Abstract
Factors regulating excystment of a toxic dinoflagellate in the genus Alexandrium were investigated in cysts from Puget Sound, Washington State, USA. Experiments were carried out in the laboratory using cysts collected from benthic seedbeds to determine if excystment is controlled by internal or environmental factors. The results suggest that the timing of germination is not tightly controlled by an endogenous clock, though there is a suggestion of a cyclical pattern. This was explored using cysts that had been stored under cold (4 °C), anoxic conditions in the dark and then incubated for 6 weeks at constant favorable environmental conditions. Excystment occurred during all months of the year, with variable excystment success ranging from 31–90%. When cysts were isolated directly from freshly collected sediments every month and incubated at the in situ bottom water temperature, a seasonal pattern in excystment was observed that was independent of temperature. This pattern may be consistent with secondary dormancy, an externally modulated pattern that prevents excystment during periods that are not favorable for sustained vegetative growth. However, observation over more annual cycles is required and the duration of the mandatory dormancy period of these cysts must be determined before the seasonality of germination can be fully characterized in Alexandrium from Puget Sound. Both temperature and light were found to be important environmental factors regulating excystment, with the highest rates of excystment observed for the warmest temperature treatment (20 °C) and in the light.
Keywords: Alexandrium, Puget Sound, Excystment, Cysts, Harmful algal bloom, Red tide
1. Introduction
Puget Sound is a fjord-type estuary in Washington State, USA. The average water depth of Puget Sound is ∼62 m, but depths approach ∼200 m in the Main Basin. It is connected to the Pacific Ocean by the Strait of Juan de Fuca, a turbulent passage ∼160 km long (Thomson, 1994). The geography of Puget Sound is complex and includes three main branching basins and a shallower southern basin that contains numerous finger inlets. Puget Sound and its adjacent inland coastal waters have a long history with toxic blooms of Alexandrium spp. that can contaminate shellfish and cause paralytic shellfish poisoning (PSP) in humans (Quayle, 1969; Kao, 1993). The first recorded cases of PSP occurred in 1793, when four people were sickened after eating mussels harvested from the central coast of British Columbia (Vancouver, 1798). Since the 1950s, toxin levels in shellfish tissues have increased in frequency, magnitude, and geographic scope and shellfish harvesting closures are now a regular occurrence in almost all of Puget Sound's basins (Trainer et al., 2003). Toxic blooms typically occur from July to November annually, but interannual variability is high (Moore et al., 2009). The species of Alexandrium thought to be responsible for the production of these toxins in Puget Sound has historically been identified as Alexandrium catenella (Whedon & Kofoid) Balech. This is synonymous with Alexandrium tamarense Group I, a provisional species name proposed by Lilly et al. (2007). However, the name Alexandrium fundyense has recently been proposed to replace all Group I strains of the A. tamarense species complex that includes A. catenella (John et al., 2014a, 2014b). In light of the recent work by John et al. (2014a) and recognizing alternative recommendations by Wang et al. (2014), we will refer here only to the genus name Alexandrium.
The life history of Alexandrium includes a resting cyst stage (Dale, 1983; Anderson, 1998). Cysts remain dormant on the seafloor when conditions are unsuitable for growth in the overlying waters (termed ‘quiescence’; Pfiester and Anderson, 1987). Both internal (endogenous) and environmental (exogenous) factors can induce excystment and provide the vegetative cell inoculum to initiate blooms. The first level of endogenous control is cyst maturation, which prevents newly formed cysts from germinating (e.g., Anderson and Morel, 1979). Another type of endogenous regulation produces an annual oscillation in excystment potential for some Alexandrium under constant environmental conditions (Anderson and Keafer, 1987). Cysts displaying this rhythm are said to possess an endogenous annual clock. The average period of the endogenous clock in resulting Alexandrium fundyense excystment in the Gulf of Maine is 11 months (Matrai et al., 2005). A function fitted to this rhythm exhibits peak excystment from March through June (Anderson et al., 2005). The presence of an endogenous clock is thought to be advantageous for cysts lying dormant at depth where seasonal variations in environmental conditions are small (i.e., cysts cannot tell when conditions at the surface are optimal for growth of the planktonic, vegetative cells). Anderson and Keafer (1987) point out that Alexandrium cysts from shallower systems in the same region did not appear to exhibit endogenous control, perhaps because they need to be more flexible due to the greater environmental variability in estuaries and small embayments. This latter response was termed ‘secondary dormancy’, a term from the vascular plant literature that describes how seeds alternately germinate or remain unresponsive in the quiescent state when exposed to the same environmental conditions (Vleeshouwers et al., 1995).
Tobin and Horner (2011) also suggest that Alexandrium from Puget Sound do not possess an endogenous clock that regulates excystment. Unlike the deep-water cyst seedbeds in the Gulf of Maine that are located at 150 m or deeper (Anderson et al., 2005), seedbeds in Puget Sound appear to be located in relatively shallow embayments (i.e., <30 m deep; Horner et al., 2011). These sediments experience considerable seasonal variation, so excystment may have some environmental regulation. However, as explained below, the method used by Tobin and Horner (2011) to determine ‘germination success’ may have masked the ability to detect the presence of an endogenous clock. This study revisits that question with improved methods.
The other mechanism that can produce an annual oscillation in excystment is secondary dormancy, as was suggested for two species of Alexandrium in Cork Harbor, Ireland (Rathaille and Raine, 2011). In Alexandrium tamarense and Alexandrium minutum from Cork Harbor, secondary dormancy (or some other as yet undescribed process that produces a similar result) prevents cysts from germinating during periods when environmental conditions are actually favorable for excystment but not for sustained growth and survival or the production of offspring. The phenomenon was observed by isolating cysts directly from the environment (i.e., no long periods of cold storage) and germinating them at the same water temperature as the environment from which the cysts were collected. Even though the temperature of Cork Harbor is the same in spring as in fall, Alexandrium cysts freshly isolated from natural sediments germinated in the spring but not in the fall. These Alexandrium spp. typically bloom during the warm season in Cork Harbor; therefore, excystment in the fall would result in offspring entering the water column when conditions are becoming unfavorable for growth of the planktonic cells. Seasonal regulation of Alexandrium excystment described by Rathaille and Raine (2011) could not be explained by the length of the mandatory dormancy period (∼2 months) or the presence of an endogenous clock. Furthermore, this seasonal rhythm produced by secondary dormancy is known to disappear with storage in constant cold conditions (Karssen, 1981). Therefore, as noted by Rathaille and Raine (2011), secondary dormancy would not be detectable during the course of a study to identify an endogenous clock because sediment is typically stored at a constant temperature of 4 °C for a period of ∼4–6 months before the experiments begin to allow for the completion of the mandatory dormancy period. Secondary dormancy may have important implications for blooms of Alexandrium in Puget Sound because it could produce a seasonal cycle in excystment that is quite similar to that produced by an endogenous clock, except that it is regulated by the external environment.
