Abstract
Leptin and oestradiol have overlapping functions in energy homeostasis and fertility, and receptors for these hormones are localised in the same hypothalamic regions. Although, historically, it was assumed that mammalian adult neurogenesis was confined to the olfactory bulbs and the hippocampus, recent research has found new neurones in the male rodent hypothalamus. Furthermore, some of these new neurones are leptin-sensitive and affected by diet. In the present study, we tested the hypothesis that diet and hormonal status modulate hypothalamic neurogenesis in the adult female mouse. Adult mice were ovariectomised and implanted with capsules containing oestradiol (E2) or oil. Within each group, mice were fed a high-fat diet (HFD) or maintained on standard chow (STND). All animals were administered i.c.v. 5-bromo-2′-deoxyuridine (BrdU) for 9 days and sacrificed 34 days later after an injection of leptin to induce phosphorylation of signal transducer of activation and transcription 3 (pSTAT3). Brain tissue was immunohistochemically labelled for BrdU (newly born cells), Hu (neuronal marker) and pSTAT3 (leptin sensitive). Although mice on a HFD became obese, oestradiol protected against obesity. There was a strong interaction between diet and hormone on new cells (BrdU+) in the arcuate, ventromedial hypothalamus and dorsomedial hypothalamus. HFD increased the number of new cells, whereas E2 inhibited this effect. Conversely, E2 increased the number of new cells in mice on a STND diet in all hypothalamic regions studied. Although the total number of new leptin-sensitive neurones (BrdU-Hu-pSTAT3) found in the hypothalamus was low, HFD increased these new cells in the arcuate, whereas E2 attenuated this induction. These results suggest that adult neurogenesis in the hypothalamic neurogenic niche is modulated by diet and hormonal status and is related to energy homeostasis in female mice.
Keywords: oestrogen, leptin, pSTAT3, obesity
The hormones leptin and oestrogen have overlapping functional roles in fertility and energy homeostasis. In 1994, the ob gene, which encodes the hormone leptin, was identified (1). Leptin, produced and secreted by adipose tissue, binds to leptin receptors (LepR) located in areas throughout the brain with a particularly high density in the hypothalamus (2). Mice deficient in leptin (ob/ob) or LepR (db/db) are obese and diabetic (3,4) and the administration of leptin to ob/ob mice results in weight loss because of decreased food intake and increased energy output (4). LepR have also been identified in hypothalamic neurones containing peptides that regulate feeding (5,6). Although oestrogens play a critical role in reproductive physiology and behaviour (7,8), they are also important in energy homeostasis. Oestrogens act as anorectics and increase activity levels in humans (9) and rodents (10–12). Postmenopausal women (13) gain fat weight and ovariectomised (OVX) rodents demonstrate a decrease in activity concurrent with an increase in feeding and weight gain (12,14). There are two oestrogen receptor (ER) subtypes (ER-α and ER-ß) (15) that have distinct functions in behaviour (16) and physiology (17). As with fertility, oestrogenic effects on feeding are predominantly mediated through ERα (18). In addition to energy homeostasis, leptin plays a critical role in fertility. Although ob/ob and db/db mice are infertile, leptin administration to ob/ob mice induces puberty and fertility (19,20), indicating that oestrogens and leptin have overlapping functions (21,22). More directly, oestrogens sensitise the effects of leptin (23,24), demonstrating cross-talk between these hormone systems. In support of this relationship, ER-α and LepR are coexpressed in neurones from hypothalamic areas involved in energy homeostasis and reproduction (25).
Adult neurogenesis in the mammalian brain has been well documented to arise from progenitor cells in the subventricular zone of the lateral ventricles and the subgranular zone of the dentate gyrus in the hippocampus (26–29). New subventricular zone neurones travel through the rostral migratory stream and a proportion of them become functional neurones in the olfactory bulb circuitry (30,31). A subpopulation of proliferating cells in the subgranular zone of the hippocampus migrates to the dentate gyrus where they differentiate into neurones. These neurones send projections to the CA3 region and are known to play a role in learning and memory (32) and mood disorders (33,34). A variety of exogenous factors, including activity level (35) and an enriched environment (36), dramatically influence the rate of cell proliferation, survival and differentiation in the adult mammalian brain. In addition, hormonal milieu (37,38), and, more specifically, oestrogens, increase proliferation in the hippocampus (39).
More recently, neurogenesis has been discovered in the hypothalamus of the adult male rodent brain (40–47). A subpopulation of these new cells in the hypothalamus differentiates into functional neurones and appears to be involved in energy homeostasis in males (40,42,44,45,47). Furthermore, hypothalamic neurogenesis in male mice is altered by a high-fat diet (HFD) (42,44,47). Administration of cilliary neurotrophic factor (CNTF) to rodents and humans leads to weight loss (48,49), which is sustained after cessation of CNTF administration (40,41,48). Interestingly, CNTF potently induces neurogenesis in the male mouse hypothalamus, and many of the new neurones respond to leptin by phosphorylation of signal transducer of activation and transcription 3 (pSTAT3). Preventing this hypothalamic neurogenesis in male mice blocks the long-term effect of CNTF on body weight (40), suggesting that CNTF-induced weight loss is the result of increased leptin signalling via new neurones.
Although the effects of diet on hypothalamic neurogenesis have been investigated in male rodents as discussed above, the effects of diet and hormones on neurogenesis in the hypothalamus have not been studied in females. Therefore, the present study tested the hypothesis that hypothalamic cell proliferation and neurogenesis occur in adult female mice and are influenced by diet and oestradiol. Moreover, we explored the possibility that diet and oestradiol would alter the number of new leptin-sensitive neurones in the hypothalamus and thus be correlated with measures of energy homeostasis. Our findings reveal that diet and oestradiol modulate neurogenesis in the female hypothalamus and that these factors also influence the number of differentiated leptin-sensitive neurones.
Materials and methods
Animals and treatment groups
C57BL6 female mice (10–12 weeks of age) from the Wellesley College breeding colony were housed two per cage and maintained under a 12 : 12 h light/dark cycle. A summary of the research design is provided in Figure 1. Mice were anaesthetised with 2.5% isoflurane, bilaterally OVX and implanted with a silastic capsule (50) containing either 50 μg of 17ß-oestradiol (E2) dissolved in 25 μl of 5% ETOH/sesame oil (51,52) or vehicle (5% ETOH/sesame oil; Veh). The silastic capsules were placed in the subcutaneous space just below the left scapular blade. Three days after OVX and capsule implantation, mice were either started on a HFD containing 58% kcal from fat in the form of lard (35.2% fat, 36.1% carbohydrate and 20.4% protein by weight) (catalogue number 03584; Harlan Teklad, Indianapolis, IN) or maintained on standard rodent chow (STND) containing 13.5% kcal from fat (catalogue number 5001; Purina, St. Louis, MO).
Fig. 1.
