Abstract
A core tenet of the original conflict-monitoring model is that regulation is triggered automatically when conflict is present and that the same regulation mechanism explains both trial-to-trial adaptation effects as well as effects of block-wise conflict manipulations. We present here results from two experiments using the Stroop task that show (a) that adaptation effects in the absence of response repetitions may occur only at the beginning of testing and that (b) robust block-wise effects can be found even in the absence of trial-by-trial effects. Furthermore, we show that the failure to eliminate target-to-distractor repetitions can produce artificial trial-to-trial adaptation effects. Based on the evidence of a weak link between conflict and conflict adaptation, we argue that a wider range of possible reasons for conflict adaptation effects needs to be taken into consideration.
The elusive link between conflict and conflict adaptation
How is cognitive control established, what are its triggering conditions, and which neural systems are involved? These are currently some of the most important questions in cognitive neuroscience. The conflict-monitoring model promises a unified answer to these questions (Botvinick, Braver, Barch, Carter, & Cohen, 2001). Its central proposal is the existence of a so-called conflict monitoring module, neuroanatomically localized in the anterior cingulate cortex (ACC), which constantly measures the level of representational conflict in the system. Once it detects conflict it automatically triggers the recruitment of attentional control activities, usually associated with lateral prefrontal cortex. What makes this model so intriguing is the fact that it promises a solution to the recursive “control-of-control” problem without having to assume an intelligent homunculus that gets to decide when control is necessary. The solution, according to the model is as simple as the thermostat in our refrigerator: Both the measurement of conflict and the triggering of control occur automatically and require no intervening intelligent agent. Obviously then, the automatic and unconditional link between conflict and control is at the heart of the conflict-monitoring model.
The data pattern that has been most commonly used in support of the conflict-monitoring model is the so-called conflict-adaptation effect (Gratton, Coles, & Donchin, 1992). Originally, this effect has been reported for the flanker task where subjects have to respond to a central stimulus (e.g., an arrow pointing either to the left or the right) surrounded by irrelevant flanker stimuli that could either point in the same or the opposite direction as the central stimulus. The critical finding from the perspective of the conflict-monitoring model is the fact that response times (RTs) are reduced on incongruent trials following incongruent trials (i.e., II) relative to incongruent trials following congruent trials (i.e., CI). Conversely, RTs on congruent trials that follow incongruent trials (i.e., IC) are longer than on congruent trials following congruent trials (i.e., CC). The conflict-monitoring explanation of this pattern is that conflict on trial n triggers recruitment of attentional control, which in turn puts the system in a better position to deal with conflict on trial n + 1 (i.e., good performance on II trials compared to CI trials), but in a worse position to use the response-consistent flanker information in case of a congruent n + 1 trial (i.e., worse performance on IC trials than on CC trials).
Another data pattern this model had been applied to occurs on the level of entire blocks of trials (e.g., Tzelgov, Henik, & Berger, 1992). Specifically, conflict effects are reduced in blocks with a high proportion of incongruent trials. According to the conflict-monitoring model, this pattern can be easily explained as the cumulative effect of trial-by-trial adaptations the frequent conflict trials.
The close relationship between local, trial-to-trial adaptation effects and global, block-wise effects as well as the claim that these effects should occur in an unconditional automatic manner, are central to the conflict-monitoring model. However, there is no work that has explicitly tested these claims. In particular with respect to the hypothesis of an automatic conflict-control link it is critical to assess how consistent the finding of a conflict-adaptation pattern actually is.
We showed in a previous paper (Mayr, Awh, & Laurey, 2003) that much of the conflict-adaptation pattern is in fact the result of a supposedly automatic effect, but one that has nothing to do with executive regulation of conflict. Specifically, we demonstrated that those trials for which fast RTs are to be expected according to the conflict-monitoring model (II and CC) are also those trials that contained a large portion of exact stimulus/response repetitions (50% in case of a 2-choice task). In other words, supposedly automatic response repetitions may be a major source behind the adaptation pattern. In fact, once such repetitions were eliminated either post-hoc (Experiment 1), or by not allowing them in the first place (Experiment 2), the conflict-adaptation effect disappeared. Our initial finding of no detectable conflict adaptation, after considering priming effects in the Flanker task, was recently replicated across five different experiments (total N = 944) that included variations in stimulus duration, speed-accuracy emphasis, and motivational incentives to regulate performance (Nieuwenhuis, Stins, Posthuma, Polderman, Boomsma, & De Geus 2006).
At this point, few would contest that lower-level repetition priming effects exist in typical conflict paradigms and that these effects probably had tainted conflict-adaptation results available at the time the conflict-monitoring model was initially established. In fact, it has now become standard practice to eliminate lower-level effects when looking for conflict adaptation. However, since our paper, there also have been a number of reports where conflict-adaptation patterns survived even though measures were taken to eliminate lower-level contributions (e.g., Freitas, Bahar, Yang, & Bahar, 2007; Kerns, Cohen, MacDonald, Cho, Stenger, & Carter, 2004). Routinely, such evidence is interpreted simply as reconfirmation of the original conflict-monitoring model.