Of the environmental factors known to regulate Alexandrium excystment, temperature, light, and oxygen have been found to exert the greatest control (Anderson et al., 1987, 2005). For some species of Alexandrium, a temperature ‘window’ has been described whereby cysts will not germinate at the warm and cold extremes outside of a favorable range (Anderson, 1980, 1998). Within the temperature window and for temperatures most likely to be encountered by Alexandrium in the wild, rates of excystment are generally higher for the warmer temperatures examined and in the light (Anderson et al., 1987, 2005). Excystment will not occur under anoxic conditions (Anderson et al., 1987).
In order to develop a predictive capacity for Alexandrium blooms in Puget Sound, a better understanding of the life history characteristics of local strains of Alexandrium is required; specifically, of the processes governing excystment and bloom initiation. Hence, the goals of this study were to investigate the nature of seasonal patterns of excystment for Alexandrium and the mechanisms responsible (i.e., endogenous clock, secondary dormancy, or a yet to be defined mechanism), and to evaluate the effects of temperature and light on the rates of Alexandrium excystment.
2. Methods
2.1. Study area
Sediment for this study was collected from two sites in Puget Sound; Quartermaster Harbor in the south (47°22.78′N, 122°28.01′W; 13 m depth) and Bellingham Bay in the north (48°43.66′N, 122°36.75′W; 9 m depth; Fig. 1). Quartermaster Harbor has a shallow inner bay with an average depth of 6 m that is connected to an outer bay with an average depth of 12 m. Quartermaster Harbor was first identified to be an important cyst bed in Puget Sound during a survey in 2005 when it was found to contain >12,000 cysts per cm3 (Horner et al., 2011). Subsequent surveys from 2011–2013 detected an order of magnitude fewer cysts in Quartermaster Harbor (Greengrove et al., 2014), though the sediment sampling methods changed from subsampling a Soutar box core in 2005 to using a Craib corer from 2011–2013. Nevertheless, Quartermaster Harbor remains an important cyst bed in Puget Sound with the highest cyst abundances relative to other sites surveyed in any given year (Greengrove et al., 2014). Quartermaster Harbor was also the site of sediment collection for the Tobin and Horner (2011) study that concluded that Alexandrium in this region do not possess an endogenous clock. Bellingham Bay is characterized by shallow tidal flats at each end that gradually deepen to the central bay floor at ∼28 m. Bellingham Bay first emerged as an important cyst bed in Puget Sound during a survey in 2011, when it was found to contain up to 4199 cysts per cm3 (Greengrove et al., 2014). This cyst bed was not detected during the earlier 2005 survey by Horner et al. (2011), suggesting that it formed more recently. The sediments at both the Quartermaster Harbor and Bellingham Bay sites consisted of soft mud.
Fig. 1.
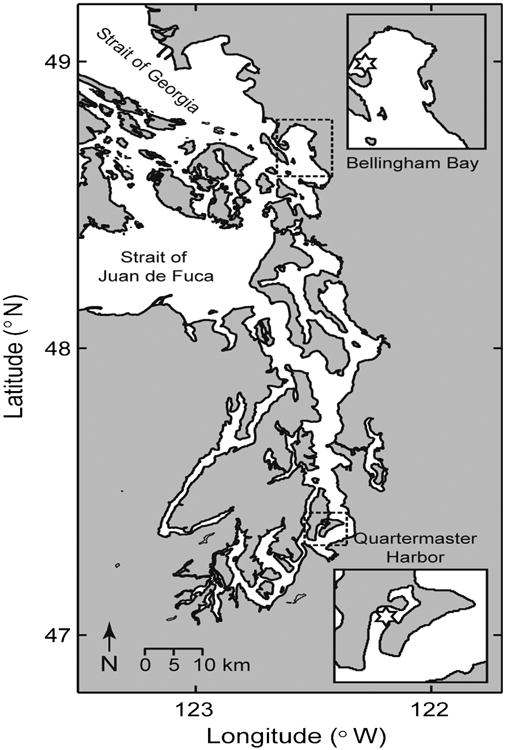
Puget Sound, Washington State, USA, showing the locations where sediment was collected in Quartermaster Harbor and Bellingham Bay (insets).
2.2. Endogenous clock
Sediment for the experiments to test for the presence of an endogenous clock was collected from Quartermaster Harbor during February 2012 from the R/V Clifford A. Barnes. The timing of the cruise was carefully chosen to occur after the formation of cysts the previous summer and fall and before cysts could germinate the following summer. Plankton samples were taken at the time of sediment collection by filtering 20 L seawater from the ship's seawater intake (∼1 m depth) through a 20-μm sieve. No vegetative cells were observed in the concentrated samples, confirming that the local population of Alexandrium was encysted in the sediment. Sediment was collected using a hydraulically dampened Craib corer (Craib, 1965) which provides short, undisturbed sediment cores with a diameter of 6.2 cm. Upon retrieval of the core, the overlying water was aspirated and sieved through a 20-μm screen. The material retained on the screen was added back into the 0–1 cm layer of sediment from the core. Six cores were required to obtain enough sediment to conduct the experiment. The sediment and material in the overlying water that was retained on the 20-mm screen were mixed together in a resealable plastic bag and stored in the dark at 4 °C until transfer to the laboratory. Within two weeks from the time of collection, the sediment was unpacked from the re-sealable plastic bags in a cold (4 °C) dimly lit room under red light and thoroughly mixed to form a slurry. Aliquots of sediment were packed into 2-ml darkened glass vials with no head space and stored at 4 °C in anoxic conditions in a light-blocking gas-sampling bag flushed with nitrogen gas.
