Abstract
Background
Dynorphin, an endogenous ligand at kappa opioid receptors (KOR), produces depressive-like effects and contributes to addictive behavior in male non-human primates and rodents. Although comorbidity of depression and addiction is greater in women than men, the role of KORs in female motivated behavior is unknown.
Methods
In adult Sprague Dawley rats, we used intracranial self-stimulation (ICSS) to measure effects of the KOR agonist (±)-trans-U-50488 methanesulfonate salt (U-50488) (0.0 – 10.0 mg/kg) on brain stimulation reward in gonadally intact and castrated males and in females at estrous cycle stages associated with low and high estrogen levels. Pharmacokinetic studies of U-50488 in plasma and brain were conducted. Immunohistochemistry was used to identify sex-dependent expression of U-50488-induced c-Fos in brain.
Results
U-50488 dose-dependently increased the frequency of stimulation (threshold) required to maintain ICSS responding in male and female rats, a depressive-like effect. However, females were significantly less sensitive than males to the threshold-increasing effects of U-50488, independent of estrous cycle stage in females or gonadectomy in males. Although initial plasma concentrations of U-50488 were higher in females, there were no sex differences in brain concentrations. Sex differences in U-50488-induced c-Fos activation were observed in CRF-containing neurons of the paraventricular nucleus (PVN) of the hypothalamus and primarily in non-CRF-containing neurons of the bed nucleus of the stria terminalis (BNST).
Conclusions
These data suggest that the role of KORs in motivated behavior of rats is sexdependent, which has important ramifications for the study and treatment of mood-related disorders including depression and drug addiction in people.
Keywords: dynorphin, female, stress, intracranial self-stimulation, pharmacokinetics, c-Fos
Women are twice as likely as men to suffer from affective disorders including major depression, anxiety disorders, and post-traumatic stress disorder (1, 2), which are often comorbid with drug addiction. Negative emotional states such as stress and depression are more common in women addicts (3–6) and more likely to trigger craving and relapse in women than men (7, 8). These findings suggest that brain mechanisms encoding aversive states may differ between the sexes.
Stress and chronic exposure to drugs of abuse promote the synthesis and release of dynorphin—an endogenous kappa opioid receptor [KOR; (9)] ligand—coincident with the emergence of depressive-like effects (10–14). KOR agonists produce negative affective states in humans and rodents (15–23), whereas KOR blockade attenuates stress- and drug-induced depressive-like states (20, 24–27). However, all studies were conducted in males, and little is known about how KORs contribute to affective states in females.
Dynorphin and KORs are found throughout the brain (28, 29), and mounting evidence suggests that mood-related effects of KOR activation are due to modulation of neurotransmission within the reciprocally connected mesocorticolimbic, extended amygdala, and hypothalamic systems (30–32). KORs are generally expressed on dopaminergic, GABArgic, and glutamatergic nerve terminals (33–35) where they inhibit transmitter release (17, 36, 37) via coupling to inhibitory Galpha subunits (38). There is also evidence for postsynaptic KOR expression (34, 39), although the functional effects are not well understood. Recent studies in guinea pigs demonstrated sex differences in KOR receptor levels and function within neural circuits that regulate motivated behavior (40, 41), and sex-specific effects of KOR antagonism on aggressive behavior in prairie voles has been reported (42). Pain studies show that females tend to be less sensitive than males to analgesic properties of KOR agonists (43–47).
Sex differences in behavior can result from activational effects of circulating gonadal hormones or organizational effects during development, and/or sex chromosome effects (48–50). We hypothesized that if depressive-like effects of KOR activation are sex-dependent, then male and female rats would have different sensitivities to the anhedonic effects of the KOR agonist U- 50488 as measured with intracranial self-stimulation (ICSS). To identify putative neural substrates for sex differences in motivated behavior, we quantified U-50488-induced c-Fos within mesolimbic, extended amygdala, and hypothalamic systems of male and female rats. Based on those results, we initiated studies characterizing the neuronal phenotypes of activated neurons in sexually dimorphic regions.
Methods and Materials
Animals
Age-matched, sexually mature female (n = 61) and male (n = 52) Sprague-Dawley rats (Charles River Laboratories) between 75 and 85 days old and weighing 300 – 325 g (female) and 380 – 410 g (male) at the start of experiments were used. Upon arrival at the facility, rats were group housed (4 rats/cage) and segregated by sex. All rats were acclimatized for one week in a 12 h/12 h light/dark cycle (lights on at 0700) with free access to food and water. All experiments were conducted during the light phase. Rats were treated according to the guidelines recommended by the Animal Care and Use Committee of McLean Hospital.
To track estrous cycles, vaginal smears were taken at the same time each day for approximately two weeks before testing. Males were simultaneously handled. On the morning of test days, vaginal cytology was examined with a light microscope to rapidly determine cycle stage in order to assign treatments. See Supplemental Methods and Fig. S1.
Intracranial self-stimulation
Rats (n = 13 female; n = 17 male) were implanted with stainless steel monopolar electrodes (0.25 mm diameter; Plastics One, Roanoke, VA) aimed at the medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to midline, 7.8 mm below dura). Rats were trained using the rate-frequency method, as in (51) and Supplemental Methods.
For drug testing, three rate-frequency functions (3 × 15 min) were determined for each rat immediately before drug injection. ICSS thresholds and maximum rates for the second and third functions were averaged and served as baseline parameters. Each rat then received an i.p. injection of drug and 4 more 15-min rate frequency trials. Drug treatment days were separated by 2–3 non-drug days during which baseline ICSS thresholds were maintained. Rats were treated with (±)-trans-U-50488 methanesulfonate salt (U-50488, Sigma-Aldrich, St. Louis, MO) at doses of 0.0, 2.5, 5.0, 10 mg/kg dissolved in water, based on weight of the salt. Our goal was to test female rats with each dose of U-50488 during estrous, diestrous, and proestrous, with doses of U-50488 administered in a randomized order. However, most females were tested with only 2 or 3 doses of U-50488 at 2 of the 3 estrous cycle stages due to loss of head cap, unstable responding during non-drug days (>10% variation from stable baseline thresholds established during training for ≥3 consecutive days), or inability to capture the rat in a particular estrous stage. Consequently, the number of animals represented in each dose and estrous stage ranges from 7 to 13. To account for repeated testing of females, males were treated twice with each dose of U-50488 in randomized order and effects of the first and second treatments compared (see Fig. S4). ICSS boxes were cleaned with isopropyl alcohol and bedding was changed between each animal. Sequentially testing males and females did not affect thresholds.
Intracranial self-stimulation: castrated males
Adult male rats (n = 7) were implanted with stimulating electrodes, trained in ICSS, and treated with U-50488 (0.0 – 10.0 mg/kg, i.p.) as described above. After completion of the U-50488 dose response, baseline ICSS thresholds were reestablished for each rat over a period of 4–5 days, and rats were castrated (see Supplemental Methods). Rats recovered for 4 days and then ICSS was performed 1hr/d, 5 d/wk for 3 wks as testosterone levels declined and other hormones stabilized. At this point, the U-50488 (0.0 – 10.0 mg/kg, i.p.) dose response test was repeated.