One particularly influential demonstration of “true” conflict-adaptation was reported by Kerns et al. (2004) in direct response to our study. These authors used a variant of the Stroop task with three response choices, 70% congruent trials, and three blocks of 80 trials each. After eliminating word-word and color-color repetitions, a sizeable conflict adaptation effect of approximately 50 ms remained. In addition, these authors provided brain imaging evidence that trial n ACC activity predicted both trial n + 1 prefrontal activity and the size of the adaptation effect. We will return to the neural evidence in the Discussion section. Our main concern here is with the behavioral adaptation pattern obtained in this study. Two aspects are noteworthy in this respect.
First, assessment of the adaptation effect was relatively short with only three blocks of testing. Why would length of testing matter? One possibility is that adaptation patterns arise from an explicit, conscious response to perceived conflict. Interestingly, this was exactly the explanation for the conflict-adaptation pattern presented in the original paper (Gratton et al., 1992). In fact, these authors provided evidence that an adaptation-like pattern is obtained when subjects receive explicit cues that signal the level of conflict for the next trial. Such conscious regulation could be expected in the early, deliberative phases of practice with a new task. Thus, it is possible that the conflict-adaptation pattern is restricted to the first few blocks of testing and disappears thereafter.
Second, while simple repetitions of relevant and irrelevant features were eliminated in this study, cross-dimensional repetitions (i.e., word-to-color and color-to-word) could still occur. In fact, with only three different stimuli, all trial-to-trial transitions that do not contain within-dimension repetitions necessarily contain across-dimension repetitions in case of II trials. A-priori it is not clear what the effect of these types of repetitions might be. Distractor-to-target repetitions (i.e., word-to-color) are negative-priming situations, which often produce particularly long response times. Including these in II trials would work against detecting a true conflict-adaptation pattern. Target-to-distractor repetitions (i.e., color-to-word) have received much less attention in the literature. However, in the few cases they have been looked at, substantial RT facilitation was obtained (e.g., May, Kane, & Hasher, 1995). Thus, including these in II trials could produce artificial adaptation patterns.
The first experiment we report here is basically a replication of Kerns et al. (2004) study. However, we use a larger number of blocks in order to examine the robustness of the any adaptation pattern as a function of within-experiment experience. In this experiment, we also look separately at distractor-to-target and at target-to-distractor repetitions to find hints for possible confounding effects of such cross-dimensional repetitions.
Experiment 1
Method
Participants
Thirty-five students of the University of Oregon participated in 10 blocks of 88 trials each.
Stimuli, tasks, and procedure
Stimuli were the words red, green, or blue, written in red, green, or blue font. Responses were executed on the computer keyboard with the colors red, green, and blue mapped onto the x key, the y key, and the z key respectively. Subjects were instructed to respond to the word color and ignore the word and to perform the task as fast as possible without sacrificing accuracy. As in Kerns et al. (2004), the congruent/incongruent ratio was 70/30 and the first four and the last four trials of each block were always incongruent and not included in the analysis.
Results and discussion
Adaptation effects
The first four and the final four trials of each block were eliminated from the analyses, as were error trials and trials following errors. In addition, we eliminated all color-repetition and all word-repetitions trials. If these had been included, large adaptation patterns would have been obtained. Table 1 shows the relevant results for both RTs and errors, along with the critical F-statistics for the prevision-trial congruent and the current-trial congruent interaction. We present both the overall results and results for the first two blocks and the remaining blocks separately. This way of defining an “early phase” of practice is somewhat arbitrary. The two blocks are a bit shorter than the three 80-trial blocks of total testing used by Kerns et al. (2004), but dividing the blocks this way consistently produced the strongest adaptation effects across the two experiments presented here.
Table 1.
Kerns et al. (2004) replication
| CC | IC | CI | II | F(1,34) | |
|---|---|---|---|---|---|
| Overall | |||||
| RTs | 642 (133) | 646 (136) | 802 (178) | 796 (170) | 1.5 |
| Errors | 2.1 (2.1) | 1.5 (2.3) | 6.6 (5.5) | 4.5 (4.8) | 2.3 |
| Blocks 1–2 | |||||
| RTs | 650 (149) | 659 (149) | 850 (205) | 791 (186) | 4.4* |
| Errors | 1.7 (2.2) | 2.3 (4.9) | 5.5 (8.7) | 3.1 (8.2) | 2.3 |
| Blocks 3–10 | |||||
| RTs | 638 (132) | 646 (137) | 789 (174) | 793 (176) | 0.2 |
| Errors | 2.2 (2.2) | 1.4 (2.6)6 | 6.8 (6.1) | 4.9 (5.6) | 0.9 |