The experiment began in June 2012 (i.e., 4 months after sediment collection) and lasted 12 months. Every 2 weeks, a darkened glass vial was retrieved from storage and 30 Alexandrium cysts were isolated using the methods outlined in Matrai et al. (2005). Sediment (1 cc) was transferred to a 50-ml polypropylene centrifuge tube (Falcon) and made up to 45 ml with 0.22 μm filtered seawater (FSW) from the Quartermaster Harbor location. The centrifuge tube was placed in an ice slurry and the sediment was sonicated for 1 min using a Branson 450 Sonifier at power output 3 on constant duty cycle. The sediment was then sieved through a 90-μm screen onto a 20-μm screen and rinsed clean with FSW. The material retained on the 20-μm screen was rinsed back into the 50-ml centrifuge tube, made up to 45 ml with FSW, placed in the ice slurry and sonicated again for 1 min using the same settings, and then sieved back onto the 20-μm screen and rinsed clean with FSW. The material on the 20-μm screen was transferred to a 15-ml polypropylene centrifuge tube (Falcon), brought up to a final volume of 10 ml, and allowed to settle for 1 h. When this step is conducted for Puget Sound sediment, cysts have been found to concentrate in the lighter flocculent material that settles at the sediment–water interface. Approximately 200 μL of this material was carefully transferred into a Sedgewick-Rafter counting chamber and dispersed evenly using 800 μL of FSW. The chamber was examined for cysts using a Zeiss Axiovert 135 inverted microscope. Cysts that visually appeared to be intact and ‘healthy’ (i.e., the presence of starch granules, particles in Brownian motion at cyst ends, and a well-defined orange-red accumulation body) were isolated into individual wells of a 96-well plate containing 200 μl of nutrient-enriched natural FSW growth media (final concentrations of nutrients in the growth media are given in Table 1). Selecting for healthy cysts may limit the potential for presumably reduced excystment success in less healthy cysts to interfere with the ability to detect the presence of an endogenous clock from the patterns of excystment. Confirmation of the presence of a single cyst per well was made using the inverted microscope and photographs of each cyst were taken using the Motic Images Plus software with a Motic live camera (Moticam MP300). Plates were incubated at 14 °C on a 14:10 h light:dark cycle at a photosynthetic photon flux density (PPFD) of ∼70–90 μE m−2 s−1 measured with a 4π scalar irradiance meter (Biospherical Instruments). Germination checks were performed every 2 weeks for 6 weeks. Preliminary studies using Alexandrium cysts from several sites in Puget Sound, including Quartermaster Harbor, found the highest excystment success to occur at a temperature near 14 °C (Hoffer et al., 2005), and this is also the same incubation temperature used by Tobin and Horner (2011). The presence of vegetative Alexandrium cells in the well were a clear indicator that excystment had occurred. Either an unger-minated cyst or a vegetative cell was located in each of the wells during every check—for this study, vegetative cell/s were always located in wells containing empty cysts. The number of germinated cysts at the end of 6 weeks divided by the total number of cysts isolated (n = 30) were used to calculate excystment success on any given isolation date.
Table 1.
Final concentrations of the nutrients added to the nutrient-enhanced natural sterile filtered seawater from Quartermaster Harbor and Bellingham Bay. Trace metals include manganese (II) sulfate, zinc sulfate, cobalt (II) sulfate, sodium Molybdate, and nickel (II) chloride. Vitamins include thiamine (B1), cyanocobalamin (B12), and biotin (H).
| Nutrient | Concentration (μM) | |
|---|---|---|
|
| ||
| Bellingham Bay | Quartermaster Harbor | |
| Nitrate | 50 | 300 |
| Phosphate | 5.4 | 30 |
| Iron | 6.6 | 6.6 |
| Trace metals | 2.86 | 2.86 |
| Vitamins | 0.3 | 0.3 |
| Copper | 0.004 | 0.004 |
| Selenium | 0.0058 | 0.0058 |
2.3. Secondary dormancy
Sediment used to test for secondary dormancy was collected fresh from Quartermaster Harbor at approximately monthly intervals between March 2012 and January 2013 from the R/V Clifford A. Barnes. Sediment was collected using the Craib corer with material >20 μm in the overlying water added back into the 0–1 cm layer and mixed together in a re-sealable plastic bag as above. The sediment was stored in the dark in a cooler with ice (4–10 °C) until it could be transported to the laboratory (<8 h), at which point it was transferred to an incubator set at the same temperature that was measured at the seafloor in Quartermaster Harbor that day. The next morning, sediment (1–2 cc) was processed as above for the endogenous clock experiment (i.e., sonicated and sieved to retain the 20–90 μm size fraction) and 50 cysts were isolated into individual wells of a 96-well plate containing 200 μl of nutrient-enriched natural FSW growth media. Unlike the endogenous clock experiment where only cysts that looked ‘healthy’ were isolated, the first 50 cysts that were encountered while scanning the Sedgwick-Rafter chamber were isolated for the secondary dormancy experiment—provided that they were intact. The results obtained from this study may therefore be more representative of the cyst seedbed at that particular location and time. Confirmation of the presence of a single cyst per well was made and photographs taken as above. Plates were incubated at the same temperature that was measured at the seafloor in Quartermaster Harbor when the sediment was collected the previous day and on a 14:10 h light:dark cycle at a PPFD of ∼70–90 μEm−2s−1. Germination was monitored 5, 14 and 28 days after isolation using the criteria described above for the endogenous clock experiment. Water temperature in Quartermaster Harbor was measured at the sediment collection site each month using a Sea-Bird Electronics conductivity-temperature-depth instrument.
2.4. Temperature and light effects
Sediment used to explore the effects of temperature and light on the rate of Alexandrium excystment was collected from Bellingham Bay in February 2012 from the R/V Clifford A. Barnes using the Craib corer. Sediment was collected using the Craib corer with material >20 μm in the overlying water added back into the 0–3 cm layer and mixed together in a re-sealable plastic bag as mentioned above. Sediment from six replicate cores were mixed together, packed into darkened glass vials with no head space, and stored in anoxic conditions in a light-blocking gas sampling bag flushed with nitrogen gas at 4 °C. Plankton samples were taken at the time of sediment collection as above and no vegetative cells were observed, confirming that the local population of Alexandrium were encysted in the sediment.
The experiment began in August 2012 (i.e., 6 months after the sediment was collected). All sediment manipulations were performed in a 4 °C cold room under dim red light following Anderson et al. (2005). Sediment was removed from the glass vials and mixed with nutrient-enriched natural FSW growth media to form a sediment slurry. The sediment slurry was mixed with a ratio of 1 part sediment to 8 parts nutrient-enriched natural FSW to yield a total volume of 1.8 L. Keeping the sediment slurry well mixed to ensure an even distribution of cysts, 10-mL aliquots were dispensed into 250-ml polycarbonate flasks containing 50 mL of nutrient-enriched natural FSW and gently swirled. Flasks were incubated at 10, 12, 15, and 20 °C. These temperatures were chosen based on data that was available at the time describing seasonal temperature variations in Puget Sound (Moore et al., 2008) and the results of a preliminary study (Hoffer et al., 2005). Flasks were exposed to a 14:10 h light:dark cycle with PPFD levels in the incubators ranging from 33–128 μE m−2 s−1. Half of the experimental flasks for each temperature treatment were wrapped in multiple layers of aluminum foil to block the light. Flasks in each incubator were gently swirled and randomly rotated once a week to ensure all cysts received consistent exposure to light, as well as oxygenated medium. Two replicate flasks were harvested at 1, 3, 5, 7, 9, 14, 21, 42, and 63 days from each temperature/light treatment (i.e., a total of 144 flasks: 9 harvest times ×2 replicate flasks per harvest ×4 temperature treatments ×2 light treatments).