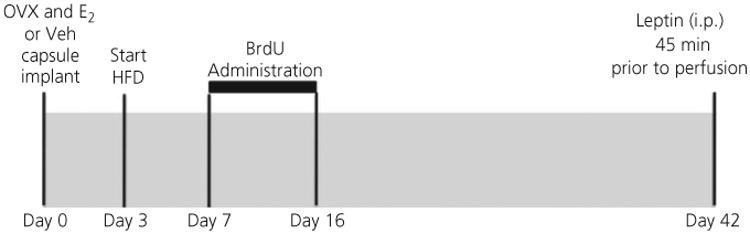
Study design. Ten- to 12-week old C57BL6 female mice were ovariectomised and implanted (s.c.) with a silastic capsule containing either 17β-oestradiol (E2) (50 μg) dissolved in 25 μl 5% ETOH/sesame oil or vehicle (5% ETOH/sesame oil). Three days after surgery, half of the mice in each hormone group were started on a high-fat diet (HFD), whereas the other half remained on standard chow. From day 7 to day 16, mice were administered i.c.v. BrdU via an osmotic minipump. Thirty-four days after the start of BrdU infusion, mice were fasted overnight and injected with leptin (5 mg/kg, i.p.), 45 min prior to perfusion the following day.
Mice were randomly assigned to one of four treatment groups: STND-Veh, STND-E2, HFD-Veh and HFD-E2. Mice were weighed every 5 days and the amount of food eaten was recorded every other day (1-2 h before lights off) throughout the study. Mice from the same treatment groups were housed two per cage for the duration of the study except in instances where a cage mate died. There were no differences in weight gain or average food intake for the five mice housed individually compared to those housed with a cage mate. All animal procedures were approved by the Institutional Animal Care and Use Committee of Wellesley College.
Intracerebroventricular 5-bromo-2′-deoxyuridine (BrdU) administration
Seven days post OVX/silastic capsule implantation, mice were anaesthetised with 2.5% isoflurane, and implanted with a cannula (2.5 mm in length) aimed at the right lateral ventricle (anteroposterior: 0.3 mm, mediolateral: 1.0 mm from bregma, (53). The cannula was attached to an Alzet osmotic pump (0.5 (μl/h, 7-day, 1007D; Durect, Cupertino, CA, USA) filled with 100 μl of home-made artificial cerebral spinal fluid containing 1 μg/μl BrdU (Sigma Aldrich, St Louis, MO, USA) and 1 μg/μl mouse serum albumin (Sigma Aldrich) via a catheter cut to 2.5 cm. Nine days post implantation of osmotic pumps, mice were re-anaesthetised and pumps were removed (in accordance with the Alzet osmotic pump protocol).
Immunohistochemistry
We were interested in whether some BrdU-labelled cells become functional as determined by their ability to phorphorylate STAT3 in response to leptin. Therefore, mice were maintained on their respective diets for 34 days after the start of BrdU infusion to allow new-born cells to become functionally mature (54,55). Thirty-four days after the start of BrdU infusion, mice were food deprived overnight. On the next day, cardiac perfusion with 4% paraformaldehyde was performed 45 min after an i.p. injection of leptin (5 mg/kg; Peprotech, Rocky Hill, NJ, USA). Leptin was administered to induce phosphorylation of STAT3 in the hypothalamus (56). Following perfusion, brains were dissected out, post-fixed in 4% paraformaldehyde for 2 h and then transferred to 20% sucrose/0.1 m phosphate buffer for 2 days until sectioning. Thirty-five micrometre thick brain sections were cut on a freezing microtome and stored in cryoprotectant at −20 °C until processing.
A representative section containing the arcuate nucleus (ARC), ventromedial nucleus of the hypothalamus (VMH) and the dorsomedial nucleus of the hypothalamus (DMH) was chosen according to figures 44–45 in Paxinos and Franklin (53). These areas were selected based on their functional importance in feeding and energy homeostasis (57–59) and the high density of leptin receptors (2,60) and leptin-nduced pSTAT3 (56). Brain sections were rinsed in 0.05 m tris-buffered saline (TBS), incubated in 0.01 m glycine for 30 min, rinsed, and then incubated in 0.05% sodium borohydride for 20 min to reduce autofluorescence as a result of aldehyde fixation. DNA was denatured for BrdU detection by incubating tissue in 2 N HCl at 40° for 40 min followed by rinses in borate buffer (pH 8.5) and TBS. Sections were treated with donkey anti-mouse immunoglobulin G (20 μg/ml; Jackson Immunoresearch, West Grove, PA, USA) to block endogenous mouse binding sites followed by a second blocking step in 0.4% Triton-X, 10% normal serum (donkey and goat; Lampire Biological, Pipersville, PA, USA) and 1% hydrogen peroxide for 30 min. Sections were incubated for 48 h at 4 °C in a primary antibody cocktail containing rat anti-BrdU (dilution 1 : 400, OBT0030G; Accurate, West-bury, NY), mouse anti-Hu (1 μg/ml, A-21271, Life Technologies, Grand Island, NY, USA) and rabbit anti-pSTAT3 (dilution 1 : 50; 9145; Cel Signaling Technology, Beverly, MA, USA). The Hu antibody is made against human neuronal protein HuC/HuD and recognises the neuronal proteins HuC, HuD and Hel-N1. The pSTAT3 antibody recognises STAT3 only when it is phosphorylated at tyrosine 705. These antibodies have been routinely used in immunohistochemistry and western analysis of mouse brain tissue (42,44,61,62). Sections were then washed with TBS and incubated for 2 h in a cocktail of fluorescently abelled secondary antibodies containing goat anti-rat (dilution 1 : 200, Cy3; Jackson Laboratories, Bar Harbor, ME, USA), donkey anti-mouse (dilution 1 : 200, Alexa Fluor 488; Life Technologies) and donkey anti-rabbit (dilution 1 : 200 Alexa Fluor 647) followed by washes in TBS. Negative controls consisting of the omission of each primary antibody were performed to confirm secondary antibody specificity. Sections were mounted on SuperFrost Plus slides (Fisher, Hampton, NH, USA), coverslips were applied with Fluorogel (Electron Microscopy Sciences, Hatfield, PA, USA) and storage was at 4 °C.
Confocal microscopy and image analysis
A total of eight (4 × 2) fields of view (FOVs) were imaged at × 400 in the left hemisphere (contralateral to BrdU administration) just lateral to the third ventricle. The FOVs were stitched together for a total imaged area of 1.4 mm × 0.73 mm. This total imaged area contained the ARC, VMH and DMH as defined in Paxinos and Franklin (53). All imaging was conducted using a TCS SP5 II confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA) equipped with an argon laser 488, a helium-neon laser 543 and a helium-neon laser 633 and a motorised stage. Gain and offset settings were optimised for each fluorescent label and kept constant across all images. A stack of 15 sections (1 μm each) was taken through the z-plane of each FOV.
BrdU/Hu/pSTAT3
Regions of interest were superimposed on each image. Each BrdU-abelled cell was tagged and clearly visualised through its entirety and manually inspected at 1-μm intervals for double (BrdU+/Hu+) and triple (BrdU+/Hu+/pSTAT3+) labelling by an experimenter who was blind to treatment. For total pSTAT3 (single-label) cell counts, all 15, 1-μm thick optical sections were collapsed and digitally analysed using imagej, version 1.46 (NIH, Bethesda, MD, USA).
