Abstract
Amplification is a hallmark of many human tumors but the role of most amplified genes in human tumor development is not yet understood. Previously, we identified a frequently amplified gene in glioma termed glioma-amplified sequence 41 (GAS41). Using the TCGA data portal and performing experiments on HeLa and TX3868, we analyzed the role of GAS41 amplification on GAS41 overexpression and the effect on the cell cycle. Here we show that GAS41 amplification is associated with overexpression in the majority of cases. Both induced and endogenous overexpression of GAS41 leads to an increase in multipolar spindles. We showed that GAS41 is specifically associated with pericentrosome material. As result of an increased GAS41 expression we found bipolar spindles with misaligned chromosomes. This number was even increased by a combined overexpression of GAS41 and a reduced expression of NuMA. We propose that GAS41 amplification may have an effect on the highly altered karyotype of glioblastoma via its role during spindle pole formation.
INTRODUCTION
Previously, we identified the glioma-amplified sequence 41 (GAS41) gene that was frequently amplified in glioblastoma and in Grade I astrocytoma (Fischer et al., 1997). Recently, we reported GAS41 amplification and overexpression in glioblastoma recurrences (Fischer et al., 2010). Amplification is a hallmark of tumor genetics but does not occur in normal human cells. While numerous amplified genes have been reported in human tumors including glioma, the downstream effects of amplified genes were mostly not yet understood. GAS41 is a highly conserved protein with an N-terminal domain found in YEATS family members. Sequence comparisons of GAS41 predict negatively charged helical structures, which are present in transcriptional activation domains of several eukaryotic transcription factors. Specifically, GAS41 shows homology to the nuclear transcription factors AF-9 and ENL which are implicated in human cancers (Marschalek, 2010). There is also strong evidence for GAS41 being involved in the process of chromatin remodeling. GAS41 is a subunit of the human TIP60 and SRCAP complexes (Doyon et al., 2004; Cai et al., 2005) that mediate the incorporation of an H2A histone variant into nucleosomes. Yaf9, which is a yeast orthologous of GAS41, is a common subunit of both the yeast NuA4HAT and the SWR1 chromatin-modifying complexes (Le Masson et al., 2003; Zhang et al., 2004). Using yeast-two-hybrid analysis, we found evidence that GAS41 interacts with the prefoldin subunit 1 (PFDN1), the ATPase KIAA1009/ QN1, and the nuclear mitotic apparatus protein (NuMA) (Munnia et al., 2001). KIAA1009 is located at the poles of the mitotic spindle and at the centrosomes during mitosis. Knockdown of KIAA1009 leads to chromosome segregation defects (Leon et al., 2006). GAS41 also interacts with the C-terminus of the human transforming acidic coiled-coil 1 protein (TACC1) (Lauffart et al., 2002). The interaction of GAS41 with NuMA has been confirmed by surface plasmon resonance spectroscopy (Harborth et al., 2000). NuMA is an abundant coiled-coil protein that is a component of the nuclear matrix and is associated with the spindle poles in mitotic cells. It locates within the nucleus during interphase and redistributes to the spindle poles during early mitosis. It is essential for the organization and stabilization of spindle poles from early mitosis until early anaphase (Haren et al., 2009; Merdes et al., 1996; Silk et al., 2009). The interaction of GAS41 with KIAA1009/QN1, TACC1, and NuMA provides evidence for an involvement of GAS41 in spindle formation. The past decade has offered a wealth of information on proteins that play a crucial role in establishing correct bipolar spindle, including Plk1, TPX2, and NuMA (Garrett et al., 2002; van Vugt et al., 2004; Oshimori et al., 2006). However, the complexity of the spindle formation process suggests that additional proteins are involved. Here, we set out to ask whether and how GAS41 expression influences spindle formation. A better understanding of downstream effects of GAS41 is also a necessary prerequisite to further understand the role of amplified GAS41 in human tumor development.
MATERIAL AND METHODS
TCGA Data Portal Screening
All glioblastoma multiforme deposited at the Cancer Genome Atlas (TCGA) data portal (last accessed October 2011) were analyzed for GAS41 amplifications and expression as well as NuMA expression. In detail, we used data from the HMS-HC-CGH-244A array for amplification analysis and data from the UNC AgilentG4502A-07 array for expression analysis. For amplification, we considered a log2Cy5/Cy3 ratio >1 as amplified. For expression, we also considered a deregulation of more than 2-fold, means a log2Cy5/Cy3 ratio >1 as overexpressed and a log2Cy5/Cy3 ratio < −1 as underexpressed. The results are in whole or part based upon data generated by pilot project established by the NCI and NHGRI. Information about TCGA and the investigators and institutions, that constitutes the TCGA research network, can be found at ‘‘http://cancergenome.nih.gov.’’
Plasmids and RNA Interference
Green fluorescent protein (GFP)-GAS41 was expressed in pEGFP-C3 (Clontech, Mountain View, CA and HA-GAS41 was expressed in pSG5 (Strata-gene, La Jolla, CA). The pcDNA3-GFP-NuMA plasmid has been kindly provided by Andreas Merdes. GAS41 siRNAs were obtained from Ambion (Foster City, CA). The sequences for GAS41 siRNA were 5′GGAUAUGUCAGCAUAUGUGtt3′ (siGAS41-1), 5′CCAAUAGUUUACGGUAAUGtt3′ (siGAS41-2), and 5′GCCUAUAAGCAUGAAACAGtt3′ (siGAS41-3). The ACTB siRNA and scrambled siRNA was obtained from Qiagen (Hilden, Germany). The 21-nucleotide siRNA sequence for the negative control was 5′UUCUCCGAACGUGUCACGUtt3′ and the sequence of siACTB was 5′AGGCUG UGCUAUCCCUGUAtt3′. NuMA siRNA (Elbashir et al., 2002) was obtained from Dharmacon (Lafayette, CO). Transfection of siRNA was performed using Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany), plasmid transfection was performed using FuGENE HD (Roche, Mannheim, Germany).
Cell Culture and Cell Cycle Synchronization
The glioblastoma cell line TX3868 was established as described by Fischer et al. (1996). HeLa cells were grown in dulbecco’s modified eagle medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Biochrom AG, Berlin, Germany). TX3868 cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS) (PAA, Pasching, Austria) and 1% penicillin/ streptomycin (Invitrogen). Cell cycle synchronization was performed via double thymidine block. Cells were seeded the day before and incubated in medium containing 2 mM thymidine (Sigma, Steinheim, Germany). After release to standard culture medium and a second thymidine exposure, cells were arrested at G1/S checkpoint. Thymidine was washed out and cells were released to standard culture medium to re-enter the cell cycle. To perform an M-phase arrest, pre-synchronized HeLa cells were incubated with 0.5 μg/ml nocodazole (Sigma) and harvested by mitotic shake off.