Ten 10-mL aliquots were processed at the beginning of the experiment to determine the initial cyst concentration. These ‘time zero’ measurements were interspersed throughout the flask preparation process to ensure that the sediment slurry remained well mixed with respect to the cyst concentration. Cysts were identified and enumerated using epifluorescence microscopy after staining with primulin according to Yamaguchi et al. (1995). Briefly, the 10-mL aliquots of sediment were sonicated for 1 min using a Branson 450 Sonifier on power output 3 at constant duty cycle and sieved to retain the 20–90 μm size fraction. This size fraction was made up to 10 ml using filtered seawater and a 5-ml subsample was fixed with 5 ml of 1% glutaraldehyde and returned to 4 °C refrigeration for at least 30 min. The sediment was then centrifuged for 10 min, the supernatant removed, 10 ml of ethanol added and returned to 4 °C refrigeration for two days. The sample was centrifuged and the supernatant removed as before, 10 ml of distilled deionized water (ddH2O) as well as 1 ml of primulin stain (2 mg ml−1) added, and left in the dark at room temperature for 1 h. The sample was then rinsed twice by sequentially centrifuging and removing the supernatant as before using ddH2O and brought up to a final volume of 10 ml with ddH2O. After vortexing, 200 μL of sample was mixed with 800 μL of ddH2O, and the resulting 1000 μL pipetted into a gridded Sedgewick Rafter counting chamber. Stained cysts of Alexandrium were counted using a Zeiss Axiostar Plus upright microscope under blue light epiflourescence. A HBO50/AC mercury short arc bulb was used for the epifluorescence with a D360/40 excitation filter, a beam splitter/dichroic of 400 DCLP, and a GG420 colored glass emission filter.
The number of cysts counted at each time point was subtracted from the initial concentration of cysts at time zero and converted to percentages to compare excystment success. It is assumed that the difference in the number of cysts observed at the two time points is due to excystment (see Anderson and Rengefors, 2006). Excystment success at each time point is used to determine rates of excystment as a function of temperature and light for Puget Sound populations of Alexandrium.
3. Results
3.1. Endogenous clock
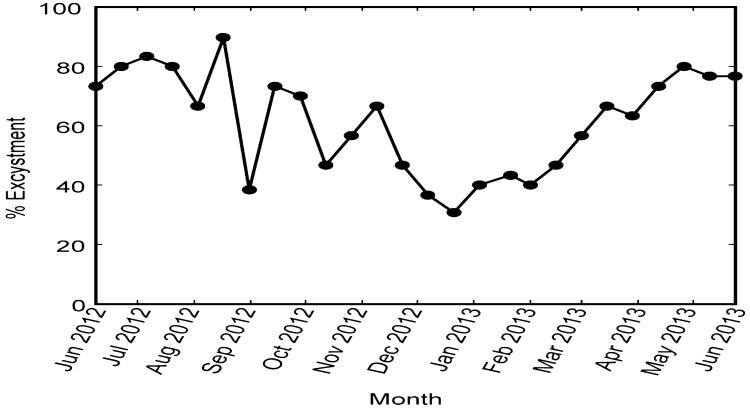
Excystment of Alexandrium from Puget Sound occurred during all months of the year, and nearly all of the cysts that germinated did so during the first 2 weeks following isolation (Fig. 2). Only three cysts germinated 2–4 weeks following isolation and no cysts germinated 4–6 weeks following isolation. Excystment success (i.e., the proportion of isolated cysts that germinated) was variable from May to December, fluctuating between 31 and 90%, but perhaps revealing a generally declining trend over that interval. From December onward, germination success steadily increased through April (Fig. 2) from 30 to 80%. This steady and consistent increase in excystment success from winter into spring is consistent with an annual cyclical pattern that might be expected for cysts that possess an endogenous clock. However, the maximum and minimum values of excystment success observed were not as high or low as what has been observed in deep water Alexandrium cysts, reaching near 100 and 10%, respectively (Anderson and Keafer, 1987; Matrai et al., 2005).
Fig. 2.
Excystment of Alexandrium from Quartermaster Harbor. Cysts (n = 30) were isolated from sediments stored in cold, dark, anoxic conditions at 2-week intervals from June 2012 until May 2013 and germinated in 96-well plates at 14 °C. The x-axis location of markers for excystment represents the day that the sediment was retrieved from cold storage for cyst isolation, and the value for excystment is the cumulative result from the three checks over the 6 weeks that plates were observed.
3.2. Secondary dormancy
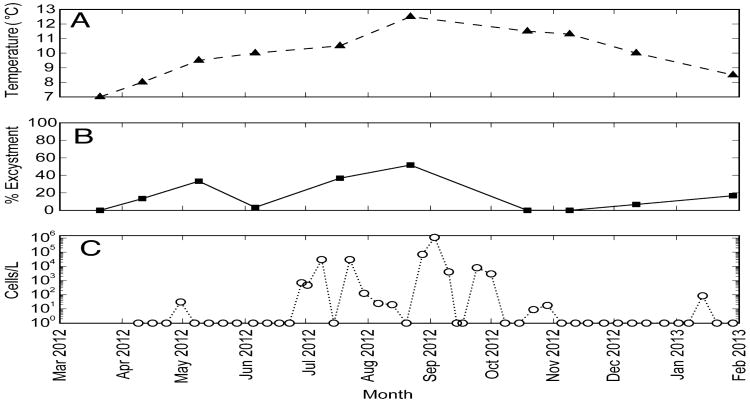
Bottom water temperature at Quartermaster Harbor varied from 7–12.5 °C during the period from March 2012 to January 2013 (Fig. 3a). The coolest and warmest temperatures were observed in March and August 2012, respectively. No observations were made in September 2012, although September is historically when the warmest water temperatures are observed in Quartermaster Harbor (King County, 2014). When cysts of Alexandrium from Quartermaster Harbor were freshly collected and incubated at in situ bottom water temperatures at monthly intervals throughout the year, excystment success varied from 0–52% in a general, seasonal pattern (Fig. 3b). Excystment success was 0% in March 2012 when the water temperature was the lowest observed (i.e., 7 °C). With the exception of June, excystment success increased from March to August 2012, coincident with warming bottom waters, and then decreased to 0% excystment success in October and November even though bottom water temperatures had only decreased slightly (i.e., 1–1.2 °C) from the maximum observed value. Excystment success increased again in December 2012 and January 2013 to 7 and 17%, respectively, even though bottom water temperature continued to decrease. The abundance of vegetative cells of Alexandrium in Quartermaster Harbor is shown in Fig. 3c. These data were collected from a dockside location nearby to the site of sediment collection at 2 week intervals and are provided courtesy of the SoundToxins program1. The timing of the bloom of Alexandrium follows the general pattern of excystment success, indicating that the results of the excystment experiments are ecologically relevant.