Pharmacokinetics
For repeated blood sampling, rats (n = 11 females, 6 males) were cannulated through the right external jugular vein and singly housed (see Supplemental Methods). After 3 days of recovery, vaginal swabs were done on female rats and male rats were handled. One hour later, rats were treated with U-50488 (10 mg/kg, i.p.) and blood samples (200 µL/sample) were collected via IV cannula at 0.083, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 hr in 1.5mL Eppendorf tubes stored on wet ice. Twenty-four hours post-U-50488 treatment, rats were decapitated and trunk blood was collected in pre-chilled 7 mL EDTA-treated blood tubes kept on wet ice. Blood was aliquoted into 1.5mL tubes and all samples were centrifuged (13,000g at 4°C for 15min). Plasma was removed, aliquoted into 1.5mL Eppendorf tubes, and frozen on dry ice before −80°C storage. For analysis of U-50488 brain levels, separate rats (n = 14 females, 9 males) were used. Rats were treated with U-50488 (10 mg/kg, i.p.) and decapitated 15 min, 1 h, or 24 h later. Brains were removed and frozen in isopentane kept on dry ice before −80°C storage. U- 50488 concentrations in plasma and brain were determined by liquid chromatography tandem mass spectrometry (LC-MS/MS). See Supplemental Methods.
Immunohistochemistry
Rats (n=19 females; 18 males) were treated with U-50488 (0.0 or 10.0 mg/kg) and perfused 2 hr later for immunohistochemistry (52; Supplemental Methods). C-Fos-positive nuclei in brain regions of interest were counted and reported as density (# c-Fos positive nuclei/area analyzed). To examine possible co-localization of c-Fos and corticotropin releasing factor (CRF) in the PVN and BNST, separate rats were treated with U-50488 (0.0 or 10.0 mg/kg; N=2 male rats/dose) and perfused 2-hr later. Double label immunohistochemistry was performed using methods described (53; Supplemental Methods).
Electrode placement histology
To compare ICSS electrode placements between males and females, a subset of rats was transcardially perfused with 4% paraformaldehyde. After fixation, coronal sections (40 µm) through the lateral hypothalamus were cut. Tissue was stained with cresyl violet and examined under the microscope. Locations of the electrode tips were mapped onto rat brain atlas plates (54).
Statistics
Dose- and time-dependent effects of U-50488 on ICSS thresholds and maximum rates of responding, as well as time-dependent effects on plasma U-50488 levels measured from repeated blood sampling were analyzed using linear mixed models with sex or cycle stage and dose or time as fixed effects and with a random effect on rat (see Supplemental Methods). Brain concentrations of U-50488 were analyzed with two-way (sex×time) ANOVA. Quantification of c-Fos expression was analyzed with two-way (sex×treatment) ANOVA for each brain region. Levels of the dependent variable “sex” include male and female, with males subdivided into pre- and post-castration (Fig. 3), females into estrous, diestrous, and proestrous (Fig. 2) or low and high E (Figs. 5, 6, 7). Significant effects and interactions were analyzed further with simple main effects tests and Bonferroni’s multiple comparisons post-hoc tests. IBM SPSS Statistics (Version 21) was used for linear mixed model analyses and GraphPad Prism 5.0 for Macintosh was used for all other statistical analyses.
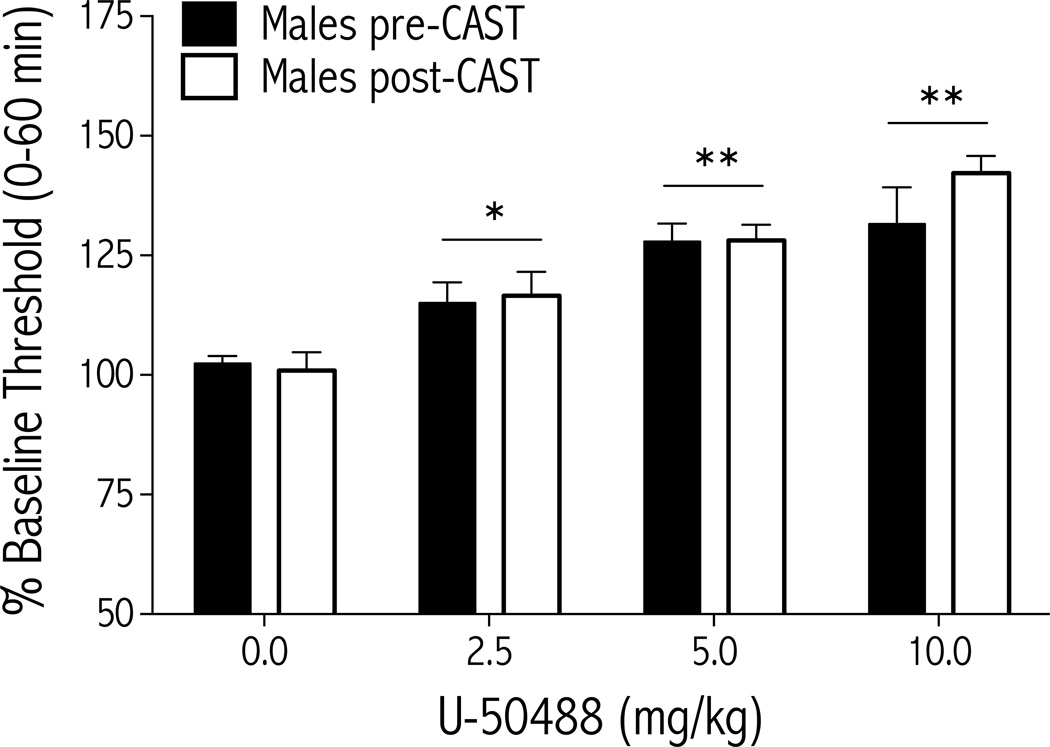
Figure 3.
The depressive-like effects of U-50488 do not depend on circulating testosterone in male rats (n=7). U-50488 (0.0, 2.5, 5.0, and 10.0 mg/kg, i.p.) was administered to rats before, and 4 weeks after, castration, and ICSS thresholds were measured immediately after each drug injection. U-50488 dose-dependently increased ICSS thresholds over the 1-hr test session to a similar extent in both gonadally intact and castrated rats (mean±SEM). *p<0.05, **p<0.01 simple main effects of dose compared to vehicle (0.0 mg/kg).