In Vitro Microtubule Assay (Taxol Assay)
For taxol experiments, cells were synchronized in metaphase with 50 μg/μl nocodazole overnight. After nocodazole was washed out, cells were incubated in medium for 1 h to re-stabilize the microtubules. Subsequently, the cells were incubated with 25 μM taxol (Sigma) for 30 min and subjected to immunofluorescence.
Coimmunoprecipitation and Western Blot Analysis
HeLa cells were collected by trypsin or mitotic shake-off and resuspended in 400 μl lysis buffer (150 mM NaCl, 50 mM Tris/HCl pH 8.0, 1% NP40, Complete Mini EDTA-free protease inhibitor cocktail and phosphatase inhibitor (Roche). After pre-clearing, the lysates were incubated with control IgG or polyclonal GAS41 antibody (Munnia et al., 2001) for 1 hr at 4° C. After addition of Protein-A-Sepharose (GE Healthcare, Freiburg, Germany), immunoprecipitation was performed over night at 4° C. Coimmunoprecipitated proteins were subjected to electrophoresis on a 15% SDS-polyacrylamide gel or an 8–13% Tris-Acetate NuPAGE (Invitrogen). A total of 10–20 μg protein per lane was separated on 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) or 8–13% Tris-Acetate NuPAGE (Invitrogen) and transferred onto a polyvinylidene fluoride (PVDF) membrane (GE Healthcare). Hybridization was performed as described previously (Munnia et al., 2001). Western blot analysis was performed by HA-tag antibody (Roche, Mannheim, Germany), monoclonal γ-tubulin antibody (GTU-88, Sigma), monoclonal NuMA antibody (Calbiochem, Merck, Darmstadt, Germany), rabbit polyclonal pericentrin antibody (Abcam, Cambridge, UK), monoclonal beta-actin antibody (Sigma), and rabbit polyclonal GAS41 antibody (Munnia et al., 2001).
Northern Blot Analysis
Northern blot hybridization was performed as described previously (Fischer et al., 1996; Munnia et al., 2001). In brief, total RNA was extracted from transfected cells using RNeasy Mini kit (Qiagen). A total of 12–15 μg RNA per lane was separated on a 1% agarose gel and blotted onto a positively charged nylon membrane (Roche). Hybridization was carried out in 500 mM phosphate buffer (pH 7.2), 7% SDS, and 1 mM EDTA. Probe labeling with 32P dCTP was performed using random primer labeling kit (Roche).
Immunofluorescence Analysis
Cells were grown on cover slips and fixed with pre-chilled methanol or 4% paraformaldehyde. After permeabilization with 0.1% Triton X-100/phosphate buffered saline (PBS), cells were blocked for 30 min using 1% normal goat serum/PBS (Dianova, Hamburg, Germany). For GAS41 staining cells were treated with ice cold pre-extraction buffer (100 mM PIPES pH 6.8, 1 mM MgCl2, 10 mM EGTA, 0.1% TritonX-100) before fixation with 4% paraformaldehyde and blocking 4% bovine serum albumin (BSA)/0.1% TritonX-100 in PBS. As primary antibody we used antibodies against GAS41 (Munnia et al., 2001), α-tubulin (Sigma), γ-tubulin (Sigma), NuMA (Calbiochem), and Centrin 20H5 (Millipore, Temecula, CA). As secondary antibodies we used goat anti-mouse IgG Alexa Fluor 594 (Invitrogen, Karlsruhe, Germany), goat anti-rabbit IgG Alexa Fluor 594 (Invitrogen), and goat anti-mouse IgG Alexa Fluor 488 (Invitrogen). For nuclear counterstaining we used Hoechst solution (Sigma). As mounting medium we used Histo-prime (Linaris, Wertheim-Bettingen, Germany). Immunofluorescence analysis was performed using an AX-70 microscope (Olympus, Hamburg, Germany) equipped with a ×60 and ×100 objective lens. Images were acquired using a DP71 camera (Olympus) and Cell F software (Olympus). ImageJ software was used for image processing.
Live Cell Imaging
HeLa H2B-GFP cells were seeded onto glass-bottom dishes (Lab-Tek Chambered Coverglass, Nunc, Langenselbold, Germany) and incubated as described above. According to the protocol for synchronizing the cells with a double thymidine block, the cells were treated with the respective media at respective times and subjected to siRNA transfection. The antioxidant TROLOX (Calbiochem) (100 μM) was added to the medium 12 hr prior to microscopy. The observation was performed at a Perkin Elmer (Waltham, MA) Ultra View VOX spinning disc system using a Zeiss ×63 PlanApochromat oil immersion objective (NA = 1.4) on a Zeiss AxioObserver (Jena, Germany). For the acquisition of the 4D data sets, a Hamamatsu EM-CCD camera and a Prior piezo stage was used taking image stacks with a z-spacing of 0.2 μm. The system was controlled by a PC running the microscope acquisition software Volocity 5.0. Images were acquired at 5–15 min intervals. Image processing was performed by ImageJ. Z stacks were compiled by maximum intensity projection for presentation.
RESULTS
Amplification and Expression of GAS41 in Glioblastoma Multiforme
First, we addressed the question whether and to what extend the amplification entails an over-expression of GAS41. To this end, we analyzed the data available through the Cancer Genome Atlas (TCGA). Gene copy number data of GAS41 were available for 258 glioblastoma. Out of the 258 glioblastoma samples we found 9 gliobla-stoma with high level GAS41 amplification as indicated by a log2Cy5/Cy3 ratio > 1. The majority of these glioblastomas with GAS41 amplification showed an overexpression of GAS41 (88.9 %). In contrast, only 4.1% of 495 glioblastoma for which expression data were available revealed an overexpression of GAS41. These data strongly indicate that gene amplification is a driving force for GAS41 overexpression. Notably, the TCGA data did not indicate overexpression of NuMA that has previously been associated with GAS41. The TCGA data also showed 26.1% of glioblastoma with a reduced expression of GAS41, indicated by a log2Cy5/Cy3 ratio < − 1. For NuMA, the TCGA data showed a reduced expression in 63.4% of glioblastoma. Thus, altered expression seems to be more frequent for NuMA than for GAS41. In total, we found 30.1% of the glioblastoma with an altered GAS41 expression including both up- and downregulation.