Mean RTs (SD) and Error percentages (SD) for the four critical trial types, congruent following congruent (CC), congruent following incongruent (IC), incongruent following congruent (CI), and incongruent following incongruent (II), across all ten blocks and separately for blocks 1–2 and blocks 3–10. Also shown are the F-statistics for the conflict-adaptation effect (*<0.05)
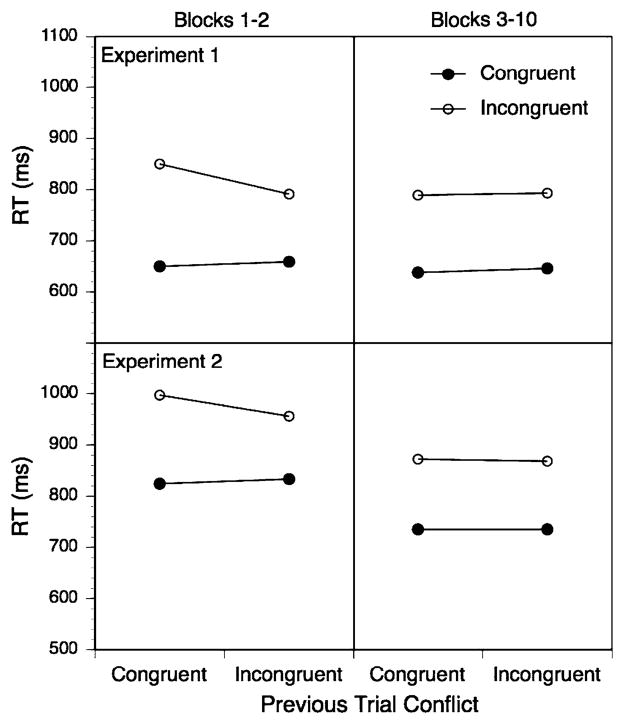
Despite a very strong congruency effect, there were no overall reliable adaptation effects, neither for RTs, where the total adaptation effect was 10 ms, nor for errors, where the total effect size was 1.5%. However, as shown in Fig. 1 (upper panel), when looking separately at the initial two blocks, a reliable RT adaptation effect of a total of 50 ms was obtained along with a numerical adaptation pattern for errors with a total effect size of 2.5%. However, for the remaining eight blocks, there was no detectable adaptation pattern (RT effect size = 4 ms, error effect size = 1.0%). The three-way interaction between phase (blocks 1–2 vs. blocks 3–10), previous-trial and current-trial congruency just failed the reliability criterion, F(1,24) = 3.42, P < 0.08.
Fig. 1.
Trial-wise RT adaptation effects separately for blocks 1–2 versus blocks 3–10 and the two experiments
Cross-dimensional repetitions
In a design with only three different responses, all II trials are either target-to-distractor or distractor-to-target (negative priming) repetitions. With the missing no-repetition control this design is not an optimal situation to look at the specific effects of either of these two types of repetitions. However, it is possible to obtain some idea whether or not such effects are present by simply comparing target-distractor and negative-priming trials. We found that target distractor trials were faster, difference score = 30 ms, t(34) = 1.7, P = 0.10, and more accurate than negative-priming trials, difference score = 2.5%, t(34) = 1.6, P = 0.13. Even though they failed the statistical significance criterion, these results suggest that in light of the usually small conflict-adaptation effects, cross-dimensional priming needs to be considered as an additional lower-level factor that can artificially produce adaptation effects. In fact, when eliminating the target-to-distractor repetitions from the analyses of the first two blocks the adaptation effect was reduced from 50 to 20 ms, and no longer reliable (P > 0.6). It needs to be pointed out though that this analysis is ambiguous as it is not clear to what degree target-to-distractor repetitions underestimate, or whether distractor-to-target repetitions overestimate the RTs for II trials. Experiment 2 will allow us to clarify this issue.
Conclusion
In our close replication of Kerns et al. (2004) design, we obtained the adaptation pattern for the first two blocks, which is roughly comparable to the three blocks total testing used in the original study. However, the adaptation pattern was not present in the remaining blocks. At first glance, this result is compatible with the idea that adaptation might be a conscious process that occurs during early, deliberate phases of dealing with a new situation. However, matters are complicated by the fact that designs with only three response options do not eliminate potential contaminations of adaptation effects through cross-dimension repetitions. In fact, detailed analyses of these repetitions suggest the possibility that they might be at least partially responsible for the observed adaptation pattern found in the first two blocks.
Experiment 2
In the second experiment, we switched to a 6-choice version of the Stroop task, which allows a sufficient number no-repetition transitions (within nor between dimensions) to look at adaptation effects in an un-confounded manner. In this experiment, we can also take another look at the potential effect of cross-dimension repetitions. Finally, we also manipulated the proportion of conflict trials with the goal to look at local, trial-to-trial adaptation effects and global, block-wise adaptation within the same experiment.
Method
Participants
Sixty students of the University of Oregon participated in 10 blocks of 80 trials each.
Stimuli, tasks, and procedure
Stimuli were the words red, green, blue, brown, gray, or yellow written in red, green, blue, brown, gray, or yellow font. Responses were executed on the computer keyboard with the colors red, green, blue, brown, gray, or yellow mapped onto the x key, the y key, and the z key respectively. Subjects were instructed to respond to the word color and ignore the word and to perform the task as fast as possible without sacrificing accuracy. Subjects were randomly assigned to three different groups differing in the ratio of congruent to incongruent trials (70/30, 50/50, 30/70). Eighteen subjects each were assigned to the 50/50 and the 30/70 group. The 70/30 group is the most interesting (i.e., it is the same condition that was used by Kerns et al. (2004) and in the Experiment 1) and the number of II trials in this condition can become very small, in particular after eliminating repetitions. Therefore we assigned 24 subjects to this condition.