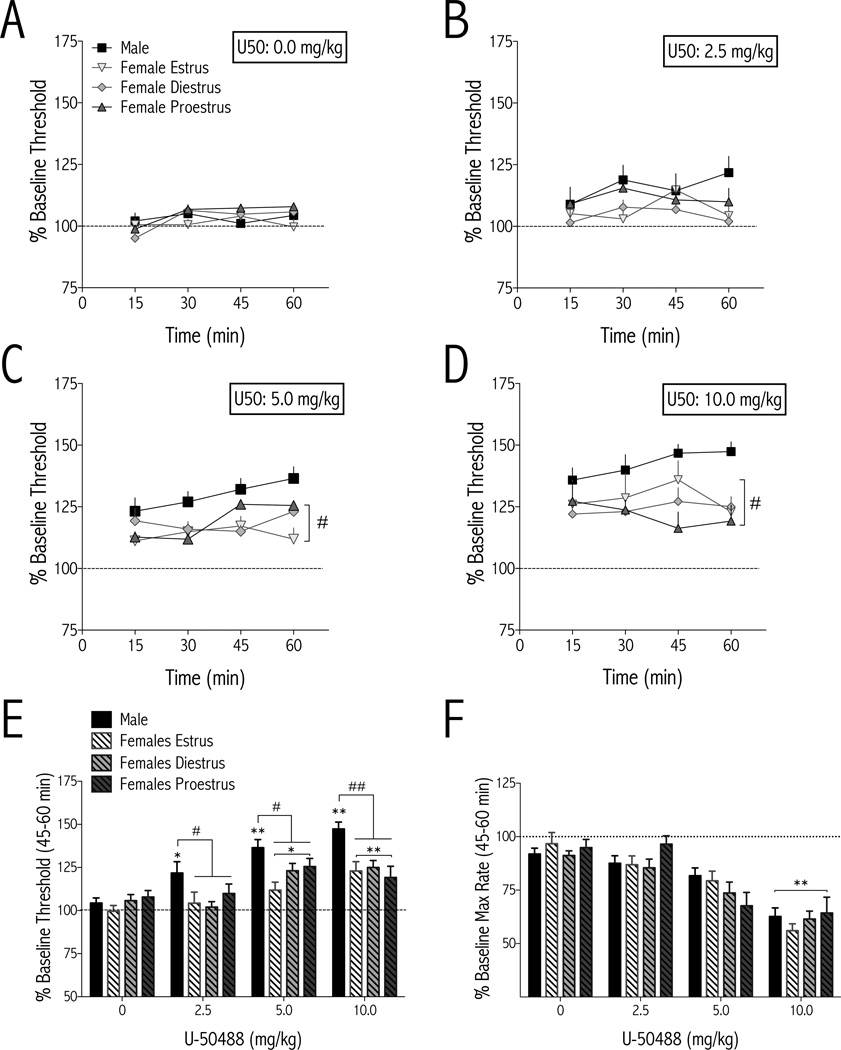
Figure 2.
Females are less sensitive than males to the threshold-increasing (anhedonic) effects of the KOR agonist U-50488 in ICSS. U-50488 or vehicle (water) was administered (i.p.) immediately after baseline thresholds were determined and testing began immediately after drug injection. Males, n=9–13/dose; females, n=7–13/dose/estrous cycle stage (mean±SEM). AD Effects of U-50488 on ICSS thresholds over time. At 5.0 and 10.0 mg/kg, the effects of U25 50488 depended on time and sex (#p<0.05 demonstrating the main effect of sex: aggregated females vs. males). E During the last 15 min of the test session, the effects of U-50488 on thresholds depended on an interaction between sex and dose. All rats responded to U-50488 with a dose-dependent increase in ICSS thresholds, but males had significantly higher thresholds than aggregated females at each dose of U-50488. F The effects of U-50488 on maximum rates of responding were dose, but not sex, dependent. C–F *p<0.05, **p<0.01 compared to vehicle (0.0 mg/kg) of the same sex; #p<0.05, ##p<0.01 comparing males and aggregated females at the indicated dose.
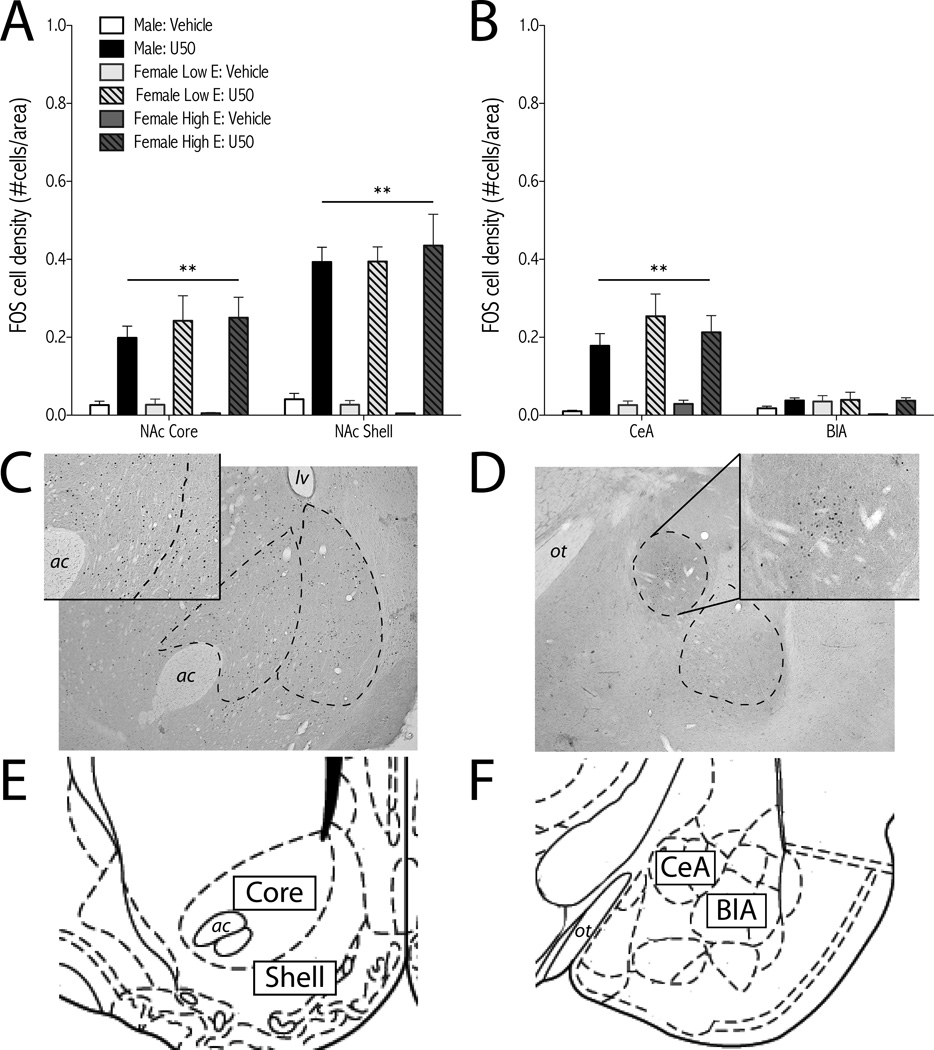
Figure 5.
U-50488 induces c-Fos expression in the NAc and amygdala. Rats were treated with U-50488 (10 mg/kg, i.p.) or vehicle (water) and perfused 2-hr later for c-Fos immunohistochemistry. Females were divided into low estrogen (E; metestrous and estrous) and high E (diestrous and proestrous) groups based on vaginal cytology. For analysis of NAc (Acore and shell) and amygdala (BCeA, and BlA), treatment groups are: male: vehicle (n=7); male: U50 (n=11); female low E: vehicle (n=6); female low E: U50 (n=4); female high E: vehicle (n=3); female high E: U50 (n=6); mean + SEM. **p<0.01 simple main effects of U50 compared to vehicle (0.0 mg/kg). Representative micrographs of U50-induced c-Fos in the NAc (C, insetmale:U50) and amygdala (D inset,CeA; male:U50) show dashed outlines of regions analyzed. Images from rat brain atlas drawings of NAc (EBregma +1.60mm)) and amygdala (FBregma − 2.80mm) adapted from (54). ac, anterior commissure; BlA, basolateral nucleus of the amygdala; CeA, central nucleus of the amygdala; E, estrogen; lv, lateral ventricle; NAc, nucleus accumbens; ot, optic tract; U50, U-50488.