Involvement of GAS41 in Spindle Pole Formation
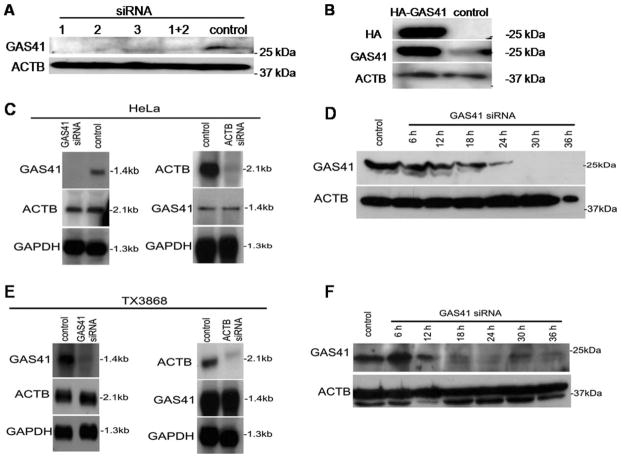
The amplification and co-overexpression of GAS41 in glioblastoma prompted us to analyze further the downstream effects of GAS41. Since there was evidence that GAS41 interacts with the NuMA that plays a role in spindle pole formation, we analyzed the possible role of GAS41 in this process. First we analyzed the effect of GAS41 depletion on the spindle phenotypes by using small interfering RNA (siRNA). We used three oligonucleotides targeting different exons of GAS41 and transfected HeLa cells with equimolar amounts of these siRNAs. As a negative-control we transfected cells with scrambled siRNA. Transfection efficiency was assessed by flow cytometry using fluorescein isothiocyanate (FITC)-labeled control siRNA. A combination of siGAS41-1 and siGAS41-2 was most efficient to deplete GAS41 in HeLa cells as shown by Western blot analysis (Fig. 1A). The extent and the specificity of GAS41 depletion were analyzed by Northern blotting. Neither the levels of GAPDH and beta-actin mRNA were affected by GAS41 siRNA, nor the level of GAS41 upon transfection with beta-actin or scrambled siRNA (Fig. 1C). GAS41 knockdown was most efficient 30 hr after transfection in HeLa cells (Fig. 1D). Immunofluorescence analysis of GAS41 depleted cells that were not synchronized provided first evidence for mitotic spindle defects. To analyze whether a loss of spindle integrity depends on the cell cycle, we synchronized cells prior to entering the M-phase by a double thymidine block. In total, we analyzed 300 metaphases to quantify the effects of GAS41 depletion. We found 20.5% of the cells with multipolar spindles, 68.5% with bipolar spindles, and 11.0% with bipolar spindles but misaligned chromosomes. HeLa cells that were transfected with control siRNA showed 91.9% normal metaphases spindles, 4.7% multipolar spindles, and 3.4% bipolar metaphases with misaligned chromosomes (Table 1).
Figure 1.
Induced altered GAS41 expression in HeLa cells and in glioblastoma derived TX3868 cells. β-Actin (ACTB) was used as control. A: A mixture of two GAS41 siRNAs caused the most efficient knockdown of GAS41 in HeLa cells. B: Overexpression of GAS41 was found in HeLa cells transfected by HA-GAS41. C/E: GAS41 siRNA did not affect ACTB expression and ACTB siRNA did not affect GAS41 expression in HeLa and TX3868 cells. GAS41 knockdown is most efficient 30 hr after transfection in HeLa cells and 24 hr after transfection in TX3868 glioblastoma cells (D,F). Scrambled siRNA was used as control.
TABLE 1.
Influence of GAS41 and NuMA Depletion and Overexpression on Spindle Phenotype in HeLa with Normal Endogenous GAS41 Expression and of GAS41 Depletion on Spindle Phenotype in TX3868 with Endogenous GAS41 Overexpression
| Spindle phenotype (%)
|
|||||
|---|---|---|---|---|---|
| Treatment | Mitotic cells | Bipolar | Multipolar | Misaligned chromosomes | Monopolar |
| HeLa | |||||
| Control siRNA | 300 | 91.9 | 4.7 | 3.4 | 0.0 |
| siGAS41–1+2 | 300 | 68.5 | 20.5 | 11.0 | 0.0 |
| siNuMA | 300 | 83.7 | 12.0 | 4.3 | 0.0 |
| siGAS41–1+2+ siNuMA | 300 | 75.9 | 12.2 | 11.9 | 0.0 |
| Control DNA | 300 | 93.7 | 3.3 | 3.0 | 0.0 |
| HA-GAS41 | 300 | 76.6 | 16.4 | 7.0 | 0.0 |
| pSG5 control | 300 | 95.5 | 4.5 | 0.0 | 0.0 |
| GFP-NuMA | 300 | 83.0 | 14.0 | 3.0 | 0.0 |
| GFP-NuMA + HA-GAS41 | 300 | 88.0 | 8.0 | 4.0 | 0.0 |
| Control siRNA + control DNA | 300 | 90.0 | 4.0 | 6.0 | 0.0 |
| HA-GAS41 + siNuMA | 300 | 66.7 | 14.3 | 19.0 | 0.0 |
| GFP-NuMA + siGAS41–1+ 2 | 300 | 74.1 | 13.8 | 12.1 | 0.0 |
| TX3868 | |||||
| siGAS41–1+2 | 100 | 70.0 | 24.0 | 0.0 | 6.0 |
| Control siRNA | 100 | 77.0 | 21.0 | 0.0 | 2.0 |
Second, we tested the effects of GAS41 over-expression by expressing HA-tagged GAS41 in synchronized HeLa cells. Overexpression of the tagged protein was confirmed by Western blotting (Fig. 1B). We found 76.6% of the cells with normal bipolar spindles, 16.4% with multipolar spindles, and 7.0% with bipolar spindles and misaligned chromosomes (Table 1). Our results show that both a reduced and an enhanced expression of GAS41 cause perturbation of spindle formation in transfected HeLa cells. Initially, GAS41 was identified in the glioblastoma cell line TX3868 that contains a stable amplicon at the chromosomal region 12q13-15 (Fischer et al., 1996). We utilized TX3868 cells to analyze endogenous GAS41 overexpression. Again, to quantify the effects of altered GAS41 expression, we synchronized TX3868 cells by a double thymidine block. Cells with increased endogenous GAS41 expression showed 21.0% mitoses with multipolar spindles and 2.0% with monopolar spindles. To determine the effect of a reduced GAS41 expression in this tumor cell line, we applied siRNA as described above for HeLa cells. Western blotting confirmed the knockdown of GAS41. Control siRNA did not affect the level of GAS41 (Fig. 1E). GAS41 knockdown was most efficient 24 hr after transfection in TX3868 glioblastoma cells (Fig. 1F). In GAS41 depleted TX3868 cells, we found 24.0% multipolar spindles and 6.0% monopolar spindles (Table 1). In summary, induced and endogenous GAS41 over-expression and induced GAS41 depletion result in altered spindle formation.