Results and discussion
Adaptation effects
Again, error trials and trials after errors were eliminated. For the main analyses we also eliminated all within-dimension and cross-dimension repetitions. One subject in the 50/50 condition was excluded as an outlier because of mean RTs that were 8 SDs larger than the mean RT in that condition. Table 2 presents relevant results for both RTs and errors across all relevant conditions. We present both the overall results, as well as results for the first two blocks and the remaining blocks separately.
Table 2.
Six-choice stroop
| CC | IC | CI | II | F-value | |
|---|---|---|---|---|---|
| 70/30 Overall | |||||
| RTs | 734 (132) | 742 (135) | 913 (136) | 900 (141) | 2.29 |
| Errors | 2.7 (2.7) | 2.2 (3.0) | 6.5 (4.9) | 7.6 (7.3) | 2.02 |
| 70/30 Blocks 1–2 | |||||
| RTs | 808 (218) | 819 (212) | 1035 (254) | 965 (199) | 6.50* |
| Errors | 3.3 (4.7) | 4.4 (9.8) | 9.0 (6.7) | 8.3 (11.7) | 0.49 |
| 70/30 Blocks 3–10 | |||||
| RTs | 716 (119) | 724 (123) | 885 (121) | 882 (139) | 0.52 |
| Errors | 2.5 (2.3) | 1.7 (2.0) | 5.9 (4.9) | 7.2 (8.0) | 2.8 |
| 50/50 Overall | |||||
| RTs | 755 (94) | 755 (106) | 879 (114) | 879 (132) | 0.0 |
| Errors | 3.1 (2.4) | 2.6 (2.1) | 6.6 (4.2) | 5.6 (4.6) | 0.1 |
| 50/50 Blocks 1–2 | |||||
| RTs | 809 (153) | 819 (169) | 929 (160) | 935 (137) | 0.0 |
| Errors | 3.8 (4.5) | 3.2 (5.8) | 8.7 (7.7) | 7.7 (8.2) | 0.2 |
| 50/50 Blocks 3–10 | |||||
| RTs | 787 (207) | 791 (242) | 907 (212) | 913 (219) | 1.59 |
| Errors | 2.9 (2.7) | 2.4 (2.3) | 5.9 (4.4) | 5.1 (4.7) | 0.0 |
| 30/70 Overall | |||||
| RTs | 775 (161) | 771 (144) | 890 (204) | 866 (154) | 1.61 |
| Errors | 1.8 (2.9) | 2.1 (2.0) | 4.4 (2.9) | 3.7 (1.9) | 0.3 |
| 30/70 Blocks 1–2 | |||||
| RTs | 858 (238) | 846 (211) | 1012 (268) | 964 (217) | 1.7 |
| Errors | 0.9 (2.8) | 2.9 (5.9) | 9.7 (7.0) | 4.4 (3.7) | 7.9* |
| 30/70 Blocks 3–10 | |||||
| RTs | 755 (147) | 750 (132) | 862 (193) | 842 (146) | 0.68 |
| Errors | 2.0 (3.1) | 1.8 (2.2) | 3.1 (3.0) | 3.5 (2.0) | 0.5 |
Mean RTs (SD) and Error percentages (SD) for the four critical trial types, congruent following congruent (CC), congruent following incongruent (IC), incongruent following congruent (CI), and incongruent following incongruent (II), separately for the three different congruency ratios, across all ten blocks, as well as separately for blocks 1–2 and blocks 3–10. Also shown are the F-statistics for the conflict-adaptation effect (*<0.05). Degrees of freedom for the F-value are 1, 23 for the 70/30 condition, 1, 16 for the 50/50 condition, and 1, 17 for the 30/70 condition
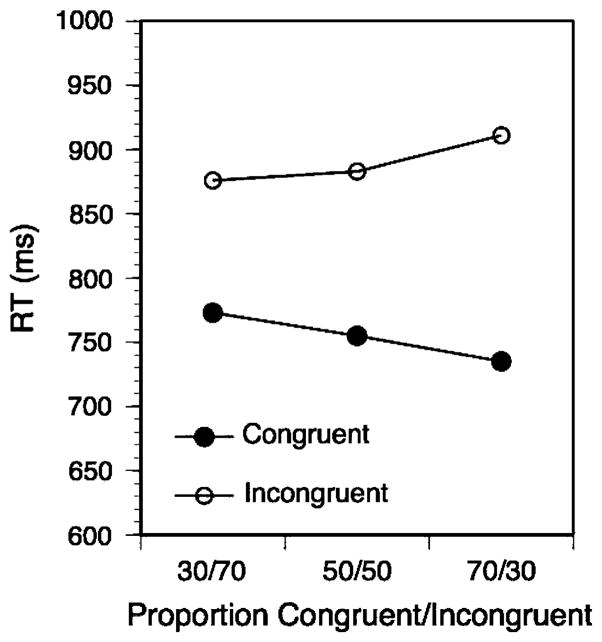
We first conducted an overall ANOVA with the factors current-trial congruency, previous-trial congruency, and phase (blocks 1–2 versus blocks 3–10) as within-subject factors and conflict-proportion as a three-level between-subject factor, specified as a linear contrast. Aside from a very large conflict effect, F(1,57) = 218.07, P < 0.01, there was a strong linear effect of conflict proportion on the congruency effect with larger conflict effects for lower conflict proportions (but no conflict-proportion main effect), F(1,57) = 8.12, P < 0.01. This effect is shown in Fig. 2. In addition, the results reveal a standard, trial-by-trial adaptation pattern in form of an interaction between prior-trial congruency and current-trial congruency, F(1,57) = 5.48, P < 0.03, and finally both a general speed-up across the two phases, F(1,57) = 47.61, P < 0.01, and a three-way interaction between the phase factor, prior-trial congruency, and current-trial congruency, F(1,57) = 4.52, P < 0.03. This three-way interaction was due to the fact that there was a substantial adaptation effect of 51 ms for the first two blocks. F(1,57) = 5.48, P < 0.03, that completely disappeared for the remaining blocks (4 ms), F(1,57) = 0.11, P > 0.7 (see Fig. 1, lower panel). Even though the adaptation pattern disappeared after the first two blocks, the effect of the conflict-proportion on the Stroop congruency effect remained highly reliable for blocks 3–10, F(1,57) = 9.0, P < 0.01. Both the clear absence of an adaptation pattern beyond the first two blocks and the fact that there was a strong congruency proportion effect in the absence of an adaptation pattern in blocks 3–10 is difficult to reconcile with the conflict-monitoring model.
