvenile females (t42=2.838; p<0.05) but not juvenile males exposed to ELS engaged in less nose-to-nose contact with a conspecific (Sex × Group interaction at P25: F1,42=8.669; p=0.005). As seen in Figure 1B, juvenile females (t43=2.768; p<0.05) but not juvenile males exposed to ELS also displayed significantly higher latencies than controls to engage in nose-to-nose contact (Sex × Group interaction at P25: F1,44=5.074; p=0.029). In adolescence however, a Sex × Group interaction (F1,35=5.519; p=0.025) is driven by higher latencies in ELS males (t18=2.32; p<0.05) but not females.
Figure 1.
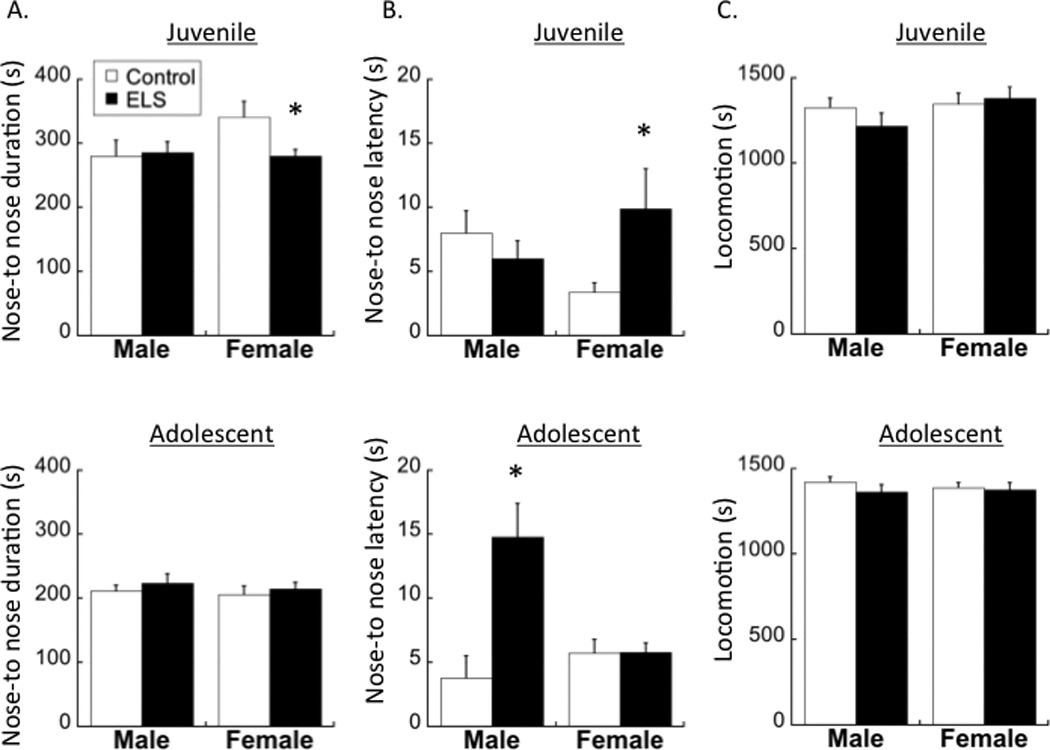
Duration of (A) and latency for (B) engagement in nose-to-nose contact with a conspecific is differentially affected by maternal separation (ELS) over development in males and females. (C) Locomotion did not differ between any group at either age. Means ± SEM are shown. *p<0.05 compared to controls.
Locomotion
In order to confirm that differences in social interaction behaviors were not due to general motor differences, horizontal locomotion was recorded during the social interaction test sessions. General locomotion was not significantly different between groups at either age (Figure 1C).
Proximity
When comparing the mean distance between an adolescent test animal and a conspecific (Figure 2), a main effect of both Sex (F1,48=14.151; p<0.001) and Group (F1,48=8.811; p=0.005) was found, with no significant Sex × Group interaction (p=0.428). While center-to-center distances were generally greater in adolescent females compared to males, ELS adolescents in general had a shorter proximity to conspecifics compared to controls. Individual t-tests reveal that the group differences in adolescent proximity were largely driven by females (t24=2.319; p<0.05), and not males (p=0.076).