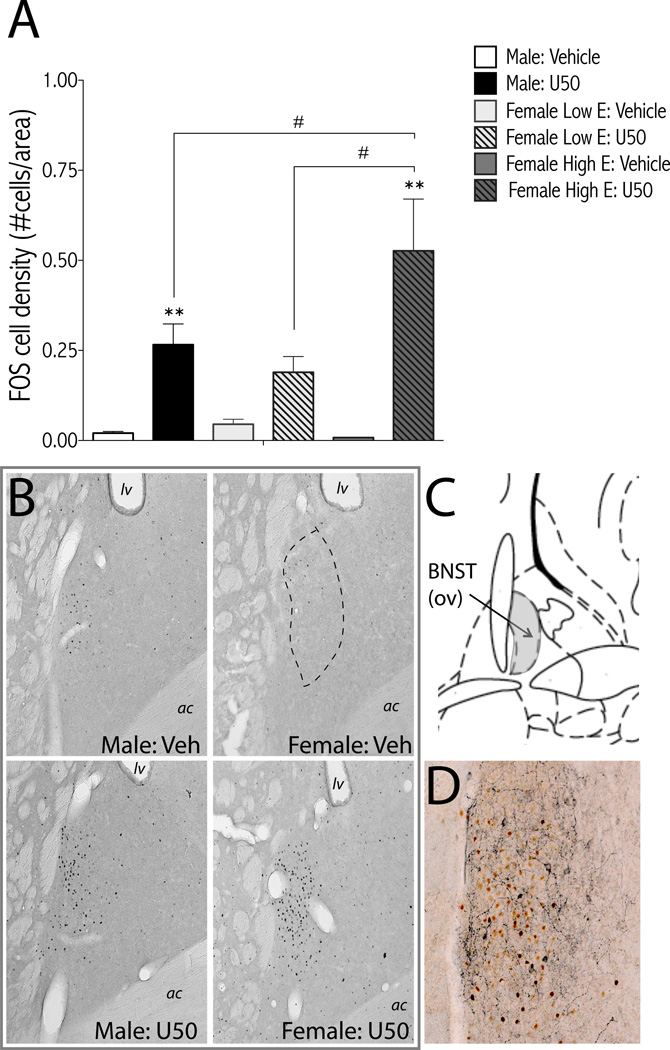
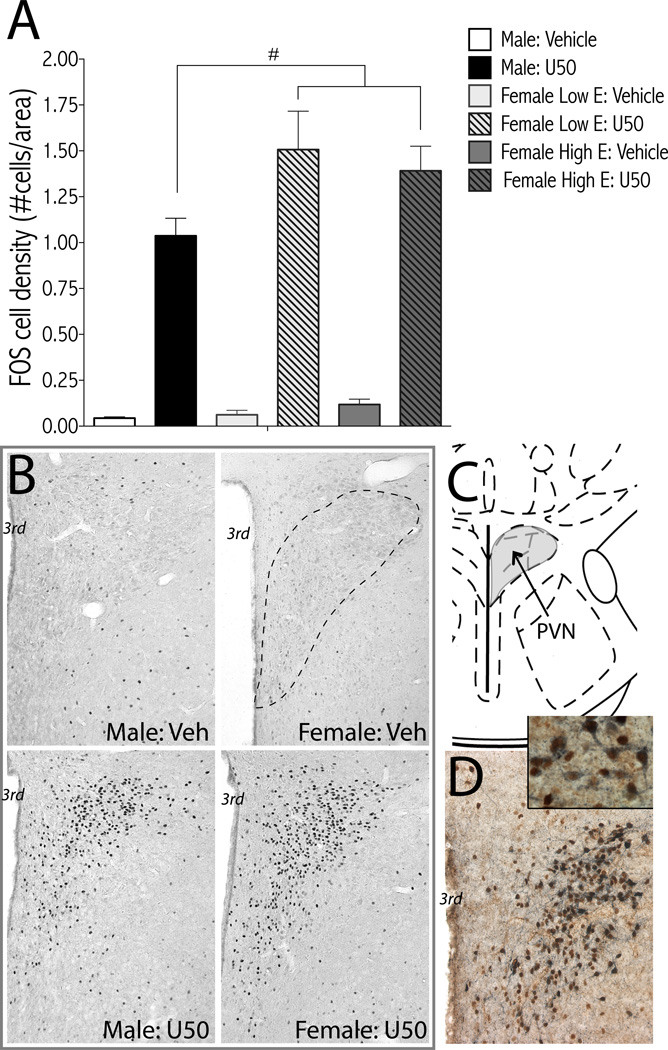
Figure 6.
U-50488-induced c-Fos expression in the BNST is sex dependent. Rats were treated with U-50488 (10 mg/kg, i.p.) or vehicle (water) and perfused 2-hr later for c-Fos immunohistochemistry. Females were divided into low estrogen (E; metestrous and estrous) and high E (diestrous and proestrous) groups based on vaginal cytology. (A) Treatment groups are: male: vehicle (n=7); male: U50 (n=11); female low E: vehicle (n=6); female low E: U50 (n=4); female high E: vehicle (n=3); female high E: U50 (n=6); mean + SEM. **p<0.01 compared to vehicle of same sex; #p<0.05 comparing groups under bars. (B) Representative micrographs of U50-induced c-Fos in the BNST. Dashed line shows region of BNSTov analyzed. (C) Image from rat brain atlas drawing of BNST (Bregma −0.26mm) adapted from (54). Oval nucleus of BNST is shaded grey. D Representative micrograph of double label immunohistochemistry for c-Fos (reddish-brown) and CRF (dark blue) from a male rat treated with U50 (10 mg/kg) shows separate populations of c-Fos- and CRF-positive neurons. ac, anterior commissure; BNSTov, bed nucleus of the stria terminalis oval nucleus; CRF, corticotropin releasing factor; E, estrogen; lv, lateral ventricle; U50, U-50488; Veh, vehicle.
Figure 7.
U-50488-induced c-Fos expression in the PVN is sex dependent. Rats were treated with U-50488 (10 mg/kg, i.p.) or vehicle (water) and perfused 2-hr later for c-Fos immunohistochemistry. Females were divided into low estrogen (E; metestrous and estrous) and high E (diestrous and proestrous) groups based on vaginal cytology. (A) Treatment groups are: male: vehicle (n=7); male: U50 (n=11); female low E: vehicle (n=6); female low E: U50 (n=4); female high E: vehicle (n=3); female high E: U50 (n=6); mean + SEM. #p<0.05 main effect of sex (female > male). (B) Representative micrographs of U50-induced c-Fos in the PVN. Dashed line shows region of PVN analyzed. (C) Image from rat brain atlas drawing of PVN (Bregma -1.80mm) adapted from (54). (D) Representative micrograph of double label immunohistochemistry for c-Fos (reddish-brown) and CRF (dark blue) from a male rat treated with U50 (10 mg/kg) shows co-expression of c-Fos and CRF. 3rd, third ventricle; E, estrogen; lv, lateral ventricle; CRF, corticotropin releasing factor; PVN, paraventricular nucleus of the hypothalamus; U50, U-50488; Veh, vehicle.