Fig. 2.
Block-wise effect of congruency proportions on the RT congruency effect in Experiment 2
With regard to errors, the pattern of adaptation effects was similar to RTs. The overall adaptation effect failed the reliability criterion, F(1,57) = 2.04, P > 0.1, but the triple interaction with the phase factor was reliable, F(1,57) = 5.14, P < 0.03 (for details, see Table 2). The fact that these block-wise and trial-to-trial adaptation effects seem to be largely dissociated (i.e., during blocks 3–10) is difficult to reconcile with the conflict-monitoring account, which sees both effects as two sides of the same coin.
Table 2 also shows the results for specific groups and separately for the initial phase and the remaining blocks. As apparent, for RTs the adaptation pattern was reliable only for the initial phase in the 70/30 group, although there was a numerical adaptation pattern also for the initial phase in the 30/70 group. For errors, the adaptation pattern was reliable only in the initial phase in the 30/70 group. The fact that the adaptation pattern was present in the first two blocks is consistent both with Experiment 1 and with the idea that it might result from conscious regulation during the early, deliberate phase of practice. Different than in Experiment 1 all repetitions could be excluded here. Thus, the adaptation pattern we observed in the initial phase cannot be attributed to lower-level priming effects.
Cross-dimensional priming
This experiment allows an additional opportunity to examine the effects of cross-dimensional priming. Looking only at II trials, and separately at no-repetition trials, target-to-distractor repetitions, and distractor-to-target repetitions, we found that target-to-distractor repetitions were highly significantly faster and more accurate than no-repetition trials, RT effect = 40 ms, t(58) = 4.0, P < 0.001, accuracy effect = 2.2%, t(58) = 2.8, P < 0.01, whereas distractor-to-target repetitions (i.e., negative priming) were not significantly slower, RT effect = 24 ms, t(58) = 1.6, P > 0.12, and actually somewhat more accurate than no-repetition trials, accuracy effect = 1.6%, t(58) = 1.7, P > 0.1. These effects were not reliably modulated by the variation in conflict proportion. Thus, in the present situation the net effect of not excluding cross-dimensional repetitions would work in favor of reducing RTs and improving accuracy rates on II trials, which in turn could lead to spurious adaptation patterns. For example, if cross-dimension trials had been included in the current experiment a significant accuracy adaptation pattern would have been obtained, F(1,57) = 4.69, P < 0.05, an effect that was not reliable after excluding cross-dimensional repetitions, F(1,57) = 2.04, P > 0.1.
General discussion
The experiments reported here were closely modeled after the paradigm used by Kerns et al. (2004), which produced one of the most-cited results in favor of the conflict-monitoring model. We find some evidence for the basic conflict-adaptation pattern when we constrain our analysis to about the same amount of testing as occurred in Kerns et al. (2004). However, in both of our experiments, we show that the adaptation effect disappears when looking beyond the initial phase of testing, even though a robust conflict effect is present thought the experiment. In Experiment 2, we also found that the trial-to-trial adaptation pattern and the block-wise modulation of the conflict effect through the proportion of conflict trials can be dissociated (for a similar result, see Fernandez-Duque & Knight, 2007). Both the transient nature of the conflict adaptation effect and the dissociation between trial-by-trial and block-wise effects are difficult to explain with current version of the conflict-monitoring model. According to this model, adaptation should arise automatically whenever there is significant response conflict. Furthermore, if trial-to-trial and block-wise effects are reflections of the same basic process, they should co-occur. We also showed that a potential confound present in the Kerns et al. 2004 paradigm, the presence of cross-dimensional repetitions, may have contributed to the adaptation pattern in that study. This result underscores the importance of taking into regard all possible low-level repetitions when the goal is to interpret more abstract trial-to-trial relationships. This result is also important with regard to studies that have used post-hoc exclusion of repetitions trials in the context of two-choice Flanker task situations (e.g., Mayr et al., 2003; Nieuwenhuis et al., 2006). In these studies, all II trials that remain after excluding target repetitions are either target-to-distractor or distractor-to-target repetitions. If negative priming was a major factor in such situations, the absence of an adaptation effect in these situations might be explained because negative priming could artificially increase RTs in II trials. However, given that we found that target-to-distractor effects were stronger than negative priming effects, such a scenario seems unlikely. In the following, we discuss some of the implications of the present and other recent results for the question of how the cognitive system deals with conflict.