Figure 2.
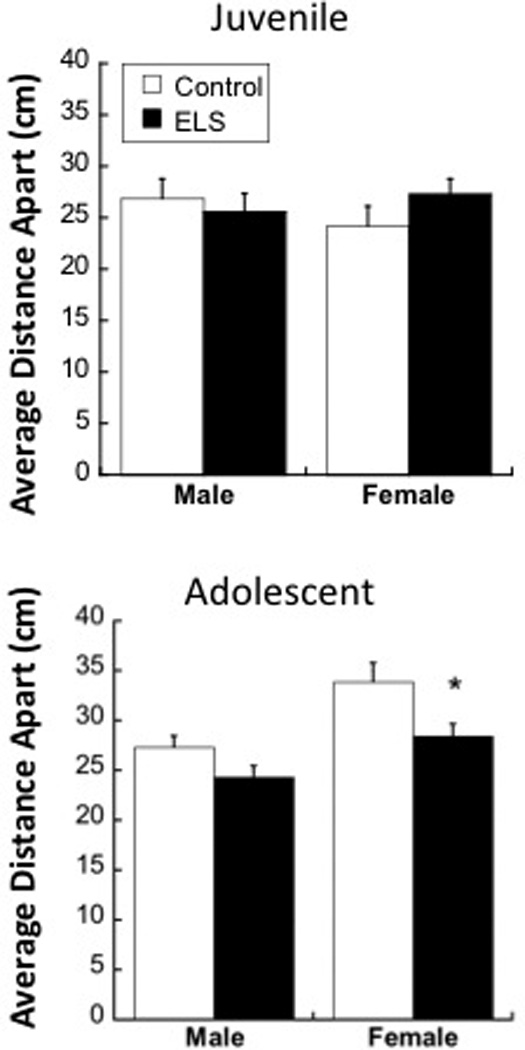
Effects of maternal separation (ELS) on mean distance apart from a conspecific in juvenility (top) or adolescence (bottom) in males and females. Means ± SEM are shown. *p<0.05 compared to controls.
Other behaviors
All other social behaviors measured were unaffected by sex or group, and are displayed in Supplemental Table 1.
3.2 PFC PVB and COX-2
ELS-exposed males expressed lower PVB in adolescence, but not earlier, since a main effect of Group (F2,28=13.3; p=0.001) was driven by a difference at P40 (t27=2.66; p<0.05) but not P25 (Figure 3A). Additionally, two-way ANOVA revealed interactive effects of Age and Group on female parvalbumin levels (Figure 3B; F1,21=4.679; p=0.042). In contrast to males, PFC PVB levels were lower in ELS exposed juvenile females compared to controls (t20=2.22; p<0.05), but were not different between groups in adolescence. COX-2 levels were higher in ELS male adolescents (t19=3.71; p<0.05) but not male juveniles compared to controls (Group×Sex Interaction: F1,19=4.72; p=0.043; Figure 3C) while females were unchanged by age (p=0.486) or group (p=0.935; see Figure 3D).
Figure 3.