Results
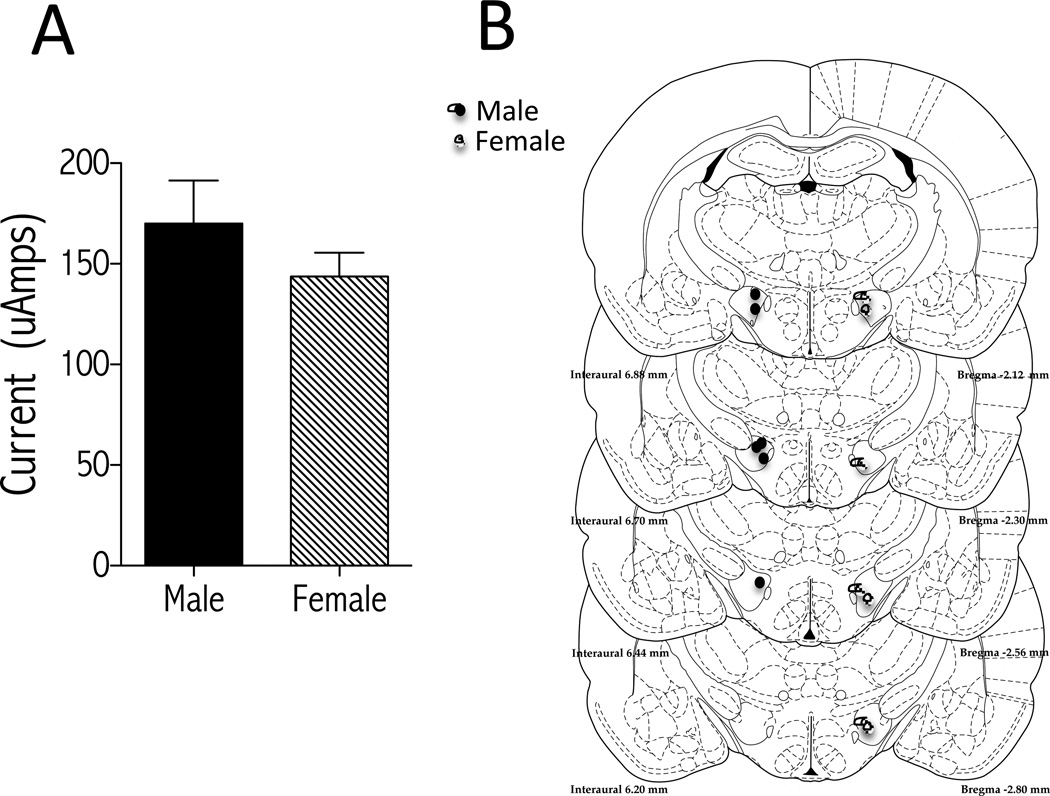
Both male and female rats responded similarly for electrical stimulation delivered through electrodes implanted in the medial forebrain bundle. There were no sex differences in baseline sensitivity to brain stimulation reward as measured by minimum currents that sustained optimal responding (≥40 presses/50s) (Fig. 1A). The time to reach stability (variation from mean of thresholds for 4 consecutive days is ≤10%) was also similar (4±1 weeks) between males and females. Electrode placements in rats that reliably pressed for stimulation and were used in the study were similar between males and females (Fig. 1B).
Figure 1.
Baseline ICSS parameters are similar in males and females. (A) Minimum currents (µAmps) that sustain high levels of responding do not differ between males (n=7) and females (n=8) (mean SEM; Student t test). (B) Summary of electrode tip placements in the medial forebrain bundle at the level of the lateral hypothalamus for a randomly chosen subset of male (black circles; n=6) and female (hatched circles; n=7) rats used in ICSS experiments shown in Figure 2. Images adapted from Paxinos and Watson (54).
Females are less sensitive than males to the reward-decreasing, but not rate-decreasing, effects of U-50488
To assess the effect of KOR activation on reward sensitivity in male and female rats, ICSS thresholds and maximum rates of responding were measured for 1 hr after U-50488 treatment. Females were treated with U-50488 at estrous, diestrous, and/or proestrous, depending on our ability to capture them in specific cycle stages. Given no clear effect of estrous cycle on ICSS, we first analyzed data from females using a linear mixed model with cycle and time (Fig. 2A–D) or cycle and dose (Fig. 2E, F) as fixed effects and with a random effect on rat. The only significant effect of cycle in female rats was with 10.0 mg/kg U-50488 on ICSS thresholds; [F(2,85) = 5.521, p<0.01 (Fig. 2D)]. However, simple main effects tests between cycles showed no significant differences. For all other analyses, we aggregated female data and performed linear mixed model analyses with sex (male and female) and time (Fig. 2A–D) or sex and dose (Fig. 2E, F) as fixed effects and rat as a random effect. We found that the effects of U-50488 (5.0 mg/kg) on ICSS thresholds over time depended on sex [F(1,20) = 11.674, p<0.01] and time [F(3,139) = 3.902, p<0.05 (Fig. 2C)]. The effects of U-50488 (10.0 mg/kg) on ICSS thresholds over time depended on cycle (“cycle” includes male, female estrous, diestrous, and proestrous) [F(3,48) = 8.459, p<0.001 (Fig. 2D)]. Main effects tests showed that each female cycle was significantly different from males (p<0.05), but not females. The effect of U-50488 on ICSS thresholds depended on an interaction between sex and dose [F(3,153) = 4.625, p<0.01 (Fig. 2E)], with U-50488-induced increases in ICSS thresholds significantly greater in males compared to females (in aggregate) at each dose. The effect of U-50488 on maximum rates of responding at 60 min depended on dose [F(3,137) = 34.788, p<0.01] but not sex [F(1,34) = 0.245, ns (Fig. 2F)], with a significant main effect of U-50488 (10.0 mg/kg) on rates. See Fig. S2 for effects of each U-50488 dose on maximum rates of responding over time. Taken together, there is a sex-dependent dissociation between the reward- versus motor-decreasing effects of KOR activation: males and females show similar KOR-mediated decreases in maximum rates of responding.
Since U-50488-mediated increases in ICSS thresholds did not significantly vary with estrous cycle stage, we hypothesized that activational effects of circulating testosterone are necessary for males’ enhanced response to U-50488. To test this, ICSS in males was measured before and after castration (Fig. 3). Baseline ICSS thresholds did not significantly differ from precastration baselines over time [F(8,48) = 1.305, ns] (see Fig. S3). However, a linear regression showed that the slope of the line through the data points is significantly nonzero [F(1,103) = 7.31, p<0.01; R2=0.066]. Two-way ANOVA with repeated measures on gonads (pre-CAST vs post-CAST) showed that effects of U-50488 depended on dose [F(3,18) = 26.38, p<0.0001], but not gonads. Pairwise comparisons of ICSS thresholds collapsed into doses showed that each dose of U-50488 tested significantly elevated thresholds, as in Fig 2E.