Fig. 3.
Monthly variation in (a) water temperature at the seafloor of Quartermaster Harbor, (b) Alexandrium excystment using freshly collected sediment from Quartermaster Harbor, and (c) the abundance of Alexandrium vegetative cells in the surface water at dockside location in Quartermaster Harbor over the period of March 2012 to February 2013. Vegetative cell abundance data are provided by the SoundToxins program. Isolated cysts (n = 50) were germinated in 96-well plates at in situ water temperatures measured at the seafloor at the time of sediment collection. The x-axis location of markers for excystment represents the day that the sediment was collected, and the value for excystment is the cumulative result from the three checks over the 28 days that plates were observed.
3.3. Temperature and light effects
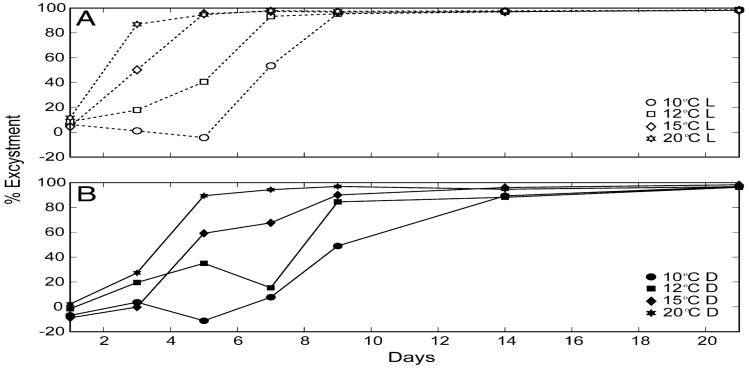
The mean concentration of Alexandrium cysts in the ten 10-mL aliquots of sediment slurry that were processed at the beginning of the experiment was 1626 (±191 standard deviation), so this was assumed to be the concentration in each flask at time zero. The number of cysts that remained in the flasks at each time point was subtracted from this value to calculate excystment success for each treatment. The numbers of cysts counted in the two replicate flasks for each treatment were generally in good agreement (not shown), with the highest ranges associated with flasks that had fewer cysts at the time of harvest. In general, excystment rates were higher for warmer temperature treatments and in the light (Fig. 4). Nearly all of the treatments had exceeded 90% excystment potential by 14 days. Excystment approached 100% for all temperature and light treatments by 21 days.
Fig. 4.
Time course of Alexandrium excystment at 10, 12, 15, and 20 °C in the (a) light and (b) dark. Cysts were germinated in flasks using sediment collected from Bellingham Bay in February 2012. Percent excystment was determined by subtracting the number of cysts that remained in the flask (i.e., did not excyst) after 1, 3, 5, 7, 9, 14, 21, 42, and 63 days from the concentration of cysts at time zero. Markers represent the mean value of duplicate flasks for each time point.
The excystment rates for each of the temperature and light treatments are given in Table 2. Following Anderson et al. (2005), these rates are derived from the slope of a linear fit to the data using only those points prior to the attainment of ∼90% germination. The fitted lines are not forced through the origin to minimize the influence of any acclimatization associated with the cysts being transferred to the experimental conditions from cold, dark, anoxic storage. Excystment rates ranged from 12–29% d−1 in the light and 8–22% d−1 in the dark for temperatures ranging from 10–20 °C.
Table 2.
Excystment rates (% d−1) for Alexandrium at 10, 12, 15, and 20°C in the light and dark. Values in parentheses indicate the range for the two replicate measurements.
| Temperature | ||||
|---|---|---|---|---|
|
| ||||
| 10°C | 12°C | 15°C | 20°C | |
| Light | 11.57 (1.93) | 13.85 (3.62) | 22.77 (1.64) | 29.10 (17.04) |
| Dark | 7.80 (2.35) | 8.40 (2.66) | 13.28 (2.00) | 21.82 (3.87) |
4. Discussion
The goal of this study was to investigate endogenous and environmental regulation of excystment in Alexandrium from Puget Sound. Combined with knowledge of the distribution and abundance of resting cysts (Horner et al., 2011; Greengrove et al., 2014), the excystment characteristics determined in this study can be used in future efforts to constrain a numerical modeling function to calculate the flux of newly germinated vegetative cells into the overlying water, as was done for the population dynamics model for Alexandrium in the Gulf of Maine (Anderson et al., 2005). Determining excystment characteristics for Alexandrium in Puget Sound will facilitate the development of a similar early warning capacity for toxic blooms.
An endogenous annual clock tightly controls the time of year when deep water cysts of Alexandrium from the Gulf of Maine can germinate, with 0–25% excystment success during the minimum phases and 80–98% during the maximum phases of the oscillation (Anderson and Keafer, 1987; Matrai et al., 2005). Such tight control was not observed in the shallow water cysts from Puget Sound in this study. More than 30% of cysts were capable of germinating at any time of year, and excystment success was variable (Fig. 2). Nevertheless, a weak annual oscillation in excystment success was observed suggesting that an endogenous clock may exist in some Puget Sound cysts. Ideally, experiments to determine the presence of an endogenous annual clock in dinoflagellate cysts would span at least two oscillations; however, this experiment was terminated after 12 months, which is unfortunate since some signs of a cyclical pattern in excystment were evident (i.e., increase in excystment success from winter to spring, and possibly a decreasing success from spring to winter). Clearly, more data are needed to resolve the uncertainty.
The suggestion of an endogenous clock in some Puget Sound cysts is at odds with the conclusions of Tobin and Horner (2011), who used different methods for investigating this phenomena. Tobin and Horner (2011) used a Van Veen grab to sample the sediments, which can result in loss of the surface layer of sediment upon retrieval. We used a Craib corer to obtain a relatively undisturbed sediment sample and allowing sediment from a known depth to be obtained for the experiment. This difference may have resulted in cysts from different depths in the sediment column being used in the studies. Further, the method used by Tobin and Horner (2011) to determine excystment success may have obscured the ability to detect an endogenous clock. Rather than isolating and tracking excystment of individual cells (i.e., one cyst per well of a 96-well plate; see Matrai et al., 2005), the authors placed ∼20 μL of sediment into wells and defined germination success as the overall percentage of wells with vegetative cells observed. The sediment was found to have 1550–1750 cysts per cm3; therefore, assuming that the sediment was well mixed, the average number of cysts deposited in each well was ∼31–35. Using this method, it is possible for 100% of wells to score as positive (i.e., 100% germination success) when only a very small percentage of cysts germinate. For example, a well could score as positive if a single cyst germinates, representing only ∼3% of the total number of cysts in the well. Furthermore, another factor that will impact the number of cells observed with this approach is the rate of division of the germling cell, which is temperature dependent. For cyst populations that have been found to possess an endogenous clock, up to 25% of cysts germinated during the minimum phases of the annual oscillation when excystment is inhibited (Anderson and Keafer, 1987; Matrai et al., 2005). Because the proportion of cysts that germinate rarely falls to zero during the minimum phases of the endogenous clock and because many cysts were deposited in each well, the method used by Tobin and Horner (2011) cannot definitively rule out the possibility that Alexandrium from Puget Sound possess an endogenous clock.