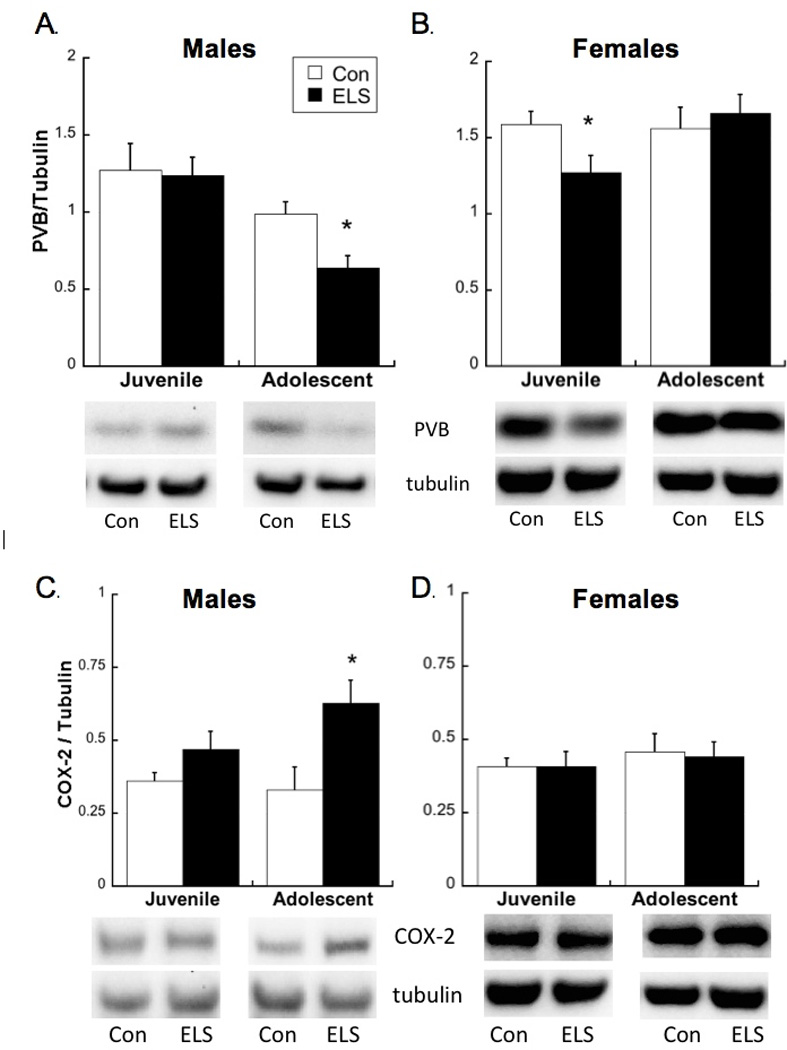
(A) As previously reported, parvalbumin (PVB) levels (expressed in relation to loading control) are decreased in maternally separated (ELS) males during adolescence, but not juvenility. (B) PVB levels (expressed in relation to loading control) are decreased in ELS females during juvenility, but not adolescence. Means ± SEM are shown. *p<0.05 compared to controls. (C) As previously reported, cyclooxygenase-2 (COX-2) levels (expressed in relation to loading control) are increased in ELS males during adolescence, but not juvenility. (D) COX-2 levels (expressed in relation to loading control) are not changed after ELS in juvenile or adolescent females. Means ± SEM are shown. Note: Male and female tissue were analyzed in separate Western blots, therefore are not compared within graphs.
4. Discussion
Here we report sexually dimorphic effects of neonatal ELS on social behaviors and PFC PVB across development. In males, ELS led to social interaction changes that first appeared in adolescence, which is consistent with our previous finding that ELS males display a delayed decrease of PFC PVB in adolescence, but not before [6]. In contrast, ELS females displayed social interaction changes earlier than males. Juvenile behavioral effects in ELS females co-occurred with decreased PFC PVB, while these changes were not apparent in adolescent females. In addition, while males exposed to ELS express higher levels of the inflammatory mediator COX-2 in the PFC (here and [6]), this was not observed in ELS females. These findings can be interpreted in the context of a number of earlier studies showing effects of early rearing conditions [22, 32] and sex [10] on later behavior. It will be important to determine in future studies whether effects of early rearing on other behaviors such as stress responsivity follows a sexually-dimorphic pattern, as well.
Notably, our observation that PVB is not deficient in adolescent ELS females contrasts with our earlier paper reporting that PVB is lower in ELS female adolescents, compared to controls. This inconsistency may be due to age differences, as our adolescent females were sacrificed for Western blots at P42-P45—up to one week later than the females reported previously [20]. We have also shown that both males and females exposed to ELS return to control PFC PVB levels by adulthood [6, 20]. Therefore, the maternal separation model of ELS in rodents appears to yield transient effects on PFC PVB that do not endure into adulthood, yet follow an earlier timeline in females than in males.