Colocalization and Binding of Endogenous GAS41 to Proteins at the Spindle Poles
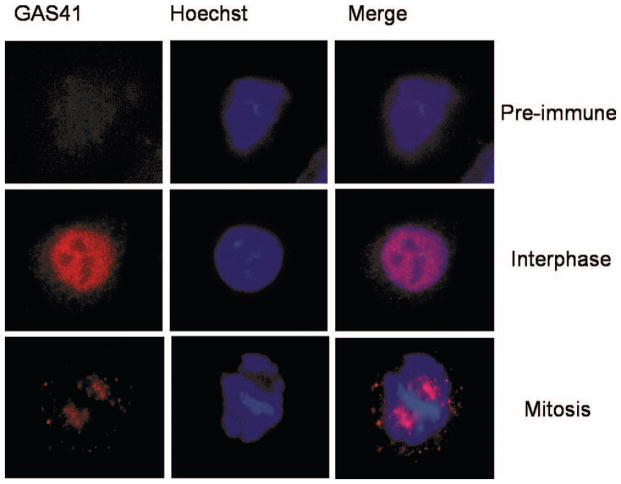
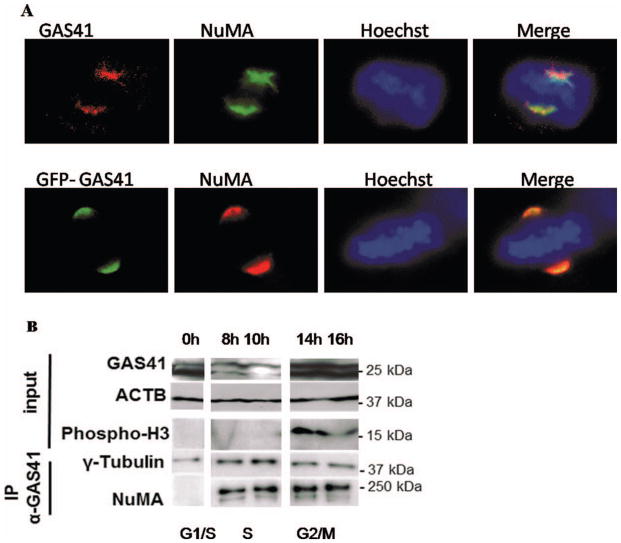
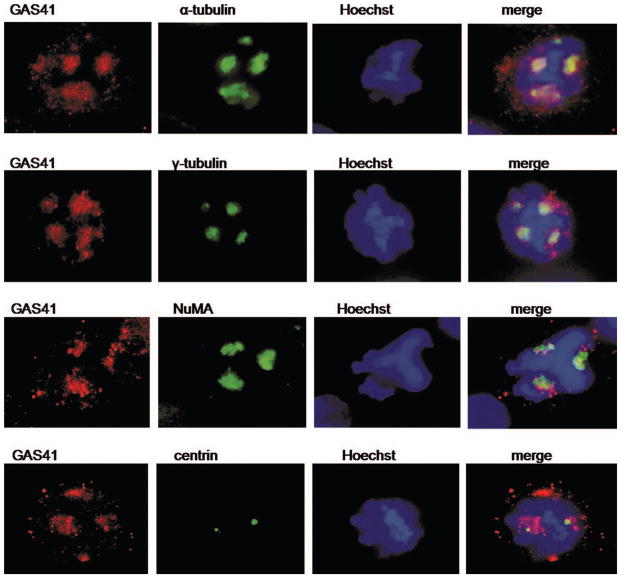
We determined the endogenous localization of GAS41 in HeLa cells using a GAS41-specific antibody that was described previously (Munnia et al., 2001; Heisel et al., 2010). No specific signal was detected with the pre-immune serum. In interphase GAS41 maps to the nucleus and in mitosis to the spindle poles (Fig. 2). These results are in agreement with previous evidence of yeast-two-hybrid studies indicating that GAS41 is a binding partner of proteins associated with spindle microtubules (Harborth et al., 2000; Munnia et al., 2001). We next mapped GAS41 localization with respect to NuMA (which is known to localize at the spindle poles during mitosis). We found colocalization of endogenous NuMA and both endogenous GAS41 as determined by a GAS41 polyclonal antibody and overexpressed GFP-GAS41 at the spindle poles in mitotic HeLa cells (Fig. 3A). In the following, we analyzed GAS41 interaction with NuMA during the cell cycle. HeLa cells were synchronized by double thymidine block followed by nocodazole treatment. Histon H3, which is phosphorylated during mitosis only, was used to indicate mitotic lysates. Western blot analysis showed elevated GAS41 expression in G1/S and in G2/M (Fig. 3B). Since the GAS41 knockdown and the GAS41 overexpression experiments affected both bands shown in Figure 3B for GAS41, we assume that the smaller band represents a variant of GAS41 possibly resulting from post-transcriptional modification. GAS41 immunoprecipitates with γ-tubulin throughout the cell cycle. The interaction of NuMA and GAS41 seems to be transient, beginning during S phase with the strongest interaction during G2/M phase. No interaction was detected in G1/S phase (Fig. 3B). By an extended exposure we also found very weak GAS41 coimmuno-precipitation with NuMA in G1/S. In conclusion, GAS41 appears to bind to NuMA throughout the cell cycle but with greatest affinity during S phase and G2/M. We were also able to detect GAS41 in a NuMA-precipitate of mitotic HeLa cells. While both the immunofluorescence analysis and the Western blotting indicate that GAS41 localizes at the spindle poles during mitosis, we next differentiated between localization at the centrosomes and pericentrosomal (PCM) material of the spindle poles. To this end, we analyzed the GAS41 localization in nocodazole-arrested cells that were subsequently treated with taxol. Taxol promotes microtubule re-growth, resulting in the assembly of pseudo spindles missing centrosomes. We found colocalizations of GAS41 with the spindle proteins NuMA, α-tubulin, and γ-tubulin, but not with centrin in the taxol treated cells (Fig. 4). The diffuse staining pattern of GAS41 was similar to the staining pattern of α-tubulin, γ-tubulin, and NuMA. By contrast, centrin that specifically stains centrosomes is indicated by a distinct signal. The different location and shape of the staining pattern of GAS41 and centrin indicates that GAS41 is most likely not a centrosome protein. Like its binding partner NuMA, GAS41 appears to be associated with the pericentrosome material (Fig. 4).