Western blotting
Ovariectomised mice were i.p. injected with either leptin (5 mg/kg; Peprotech) or vehicle (authoclaved purified water) 45 min prior to sacrificed via CO2 inhalation. The hypothalamus was dissected out and immediately frozen on dry ice and stored at −80°C until processing. Tissue was homogenised in a buffer containing 10 mm ethylenediaminetetraacetic acid, 2 mm ethylene glycol tetraacetic acid, 10 mm Tris, 400 mm NaCl, 1 mm dithiothreitol, 10% glycerol, 10 mm α monothioglycerol, 1 mm Na3VO4, 50 mm NaF and 50 mm KPO4 and a 1 : 10 dilution of protease inhibitor cocktail (P2714-1BTL; Sigma). Homogenised hypothalamic protein extract (60 μg of total protein) was run on a 7.5% Criterion TGX gel (Bio-Rad, Hercules, CA, USA). Protein extracted from HeLa cells treated with interferon (IFN)α (9133S; Cell Signaling Technology) was used for the pSTAT3 positive control. Western blots were performed as described previously (63). Briefly, proteins were denatured by boiling in sample buffer for 5 min prior to loading. Proteins were transferred (100 V for 1 h) to a polyvinylidene difluoride membrane (Bio-Rad). After transfer, the membrane was washed with Tris-buffered saline with 0.05% tween (TBS-T) and then blocked in TBS-T containing 5% bovine serum albumin (BSA) for 1 h followed by washes in TBS-T. The blot was incubated overnight at 4°C in primary antibody solution containing either monoclonal rabbit anti-pSTAT3 (dilution 1 : 200; 9145; Cel Signaling Technology) or polyclonal rabbit anti-STAT3 (dilution 1 : 200; sc-482; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in TBS-T with 0.02% NaN3 and 3% BSA. Blots were washed and incubated in horseradish protein (HRP) conjugated donkey anti-rabbit (dilution 1 : 5000; Jackson Immunoresearch) and Precision-Protein HRP conjugated StrepTactin (dilution 1 : 5000) for 1 h. Blots were washed and proteins were detected with incubation in Clarity Western ECL Substrate (170-5060; Bio-Rad) for 5 min. Finally, blots were maged on a ChemiDoc MP system (170-8280; Bio-Rad).
Statistical analysis
After excluding animals that did not receive the full extent of BrdU nfusion because of a loss of cannula and animals whose cannula placement was not confirmed to the lateral ventricle, there were 28 mice in total that were analysed for food intake and body weight: STND-Veh (n = 5), STND-E2 (n = 6), HFD-Veh (n = 9) and HFD-E2 (n = 8). All animals were used for cell counts analyses with the exception of the loss of ARC data from one animal as a result of torn brain tissue.
For weight and food intake measures, a repeated anova with two between factors (diet and hormone) was run. For cell count analyses, a two-way anova (diet and hormone) was run for each brain area separately. Where there were significant effects, a Tukey's honestly significant difference (HSD) post-hoc test was used for comparisons between groups. spss, version 21 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. P < 0.05 was considered statistically significant.
Results
Food intake and weight gain
The overall ANOVA for food intake indicated main effects for both diet (F1,24 = 19.8, P < 0.001) and hormone (F1,24 = 10.4, P < 0.005) with no significant interaction between the two factors (Fig. 2A). Mice on STND diet and mice treated with Veh consumed significantly more kcal throughout the study than those on a HFD and those treated with E2, respectively (Fig. 2A). Given that there are 5.4 kcal/g in the HFD and 4.07 kcal/g in the STND diet, the number of grammes consumed differs to an even greater extent.
Fig. 2.
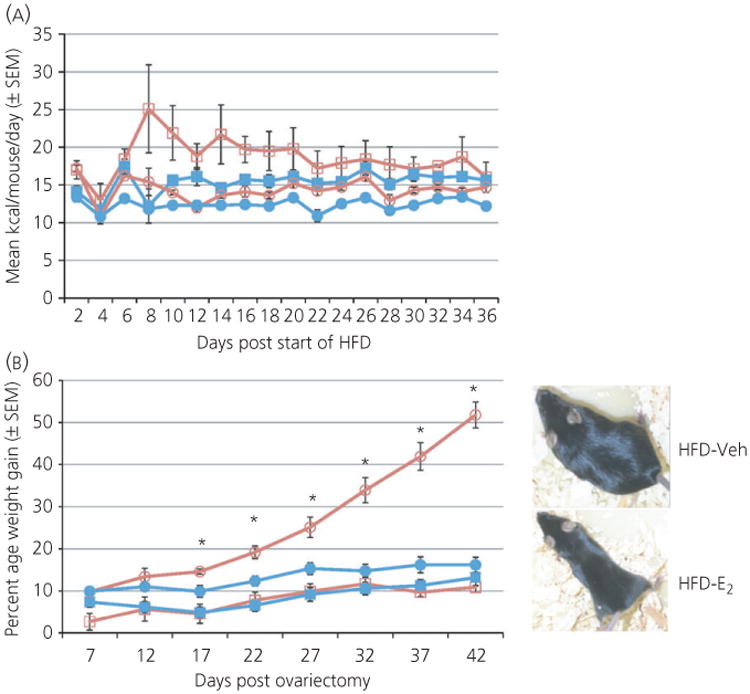
Hormone and diet altered energy intake and weight gain in adult female mice. (a) Oestradiol (E2) treatment and high-fat diet (HFD) decreased energy intake compared to vehicle treatment and standard diet (STND), respectively. (b) Mice on a HFD weighed more than mice on a STND. Vehicle (Veh)-treated mice on a HFD gained significantly more weight than any other group starting 17 days after ovariectomy.
 =STND-Veh,
=STND-Veh,
 = STND-E2,
= STND-E2,
 = HFD-Veh,
= HFD-Veh,
 = HFD-E2. *Statistically different compared to all other groups (P < 0.05)
= HFD-E2. *Statistically different compared to all other groups (P < 0.05)
The overall ANOVA for weight gain found main effects for both diet (F1,24 = 53.1, P < 0.001) and hormone (F1,24 = 15.6, P < 0.001) and an interaction between diet and hormone (F1,24 = 20.1, P < 0.001). Overall, mice on a HFD weighed more than mice on a STND diet, regardless of hormone treatment and mice treated with Veh weighed more than mice treated with E2 regardless of diet (Fig. 2B). The greatest effect, however, was seen in the HFD-Veh treatment group that gained more weight than any other group beginning 17 days after the start of HFD (Tukey's HSD, P < 0.05) and maintained a greater weight gain throughout the study (Fig. 2B).