Social behaviors arise through the activity of complex cortical and subcortical circuitries [12, 16, 38]. Here, we report that PFC PVB loss after ELS co-occurs with appearance of social interaction deficits. These deficits were specific to nose-to-nose contacts, since the latency, frequency, and duration of nose-to-tail contacts were unaffected by ELS (Supplemental Table 1). While anogenital contacts are important for initial identification of a conspecific [28], facial interactions have been shown to convey social information spanning from aggressive intent to transmission of food preferences [39]. Therefore, ELS exposure was shown here to impair social interaction, but not decrease general social behavior, in juvenile females and adolescent males. Cortical PVB interneurons have been shown to regulate social behaviors since increased activity of these cells can rescue social deficits after experimentally-induced excitatory/inhibitory imbalance [40]. Additionally, nose-to-nose sniffing is reduced in animals with genetic knockouts that also reduce cortical PVB interneurons [31]. Therefore, early PVB loss in the female PFC after ELS was consistent with social behavior deficits that occurred earlier in females than in males.
In adolescence, females no longer displayed deficits in latency for nose-to-nose contact, but rather kept closer proximity to a conspecific. In other words, while adolescent ELS males were beginning to show longer latencies for social interaction, females were shifting to a greater engagement in social interaction compared to controls. These differences in female proximity further illustrate developmental changes in social behavior after ELS. While the neural mechanisms controlling this change are unclear, increased social interaction in ELS females could reflect altered reactivity controlled by regions outside the PFC as well, such as the hippocampus and where other sex-specific ELS effects have been found [17].
Our observation that COX-2 changes in ELS males but not females points to the possibility that the mechanism, as well as the timing, for PFC deficits could differ between sexes. While we previously reported in males that increased PFC COX-2 correlated directly with decreased PFC PVB [6], the cause of PVB loss after ELS has yet to be determined. Further investigation into the causation of PVB loss and PFC dysfunction after ELS is needed, with particular attention to the emerging evidence that these causative mechanisms could differ between males and females. In addition, the timing of assessment appears to influence ELS effects, which may partially explain conflicting reports of ELS effects in the literature [26]. Behavioral or neurochemical measures taken in adulthood could overlook transient effects that might have resolved by adulthood in this ELS model. Importantly, the transient nature of ELS effects reported here and elsewhere [9] speaks to the careful nature by which animal models should be translated to clinical relevance, since animal models could lack important mediating effects of human experience that might lead to enduring dysfunction. Taken together, we report here that neonatal ELS alters later social interaction and PFC PVB, which is seen earlier in females than males and may occur through sexually dimorphic mechanisms.
Supplementary Material
Highlights.
We examined divergent sex profiles in early-life stress (ELS) exposed rats.
ELS females showed social interaction deficits earlier in development than males.
Parvalbumin levels were decreased after ELS earlier in females than males.
COX-2 expression remained unchanged in ELS females throughout development but was increased in ELS male adolescents.