Conflict-adaptation in Kerns et al. (2004) study?
Our results suggest that the conflict-adaptation effects found in Kerns et al. (2004) study may have been rather limited with regard to amount of testing and may have been affected by a cross-dimensional repetition confound. One could counter that the behavioral effects presented by Kerns et al. (2004) are only half of the story and that the more interesting results relevant for the conflict-monitoring model are actually in the brain-imaging results from that study. Specifically, these authors reported that the conflict-related ACC response on trial n was predictive of the amount of prefrontal activity and the behavioral conflict effect on trial n + 1. This result seems to directly support the claim that ACC registers conflict and initiates activity in regulatory areas (i.e., lateral prefrontal cortex).
However, the analyses that this conclusion is based on were geared towards confirming the one predicted association (i.e., between trial n ACC activity and trial n + 1 PFC activity), while ignoring all other possible associations between two areas across two time points. To be more specific, the data-analytic situation is that of a cross-lagged panel analysis where inferences about variable A at time point 1 predicting variable B at time point 2 would have to be examined after controlling for all possible synchronous and asynchronous relations (e.g., Kenny, 1975). For example, consider the possibility that ACC and PFC activity covary across trials, such that both regions tend to be both more active on some trials and less active on others, and that activity on one trial predicts activity on the next trial. In such a case, the correlation between trial n ACC activity and trial n + 1 PFC activity could arise simply because activity in these areas is correlated, rather than because of a causal effect of ACC on PFC activity. The critical question is whether ACC activity is a “leading indicator” of prefrontal activity and/or behavioral adjustments. Until such results are provided, any verdict on whether or not there is strong neural-level support for the conflict-adaptation model needs to be considered with caution.
In this context it is interesting to note that data from patients with profound ACC damage do not corroborate the conflict-monitoring model. Specifically, Fellows and Farah reported no change in Stroop effect and block-wise adaptation patterns in patients with substantial ACC lesions. In addition, in a particularly careful neuroimaging study by Egner and Hirsch (2005) there was clear indication of conflict adaptation and of prefrontal involvement in this effect, but no evidence for ACC involvement.
The role of practice: do adaptation effects depend on explicit regulation?
In those cases where we did find evidence for conflict-adaptation patterns in our study they were all constrained to the first two blocks of practice in the task. One possible explanation for this pattern is that these types of early adaptation effects are the result of deliberate and conscious regulation. It has long been thought that early phases of dealing with a new task are characterized by deliberate regulation (e.g., Schneider & Shiffrin, 1977).
Interestingly, some of the clearest results regarding “true” conflict-adaptation effects come from studies that have used relatively short testing episodes. For example, Egner and Hirsch (2005) had used runs of runs of 148 trials for each of their two different task conditions. Freitas et al. (2007) has used three blocks with a total of 288 trials. The Freitas et al. study had used a mixed-dimension situation very similar to Experiment 2 in Mayr et al. (2003), in which subjects alternated between two different tasks after each trial. Under these conditions, no trial-to-trial adaptation effects were observed. Situations in which two different task sets are mixed within a block are particularly revealing because adaptation effects that survive trial-by-trial dimension cannot be explained by low-level repetition effects, but rather point to a relatively abstract, form of regulation that generalizes across dimensions. In Mayr et al. (2003), subjects worked through 448 test trials after an initial 192 practice trials. We reanalyzed these 192 practice trials and did observe a numerical adaptation effect that was about similar in size as the one obtained in the corresponding Experiment 1 by Freitas et al., 14 ms, F(1,18) = 1.5, P < 0.25, and that may have reached the reliability criterion if we had the same statistical power as these authors.
Is there more direct evidence that trial-to-trial adaptation patterns can arise when subjects exert deliberate control? In fact, even the original paper on conflict adaptation effect by Gratton et al. (1992) reports that explicit cues that signal either conflict or non-conflict trials produce adaptation patterns. Interestingly, these authors make no distinction between the type of regulation that might occur as the result of an explicit cue and the regulation resulting from the experience of conflict. It is only since the conflict-monitoring model was introduced that such adaptation patterns are almost exclusively discussed as a result of automatic regulation.
A very interesting recent paper by Fernandez-Duque and Knight (2007) looked at cross-task, trial-by-trial adaptation patterns as well as cross-task adaptation as the result of explicit cues. Regarding trial-to-trial patterns, these authors replicated Mayr et al. in finding no trial-to-trial cross-task adaptation, but n − 2 within-task adaptation that was contingent on stimulus repetitions. For the explicit cuing situation, these authors used a novel setup where the cues referred to the difficulty of a pair of trials (e.g., a color-word Stroop task) between which a single trial from a different task (e.g., a number Stroop task) was sandwiched. The cues were 100% valid with regard to the level of conflict in the targeted task, but the sandwiched trial was unpredictably either congruent or incongruent. Interestingly, conflict effects for the sandwiched trials were modulated by the conflict in the “surrounding” task, but only when explicitly cued. Thus, from these results we can derive the interesting suggestion that cross-task adaptation patterns might be a behavioral signature of explicit regulation.