Effects of leptin on STAT3 phosphorylation
To confirm up-regulation of pSTAT3 in brain by leptin and the specificity of our pSTAT3 antibody, western blot analysis of hypothalamic cell extracts was performed. Extracts from leptin-treated mice revealed a strong immunoreactive band for pSTAT3, whereas extracts from vehicle-treated mice had a weak band (Fig. 3). STAT3 expression was similar between leptin-treated and vehicle-treated mouse hypothalamic extracts. Consistent with the literature (56), these data indicate that leptin up-regulates the phosphorylation of STAT3 in OVX mice, whereas total STAT3 remains the same.
Fig. 3.
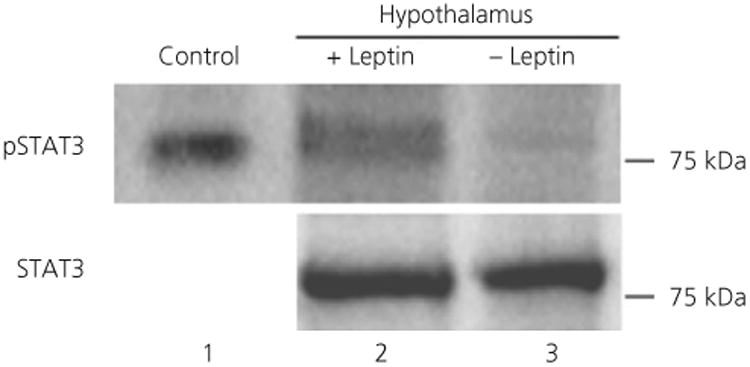
Leptin treatment increased signal transducer of activation and transcription 3 (STAT3) phosphorylation in ovariectomised (OVX) mice. Hypothalamic protein extracts from OVX mice injected with leptin (lane 2) or vehicle (lane 3), 45 min prior to sacrifice were probed for phosphorylated STAT3 (pSTAT3) and STAT3 by western blotting. Protein extracted from HeLa cells treated with interferon (IFN)α (lane 1) served as a pSTAT3 positive control. Leptin treatment up-regulated pSTAT3 expression (lane 2 compared to lane 3), whereas STAT3 expression was similar between treatment groups.
New cells in the hypothalamus
Effects of diet
The total number of BrdU-labelled cells through all z-planes was counted in the ARC, VMH and DMH (Fig. 4). Although there was no main effect of diet on BrdU cell number, there was a significant interaction between diet and hormone in all hypothalamic brain regions (ARC: F1,23 = 29.3, P < 0.001; VMH: F1,24 = 29.5, P < 0.001, DMH: F1,24 = 38.8, P < 0.001). Post-hoc analysis found that HFD increased BrdU-labelled cells in the ARC, VMH and DMH (HFD-Veh greater than STND-Veh, P < 0.005 in all regions) (Fig. 5).
Fig. 4.
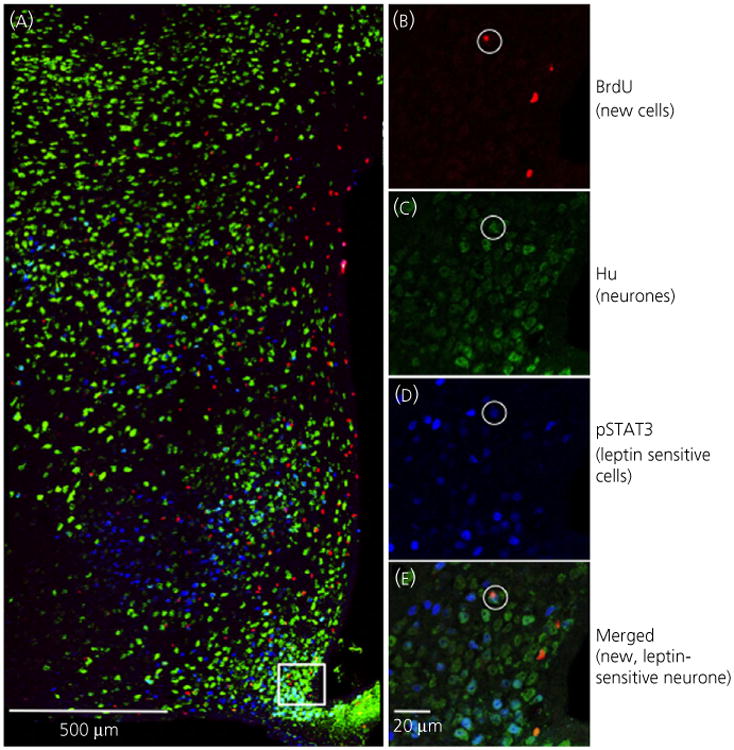
Newly born neurones in the hypothalamus are leptin-sensitive. (a) The arcuate nucleus (ARC), ventromedial nucleus of the hypothalamus (VMH) and dorsomedial nucleus of the hypothalamus (DMH) was immunohistochemically labelled for 5-bromo-2′-deoxyuridine (BrdU), Hu and phosphorylated signal transducer of activation and transcription 3 (pSTAT3) and imaged at × 400 magnification. (b–e) Confocal images (× 630, 1 μm thick) of the box outlined in (a) showing a cell triple-labelled for BrdU, Hu and pSTAT3.
Fig. 5.
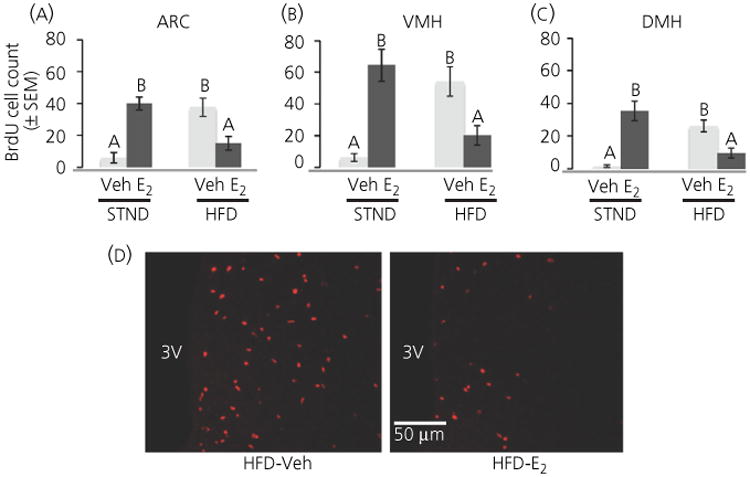
The number of newly born cells in the adult female mouse was affected by both diet and hormone treatment. (a–c) Number of 5-bromo-2′-deoxyuri-dine (BrdU)+ cells in the arcuate nucleus (ARC), ventromedial nucleus of the hypothalamus (VMH) and dorsomedial nucleus of the hypothalamus (DMH). A high-fat diet (HFD) increased the number of BrdU+ cells in the ARC, VMH and DMH, whereas oestradiol (E2) treatment suppressed this HFD-induced increase. Conversely, E2 increased BrdU cell number in female mice on a standard (STND) diet. (d) Photomicrographs of BrdU in the ARC. A HFD increased cell proliferation in the ARC (left), whereas oestradiol administration inhibited this effect (right). Light bars, vehicle-treated; dark bars, E2-treated. Different letters indicate significant differences between groups (P < 0.05). 3V, third ventricle.