Acknowledgements
This work was supported by NIMH 5R21MH097182-02. We appreciate the technical assistance of Thomas Backus and Noa Golan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: an analysis utlizing reversible cryogenic depression. Brain research. 1978;143:233–249. doi: 10.1016/0006-8993(78)90566-8. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and biobehavioral reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 3.Andersen SL, Lyss PJ, Dumont NL, Teicher MH. Enduring neurochemical effects of early maternal separation on limbic structures. Annals of the New York Academy of Sciences. 1999;877:756–759. doi: 10.1111/j.1749-6632.1999.tb09317.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 5.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neuroscience and biobehavioral reviews. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry. 2011;70:434–440. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabungcal J-H, Nicolas D, Kraftsik R, Cuenod M, Do K, Hornung J-P. Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: Relevance to schizophrenia. Neurobiology of disease. 2006;22:624–637. doi: 10.1016/j.nbd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA., Jr Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26:6450–6457. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chocyk A, Przyborowska A, Dudys D, Majcher I, Mackowiak M, Wedzony K. The impact of maternal separation on the number of tyrosine hydroxylase-expressing midbrain neurons during different stages of ontogenesis. Neuroscience. 2011;182:43–61. doi: 10.1016/j.neuroscience.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Frankola KA, Flora AL, Torres AK, Grissom EM, Overstreet S, Dohanich GP. Effects of early rearing conditions on cognitive performance in prepubescent male and female rats. Neurobiology of learning and memory. 2010;94:91–99. doi: 10.1016/j.nlm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Giachino C, Canalia N, Capone F, Fasolo A, Alleva E, Riva MA, Cirulli F, Peretto P. Maternal deprivation and early handling affect density of calcium binding protein-containing neurons in selected brain regions and emotional behavior in periadolescent rats. Neuroscience. 2007;145:568–578. doi: 10.1016/j.neuroscience.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain research bulletin. 2002;57:651–659. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- 13.Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, Luo F. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. European journal of pharmacology. 2009;612:54–60. doi: 10.1016/j.ejphar.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 14.Heidbreder C, Weiss I, Domeny A, Pryce C, Homberg J, Feldon J, Moran M, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrom. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 15.Helmeke C, Ovtscharoff WJ, Poeggel G, Braun K. Imbalance of immunohistochemically characterized interneuron populationsn in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience. 2008;152:18–28. doi: 10.1016/j.neuroscience.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosten TA, Miserendino MJ, Bombace JC, Lee HJ, Kim JJ. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav Brain Res. 2005;157:235–244. doi: 10.1016/j.bbr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Kunzler J, Braun K, Bock J. Early life stress and sex-specific sensitivity of the catecholaminergic systems in prefrontal and limbic regions of Octodon degus. Brain structure & function. 2013 doi: 10.1007/s00429-013-0688-2. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 20.Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- 21.Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL. Depressive-Like Behavior in Adolescents after Maternal Separation: Sex Differences, Controllability, and GABA. Dev Neurosci. 2012;34:210–217. doi: 10.1159/000339162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine S. Infantile experience and resistance to physiological stress. Science (New York, N.Y.) 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 23.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Archives of neurology. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 24.Liang X, Wu L, Wang Q, Hand T, Bilak M, McCullough L, Andreasson K. Function of COX-2 and prostaglandins in neurological disease. J Mol Neurosci. 2007;33:94–99. doi: 10.1007/s12031-007-0058-8. [DOI] [PubMed] [Google Scholar]
- 25.Lin A, Wood SJ, Yung AR. Measuring psychosocial outcome is good. Current opinion in psychiatry. 2013;26:138–143. doi: 10.1097/YCO.0b013e32835d82aa. [DOI] [PubMed] [Google Scholar]
- 26.Marco EM, Macri S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotoxicity research. 2011;19:286–307. doi: 10.1007/s12640-010-9205-z. [DOI] [PubMed] [Google Scholar]
- 27.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miczek KA, Boer SF. Agressive, Defensive, and Submissive Behavior. New York: Oxford University Press, New York; 2005. [Google Scholar]
- 29.Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 33.Read J, Perry BD, Moskowitz A, Connolly J. The contribution of early traumatic events to schizophrenia in some patients: a traumagenic neurodevelopmental model. Psychiatry. 2001;64:319–345. doi: 10.1521/psyc.64.4.319.18602. [DOI] [PubMed] [Google Scholar]
- 34.Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, Taylor R, Cain DP. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54:901–911. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson CW, Marsden CA, Mason R. Early life stress causes FG-7142-induced corticolimbic dysfunction in adulthood. Brain Res. 2008;1193:43–50. doi: 10.1016/j.brainres.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 36.Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin Psychiatry. 2009;70:684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell and tissue research. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- 38.Wall VL, Fischer EK, Bland ST. Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiol Behav. 2012;107:440–450. doi: 10.1016/j.physbeh.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfe J, Mende C, Brecht M. Social facial touch in rats. Behavioral neuroscience. 2011;125:900–910. doi: 10.1037/a0026165. [DOI] [PubMed] [Google Scholar]
- 40.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