Pharmacokinetics of U-50488 in male and female rats
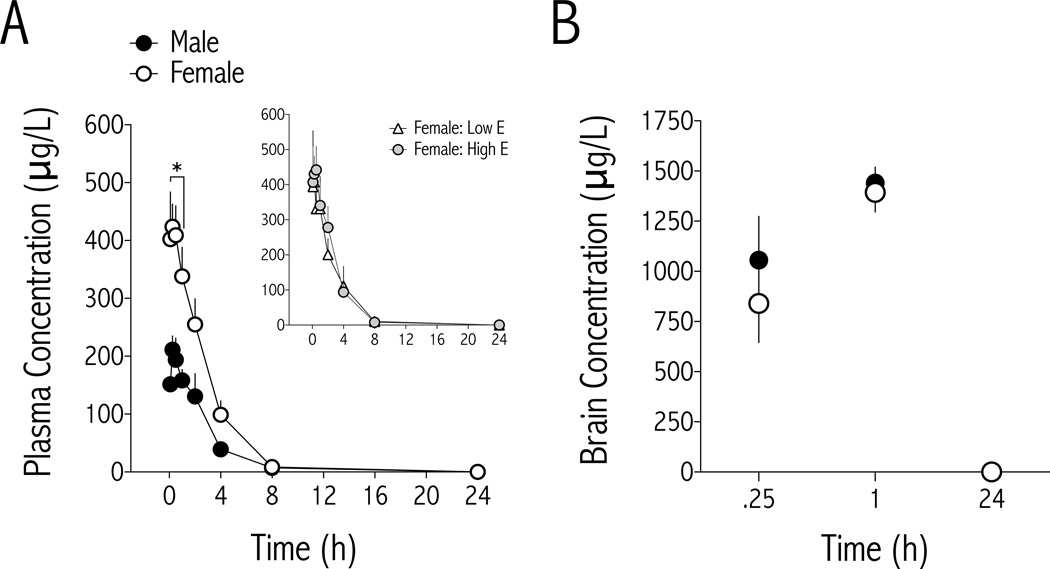
Plasma and brain concentrations of U-50488 were determined following U-50488 (10 mg/kg i.p.) administration to male and female rats (Fig. 4). Data were analyzed with a linear mixed model in which sex and time were fixed effects and rat was a random effect, to account for the fact that plasma samples were not obtained from all rats at each time point. Plasma concentrations of U- 50488 (Fig. 4A) depended on an interaction between sex and time [F(7,88) = 2.928, p<0.01], with U-50488 significantly greater in females compared to males for the first 60 minutes after treatment. Maximum U-50488 concentration (Cmax) and total area under the concentration×time curve (AUC) were higher in females (Table 1). Pharmacokinetic analyses of plasma concentrations clearance (CL/F) and terminal distribution (Vz/F) of U-50488 show that they are lower in female rats than males, raising the possibility that females have a slightly higher bioavailability than males. No sex difference in terminal plasma half-life U-50488 was observed (Table 1). Despite higher levels of U-50488 in female plasma, there were no sex differences in whole brain concentrations of U-50488 at times when behavioral differences were observed (Fig. 4B): Main effect of time [F(1,101) = 26.32, p<0.0001], but not sex [F(1,17) = 1.08, ns]. These data show that males have lower plasma, but similar brain, levels of U-50488 at times when males show greater increases in ICSS thresholds than females.
Figure 4.
Plasma and brain levels of U-50488. (A) U-50488 levels are significantly higher in female (n=8–11/time point) compared to male (n=4–6/time point) plasma for the first 60 min after drug injection (10.0 mg/kg, i.p.), and remain higher than males for at least 4 hr (mean±SEM). *p<0.05 comparing males and females at each time point. Inset Separating females into either high or low estrogen groups reveals no difference in time course of plasma U-50488 levels. (B) Brain concentrations of U-50488 are similar between males (n=3/time point) and females (n=3- 8/time point) at 15 min, 1- and 24 hr after drug injection.
Table 1.
Pharmacokinetics of U-50488 (10 mg/kg, i.p.) in plasma from male and female rats. Values are Mean ± S.D.
| Males | Females | |
|---|---|---|
| Cmax (µg/L) | 236 ± 79 | 532 ± 191** |
| Tmax (h) | 0.313 ± 0.125 | 0.400 ± 0.576 |
| AUC (µg* h/L) | 644 ± 278 | 1350 ± 570* |
| CL/F (L/h* kg) | 16.9 ± 5.3 | 8.71 ± 3.82** |
| Vz/F (L/kg) | 68.6 ± 27.9 | 30.5 ± 15.5** |
| t1/2 (h) | 2.75 ± 0.42 | 2.38 ± 0.42 |
p<0.05,
p<0.01,
unpaired, 2-tailed t-test. N=6 male, 11 female
Sex-dependent patterns of c-Fos activation in limbic brain regions
To begin to understand how KORs modulate neural circuits within the mesocorticolimbic system, amygdala, and hypothalamus in males and females, we measured c-Fos expression in response to U-50488. Given no overall effect of estrous cycle stage on ICSS behavior (Fig. 2), we used vaginal epithelial cytology to separate females into “low estrogen” (estrous, metestrous) and “high estrogen” (diestrous, proestrous) groups. There were main effects of treatment for U-50488-induced c-Fos in the NAc core [F(1,31) = 72.79, p<0.0001], NAc shell [F(1,31) = 153.15, p<0.0001] (Fig. 5A,C), central nucleus of the amygdala [CeA; [F(1,31) = 72.83, p<0.001], (Fig. 5B,D), BNST [F(1,31) = 39.25, p<0.0001] (Fig. 6), and PVN [F(1,31) = 247.69, p<0.0001] (Fig. 7). However, there were only significant sex differences in c-Fos induction in the BNSTov (Fig. 6A), with a sex×treatment interaction [F(2,31) = 4.37, p<0.05] and the PVN (Fig. 7A), with main effects of sex [F(2,31) = 3.34, p<0.05] and treatment [F(1,31) = 181.8, p<0.01]. There was significantly more c-Fos in the BNST of females with high, compared to low, estrogen, and significantly more c-Fos in the PVN of both high and low estrogen females compared to males.
Both the BNSTov and parvocellular cells of the PVN produce CRF (55, 56), so we used doublelabel immunohistochemistry to determine if c-Fos expression overlapped with CRF. Although c- Fos and CRF expression were both strong in the BNSTov, we observed little double labeling (Fig. 6D). In the PVN, c-Fos expression appeared confined to parvocellular regions, and qualitatively there was clear overlap between c-Fos and CRF expressing cells (Fig. 7C).
Discussion
From mice to humans, KORs are involved in stress-, fear-, anxiety-, and depressive-like behaviors in males (15, 30, 31). However, few studies have examined the contribution of KORs to these behaviors in females. Here we demonstrate that female rats are less sensitive than males to the depressive-like effects of the KOR agonist U-50488 using ICSS. Importantly, these effects are not due to sex differences in pharmacokinetics, since brain levels of U-50488 are similar between males and females at times when ICSS behavior differs. Our data suggest that circulating gonadal hormones are not a direct cause of females’ decreased sensitivity to U- 50488 because 1) females show a uniformly reduced behavioral response to the KOR agonist, regardless of estrous cycle stage, and 2) the level of U-50488-induced anhedonia in males is unchanged after castration. Finally, using c-Fos activation to identify regions of increased neural activity (57), we identify the BNST and PVN as potential sexually dimorphic substrates for the depressive-like effects of KOR activation.
Our finding that females are less sensitive than males to the threshold-increasing effects of U- 50488 raises the possibility that KORs play a more significant role in drug- and stress-induced depressive-like states in males. Consistent with this, KORs are necessary for selective aggression in pair-bonded male, but not female, prairie voles (42). One explanation for the observed sex difference in U-50488-induced anhedonia is that females find the stimulation itself more rewarding, offsetting negative effects of KOR activation. However, there was no difference in minimum currents required to sustain operant responding, consistent with prior studies using the discrete trial method of ICSS (58, 59). Electrical stimulation of the medial forebrain bundle at frequencies similar to those used in our ICSS studies produces equivalent levels of dopamine overflow in the striata of male and female rats (60), suggesting comparable levels of baseline dopaminergic function. Finally, histological analysis of electrode placements showed no overt differences in the optimal location of electrode tip within the lateral hypothalamus between males and females.