Figure 2.
Endogenous GAS41 localization in HeLa cells as determined by immunefluorescence analysis. Preimmune serum was used as control. GAS41 maps at the nucleus excluding the nucleoli in inter-phase and at the spindle poles in mitosis.
Figure 3.
A: Colocalization of GAS41 and NuMA as well as GFP-GAS41 and NuMA at the spindle poles during mitosis. B: Binding partners of GAS41. A double thymidine block was used to synchronize HeLa cells in G1/S phase, followed by nocodazole treatment to accumulate cells in G2/M phase. Coimmunoprecipitation of GAS41 with γ-tubulin and NuMA during cell cycle are shown. The upper part of the figure shows the amount of endogenous GAS41, β-actin (ACTB) used as control and phosphorylated histon H3 used for indication of the mitotic fractions. While GAS41 consistent binding to tubulin was found throughout the cell cycle, GAS41 binding to NuMA was very weak in G1/S phase (found by an extended exposure, data not shown) but preferentially in G2/M.
Figure 4.
GAS41 localization in HeLa cells treated by taxol to induce pseudo-spindles without cen-trosomes. GAS41 colocalizes with α-tubulin, γ-tubulin, and NuMA at induced pseudo-spindle poles. The localization of the centrosome is indicated by two distinct signals found for centrin. Since GAS41 does not colocalize with centrin, the results indicate a localization of GAS41 at the spindle poles but specifically at the centrosomes.
Interference of GAS41 and NuMA on Spindle Formation
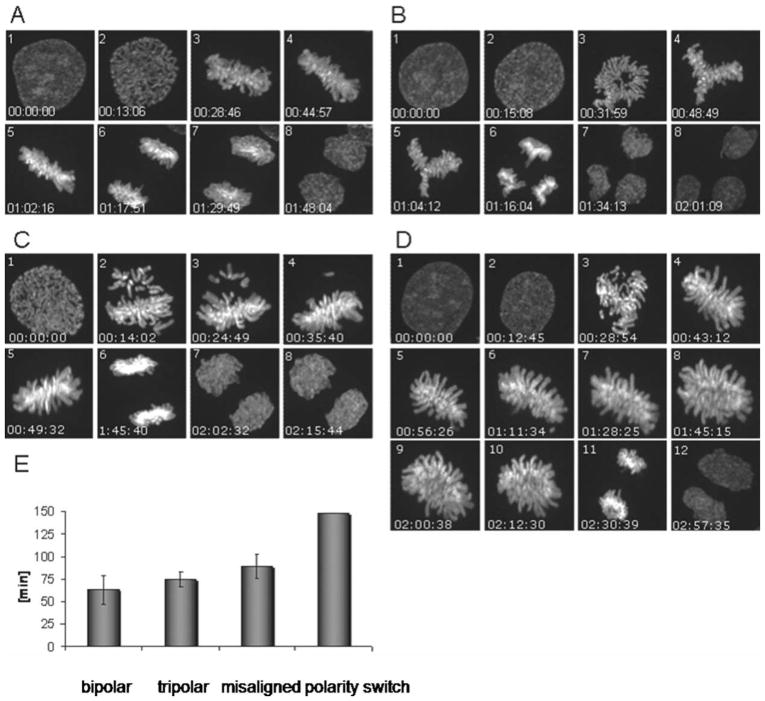
As aforementioned, coimmunoprecipitation demonstrated transient binding of GAS41 and NuMA during cell cycle. We set out to analyze the combined effects of altered NuMA and altered GAS41 expression on the spindle formation. The simultaneous depletion of GAS41 and NuMA by siRNAs leads to 12.2% of multipolar spindles in HeLa cells. Almost the same percentage (12%) of multipolar spindles was found in HeLa cells that were treated by siRNA for NuMA only. These results show that the effects of a reduced expression of NuMA and GAS41 do not add up (Table 1). The effect of a reduced GAS41 expression that leads to 20% multipolar spindles was rescued by a simultaneous reduction of both the GAS41 and the NuMA expression. This rescue effect is also mirrored in the number of bipolar HeLa cells: While HeLa cells depleted for GAS41 have 68.5% bipolar spindles, cells depleted for both proteins have 75.9% of bipolar spindles (Table 1). Next, we analyzed the effect of simultaneous overexpression of GAS41 and NuMA. First, we confirmed that the overexpression of NuMA results in multipolar spindle formation (Quintyne et al., 2005). Upon transfection with GFP-NuMA, we found 14% of multipolar spindles. As stated above, GAS41 overexpression results in up to 16% of multipolar spindles. The simultaneous overexpression of both proteins by cotransfection of HA-GAS41 and GFP-NuMA in HeLa cells results in a considerable reduction of multipolar spindles (8%). Again, this rescue effect is also mirrored in the number of bipolar HeLa cells: While HeLa cells with GAS41 overexpression have 76.1% bipolar spindles and cells with NuMA overexpression have 83% bipolar spindles, cells overexpressing both proteins have 88% of bipolar spindles (Table 1). This rescue effect was not observed in cells in which NuMA and GAS41 expression was altered in opposite directions: HeLa cells with combined GAS41 overexpression and reduced NuMA expression have only 66.7% bipolar spindles. This number is smaller than the numbers found for cells either with GAS41 over-expression only or reduced NuMA expression only. We found a limited rescue effect in HeLa cells with combined reduced GAS41 expression and NuMA overexpression. These cells showed 74.1% of bipolar spindles. As shown above cells with reduced GAS41 expression have 68.5% bipolar spindles. Cells with reduced NuMA expression show however a higher percentage of bipolar spindles (83.7%) than cells with reduced expression of both proteins (Table 1). As expected, the percentage of spindles with misaligned chromosomes was significantly lower than the number of cells with multipolar spindles in all experiments with altered GAS41 and/or NuMA expression. The percentage of spindles with misaligned chromosomes varied between 3% and 11%. Notably, we did not observe a very strong rescue effect when the expression of both proteins was either simultaneously reduced or elevated. Unequal expression of NuMA and GAS41, e.g., increased expression of one and reduced expression of the other protein, leads to an increase of spindles with misaligned chromosomes (Table 1). This effect was most obvious for the simultaneous overexpression of GAS41 and