Effects of hormone
There was a main effect of hormone on BrdU cell number in the DMH (F1,24 = 489.5, P < 0.05) with oestradiol increasing the number of new cells (Fig. 5C). There was no main effect of hormone on BrdU cell number in the ARC or VMH. There was, however, a significant interaction between hormone and diet in all brain regions (see above). Consistent with findings of others (38,39,64), E2 increased BrdU-labelled cells in animals maintained on a standard diet (STND-E2 greater than STND-Veh in all hypothalamic regions, P < 0.005) (Fig. 5). Interestingly, however, E2 had the opposite effect in mice fed a HFD. In animals fed a HFD, E2 decreased the number of new cells compared to mice treated with Veh in all brain areas studied (P < 0.02) (Fig. 5). The majority of sections from HFD-E2-treated mice appeared to have a different distribution of BrdU-labelled cells than HFD-Veh treated mice (Fig. 5d), which may partially account for the difference in cel number. This difference in distribution of new cells warrants further investigation.
New hypothalamic neurones (BrdU+/Hu+)
Effects of diet
Newly born cells, as indicated by BrdU labelling, were inspected through all z-planes (15 μm in total) for double labelling with Hu to determine neurogenesis (Fig. 6). Although there was no main effect of diet, there was an interaction between diet and hormone on neurogenesis in all brain areas studied (ARC: F1,23 = 10.2, P < 0.005; VMH: F1,24=11.5, P < 0.005; DMH: F1,24 = 25.2, P < 0.001). As with BrdU cell number, mice maintained on a HFD had a higher rate of neurogenesis in the ARC, VMH and DMH (HFD-Veh greater than STND-Veh, P < 0.01 in all brain areas) (Fig. 6).
Fig. 6.
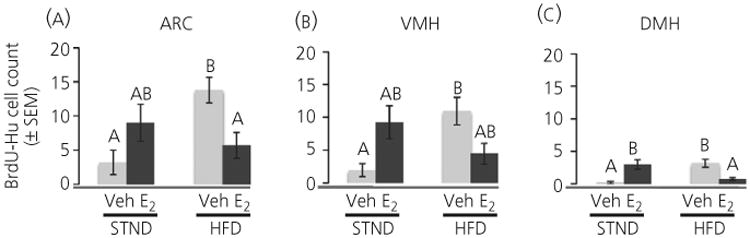
A high-fat diet (HFD) increased the number of new neurones in the hypothalamus of the adult female mouse. A HFD increased 5-bromo-2′-deoxyuridine (BrdU)-Hu cell number in vehicle-treated mice (Veh) in all hypothalamic regions analysed. This diet-induced increase in neurogenesis was attenuated by oestradiol (E2) treatment in the arcuate nucleus (ARC) and the dorsomedial nucleus of the hypothalamus (DMH). Light bars, vehicle-treated; dark bars, E2-treated. Different letters indicate significant differences between groups (P < 0.05). VMH, ventromedial nucleus of the hypothalamus; STND, standard diet.
Effects of hormone
Although there was no main effect of hormone on neurogenesis, post-hoc analysis on the interaction of hormone and diet (for nteraction statistics, see above) found that E2 increased neurogenesis in the DMH of mice on a STND diet (STND-E2 greater than STND-Veh, P < 0.05). Although not significant, there was a trend for an E2-induced increase in neurogenesis in the ARC and VMH of mice on a STND diet. As with newly born cells, E2 decreased neurogenesis in the ARC and DMH (HFD-E2 less than HFD-Veh, P < 0.05 and P < 0.005, respectively) when mice were maintained on a HFD.
New leptin-sensitive hypothalamic neurones (BrdU+/Hu+/ pSTAT3+)
Effects of diet
BrdU-Hu cells were further analysed for pSTAT3 labelling to determine new, leptin-sensitive neurones (Fig. 7). There was an interaction of diet and hormone in the ARC (F1, 23 = 17.7, P < 0.001) and the VMH (F1, 24 = 6.1, P < 0.05) but no differences were detected in the DMH. HFD-Veh mice had more newly born leptin-sensitive neurones in the ARC compared to STND-Veh mice (P < 0.001).
Fig. 7.
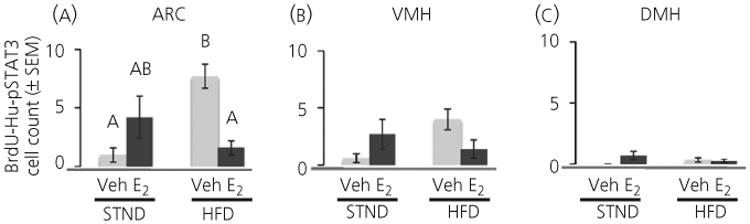
A high-fat diet (HFD) increased the number of new phosphorylated signal transducer of activation and transcription 3 (pSTAT3) neurones in the arcuate nucleus of the adult female mouse. A HFD increased the number of 5-bromo-2′-deoxyuridine (BrdU)-Hu-pSTAT3 cells in the arcuate nucleus (ARC) of vehicle (Veh)-treated mice. No effect was detected in the ventromedial nucleus of the hypothalamus (VMH) and dorsomedial nucleus of the hypothalamus (DMH) where there were very few triple-labelled cells. Oestradiol (E2) decreased the diet-induced increase in triple-labelled cells in the arcuate nucleus. Light bars, vehicle-treated; dark bars, E2-treated. Different letters indicate significant differences between groups (P < 0.05). STND, standard diet.
Effects of hormone
As with the number of new cells and neurogenesis, E2 decreased the number of new leptin-sensitive neurones in the ARC in mice kept on a HFD (HFD-E2 less than HFD-Veh, P < 0.001). E2 had no effect on new leptin-sensitive neurones in mice maintained on a standard diet. There were no differences among the four groups in the VMH but the trend was comparable to that in the ARC (Fig. 7b compared to Fig. 7a).
Sections were also analysed for single-labelled pSTAT3 to investigate whether pSTAT3 expression after leptin injection differed with hormone and/or diet. There were no differences between groups in number of pSTAT3 labelled cells in any brain area examined (data not shown).
Controls were performed to confirm the specificity of the triple-label immunohistochemistry technique. Omission of each individual primary antibody resulted in no detectable immunoreactivity of the respective label (data not shown). In further confirmation of the specificity of the triple-label technique, intensely-labelled cells with only BrdU or Hu immunoreactivity were observed.
Discussion
The present study examined the effects of diet and oestradiol on weight, energy intake and hypothalamic neurogenesis in female mice. Vehicle-treated mice on a HFD became obese, whereas oestradiol treatment protected female mice from diet-induced obesity. Consistent with previous findings in female rodents (10,11,65), oestradiol decreased energy intake and weight gain. Newly born cells and cell differentiation in hypothalamic areas were altered by diet and hormone. HFD increased the number of new cells and neurogenesis in the hypothalamus of female mice, whereas oestradiol decreased this diet-induced effect. Furthermore, vehicle-treated mice on a HFD became obese and had the largest number of new leptin-sensitive hypothalamic neurones.