Earlier studies suggest that women are more sensitive than men to the psychomotor stimulant, rewarding, and reinforcing properties of cocaine (for review see 61). Many of these effects are reported to be dependent on circulating gonadal hormones, with estrogen potentiating, and testosterone blunting, behavioral effects of psychostimulants (61, 62, 63, 64). Since KORs produce depressive-like effects, in part, through inhibition of dopamine release in the NAc (17, 21), females may be less sensitive to the reward-decreasing effects of U-50488 because estrogen counteracts—while testosterone potentiates—the inhibitory effects of KORs on dopamine release. Consistent with this, it has been reported that castration decreases dynorphin release in the hypothalamus (65). Despite these findings, it is unlikely that circulating gonadal hormones are a major factor in our study since the depressive-like effects of U-50488 do not significantly vary with the estrous cycle or after castration. Furthermore, after castration ICSS thresholds did not significantly change from pre-castration baseline values, although the slope of the linear regression line through each day’s percent baseline threshold values was greater than zero. These data suggest that—in the present study—brain stimulation reward per se was unaffected, which is consistent with an early study showing that castration did not affect glucose preference (66). It has been shown that rates of responding for electrical stimulation are highest between proestrous and estrous (67, 68). However, in the current study, the rate-decreasing effects of U-50488 are similar between males and females (regardless of cycle stage), suggesting that neural circuits regulating KOR effects on hedonic state are sexually dimorphic whereas those that regulate KOR effects on motor function are not (59). Taken together, our data raise the possibility that decreased sensitivity of females to the anhedonic effects of KOR activation enables increased sensitivity to cocaine reward.
Sex differences in pharmacokinetics have been reported in many species (69). We found that plasma concentrations of U-50488 were significantly higher in female compared to male rats for the first 60-min after injection, although terminal half-life was the same in both sexes. In addition, clearance of drug from plasma was significantly lower in females, suggesting a higher bioavailability of U-50488 in females. However, there were no sex differences in brain concentrations of U-50488 at 15 min and 1 hr, times at which females are less sensitive than males to the threshold-increasing effects of U-50488. This suggests that pharmacokinetics do not play a significant role in sex differences in KOR-mediated depressive-like effects. The reason for this discrepancy between plasma and brain levels is unknown, but may involve sex differences in uptake or efflux transporters (70, 71). However, the structurally similar KOR agonist U69,593 is not a substrate for the efflux transporter P-gp (72).
To identify putative neural substrates for sex differences in depressive-like effects of KOR activation, we measured U-50488-induced c-Fos expression in the NAc, CeA, BLA, PVN and BNST, sexually dimorphic regions previously shown to regulate mood (60, 73, 74). The NAc is a well-established site of KOR and dopamine interactions (17, 75), and it has been shown that direct infusion of U-50488 into the NAc shell is aversive (21, 76). KOR activation in the amygdala is anxiolytic and critical for fear memory (77), raising the possibility that it contributes to pro-depressive consequences of stress. The CeA sends projections to the BNST that coexpress GABA and dynorphin, and it has been shown that dynorphin acts to inhibit GABA release and hence facilitate CRF activation of the BNST (78). The BNST projects to multiple regions, including the VTA and PVN (79), a gateway to hypothalamic pituitary adrenal (HPA) axis activation. We found sex-dependent effects in the BNST and PVN.
In the BNST, U-50488-induced c-Fos was limited to the oval nucleus in the anterior dorsolateral region (BNSTov). In females, the number of c-Fos positive nuclei was greatest during high estrogen cycle stages. Previously it was shown that the total number of cells and volume of the anterior dorsolateral BNST is greater in females (80, 81), with testosterone suppressing cell number in males (80). Taken together, it is possible that the greater c-Fos expression observed in high estrogen females is a result of hormone-mediated fluctuations in BNST volume and cell number. Future studies using stereology could confirm this. The BNSTov is rich in GABAergic and CRF-containing neurons and fibers, but our initial studies suggest no overlap between c- Fos and CRF expression, consistent with previous findings (82). Given that U-50488-induced c- Fos expression in the BNST appears to be hormone status-dependent whereas ICSS behavior is not, the BNST may not play a critical role in anhedonia per se, but may be more relevant to sex differences in stress- and anxiety-related disorders (83, 84).
U-50488-induced c-Fos in the PVN was higher in females than males, regardless of estrous cycle stage. The PVN is necessary for the net stress response that ultimately controls glucocorticoid output of the HPA axis (56). Similar to a prior study (85), U-50488-induced c-Fos overlapped with CRF expression in the medial parvocellular zone of the PVN and was segregated from oxytocin-expressing neurons of the magnocellular division (86) (data not shown). Although the functional consequences of U-50488-induced c-Fos in the PVN and sex differences therein are not known, the observation in females that U-50488 elicits greater activation of parvocellular PVN neurons but decreased depressive-like effects compared to males suggests that KOR-mediated activation of the HPA axis counteracts the depressive-like effects of KOR activation in other brain regions [e.g. NAc, (21)]. This is supported by findings that ACTH and corticosterone, which are triggered by CRF, can facilitate brain stimulation reward (87, 88) and are required for acquisition and maintenance of cocaine self-administration (89).
Results from this study add to an emerging literature on sex differences in dynorphin-KOR systems and provide the first evidence that female rats are less sensitive than males to the depressive-like effects of KOR activation. These findings raise important questions: Does this translate to sex differences in sensitivity to endogenous dynorphin? Do sex differences occur at the level of KORs or from engagement of downstream, sexually dimorphic substrates? Future studies are needed to determine the role of the PVN and BNST, as well as afferent aminergic nuclei such as the locus coeruleus and dorsal raphe, in KOR-mediated behaviors, with particular emphasis on differentiating anhedonia, anxiety, and the HPA axis stress response. Regardless, the present studies suggest that distinct mechanisms underlie stress- and druginduced depressive-like states in males and females and raise the possibility that KOR-based pharmacotherapies for mood disorders will have different efficacies in men and women.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Drug Abuse (Grant Number DA033526 to EHC). We thank Dr. Garrett Fitzmaurice for assistance with statistical analysis. A portion of this work has appeared in poster form at the Society for Neuroscience annual meeting (Russell et al., Society for Neuroscience 2012; Program No. 400.03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
Dr. Elena Chartoff, Dr. Karen Smith, Ms. Shayla Russell, Ms. Anna Rachlin, Mr. David Potter, Dr. John Muschamp, Mr. Loren Berry, and Dr. Zhiyang Zhao report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Holden C. Sex and the suffering brain. Science. 2005;308:1574. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psych Clin No Amer. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch WJ. Sex differences in vulnerability to drug self-administration. Exper Clin Psychopharm. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- 5.Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharm. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- 6.Griffin ML, Weiss RD, Mirin SM, Lange U. A Comparison of Male and Female Cocaine Abusers. Arch Gen Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- 7.Fox HC, Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 10.Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurd YL, Herkenham M. Molecular Alterations in the Neostriatum of Human Cocaine Addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 12.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, et al. Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 16.Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- 17.Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlezon WA, Jr, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-Like Effects of the kappa-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 19.DiNieri JA, Nemeth CL, Parsegian A, Carle T, Gurevich VV, Gurevich E, et al. Altered Sensitivity to Rewarding and Aversive Drugs in Mice with Inducible Disruption of cAMP Response Element-Binding Protein Function within the Nucleus Accumbens. J Neurosci. 2009;29:1855–1859. doi: 10.1523/JNEUROSCI.5104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 21.Muschamp JW, Van't Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, et al. Activation of CREB in the Nucleus Accumbens Shell Produces Anhedonia and Resistance to Extinction of Fear in Rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappaopioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 23.Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 29.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- 30.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fallon JH, Leslie FM, Cone RI. Dynorphin-containing pathways in the substantia nigra and ventral tegmentum: a double labeling study using combined immunofluorescence and retrograde tracing. Neuropeptides. 1985;5:457–460. doi: 10.1016/0143-4179(85)90053-8. [DOI] [PubMed] [Google Scholar]