the reduced expression of NuMA leading to almost 20% of cells with misaligned chromosomes. The results indicate that the altered NuMA and GAS41 expression may exerts their effect on either multipolar spindles or spindles with misaligned chromosomes via two different pathways. To understand better the mechanism of multipolar and misaligned spindles, we performed live cell analysis in HeLa cells with GAS41 knockdown. For this we used HeLa cells that stably expressed histone H2B-GFP for real time analysis. First, we verified that HeLa H2B-GFP show the same phenotypes as the HeLa used for the other experiments. We performed an RNAi mediated knockdown in the HeLa H2B-GFP cells and confirmed the formation of multipolar spindles and bipolar spindles with misaligned chromosomes by immunofluorescence using an α-tubulin antibody. The majority of HeLa H2B-GFP cells showed normal bipolar spindles (Fig. 5A). We found an average duration of 63 ± 16 min from prometaphase to the inter-phase of the resulting daughter cells (Fig. 5E). Most of the multipolar spindles had three spindle poles. In the majority of the tripolar cells, we observed spindles forming at an angle of 120° (Fig. 5B). GAS41 depleted cells with these symmetric tripolar spindles divided into three daughter cells without significant mitotic delay (75 ± 8 min) (Fig. 5E). In a few cases, we observed multipolar spindles with asymmetric spindle orientation (Fig. 5D). A polarity switch allowed to turning the asymmetric tripolar prometaphase into a bipolar spindle. The formation of the resulting bipolar spindle was accompanied by a prolonged arrangement of chromosomes in the metaphase plate and a delayed onset of anaphase, both of which causing a mitotic delay of more than 2 hr (Fig. 5E). The few cells that show this rather extended mitosis did not, however, cause an overall prolonged duration of mitosis of the cell population as result of GAS41 deregulation. In cells that showed spindles with misaligned chromosomes (Fig. 5C), the onset of anaphase was delayed until each kinetochore was attached to spindle microtubules and arranged in the equatorial plane. This reorganization process leads to a prolonged mitosis (89 ± 13 min) (Fig. 5E). Our live cell imaging of GAS41 depleted HeLa H2B-GFP showed that cells with altered spindles are able to exit from mitosis and give rise to bipolar daughter cells.
Figure 5.
Cell cycle analysis of GAS41 depleted HeLa H2B-GFP cells. The formation of a normal bipolar spindle (A), of a tripolar spindle (B), of a spindle with misaligned chromosomes (C), and of a spindle with a polarity switch to a bipolar metaphase after a tripolar prometaphase (D) are shown. The duration of metaphase to the next interphase in the different phenotypes is shown in (E).
DISCUSSION
In agreement with previous preliminary evidence, our study shows that GAS41 is a binding partner of proteins associated with spindle micro-tubules. Among the coimmunoprecipitated proteins was γ-tubulin that has been shown by both genetic and biochemical approaches to be important for microtubule nucleation during mitosis (Oakley and Oakley, 1989; Joshi et al., 1992). Specifically, γ-tubulin shows a high affinity to microtubule minus ends (Dammermann et al., 2003). Although γ-tubulin is concentrated at the centrosomes, a huge amount is not associated with the centrosomes (Stearns and Kirschner, 1994; Vassilev et al., 1995; Moudjou et al., 1996). The cytoplasmic γ-tubulin forms a complex of unknown function (Thomasa et al., 2010). Since GAS41 binds to γ-tubulin throughout the cell cycle, it remains to be seen what role GAS41 plays in the cytoplasmic complex outside mitosis. A second coimmunoprecipitated protein was pericentrin that is localized at the pericentriolar matrix (PCM). As previously shown, pericentrin antibodies stain centrosomes and acentriolar microtubule-organizing centers (Doxsey et al., 1994). The third coimmunoprecipitated protein was NuMA. Previously, we showed that GAS41 has a similar localization pattern to NuMA (Munnia et al., 2001). In mitosis, NuMA is recruited at the spindle poles by the minus end-directed motor protein dynein (Compton et al., 1992; Merdes et al., 2000; Wong et al., 2006). The dynein/dynactin complex binds to the N-terminus of NuMA. NuMA is discussed as divalent cross-linker of microtubules on the spindle pole (Wong et al., 2006). We observed an interaction of NuMA and GAS41 beginning in S phase and being the strongest in G2/M phase. Since no interaction has been observed in G1/S, we suggest that the interaction of GAS41 and NuMA may be important for mitosis. As discussed below, GAS41 and NuMA are even likely to function in the same pathway.