Weight and feeding
Mice on a HFD weighed more than mice on a standard diet, confirming previous findings obtained in male and female mice with diet-induced obesity (66, 67). Interestingly, despite an increase in weight, the mice in the HFD groups had a lower energy intake (kcal) than those on a standard diet. These data indicate that weight gain in the HFD group was a result of factors other than energy intake. Although some studies have found a positive correlation between diet fat content and calories consumed (68), others have found a negative correlation and that HFD leads to a high food efficiency (body weight gain/kcal consumed) compared to diets rich in protein or carbohydrates (69,70). The negative correlation between intake and weight gain has been proposed to arise from alterations in thermogenesis (69). It should also be noted that other factors, such as activity level or altered metabolism, could contribute to the discrepancy between intake and weight.
Oestradiol treatment reduced the average kcal/day consumed on both HFD and standard diet. In support of these findings, oestrogens are well known anorectics (10–12,71–73). Oestradiol treatment decreased percent body weight gain in mice on a HFD. This protective effect of oestradiol against HFD-induced obesity in female mice has been reported previously (65). The mechanism by which oestradiol prevents diet-induced obesity is not yet well understood (74).
Hypothalamic neurogenesis
Hypothalamic neurogenesis is a recently discovered phenomenon in adult males (40,41). Subsequently, research on the involvement of hypothalamic neurogenesis in energy homeostasis has been growing (42,44,45,47,75,76). To the best of our knowledge, the present study is the first to show hypothalamic cell proliferation and neurogenesis in the adult female rodent. Hypothalamic BrdU cell number was increased in female mice fed a HFD when deprived of oestrogens. These results are in agreement with those of Lee et al. (42) who found that HFD increased BrdU cell labelling in the adult male mouse median eminence of the hypothalamus. Additionally, Gouaze et al. (47) found that, in male mice, HFD led to a biphasic increase in hypothalamic cell proliferation followed by a decrease compared to males on a standard diet. Interestingly, blocking cellular proliferation throughout the brain resulted in an increase in body weight indicating the important mechanistic role of the new cells, including neurones, in energy homeostasis (47). However, contrary to the present results, McNay et al. (44) and Li et al. (77) found a decrease in newborn cells in the hypothalamus of adult male mice fed a HFD compared to mice on a standard diet. There are a few differences in study design that may explain these discrepancies. First, male mice were used in the previous studies (44,77) in contrast to females in the present study. In addition, in the previous studies, mice were exposed to a long-term HFD paradigm of 2 months (44) and 4 months (77) leading to obesity prior to BrdU administration. However, in the present study, BrdU was administered during the first week of HFD prior to the onset of obesity. It will be important for future studies to compare differences in BrdU labelling between mice administered BrdU at the beginning of a HFD trial and administration after obesity has been established.
In addition to increasing newly born cells, HFD increased the number of new neurones (BrdU-Hu cells) in the ARC, VMH and DMH. Hu is an early neuronal marker (78,79) and was therefore chosen because of the relatively early time point during differentiation examined in the present study. Two previous studies found that HFD induced a decrease in hippocampal neurogenesis in male mice (80) and male rats (81). Lindqvist et al. (82) also found a decrease in hippocampal neurogenesis in response to a HFD in adult male rats with no alteration in adult female rats (82). Overall, these data suggest that there are sex differences in the neurogenic response in the hypothalamus to HFD.
Oestradiol treatment blocked the increase of newly born hypo-thalamic cells induced by HFD. There are many factors that contribute to the rate of neurogenesis, including cell proliferation, cell survival/cell death and cellular differentiation. Interestingly, HFD and oestradiol have frequently been noted to have opposing effects on each of these factors. In the hippocampus, HFD inhibits both cell proliferation and differentiation (81,83), at the same time as increasing cell death (84). By contrast, oestrogens have been shown to increase cell proliferation, differentiation and cell survival in the hippocampus (85). It will be important to continue to explore the differential effects of HFD and E2 on the components of neurogenesis in other brain areas, including the hypothalamus. Obesity has known inflammatory effects in brain and specifically within the hypothalamus (86–89). Inflammation in the brain leads to activation of microglia (90). Although a subpopulation of BrdU+ cells in the present study were co-labelled with Hu (a neuronal marker), a large proportion of BrdU+ cells were negative for Hu, suggesting that the majority of these HFD-induced new cells are not neurones. It is possible that these new non-neuronal cells express microglial markers. Given that oestrogens are known suppressors of inflammation in brain (91,92), it may be that oestradiol treatment alters microglia expression and/or function in animals on a HFD.
By contrast to the oestradiol-induced attenuation of BrdU incorporation in the hypothalamus of mice on a HFD, oestradiol administration increased BrdU-labelled cell counts in the ARC, VMH and DMH in mice on a standard diet. These findings are consistent with previous findings showing that acute oestradiol treatment increases cell proliferation in the adult female rat hippocampus (64) and in the amygdala of adult female meadow voles (93). In the present study, the administration of oestradiol increased neurogenesis in the DMH of female mice maintained on a standard diet and there was a trend towards an increase in the ARC and VMH. Although the effects of oestradiol on hippocampal neurogenesis has been well-studied in female rats and voles (37,85), the few studies that have examined oestradiol effects on neurogenesis in the adult female mouse have found conflicting results: no effect of oestradiol on hippocampal neurogenesis (94) and a decrease in SVZ/olfactory bulb neurogenesis after oestradiol administration (95). Taken together with the findings of present study, it is suggested that oestradiol affects neurogenesis in a brain region specific manner in the adult female mouse.
Oestradiol, diet and pSTAT3
Interestingly, in the present study, the number of new pSTAT3 neurones (BrdU-Hu-pSTAT3) in the arcuate was greatest in the HFD-Veh group; the group that weighed significantly more than all other groups. This HFD-induced increase in new pSTAT3 neurones may be a compensatory mechanism that increases leptin sensitivity in the brain.
Although the number of studies exploring adult hypothalamic neurogenesis has increased considerably in the last decade (75), most neuroanatomical analyses have not distinguished between different hypothalamic nuclei (40,41,43–47). In the present study, cell proliferation was affected by diet and hormone similarly in all three hypothalamic nuclei studied. However, alteration in neurogenesis was observed only in the arcuate and DMH, whereas new leptin-sensitive neurones were only affected in the arcuate. ER and leptin are co-expressed in cells of the arcuate and DMH and no such co-expression is observed in the VMH, providing neuroanatomical evidence that oestradiol and leptin may have a direct interaction in the arcuate and DMH but not in the VMH (96). Furthermore, it is interesting that the interaction between hormone and diet on new leptin-sensitive neurones was seen distinctly in the arcuate because this hypothalamic region is most strongly associated with neuroendocrine effects on energy homeostasis (97).