Another interesting line of work that speaks to the issue of explicit, conscious regulation looks at the role of conflict awareness on conflict regulation. Clearly, from the perspective of the conflict-monitoring model awareness should not be an issue. All that should count is the level of conflict “measured” by ACC. However, Kunde (2003) manipulated conflict-awareness through metacontrast masking of either congruent or incongruent primes. He found an adaptation pattern only if participants were aware of primes, even though conflict effects were present both for the aware and the unaware situation. The few papers that have looked at the question of how of conscious awareness is related to regulation have produced somewhat mixed results (for a short review, see Mayr, 2004). Thus, more work on this issue is likely to provide critical results with regard to the theoretically important question of the necessary and sufficient triggering conditions for conflict-adaptation effects.
Taken together, we believe that there is good evidence to suggest that adaptation patterns can be produced through conscious, deliberate regulation. This is important because all evidence regarding claims of automatic adaptation would have to be scrutinized relative to the alternative hypothesis that it might have been produced through explicit regulation. The results by Fernandez-Duque and Knight (2007) are particularly interesting in this regard as they suggest a possible indicator of explicit regulation: the presence of cross-task adaptation effects.
Is there an automatic conflict-regulation feedback loop?
While there can be no question that conflict-adaptation patterns can be obtained under certain circumstances (e.g., Egner & Hirsch, 2005; Freitas et al., 2007; Ullsperger, Bylsma, & Botvinick, 2005; Verbruggen, Notebaert, Liefooghe, & Vandierendonck, 2006) we believe that the effect is not as consistent as the notion of an automatic link between conflict and control would require. Aside from the present results, a number of other recent papers have failed to obtain conflict adaptation effects in specific conditions (Fernandez-Duque & Knight 2007; Kunde, 2003; Mayr et al., 2003; Nieuwenhuis et al., 2006). An elusive, context-dependent link between conflict and conflict-adaptation is difficult to handle for the conflict-monitoring model, which predicts a uniform and automatic adaptive response whenever there are strong levels of response conflict. While it might be possible to eliminate from the model the idea of an automatic link between conflict and adaptation, such a change would take away from the allure of this hypothesis. After all, the model has been so influential because an automatic conflict-monitoring device provides a mechanistic account of how cognitive control is controlled. If the conflict adaptation turns out to be context sensitive, we need to reopen the search for the origins of this control process.
We believe the current empirical situation calls for serious attempts to explore the entire range of theoretical alternatives that might produce conflict-adaptation effects. Specifically, we see the following possible alternatives to the conflict-monitoring model:
Deliberate regulation
As already argued above, some of our and others’ results could point to the role of explicit, deliberate regulation. We also discussed evidence suggesting that explicit, cue-driven regulation can produce the typical adaptation pattern. One argument here could be that it is exactly the unconscious conflict-detection mechanism that results in subjects becoming aware of the fact that conscious regulation may be called for. While this is a viable possibility it is equally true that in all standard conflict paradigms, the presence of conflict can be easily detected by the “conscious subject” (e.g., the fact that arrows point in different directions or color and color word do not match). In this context it is particularly interesting that when conflict cannot be detected consciously, adaptation effects disappear (e.g., Kunde, 2003).
Passive carry-over of control settings
A simple account of the adaptation pattern that, curiously, has received little attention relies on the idea of passive carry-over or priming of control settings. This account can be illustrated by appluing the Gilbert and Shallice (2002) model of task switching to trial-to-trial adaptations to conflict. This model is a rather straightforward extension of the same model of the Stroop effect (Cohen, Dunbar, & McClelland, 1990) that also served as the starting point for the Botvinick et al. conflict-monitoring model. A core assumption in that model is that nodes representing the current task goals provide a constant source of activity into the corresponding task-demand node, which ultimately controls which of several possible tasks are executed. If there is conflict from incongruent stimulus information, it takes the system longer to use both task-demand input and input from the correct stimulus information to come up with the correct response. This in turn, gives the task-goal representation more opportunity to feed activity into the task demand node. Thus, in “surviving” a high-conflict trial the system automatically settles into a more controlled state than in “surviving” a low-conflict trial As a result it will be better prepared for conflict on the following trial In fact, in initial simulations we were able to get this model to produce the basic trial-to-trial adaptation pattern without requiring an explicit conflict-adaptation device (Mayr, 2007).
Instance-based episodic learning
Logan’s (1988) instance model assumes automatic encoding of aspects relevant for specific selection episodes into long-term memory (LTM). In addition, the more similar the current selection episode is to recent selection episodes stored in LTM, the greater the probability of automatic retrieval of this episode. There is now growing evidence that LTM encoding of selection episodes can include internal control states relevant to perform a particular action (e.g., Awh, Kliestik, & Sgarlata, 2005; Bryck & Mayr, in press; Mayr & Bryck, 2005, 2006, Schmidt, Crump, Cheesman, & Besner, 2007). Thus, one possible explanation of the conflict-adaptation effect is that the attentional setting stored on a high-conflict trial is the dominant episodic instance retrieved when the next trial again is a high-conflict trial. Consistent with this view, recent evidence suggests that adaptation effects have a strong item-specific component (e.g, Jacoby, Lindsay, & Hessel, 2003; Schmidt et al. 2007). There have been recent modeling attempts to account for the item-specific effects by adding an item-specific learning component to the basic conflict-detection loop (Blais, Robidoux, Risko, & Besner, 2007).