Further evidence for a role of GAS41 at the spindle poles stems from the analysis of the acentrosomal pseudo-spindles induced by taxol. GAS41 was colocalized with NuMA, α- and γ-tubulin at the induced spindle poles. However, GAS41 does not colocalize with centrin that is a member of the calcium-binding EF-hand protein superfamily and plays an important role in the organization of centrosomes (Salisbury, 1995; Schiebel and Bornens, 1995). The centrosome-associated centrin is localized at the distal region of each of the two centrioles of the centrosomes (Paoletti et al., 1996). Although a pericentriolar staining of centrin has been reported in calf thymocytes centrosomes (Komesli et al., 1989), a strict centriolar localization of centrin is found in HeLa cells (Paoletti et al., 1996). Based on these studies, our data indicate that GAS41 is spindle pole protein but not necessarily a centrosome protein. This conclusion is also supported by the similarity of the staining pattern of GAS41 and the spindle pole protein NuMA. As for γ-tubulin a major portion of centrin is not associated with the centrosome. It remains to be seen whether GAS41 also binds to centrin when this is not localized at the centrosomes. Our finding of GAS41 at the spindle pole during mitosis is in contrast to the results of Harborth et al. (Harborth et al., 2000) who reported localization of endogenous GAS41 throughout the cell in mitosis in the glioma cell line U333CG/343MG. While we consistently found strong GFP-signals for GFP-GAS41 at the spindle poles, the signals with the polyclonal GAS41 antibody were less pronounced. Possibly, detection of endogenous GAS41 may be hampered due to an inherent instability that has previously been reported in the study of Llanos et al. (2006) who also used GFP-GAS41 for immune-fluorescent analysis. Independent of this difficulty, our results show that both endogenous GAS41 like GFP-GAS41 map at the spindle poles together with NuMA during mitosis. Analyzing HeLa cells we found evidence that both a reduced and an enhanced expression of GAS41 cause perturbation of spindle formation. The altered expression caused an increased number of multipolar cells and of cells with misaligned chromosomes. While these results stem from experiments with induced altered expression of GAS41, comparable effects were also obtained for a glioblastoma derived cell line with endogenous GAS41 overexpression. Together these results show that GAS41 is not only localized at the spindle pole but also influences spindle formation. Our results also show that the combined effects of altered NuMA and GAS41 expression on the spindle formation are rather complex. The number of multipolar cells that we found as result of altered GAS41 expression is significantly reduced when GAS41 and NuMA are simultaneously overexpressed. Likewise, the number of multipolar cells as result of altered GAS41 expression is significantly reduced when GAS41 and NuMA expression is simultaneously reduced. The observed rescue effects indicated that NuMA and GAS41 do not seem to influence independently spindle formation. The rescue effect even indicates that both proteins influence the number spindles via the same pathway. A comparable ‘‘rescue effect’’ was previously described as result of the simultaneous deregulation of NuMA and Rae1 which is a messenger RNA export factor binding to microtubules (Wong et al., 2006). The effect of an altered GAS41 and NuMA expression on spindles with misaligned chromosomes cannot readily be compared with their effects on the number of multipolar spindles. First, we did not find an obvious rescue effect when the expression of both proteins was either simultaneously reduced or elevated. Second, increased expression of NuMA and reduced expression of GAS41, or vice versa lead to an increase of spindles with misaligned chromosomes but not to an obvious increase of multipolar spindles. The effects of a GAS41 over-expression combined with reduced NuMA expression on the number of spindles with misaligned chromosomes were significantly higher than the sum of each single event. The combined overexpression of NuMA and the reduced GAS41 expression lead to almost twice the number of spindles with misaligned chromosomes as compared with the number that was found in cells that had either increased NuMA of reduced GAS41 expression. These results show that altered NuMA and GAS41 expression are likely to influence the number spindles with misaligned chromosomes by a pathway different from the pathway that leads to multipolar spindles. It is important to realize that GAS41 has numerous other functions some of which may also interfere with the alteration in spindle formation via pathways other than those addressed in this study. Loss of GAS41 function induces upregulation not only of the tumor suppressor proteins TP53 but also of 14ARF. Mutations in the C-terminus of GAS41 result in the phosphorylation of TP53 serine-15 residue. Upregulation and phosphorylation of TP53 are in turn sufficient to activate CDKN1A gene expression (Park and Roeder, 2006). Furthermore, GAS41 is required for dephosphorylation of serine 366 on TP53 that forms a complex with PP2CB. The cell survival upon genotoxic DNA damage was increased after ectopic expression of GAS41 and PP2CB by reducing the TP53 upregulation (Park et al., 2011). NuMA appears to be a key to further understanding the role of GAS41 in mitosis. NuMA colocalizes in a cell cycle dependent manner with dynein, most significantly during mitosis (Merdes et al., 2000). The binding of dynein to NuMA requires dynactin as adaptor. The dynein/dynactin complex binds to NuMA and is believed to pull NuMA aggregates along microtubule fibers towards the poles (Merdes et al., 2000; Wong et al., 2006). Since GAS41 binds to NuMA beginning in the S-phase and during mitosis, we suppose that GAS41 is also transported to the spindle poles by the dynein/dynactin complex. Notably, GAS41 has no microtubule-binding site. In this scenario NuMA and GAS41 are acting in the same pathway with NuMA downstream from GAS41. The hypothesis that GAS41 is acting upstream from NuMA is also in keeping with our findings that the number of multipolar spindles (20%) induced by reduced GAS41 expression is significantly lowered (12%) by simultaneous reduced NuMA expression. Vice versa a reduced GAS41 expression does not lower the number of multipolar spindles that are found in cells with reduced NuMA expression. Although not as obvious, a similar observation can be made for GAS41 and NuMA overexpression. NuMA over-expression seems to have a stronger effect than GAS41 overexpression on the rescue effect in cells that overexpress both proteins. Finally, we would like to acknowledge that many other proteins and pathways are involved in spindle pole formation. Other pathways may compensate any NuMA–GAS41 imbalance. This might hold especially true for tumor cells with multiple genetic alterations leading in turn to largely altered pathways.
Acknowledgments
Supported by: Homfor.
Authors thank Andreas Merdes for kindly providing the pcDNA3-GFP-NuMA plasmid.