Although the findings of the present study suggest that oestradiol may protect against HFD-induced obesity through alterations in neurogenesis, oestrogens affect weight through multiple channels. Previous research has shown that the effects of oestrogens on energy homeostasis are mediated through both the pro-opiomelanocortin (71,73) and neuropeptide Y (98) systems. Additionally, oestrogens affect thermogenesis (99,100), activity (9–12) and have peripheral effects on adiposity (101). Therefore, in the present study, oestradiol may protect against obesity independent of its effects on neurogenesis.
The mechanism and functional importance of STAT3 phosphorylation in leptin action has been well defined. Leptin treatment results in STAT3 phosphorylation in hypothalamic brain areas that express leptin receptors (102) and pSTAT3 in brain is distinctly and specifically activated by leptin (56). Furthermore, phosphorylation of STAT3 is required for the effects of leptin on energy homeostasis (103,104). Total STAT3 deletion in brain leads to obesity and infertility (105) and STAT3 deletion in leptin receptor neurones results in obesity (106). All of these findings support a critical role for STAT3 signalling in the effects of leptin on energy homeostasis. Diet-induced obesity in mice has been associated with leptin insensitivity (107). In support, obese animals have a higher level of circulating leptin levels (as a result of increased adiposity) but are insensitive to leptin signalling. Although normal-weight mice respond to leptin injections with a decrease in food intake and an increase in activity, diet-induced obese mice show no (or attenuated) alteration in either measure in response to leptin administration (108–110). Additionally, ovariectomy leads to leptin insensitivity in rats (23), which can be reversed with oestradiol supplementation (111). The number of new leptin-sensitive neurones found in the present study was relatively low. Although the number of newly differentiated neurones appears to stabilise by 4 weeks after BrdU administration, neuronal maturation has been shown to continue for 4 months (54,112). It is possible that a larger number of BrdU-Hu-pSTAT3 labelled cells would have been detected if a longer survival time (e.g. 4 months) had been studied.
In the present study, leptin-induced phosphorylation of STAT3 was not altered by diet or hormone treatment in female mice. In support of these findings, previous research has found no alteration in pSTAT3 in response to leptin administration in the ARC of leptin-insensitive obese male rats (68), suggesting that leptin resistance does not arise from an inability to phosphorylate STAT3 in response to leptin. Conversely, other studies (113) have noted a small (10%) decrease in leptin-induced pSTAT3 in the ARC of male mice after 4 weeks on a HFD. The discrepancy with the results of the present study could be a result of sex differences or the method of analysis.
The present study further supports the concept that oestradiol profoundly affects energy homeostasis in female mice and provides evidence for a novel mechanism through which oestradiol modulates energy homeostasis by altering hypothalamic structure through a regulation of adult neurogenesis in this region. As with the findings that hypothalamic neurogenesis plays a significant role in energy homeostasis in the adult male mouse (42,47), it will be important for future studies to investigate the functional significance of these new cells that are regulated by both hormone and diet in the female mouse. Finally, these findings on the action of oestradiol in brain and in the modulation of energy homeostasis enhance our understanding of disorders of metabolic homeostasis in women with ovarian dysfunction (114).
Acknowledgments
We thank Derek Daniels for helpful comments on the manuscript. This work was supported by NIH RO1 DK061935 (MJT) and an NIDDK Reentry Award (EPB).
Footnotes
The authors have nothing to disclose.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homo-logue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 3.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 4.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 5.Hakansson ML, Hulting AL, Meister B. Expression of leptin receptor mRNA in the hypothalamic arcuate nucleus–relationship with NPY neurones. NeuroReport. 1996;7:3087–3092. doi: 10.1097/00001756-199611250-00059. [DOI] [PubMed] [Google Scholar]
- 6.Weigle DS, Kuijper JL. Obesity genes and the regulation of body fat content. BioEssays. 1996;18:867–874. doi: 10.1002/bies.950181105. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Pfaff DW. Hormone effects on specific and global brain functions. J Physiol Sci. 2008;58:213–220. doi: 10.2170/physiolsci.RV007008. [DOI] [PubMed] [Google Scholar]
- 8.Pfaff D, Waters E, Khan Q, Zhang X, Numan M. Minireview: estrogen receptor-initiated mechanisms causal to mammalian reproductive behaviors. Endocrinology. 2011;152:1209–1217. doi: 10.1210/en.2010-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 11.Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- 12.Clegg DJ. Minireview: the year in review of estrogen regulation of metabolism. Mol Endocrinol. 2012;26:1957–1960. doi: 10.1210/me.2012-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr. 1999;70:405–411. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- 14.Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetel MJ, Pfaff DW. Contributions of estrogen receptor-alpha and estrogen receptor-ss to the regulation of behavior. Biochim Biophys Acta. 2010;1800:1084–1089. doi: 10.1016/j.bbagen.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- 18.Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ERalpha is necessary for estradiol's anorexigenic effect in female rats. Horm Behav. 2010;58:872–877. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 20.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 21.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Schneider JE, Wise JD, Benton NA, Brozek JM, Keen-Rhinehart E. When do we eat? Ingestive behavior, survival, and reproductive success Horm Behav. 2013;64:702–728. doi: 10.1016/j.yhbeh.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- 24.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 25.Diano S, Kalra SP, Sakamoto H, Horvath TL. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Res. 1998;812:256–259. doi: 10.1016/s0006-8993(98)00936-6. [DOI] [PubMed] [Google Scholar]
- 26.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 27.Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 29.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Sub-ventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 31.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 32.Epp JR, Chow C, Galea LA. Hippocampus-dependent learning influences hippocampal neurogenesis. Front Neurosci. 2013;7:57. doi: 10.3389/fnins.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepres-sants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 34.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 36.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 37.Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- 38.Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Barha CK, Lieblich SE, Galea LAM. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 40.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 41.Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 42.Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cifuentes M, Perez-Martin M, Grondona JM, Lopez-Avalos MD, Inagaki N, Granados-Duran P, Rivera P, Fernandez-Llebrez P. A comparative analysis of intraperitoneal versus intracerebroventricular administration of bromodeoxyuridine for the study of cell proliferation in the adult rat brain. J Neurosci Methods. 2011;201:307–314. doi: 10.1016/j.jneumeth.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 44.McNay DE, Briancon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010;30:723–730. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33:6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gouaze A, Brenachot X, Rigault C, Krezymon A, Rauch C, Nedelec E, Lemoine A, Gascuel J, Bauer S, Penicaud L, Benani A. Cerebral cell renewal in adult mice controls the onset of obesity. PLoS One. 2013;8:e72029. doi: 10.1371/journal.pone.0072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert PD, Anderson KD, Sleeman MW, Wong V, Tan J, Hijarunguru A, Corcoran TL, Murray JD, Thabet KE, Yancopoulos GD, Wiegand SJ. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc Natl Acad Sci USA. 2001;98:4652–4657. doi: 10.1073/pnas.061034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ettinger MP, Littlejohn TW, Schwartz SL, Weiss SR, McIlwain HH, Heymsfield SB, Bray GA, Roberts WG, Heyman ER, Stambler N, Heshka S, Vicary C, Guler HP. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: a randomized, dose-ranging study. JAMA. 2003;289:1826–1832. doi: 10.1001/jama.289.14.1826. [DOI] [PubMed] [Google Scholar]
