Abstract
Neurofeedback that tracks attentional focus in real time using fMRI and alerts subjects to impending lapses by modulating the difficulty of the task itself has been demonstrated to improve behavioral performance.
Brief lapses of attention while performing daily tasks are ubiquitous. Whether it’s adding salt instead of sugar to your coffee or missing a stop sign, these attentional lapses can result in unintended consequences ranging from minor nuisances to outright catastrophes1. A challenge for controlling such lapses is that humans often are not very good at immediately noticing when their mind has drifted off from the task at hand2. However, deBettencourt et al.3 have now developed an approach that uses fMRI in real time to detect when the subject’s brain is no longer in an attentive state and provides them with continuous feedback to get them back on track. This neurofeedback approach yielded reliable increases in behavioral performance relative to a sham feedback condition, demonstrating the value of online feedback for optimizing performance in attention-demanding situations.
The authors required subjects to attend to either the face or scene aspect of a composite stimulus (Fig. 1) while tracking the strength of task-relevant information in each subject’s brain. The task required them to make a response on 90% of trials, but to withhold that response on the rare trials in which non-target stimuli were presented; this task is well known to tax one’s ability to sustain attention over time and to inhibit prepotent responses. As the subjects performed this attentionally demanding task, the authors used the ongoing neural signals from each subject’s brain to provide moment-to-moment feedback using a clever and direct method: the weight of each image in the composite stimulus started out equal, but when ongoing neural activity indicated that attention to the relevant stimulus was waning, the percentage of the task-relevant aspect (face or scene) in the composite mixture was reduced. Conversely, when neural activity indicated increasing attentional focus, the relevant face or scene aspect of the physical stimulus was amplified (Fig. 1). Thus, the feedback signal that informed subjects of their current attentional state was integrated into the very stimulus subjects were attempting to attend. deBettencourt et al.3 suggest that this feedback scheme served to reward subjects with an easier stimulus display when they were on task and punish them when their attention began to stray.
Figure 1.
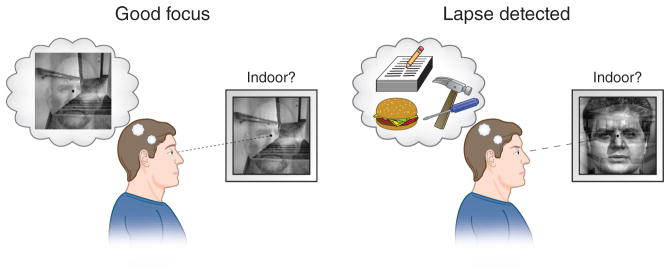
Real-time neurofeedback. Ongoing neural activity was used to monitor attentional focus. During moments of good focus, the weight of the salient stimulus (here, the scene) was amplified in the physical display, making the task easier. By contrast, if attention toward the salient stimulus waned, its weight in the physical display was reduced. Stimuli reprinted from ref. 3 with permission.
Remarkably, this neurofeedback procedure produced reliable improvements in behavioral performance after a single training session. Other participants who spent an equivalent amount of time practicing the task with sham feedback did not show reliable improvement, suggesting that accurate feedback based on ongoing neural activity was responsible for the improvement in behavioral sensitivity. Indeed, those subjects whose neurofeedback signal improved the most over time were the ones who showed the greatest improvement from the pre-training to the post-training assessment of attentional function. deBettencourt et al.3 also found that neural activity discriminating between the two attentional states (face versus scene) was sharpened by neurofeedback, such that distinct attentional states evoked more differentiated patterns of activity following neurofeedback training. This effect was most pronounced in a distributed network of brain regions that included ones, such as frontoparietal cortex, outside of the traditional sensory areas, suggesting that neurofeedback may have influenced processing in attentional control regions that extend beyond category-selective visual regions. These findings suggest that individually tailored neurofeedback can be an effective approach for helping individuals to avoid lapses of attention and take full advantage of their existing ability to engage in goal-driven selection of relevant information.