Anderson and Keafer (1987) suggest that the inflexibility of an endogenous clock that tightly controls the time of year when cysts can germinate may be disadvantageous for shallow water cysts. This is because cysts from shallow estuaries are exposed to large seasonal variations in environmental conditions, such as light and temperature. The ability to germinate rapidly in response to favorable environmental conditions may therefore be a more competitive strategy (Anderson and Keafer, 1987). The absence of a strict endogenous clock in Alexandrium cysts from a shallow coastal estuary in Ireland is consistent with this theory (Rathaille and Raine, 2011). In contrast, cysts in deeper ocean waters are far removed from the surface waters where the vegetative cells will grow. Without an internal clock to regulate germination timing, excystment might occur at times of the year when conditions in the surface waters are not favorable for vegetative growth. Water temperatures in Quartermaster Harbor are generally conducive to excystment at almost all times of the year; therefore, if Puget Sound Alexandrium do not possess an endogenous clock, cysts could potentially germinate at any time of the year if presented with appropriate environmental conditions—provided that the mandatory dormancy requirements of newly formed cysts have been fulfilled. This would challenge monitoring and prediction efforts since the bloom season could be significantly influenced by environmental variability. Likewise, modeling and forecasting would be difficult, as the observed seasonality of blooms would be difficult to reproduce if cyst germination occurred throughout the year.
When Alexandrium cysts were isolated directly from freshly collected sediments every month and incubated at in situ bottom water temperatures, a general seasonal pattern in excystment was observed. Maximum excystment coincided with the maximum water temperature in September (Fig. 3a), but no excystment occurred during the 2 months that immediately followed (i.e., October and November) even though the next two warmest water temperatures were observed during those months (Fig. 3b). This pattern of minimum excystment occurring when the water temperature is decreasing from its seasonal maximum is consistent with secondary dormancy, which prevents excystment during periods that are favorable for excystment but not for sustained vegetative growth (Rathaille and Raine, 2011). However, this pattern may also be produced by a combination of a temperature window for excystment and the mandatory dormancy period of newly formed cysts (see Itakura and Yamaguchi, 2001). In this case, the seasonal minimum values of excystment observed in October and November may have resulted from sampling newly formed cysts that had not yet completed their mandatory dormancy period. These sediment samples were collected at the end of the seasonal bloom (Fig. 3c); therefore it is likely that the immature cysts would have been unable to germinate during this time despite the favorable environmental conditions. Outside of the mandatory dormancy period, excystment may be more strongly regulated by in situ bottom water temperatures.
The mandatory dormancy period is typically thought of as the time required for the cysts to ‘mature’ (Dale, 1977; Anderson, 1980). Excystment cannot be induced during the mandatory dormancy period, even if cysts are presented with appropriate environmental conditions, and metabolic activity is low (Binder and Anderson, 1990). The duration of the mandatory dormancy period varies and has been documented to last from <15 to 180 d among Alexandrium species (Anderson, 1980; Bravo and Anderson, 1994; Genovesi-Giunti et al., 2006; Joyce and Pitcher, 2006). Temperature also affects the duration of the mandatory dormancy period of Alexandrium species (Anderson, 1980; Rathaille and Raine, 2011). The mandatory dormancy period of Alexandrium in Puget Sound is unknown. During the course of this study, efforts to induce cyst formation using Puget Sound bloom water and collect newly formed cysts following the methods described in Anderson and Rengefors (2006) and Rathaille and Raine (2011) did not yield a sufficient number of cysts to conduct the necessary laboratory experiments. Until the duration of the mandatory dormancy period can be determined for Alexandrium in Puget Sound, it is not possible to determine whether excystment is cued to the environment through secondary dormancy or whether the seasonal pattern suggested in Fig. 3 is simply a product of the mandatory dormancy period followed by suppression of germination (e.g., no germination at unfavorable high or low temperatures; Anderson, 1998; Anderson and Rengefors, 2006). The mandatory dormancy period and the permissive temperature window for Puget Sound Alexandrium need to be determined, as they are both important parameters that influence the timing of blooms.
Both temperature and light were found to be key environmental factors regulating excystment of Alexandrium in Puget Sound sediments. Excystment occurred at all of the experimental temperatures examined in this study (i.e., 10–20 °C) in both the light and dark, with the highest rates of excystment occurring at the warmest temperature treatment and in the light. The experimental temperatures were based on in situ water temperatures measured at the seafloor over a typical seasonal cycle from long-term monitoring throughout Puget Sound by the Washington State Department of Ecology (Moore et al., 2008). Water temperatures in small and shallow bays are not captured by that department's monitoring program, and can sometimes fall below 10 °C at the seafloor in winter/early spring (i.e.,∼7 °C in Quartermaster Harbor; Fig. 3a). Therefore, this experimental design did not determine whether excystment of Alexandrium in Puget Sound is inhibited by in situ water temperatures during the coldest months of the year. However, no excystment was observed at 7 °C in cysts isolated to explore secondary dormancy (Fig. 3b), suggesting that the lower limit of the temperature window for excystment of Puget Sound Alexandrium is between 7–10 °C. The highest rates of excystment observed in this study occurred at the warmest water temperature examined (i.e., 20 °C). Therefore, excystment is not likely to be inhibited by present-day in situ water temperatures at the seafloor during the warmest months of the year. More work is required over a broader range of temperatures to determine a definitive temperature window for excystment of Alexandrium in Puget Sound and to constrain a function describing the flux of cells from the sediment.
Excystment rates were ∼1.5 times greater for cysts exposed to light compared to the dark treatment, similar to the findings of Anderson et al. (2005) for Gulf of Maine A. fundyense. This suggests that excystment may be facilitated by light in shallow seedbeds compared to deep seedbeds located below the photic zone. However, even for shallow seedbeds, rapid light attenuation within the sediment results in only a small proportion of cysts (i.e., those situated within the uppermost few millimeters of sediment) being exposed to light (Anderson et al., 2005). Therefore, even though light is an important factor regulating excystment of Alexandrium spp., care must be taken using light to further constrain a function describing the flux of cells from the sediment. Other factors not considered in this study may further complicate any such function, such as rapid oxygen depletion within the sediment that may inhibit excystment (Anderson et al., 1987; Keafer et al., 1992). Efforts to determine in situ rates of excystment for Alexandrium spp. may help to alleviate these uncertainties (e.g., Anglés et al., 2012a, 2012b).