- 50.Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17beta-estradiol administration to mice. Gen Comp Endocrinol. 2012;175:188–193. doi: 10.1016/j.ygcen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci USA. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kudwa AE, Harada N, Honda SI, Rissman EF. Regulation of progestin receptors in medial amygdala: estradiol, phytoestrogens and sex. Physiol Behav. 2009;97:146–150. doi: 10.1016/j.physbeh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates Amsterdam. Boston, MA: Elsevier; Academic Press; 2004. [Google Scholar]
- 54.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YF, Sandeman DC, Benton JL, Beltz BS. Birth, survival and differentiation of neurons in an adult crustacean brain. Dev Neurobiol. 2014;74:602–615. doi: 10.1002/dneu.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frontini A, Bertolotti P, Tonello C, Valerio A, Nisoli E, Cinti S, Giordano A. Leptin-dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res. 2008;1215:105–115. doi: 10.1016/j.brainres.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 57.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypo-thalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki Y, Shimizu H, Ishizuka N, Kubota N, Kubota T, Senoo A, Kageyama H, Osaka T, Hirako S, Kim HJ, Matsumoto A, Shioda S, Mori M, Kadowaki T, Inoue S. Vagal hyperactivity due to ventromedial hypothalamic (VMH) lesions increases adiponectin production and release. Diabetes. 2014;63:1637–1648. doi: 10.2337/db13-0636. [DOI] [PubMed] [Google Scholar]
- 59.Kim YM, An JJ, Jin YJ, Rhee Y, Cha BS, Lee HC, Lim SK. Assessment of the anti-obesity effects of the TNP-470 analog, CKD-732. J Mol Endocrinol. 2007;38:455–465. doi: 10.1677/jme.1.02165. [DOI] [PubMed] [Google Scholar]
- 60.Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. NeuroReport. 1996;7:2635–2638. doi: 10.1097/00001756-199611040-00045. [DOI] [PubMed] [Google Scholar]
- 61.Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Horm Behav. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. Both estrogen receptor alpha and estrogen receptor beta agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 65.Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 66.Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes Relat Metab Disord. 2004;28:879–885. doi: 10.1038/sj.ijo.0802640. [DOI] [PubMed] [Google Scholar]
- 67.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 68.deLartigue G, de Barbier la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab. 2011;301:E187–E195. doi: 10.1152/ajpendo.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill JO, Melanson EL, Wyatt HT. Dietary fat intake and regulation of energy balance: implications for obesity. J Nutr. 2000;130:284S–288S. [PubMed] [Google Scholar]
- 70.Warwick ZS, Schiffman SS. Role of dietary fat in calorie intake and weight gain. Neurosci Biobehav Rev. 1992;16:585–596. doi: 10.1016/s0149-7634(05)80198-8. [DOI] [PubMed] [Google Scholar]
- 71.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 73.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 74.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res. 2010;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sousa-Ferreira L, Almeida LP, Cavadas C. Role of hypothalamic neurogenesis in feeding regulation. Trends Endocrinol Metab. 2014;25:80–88. doi: 10.1016/j.tem.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Cheng MF. Hypothalamic neurogenesis in the adult brain. Front Neuroendocrinol. 2013;34:167–178. doi: 10.1016/j.yfrne.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28:82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- 79.Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- 80.Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. 2008;1241:1–6. doi: 10.1016/j.brainres.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 81.Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett. 2010;482:235–239. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 82.Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 83.Huang XF, Xin X, McLennan P, Storlien L. Role of fat amount and type in ameliorating diet-induced obesity: insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro-opiomelanocortin mRNA expression. Diabetes Obes Metab. 2004;6:35–44. doi: 10.1111/j.1463-1326.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- 84.Park S, da Kim S, Kang S, Kwon DY. Ischemic hippocampal cell death induces glucose dysregulation by attenuating glucose-stimulated insulin secretion which is exacerbated by a high fat diet. Life Sci. 2011;88:766–773. doi: 10.1016/j.lfs.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 85.Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 86.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 87.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velloso LA, Araujo EP, de Souza CT. Diet-induced inflammation of the hypothalamus in obesity. NeuroImmunoModulation. 2008;15:189–193. doi: 10.1159/000153423. [DOI] [PubMed] [Google Scholar]
- 90.Badoer E. Microglia: activation in acute and chronic inflammatory states and in response to cardiovascular dysfunction. Int J Biochem Cell Biol. 2010;42:1580–1585. doi: 10.1016/j.biocel.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29:507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann NY Acad Sci. 2006;1089:302–323. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- 93.Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 95.Brock O, Keller M, Veyrac A, Douhard Q, Bakker J. Short term treatment with estradiol decreases the rate of newly generated cells in the sub-ventricular zone and main olfactory bulb of adult female mice. Neuroscience. 2010;166:368–376. doi: 10.1016/j.neuroscience.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 96.Del Bianco-Borges B, Cabral FJ, Franci CR. Co-expression of leptin and oestrogen receptors in the preoptic-hypothalamic area. J Neuroendocrinol. 2010;22:996–1003. doi: 10.1111/j.1365-2826.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 97.Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci USA. 2009;106:15932–15937. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uchida Y, Tokizawa K, Nakamura M, Mori H, Nagashima K. Estrogen in the medial preoptic nucleus of the hypothalamus modulates cold responses in female rats. Brain Res. 2010;1339:49–59. doi: 10.1016/j.brainres.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 100.Clarke SD, Clarke IJ, Rao A, Evans RG, Henry BA. Differential effects of acute and chronic estrogen treatment on thermogenic and metabolic pathways in ovariectomized sheep. Endocrinology. 2013;154:184–192. doi: 10.1210/en.2012-1758. [DOI] [PubMed] [Google Scholar]
- 101.Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int J Obes. 1985;9(Suppl. 1):83–92. [PubMed] [Google Scholar]
- 102.Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518:459–476. doi: 10.1002/cne.22219. [DOI] [PubMed] [Google Scholar]
- 103.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 104.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piper ML, Unger EK, Myers MG, Jr, Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol. 2008;22:751–759. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 108.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Widdowson PS, Upton R, Buckingham R, Arch J, Williams G. Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes. 1997;46:1782–1785. doi: 10.2337/diab.46.11.1782. [DOI] [PubMed] [Google Scholar]
- 110.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R941–R948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- 111.Matyskova R, Zelezna B, Maixnerova J, Koutova D, Haluzik M, Maletinska L. Estradiol supplementation helps overcome central leptin resistance of ovariectomized mice on a high fat diet. Horm Metab Res. 2010;42:182–186. doi: 10.1055/s-0029-1243250. [DOI] [PubMed] [Google Scholar]
- 112.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 113.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 114.Torre SD, Benedusi V, Fontana R, Maggi A. Energy metabolism and fertility-a balance preserved for female health. Nat Rev Endocrinol. 2014;10:13–23. doi: 10.1038/nrendo.2013.203. [DOI] [PubMed] [Google Scholar]