However, from these modeling attempts it is not clear whether the conflict-adaptation component is actually necessary or whether episodic learning models without conflict-adaptation can also produce adaptation patterns.
Currently, any of these accounts (or combination of accounts) provides a viable explanation for any given adaptation pattern reported in the literature. Moreover, in each of these accounts, conflict-adaptation results from well-established mechanisms that explain also other important phenomena. In contrast, the conflict-monitoring model explains adaptation patterns as the result of an extra process that is specifically designed to detect and respond to conflict. Whether or not the assumption of this extra process is necessary needs to be addressed in future research. Until then we should exercise caution when interpreting adaptation patterns.
References
- Awh E, Sgarlata AM, Kliestik J. Resolving visual interference during covert spatial orienting: online attentional control through static records of prior visual experience. Journal of Experimental Psychology: General. 2005;134(2):192–206. doi: 10.1037/0096-3445.134.2.192. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Blais C, Dobidoux S, Risko EF, Besner D. Item-specific adaptation and the conflict-monitoring hypothesis: A computational model. Psychological Review. 2007;114:1076–1086. doi: 10.1037/0033-295X.114.4.1076. [DOI] [PubMed] [Google Scholar]
- Bryck RL, Mayr U. Task selection cost asymmetry without task switching. Psychological Bulletin and Review. doi: 10.3758/pbr.15.1.128. in press. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing model of the Stroop effect. Psychological Review. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. NeuroImage. 2005;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Knight MB. Cognitive control: Dynamic, sustained, and voluntary influences. Journal of experimental psychology. Human perception and performance. 2007 doi: 10.1037/0096-1523.34.2.340. [DOI] [PubMed] [Google Scholar]
- Freitas AL, Bahar M, Yang S, Bahar R. Contextual adjustments in cognitive control across tasks. Psychological Science. 2007;18:1040–1043. doi: 10.1111/j.1467-9280.2007.02022.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Shallice T. Task switching: a PDP model. Cognitive Psychology. 2002;44:297–333. doi: 10.1006/cogp.2001.0770. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Lindsay DS, Hessels S. Item-specific control of automatic processes: Stroop process dissociations. Psychonomic Bulletin & Review. 2003;10:638–644. doi: 10.3758/bf03196526. [DOI] [PubMed] [Google Scholar]
- Kenny DA. Cross-lagged panel correlation: A test for spuriousness. Psychological Bulletin. 1975;82:887–903. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kunde W. Sequential modulations of stimulus-response correspondence effects depend on awareness of response conflict. Psychonomic Bulletin and Review. 2003;10:198–205. doi: 10.3758/bf03196485. [DOI] [PubMed] [Google Scholar]
- Logan GD. Toward an instance theory of automatization. Psychological Review. 1988;95:492–527. [Google Scholar]
- May CP, Kane HJ, Hasher L. Determinants of negative priming. Psychological Bulletin. 1995;118:35–54. doi: 10.1037/0033-2909.118.1.35. [DOI] [PubMed] [Google Scholar]
- Mayr U. Consciousness and control. Trends in Cognitive Science. 2004;8:145–148. doi: 10.1016/j.tics.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Mayr U. Conflict and Control. Presentation at the Workshop “Conclicts as Signals”; Binz, Germany. October 2007.2007. [Google Scholar]
- Mayr U, Bryck RL. Sticky rules: Integration between abstract rules and specific actions. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:337–350. doi: 10.1037/0278-7393.31.2.337. [DOI] [PubMed] [Google Scholar]
- Mayr U, Bryck RL. Outsourcing control to the environment: effects of stimulus/response locations on task selection. Psychological Research. 2006;71:107–116. doi: 10.1007/s00426-005-0039-x. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Stins JF, Posthuma D, Polderman TJC, Boomsma DI, De Geus EJ. Accounting for sequential trial effects in the flanker task: Conflict adaptation or associative priming? Memory & Cognition. 2006;34:1260–1272. doi: 10.3758/bf03193270. [DOI] [PubMed] [Google Scholar]
- Schmidt JR, Crump MJ, Cheesman J, Besner D. Contingency learning without awareness: Evidence for implicit control. Consciousness and Cognition. 2007;16:421–435. doi: 10.1016/j.concog.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending, and a general theory. Psychological Review. 1977;84:127–190. [Google Scholar]
- Tzelgov J, Henik A, Berger J. Controlling Stroop effects by manipulating expectations for color words. Memory & Cognition. 1992;20:727–735. doi: 10.3758/bf03202722. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Bylsma LM, Botvinick M. The conflict-adaptation effect: It’s not just priming. Cognitive, Affective and Behavioral Neuroscience. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Notebaert W, Liefooghe B, Vandierendonck A. Stimulus and response conflict-induced cognitive control in the flanker task. Psychonomic Bulletin & Review. 2006;13:328–333. doi: 10.3758/bf03193852. [DOI] [PubMed] [Google Scholar]