- 34.Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- 36.Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- 37.Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 39.Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, et al. The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci U S A. 1995;92:5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY. Sex difference in kappa-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5'-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J Pharmacol Exp Ther. 2011;339:438–450. doi: 10.1124/jpet.111.183905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasakham K, McGillivray KL, Liu-Chen LY. Sex differences in U50,488H-induced phosphorylation of p44/42 mitogen-activated protein kinase in the guinea pig brain. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. kappa-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Negus SS, Mello NK. Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther. 1999;290:1132–1140. [PubMed] [Google Scholar]
- 44.Negus SS, Mello NK. Effects of gonadal steroid hormone treatments on opioid antinociception in ovariectomized rhesus monkeys. Psychopharmacology (Berl) 2002;159:275–283. doi: 10.1007/s002130100912. [DOI] [PubMed] [Google Scholar]
- 45.Negus SS, Zuzga DS, Mello NK. Sex differences in opioid antinociception in rhesus monkeys: antagonism of fentanyl and U50,488 by quadazocine. J Pain. 2002;3:218–226. doi: 10.1054/jpai.2002.124734. [DOI] [PubMed] [Google Scholar]
- 46.Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005;6:261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol. 2008;16:376–385. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- 48.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy MM. How it's made: organisational effects of hormones on the developing brain. J Neuroendocrinol. 2010;22:736–742. doi: 10.1111/j.1365-2826.2010.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 51.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat prot. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 52.Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith KL, Roche M, Jessop DS, Finn DP. The effects of synthetic and endogenous imidazoline binding site ligands on neuronal activity in discrete brain regions of naive and restraint-stressed rats. Eur Neuropsychopharmacology. 2009;19:371–380. doi: 10.1016/j.euroneuro.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Third ed. Academic Press; 1996. [Google Scholar]
- 55.Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 57.Morgan JI, Curran T. Stimulus-Transcription Coupling in the Nervous-System - Involvement of the Inducible Protooncogenes Fos and Jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 58.Kornetsky C, Bain G. Brain-stimulation reward: a model for the study of the rewarding effects of abused drugs. NIDA Res Monogr. 1992;124:73–93. [PubMed] [Google Scholar]
- 59.Stratmann JA, Craft RM. Intracranial self-stimulation in female and male rats: no sex differences using a rate-independent procedure. Drug Alcohol Depend. 1997;46:31–40. doi: 10.1016/s0376-8716(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 60.Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- 61.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 62.White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol, biochem, and behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- 63.Beatty WW, Dodge AM, Traylor KL. Stereotyped behavior elicited by amphetamine in the rat: influences of the testes. Pharmacol, biochem, and behav. 1982;16:565–568. doi: 10.1016/0091-3057(82)90416-6. [DOI] [PubMed] [Google Scholar]
- 64.Long SF, Dennis LA, Russell RK, Benson KA, Wilson MC. Testosterone implantation reduces the motor effects of cocaine. Behav Pharmacol. 1994;5:103–106. doi: 10.1097/00008877-199402000-00012. [DOI] [PubMed] [Google Scholar]
- 65.Almeida OFX, Nikolarakis KE, Herz A. Significance of Testosterone in Regulating Hypothalamic Content and In Vitro Release of β-Endorphin and Dynorphin. J Neurochem. 1987;49:742–747. doi: 10.1111/j.1471-4159.1987.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 66.Gabric D, Soljacic M. Effect of gonadectomy on taste preference for glucose solutions in rats. Physiol Behav. 1975;15:145–148. doi: 10.1016/0031-9384(75)90227-9. [DOI] [PubMed] [Google Scholar]
- 67.Prescott RG. Estrous cycle in the rat: effects on self-stimulation behavior. Science. 1966;152:796–797. doi: 10.1126/science.152.3723.796. [DOI] [PubMed] [Google Scholar]
- 68.Steiner M, Katz RJ, Baldrighi G, Carroll BJ. Motivated behavior and the estrous cycle in rats. Psychoneuroendocrinology. 1981;6:81–90. doi: 10.1016/0306-4530(81)90051-2. [DOI] [PubMed] [Google Scholar]
- 69.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 70.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dagenais C, Zong J, Ducharme J, Pollack GM. Effect of mdr1a P-glycoprotein gene disruption, gender, and substrate concentration on brain uptake of selected compounds. Pharm Res. 2001;18:957–963. doi: 10.1023/a:1010984110732. [DOI] [PubMed] [Google Scholar]
- 72.Butelman ER, Caspers M, Lovell KM, Kreek MJ, Prisinzano TE. Behavioral effects and central nervous system levels of the broadly available kappa-agonist hallucinogen salvinorin A are affected by P-glycoprotein modulation in vivo. J Pharmacol Exp Ther. 2012;341:802–808. doi: 10.1124/jpet.112.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 74.Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- 77.Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, et al. Kappa Opioid Receptor Signaling in the Basolateral Amygdala Regulates Conditioned Fear and Anxiety in Rats. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, et al. Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol Psychiatry. 2012;71:725–732. doi: 10.1016/j.biopsych.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 80.Guillamon A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res. 1988;44:281–290. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- 81.del Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987;429:295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- 82.Kozicz T. Met-enkephalin immunoreactive neurons recruited by acute stress are innervated by axon terminals immunopositive for tyrosine hydroxylase and dopamine-alphahydroxylase in the anterolateral division of bed nuclei of the stria terminalis in the rat. Eur J Neurosci. 2002;16:823–835. doi: 10.1046/j.1460-9568.2002.02129.x. [DOI] [PubMed] [Google Scholar]
- 83.Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Roubos EW, et al. Sex-dependent and differential responses to acute restraint stress of corticotropinreleasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res. 2012;90:179–192. doi: 10.1002/jnr.22737. [DOI] [PubMed] [Google Scholar]
- 84.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Posttraumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laorden ML, Castells MT, Martinez MD, Martinez PJ, Milanes MV. Activation of c-fos expression in hypothalamic nuclei by mu- and kappa-receptor agonists: correlation with catecholaminergic activity in the hypothalamic paraventricular nucleus. Endocrinology. 2000;141:1366–1376. doi: 10.1210/endo.141.4.7407. [DOI] [PubMed] [Google Scholar]
- 86.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 87.Katz RJ. Effects of an ACTH 4-9 related peptide upon intracranial self stimulation and general activity in the rat. Psychopharmacology (Berl) 1980;71:67–70. doi: 10.1007/BF00433254. [DOI] [PubMed] [Google Scholar]
- 88.Barr AM, Brotto LA, Phillips AG. Chronic corticosterone enhances the rewarding effect of hypothalamic self-stimulation in rats. Brain Res. 2000;875:196–201. doi: 10.1016/s0006-8993(00)02652-4. [DOI] [PubMed] [Google Scholar]
- 89.Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–348. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.