The excystment rates obtained from this study for Alexandrium are approximately an order of magnitude higher than those obtained by Anderson et al. (2005) for Alexandrium fundyense cysts from the western and eastern Gulf of Maine. These differences presumably reflect the warmer temperatures experienced in Puget Sound, resulting in the range of ecologically relevant temperature treatments used in this study being much higher (i.e., 10–20 °C) compared to Anderson et al. (2005; 2–15 °C). However, even for the common temperature treatment of 15 °C used in both studies, excystment rates calculated in this study for Puget Sound Alexandrium are ∼3 times higher than Gulf of Maine Alexandrium. Based on the seasonal range of in situ water temperatures observed in Quartermaster Harbor at the seafloor in this study (Fig. 3a), the our 10 °C treatment provides the most environmentally relevant rates of excystment. These rates are still 2–3 times than those observed for the closest temperature treatment of 8 °C in Anderson et al. (2005). At 10 °C, Puget Sound Alexandrium can excyst at 12% d−1 in the light and at 8% d−1 in the dark (Table 2). Excystment rates this high could result in all of the mature cysts in the surface sediments of a seed bed germinating in only a week or two, depleting the surface sediment later of cysts, and leading to very tight seasonality of blooms. This seems unrealistic given the observed extended bloom pattern in Quartermaster Harbor, consisting of a small spring bloom followed by larger recurrent summer and fall blooms (Fig. 3c). It is more likely that excystment is slowed down or regulated by factors other than temperature. Seasonality in excystment via an endogenous clock or secondary dormancy would provide such regulation, but other physical mechanisms might also contribute such as cyst burial below the oxygenated surface sediment layer, and bioturbation or other sediment disturbance events that replenish the surface layer with cysts.
In conclusion, this study finds that germination of Alexandrium spp. cysts within Puget Sound is more dependent on environmental conditions, in contrast to those of the Gulf of Maine. For this reason, it is important to pursue a deeper understanding of the conditions that lead to excystment in order to predict blooms and prevent undue economic loss to shellfish growers in Puget Sound. It is imperative that future experiments address more specifically the microenvironments that the cysts are exposed to, including the frequency and extent of mixing of sediment on the ocean floor, along with the length of the mandatory dormancy period, and the maximum and minimum temperatures at which these cysts are viable.
Acknowledgments
The authors thank the Captain and crew of the R/V Clifford A. Barnes; D. Kulis, B. Keafer and J. Kleindinst at Woods Hole Oceanographic Institution; V. Trainer at the National Oceanic and Atmospheric Administration (NOAA) Northwest Fisheries Science Center; and K. Rickerson from the SoundToxins program. This research was supported in part by NOAA ECOHAB funding to the NOAA Northwest Fisheries Science Center, University of Washington (NA10NOS4780158) and Woods Hole Oceanographic Institution (NA10NOS4780159); and a grant from Washington Sea Grant, University of Washington, pursuant to NOAA Award No. NA10OAR4170057, Project R/OCEH-9, to the University of Washington, Tacoma. The views expressed herein are those of the authors and do not necessarily reflect the views of NOAA or any of its sub-agencies. Support for D. M. Anderson was provided by NOAA ECOHAB through the Woods Hole Center for Oceans and Human Health, National Science Foundation Grant OCE-1314642 and National Institute of Environmental Health Sciences Grant 1-P01-ES021923-01. This is ECOHAB contribution number 793.[SS]
Footnotes
The SoundToxins program is a cooperative partnership for early warning of harmful algal blooms in Puget Sound, jointly managed by the Northwest Fisheries Science Center and Washington Sea Grant. Cell counts are courtesy of Karlista Rickerson.
References
- Anderson DM. Effects of temperature conditioning on development and germination of Gonyaulax tamarensis (Dinophyceae) hypnozygotes. J Phycol. 1980;16:166–172. [Google Scholar]
- Anderson DM. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological Ecology of Harmful Algal Blooms. Springer-Verlag; Berlin: 1998. pp. 29–48. [Google Scholar]
- Anderson DM, Keafer BA. An endogenous annual clock in the toxic marine dinoflagellate Gonyaulax tamarensis. Nature. 1987;325:616–617. doi: 10.1038/325616a0. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Morel FMM. The seeding of two red tide blooms by the germination of benthic Gonyaulax tamarensis hypnocysts. Estuar Coast Mar Sci. 1979;8:279–293. [Google Scholar]
- Anderson DM, Rengefors K. Community assembly and seasonal succession of marine dinoflagellates in a temperate estuary: the importance of life cycle events. Limnol Oceanogr. 2006;51(2):860–873. [Google Scholar]
- Anderson DM, Stock CA, Keafer BA, Bronzino Nelson A, Thompson B, McGillicuddy DJ, Jr, Keller M, Matrai PA, Martin J. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep-Sea Res Pt II. 2005;52:2522–2542. [Google Scholar]
- Anderson DM, Taylor CD, Armbrust EV. The effects of darkness and anaerobiosis on dinoflagellate cyst germination. Limnol Oceanogr. 1987;32(2):340–351. [Google Scholar]
- Anglés S, Garcés E, Hattenrath-Lehmann TK, Gobler CJ. In situ life-cycle stages of Alexandrium fundyense during bloom development in Northport Harbor (New York, USA) Harmful Algae. 2012a;16:20–26. [Google Scholar]
- Anglés S, Garcés E, Reñé A, Samperdo N. Life-cycle alterations in Alexandrium minutum natural populations from the NW Mediterranean Sea. Harmful Algae. 2012b;16:1–11. [Google Scholar]
- Binder JB, Anderson DM. Biochemical composition and metabolic activity of Scripsiella trochoidea (Dinophyceae) resting cysts. J Phycol. 1990;26:289–298. [Google Scholar]
- Bravo I, Anderson DM. The effects of temperature, growth medium and darkness on excystment and growth of the toxic dinoflagellate Gymnodinium catenatum from northwest Spain. J Plankton Res. 1994;16(5):513–525. [Google Scholar]