The focus of deBettencourt et al.3 on attentional lapses may also provide a productive perspective for understanding individual differences in attentional ability. It has long been known that an individual’s ability to voluntarily select the relevant over the irrelevant aspects of an environment predicts broad measures of intellectual function, such as fluid intelligence4,5 and scholastic aptitude6. These links with success in a wide variety of contexts motivate a search for explanations of why attentional efficiency varies across individuals. An intuitive idea is that individuals vary in the maximal efficiency of attention, leading to consistent differences in their ability to voluntarily select the most relevant aspects of a stimulus. It is also possible, however, that individual differences in attentional control reflect the probability that an individual will avoid lapses and make full use of their attentional ability. In this case, the frequency of attentional lapses could have a powerful effect on performance in attentionally demanding tasks even if there are no differences in the maximal efficiency of attention. Indeed, both the frequency of ‘mind wandering’1,7 and attentional lapses8 predict individual differences in executive control and fluid intelligence, showing that broad measures of cognitive function are shaped by the prevalence of inattentive episodes. Thus, a fuller appreciation of how ability varies from moment to moment may sharpen our understanding of individual differences in cognitive control.
Finally, the findings of deBettencourt et al.3 may have implications for ‘brain training’ approaches that seek to improve general cognitive function in humans. Because attentional control is a core facet of cognitive ability, there has been a longstanding interest in whether it is possible to enhance attentional ability via training exercises. Most of these attention training interventions involve attempts at boosting the native capacity of the attentional system through extensive attentional control practice9. However, after over 100 years of attempts, this approach has yielded only minimal success and much controversy10–14, with some arguing that it is unrealistic to expect permanent changes in native cognitive ability following relatively short-lived exposure to a behavioral intervention14. Consistent with other recent work15, the findings of deBettencourt et al.3 highlight a qualitatively different approach. Instead of attempting to boost the maximal efficiency of attention, this strategy seeks to optimize the individual’s existing attentional capacity through the detection and correction of lapses. Thus, rather than trying to make the individual ‘smarter’, the more tractable training goal may be to make the individual ‘stupid less often’.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Edward Awh, Email: awh@uoregon.edu.
Edward K Vogel, Email: vogel@uoregon.edu.
References
- 1.Kane MJ, et al. Psychol Sci. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 2.Schooler JW, et al. Trends Cogn Sci. 2011;15:319–326. doi: 10.1016/j.tics.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 3.deBettencourt MT, Cohen JD, Lee RF, Norman KA, Turk-Browne NB. Nat Neurosci. 2015;18:470–475. doi: 10.1038/nn.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane MJ, Engle RW. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 5.Unsworth N, Fukuda K, Awh E, Vogel EK. Cogn Psychol. 2014;71:1–26. doi: 10.1016/j.cogpsych.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner ML, Engle RW. J Mem Lang. 1989;28:127–154. [Google Scholar]
- 7.Mrazek MD, et al. J Exp Psychol Gen. 2012;141:788–798. doi: 10.1037/a0027968. [DOI] [PubMed] [Google Scholar]
- 8.Unsworth N, Redick TS, Lakey CE, Young DL. Intelligence. 2010;38:111–122. [Google Scholar]
- 9.Morrison AB, Chein JM. Psychon Bull Rev. 2011;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- 10.Thorndike EL, Woodworth RS. Psychol Rev. 1901;8:247–261. [Google Scholar]
- 11.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Proc Natl Acad Sci USA. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingberg T, Forssber H, Westerberg H. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 13.Caroll JB. Human Cognitive Abilities: a Survey of Factor-Analytic Studies. Cambridge University Press; 1993. [Google Scholar]
- 14.Redick TS, et al. J Exp Psychol Gen. 2013;142:359–379. doi: 10.1037/a0029082. [DOI] [PubMed] [Google Scholar]
- 15.Mrazek MD, Franklin MS, Phillips DT, Baird B, Schooler JW. Psychol Sci. 2012;24:776–781. doi: 10.1177/0956797612459659. [DOI] [PubMed] [Google Scholar]