References
- Cai Y, Jin J, Florens L, Swanson SK, Kusch T, Li B, Workman JL, Washburn MP, Conaway RC, Conaway JW. The mammalian YL1 protein is a shared subunit of the TRRAP/ TIP60 histone acetyltransferase and SRCAP complexes. J Biol Chem. 2005;280:13665–13670. doi: 10.1074/jbc.M500001200. [DOI] [PubMed] [Google Scholar]
- Compton DA, Szilak I, Cleveland DW. Primary structure of NuMA, an intranuclear protein that defines a novel pathway for segregation of proteins at mitosis. J Cell Biol. 1992;116:1395–1408. doi: 10.1083/jcb.116.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A, Desai A, Oegema K. The minus end in sight. Curr Biol. 2003;13:R614–R624. doi: 10.1016/s0960-9822(03)00530-x. [DOI] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Doyon Y, Selleck W, Lane WS, Tan S, Côté J. Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol Cell Biol. 2004;24:1884–1896. doi: 10.1128/MCB.24.5.1884-1896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- Fischer U, Meltzer P, Meese E. Twelve amplified and expressed genes localized in a single domain in glioma. Hum Genet. 1996;98:625–628. doi: 10.1007/s004390050271. [DOI] [PubMed] [Google Scholar]
- Fischer U, Heckel D, Michel A, Janka M, Hulsebos T, Meese E. Cloning of a novel transcription factor-like gene amplified in human glioma including astrocytoma grade I. Hum Mol Genet. 1997;6:1817–1822. doi: 10.1093/hmg/6.11.1817. [DOI] [PubMed] [Google Scholar]
- Fischer U, Leidinger P, Keller A, Folarin A, Ketter R, Graf N, Lenhof HP, Meese E. Amplicons on chromosome 12q13–21 in glioblastoma recurrences. Int J Cancer. 2010;126:2594–2602. doi: 10.1002/ijc.24971. [DOI] [PubMed] [Google Scholar]
- Garrett S, Auer K, Compton DA, Kapoor TM. hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr Biol. 2002;12:2055–2059. doi: 10.1016/s0960-9822(02)01277-0. [DOI] [PubMed] [Google Scholar]
- Harborth J, Weber K, Osborn M. GAS41, a highly conserved protein in eukaryotic nuclei, binds to NuMA. J Biol Chem. 2000;275:31979–31985. doi: 10.1074/jbc.M000994200. [DOI] [PubMed] [Google Scholar]
- Haren L, Gnadt N, Wright M, Merdes A. NuMA is required for proper spindle assembly and chromosome alignment in prometaphase. BMC Res Notes. 2009;2:64. doi: 10.1186/1756-0500-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisel S, Habel NC, Schuetz N, Ruggieri A, Meese E. The YEATS family member GAS41 interacts with the general transcription factor TFIIF. BMC Mol Biol. 2010;11:53. doi: 10.1186/1471-2199-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi HC, Palacios MJ, McNamara L, Cleveland DW. Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Komesli S, Tournier F, Paintrand M, Margolis RL, Job D, Bornens M. Mass isolation of calf thymus centrosomes: Identification of a specific configuration. J Cell Biol. 1989;109:2869–2878. doi: 10.1083/jcb.109.6.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffart B, Howell SJ, Tasch JE, Cowell JK, Still IH. Interaction of the transforming acidic coiled-coil 1 (TACC1) protein with ch-TOG and GAS41/NuBI1 suggests multiple TACC1-containing protein complexes in human cells. Biochem J. 2002;363:195–200. doi: 10.1042/0264-6021:3630195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Masson I, Yu DY, Jensen K, Chevalier A, Courbeyrette R, Boulard Y, Smith MM, Mann C. Yaf9, a novel NuA4 histone acetyltransferase subunit, is required for the cellular response to spindle stress in yeast. Mol Cell Biol. 2003;23:6086–6102. doi: 10.1128/MCB.23.17.6086-6102.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon A, Omri B, Gely A, Klein C, Crisanti P. QN1/ KIAA1009: a new essential protein for chromosome segregation and mitotic spindle assembly. Oncogene. 2006;25:1887–1895. doi: 10.1038/sj.onc.1209215. [DOI] [PubMed] [Google Scholar]
- Llanos S, Efeyan A, Monsech J, Dominguez O, Serrano M. A high-throughput loss-of-function screening identifies novel p53 regulators. Cell Cycle. 2006;5:1880–1885. doi: 10.4161/cc.5.16.3140. [DOI] [PubMed] [Google Scholar]
- Marschalek R. Mixed lineage leukemia: roles in human malignancies and potential therapy. FEBS J. 2010;277:1822–1831. doi: 10.1111/j.1742-4658.2010.07608.x. [DOI] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Merdes A, Heald R, Samejima K, Earnshaw WC, Cleveland DW. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J Cell Biol. 2000;149:851–862. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudjou M, Bordes N, Paintrand M, Bornens M. Gamma-Tubulin in mammalian cells: The centrosomal and the cytosolic forms. J Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- Munnia A, Schütz N, Romeike BF, Maldener E, Glass B, Maas R, Nastainczyk W, Feiden W, Fischer U, Meese E. Expression, cellular distribution and protein binding of the glioma amplified sequence (GAS41), a highly conserved putative transcription factor. Oncogene. 2001;20:4853–4863. doi: 10.1038/sj.onc.1204650. [DOI] [PubMed] [Google Scholar]
- Oakley CE, Oakley BR. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Oshimori N, Ohsugi M, Yamamoto T. The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat Cell Biol. 2006;8:1095–1101. doi: 10.1038/ncb1474. [DOI] [PubMed] [Google Scholar]
- Paoletti A, Moudjou M, Paintrand M, Salisbury JL, Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- Park JH, Roeder RG. GAS41 is required for repression of the p53 tumor suppressor pathway during normal cellular proliferation. Mol Cell Biol. 2006;26:4006–4016. doi: 10.1128/MCB.02185-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Smith RJ, Shieh SY, Roeder RG. The GAS41–PP2C{beta} Complex dephosphorylates p53 at serine 366 and regulates its stability. J Biol Chem. 2011;286:10911–10917. doi: 10.1074/jbc.C110.210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–129. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7:39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Schiebel E, Bornens M. In search of a function for centrins. Trends Cell Biol. 1995;5:197–201. doi: 10.1016/s0962-8924(00)88999-0. [DOI] [PubMed] [Google Scholar]
- Silk AD, Holland AJ, Cleveland DW. Requirements for NuMA in maintenance and establishment of mammalian spindle poles. J Cell Biol. 2009;184:677–690. doi: 10.1083/jcb.200810091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Kirschner M. In vitro reconstitution of centro-some assembly and function: The central role of gamma-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Thomas NE, Shashikala S, Sengupta S. Cytoplasmic gamma-tubulin complex from brain contains nonerythroid spectrin. J Cell Biochem. 2010;110:1334–1341. doi: 10.1002/jcb.22647. [DOI] [PubMed] [Google Scholar]
- van Vugt MA, van de Weerdt BC, Vader G, Janssen H, Calafat J, Klompmaker R, Wolthuis RM, Medema RH. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J Biol Chem. 2004;279:36841–36854. doi: 10.1074/jbc.M313681200. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kimble M, Silflow CD, LaVoie M, Kuriyama R. Identification of intrinsic dimer and overexpressed monomeric forms of gamma-tubulin in Sf9 cells infected with baculovirus containing the Chlamydomonas gamma-tubulin sequence. J Cell Sci. 1995;108:1083–1092. doi: 10.1242/jcs.108.3.1083. [DOI] [PubMed] [Google Scholar]
- Wong RW, Blobel G, Coutavas E. Rae1 interaction with NuMA is required for bipolar spindle formation. Proc Natl Acad Sci USA. 2006;103:19783–19787. doi: 10.1073/pnas.0609582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Richardson DO, Roberts DN, Utley R, Erdjument-Bromage H, Tempst P, Côté J, Cairns BR. The Yaf9 component of the SWR1 and NuA4 complexes is required for proper gene expression, histone H4 acetylation, and Htz1 replacement near telomeres. Mol Cell Biol. 2004;24:9424–9436. doi: 10.1128/MCB.24.21.9424-9436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]