- Craib JS. A sampler for taking short undisturbed marine cores. J Cons. 1965;30(1):34–39. [Google Scholar]
- Dale B. Cysts of the toxic red-tide dinoflagellate Gonyaulax excavata (Braarud) Balech from Oslofjorfen, Norway. Sarsia. 1977;63:29–34. [Google Scholar]
- Dale B. Dinoflagellate resting cysts: ‘benthic plankton’. In: Fryxell GA, editor. Survival Strategies of the Algae. Cambridge University Press; Cambridge: 1983. pp. 69–136. [Google Scholar]
- Genovesi-Giunti B, Laabir M, Vaquer A. The benthic resting cyst: a key actor in harmful dinoflagellate blooms—a review. Vie Milieu. 2006;56:327–337. [Google Scholar]
- Greengrove CL, Masura JE, Moore SK, Bill BD, Hay LR, Banas NS, Salathé EP, Jr, Mantua NJ, Anderson DM, Trainer VL, Stein JE. Alexandrium catenella cyst distribution and germination in Puget Sound, WA USA. In: Kim HG, Reguera B, Hallegraeff GM, Lee CK, Han MS, Choi JK, editors. Harmful Algae, Proceedings of the 15th International Conference on Harmful Algae International Society for the Study of Harmful Algae.2014. [Google Scholar]
- Hoffer S, Horner RA, Greengrove CL. Germination Experiments with Alexandrium catenella Cysts Collected from Surface Sediments in Puget Sound, Washington. Pacific Grove; California: 2005. [Google Scholar]
- Horner RA, Greengrove CL, Davies-Vollum KS, Gawel JE, Postel JR, Cox A. Spatial distribution of benthic cysts of Alexandrium catenella in surface sediments of Puget Sound, Washington, USA. Harmful Algae. 2011;11:96–105. [Google Scholar]
- Itakura S, Yamaguchi M. Germination characteristics of naturally occurring cysts of Alexandrium tamarense (Dinophyceae) in Hiroshima Bay, Inland Sea of Japan. Phycologia. 2001;40(3):263–267. [Google Scholar]
- John U, Litaker RW, Montresor M, Murray S, Brosnahan ML, Anderson DM. Formal revision of the Alexandrium tamarense species complex (Dinophyceae) taxonomy: The introduction of five species with emphasis on molecular-based (rDNA) classification. Protist. 2014a;165:779–804. doi: 10.1016/j.protis.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Litaker W, Montresor M, Murray S, Brosnahan ML, Anderson DM. Proposal to reject the name Gonyaulax catenella (Alexandrium catenella) (Dinophyceae) Taxon. 2014b;63(4):932–933. doi: 10.12705/634.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce LB, Pitcher GC. Cysts of Alexandrium catenella on the west coast of South Africa: distribution and characteristics of germination. Afr J Mar Sci. 2006;28(2):295–298. [Google Scholar]
- Kao CY. Paralytic shellfish poisoning. In: IR F, editor. Algal Toxins in Seafood and Drinking Water. Academic Press; London: 1993. [Google Scholar]
- Karssen CM. Environmental conditions and endogenous mechanisms involved in secondary dormancy of seeds. Israel J Bot. 1981;29(1981):45–64. [Google Scholar]
- Keafer BA, Buesseler KO, Anderson DM. Burial of living dinoflagellate cysts in estuarine and nearshore sediments. Mar Micropaleontol. 1992;20:147–161. [Google Scholar]
- King County, 2014 Quartermaster Harbor Marine Water Quality Data Report 2007-2011. Prepared by Kimberle Stark (King County Water and Land Resources Division), C. Greengrove, N., Schlafer, N. Huber, and J. Masura (University of Washington-Tacoma). Submitted by King County Dept. of Nat. Resources & Parks, Seattle, Washington.
- Lilly EL, Halanych KM, Anderson DM. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae) J Phycol. 2007;43:1329–1338. [Google Scholar]
- Matrai P, Thompson B, Keller M. Circannual excystment of resting cysts of Alexandrium spp. from eastern Gulf of Maine populations. Deep-Sea Res Pt II. 2005;52:2560–2568. [Google Scholar]
- Moore SK, Mantua NJ, Newton JA, Kawase M, Warner MJ, Kellogg JP. A descriptive analysis of temporal and spatial patterns of variability in Puget Sound oceanographic properties. Estuar Coast Shelf Sci. 2008;80:545–554. http://dx.doi.org/10.1016/j.ecss.2008.09.016. [Google Scholar]
- Moore SK, Mantua NJ, Trainer VL, Hickey BM. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae. 2009;8(3):463–477. http://dx.doi.org/10.1016/j.hal.2008.1010.1003. [Google Scholar]
- Pfiester LA, Anderson DM. Dinoflagellate reproduction. In: Taylor FJR, editor. The Biology of Dinoflagellates. Blackwell Scientific Publications Ltd; Oxford: 1987. pp. 611–648. [Google Scholar]
- Quayle DB. Paralytic shellfish poisoning in British Columbia. Fish Res Board Can Bull. 1969;168:1–68. [Google Scholar]
- Rathaille AN, Raine R. Seasonality in the excystment of Alexandrium minutum and Alexandrium tamarense in Irish coastal waters. Harmful Algae. 2011;10(6):629–635. [Google Scholar]
- Thomson RE. Physical oceanography of the Strait of Georgia-Puget Sound-Juan de Fuca Strait System. In: Wilson R, Beamish R, Aitkens F, Bell J, editors. Review of the Marine Environment and Biota of Strait of Georgia, Puget Sound and Juan de Fuca Strait, Proceedings of the BC/Washington Symposium on the Marine Environment. 1994. Jan 13–14, pp. 36–100. 1994. [Google Scholar]
- Tobin ED, Horner RA. Germination characteristics of Alexandrium catenella cysts from surface sediments in Quartermaster Harbor, Puget Sound, Washington, USA. Harmful Algae. 2011;10:216–223. , http://dx.doi.org/10.1016/j.hal.2010.1010.1002. [Google Scholar]
- Trainer VL, Eberhart BTL, Wekell JC, Adams NG, Hanson L, Cox F, Dowell J. Paralytic shellfish toxins in Puget Sound, Washington State. J Shellfish Res. 2003;22(1):213–223. [Google Scholar]
- Vancouver G. In: A Voyage of Discovery to the North Pacific Ocean and Round the World, 1791–1795. Robinson GG, Robinson J, editors. Vol. 2. Paternoster-Row and J. Edwards; London: 1798. pp. 260–287. Fourth book (Chapter 2) [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. Redefining seed dormancy: an attempt to integrate physiology and ecology. J Ecol. 1995;83:1031–1037. [Google Scholar]
- Wang L, Zhuang Y, Zhang H, Lin X, Lin S. DNA barcoding species in Alexandrium tamarense complex using ITS and proposing designation of five species. Harmful Algae. 2014;31:100–113. doi: 10.1016/j.hal.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Itakura S, Imai I, Ishida Y. A rapid and precise technique for enumeration of resting cysts of Alexandrium spp. (Dinophyceae) in natural sediments. Phycologia. 1995;34(3):207–214. [Google Scholar]