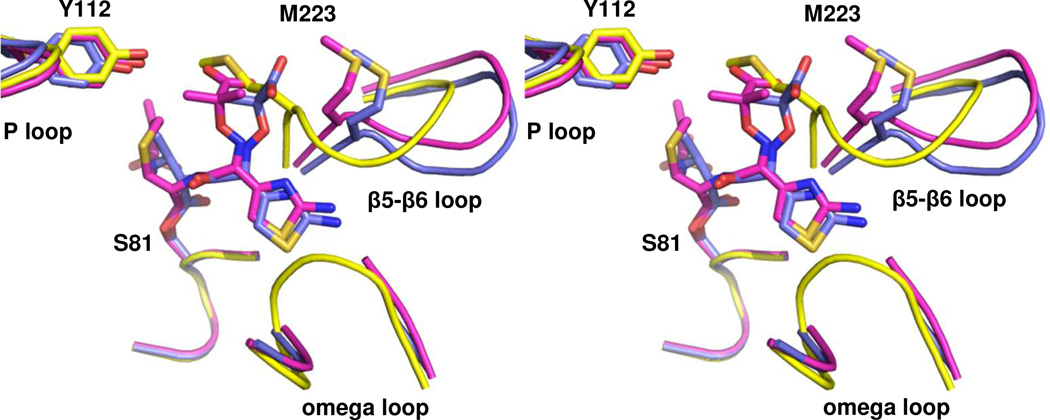
Figure 4. Comparison of acyl-enzyme intermediates in the active site of OXA-160.
Stereoview of the structures of OXA-160 V130D with ceftazidime (magenta; PDB 4X56) and aztreonam (blue; PDB 4X53) bound. To generate the structural alignment, key active site residues from OXA-160 V130D/ceftazidime (S81, S128, K218 and G220) were superposed with the same residues of OXA-24/40 K84D (yellow; PDB 3PAE) using the align function of PyMOL generating a relative mean square deviation (RMSD) of 0.174 Å. This process was repeated for OXA-160 V130D/aztreonam and OXA-24/40 K84D (RMSD, 0.321 Å).