Abstract
Objective
The central nervous system can regulate peripheral inflammation, but the efferent neuronal routes and the mediators remain poorly defined. One candidate is the cholinergic pathway, which releases acetylcholine (ACh). This neurotransmitter can bind to the α7 cholinergic receptor (α7R) expressed by nonneuronal cells and reduce inflammation. To test this possibility, we evaluated the expression of α7R and its potential role as a target in rheumatoid arthritis (RA).
Methods
The expression of α7R in human synovium and fibroblast-like synoviocytes (FLS) was determined using immunohistochemical, Western blot, and quantitative polymerase chain reaction (PCR) analyses. The effects of ACh in vitro were determined in interleukin-1 (IL-1)–stimulated FLS using immunoassays for protein, quantitative PCR for messenger RNA (mRNA), luciferase reporter constructs for IL-6 and NF-κB promoter activity, and electrophoretic mobility shift assays. Expression of α7R was knocked down with small interfering RNA (siRNA) or was inhibited with the selective α7R antagonist methyllycaconitine (MLA).
Results
Protein and mRNA for α7R were demonstrated in RA and osteoarthritis synovium and cultured synoviocytes. Expression in synovium was mainly in the intimal lining. ACh significantly reduced the production of IL-6, CXCL8, CCL2, CCL3, CCL5, and granulocyte colony-stimulating factor by IL-1–stimulated FLS. This effect was blocked by the α7R antagonist MLA or by using α7R siRNA to knock down receptor expression. The selective α7R agonist PNU-282,987 decreased the production of IL-6 by IL-1–stimulated FLS. ACh did not reduce IL-6 transcription, but it decreased IL-6 mRNA half-life and reduced IL-6 mRNA steady-state levels.
Conclusion
The α7 receptor is expressed in the synovium and by synoviocytes. Receptor ligation inhibits cytokine expression in FLS through a posttranscriptional mechanism. Therefore, α7R is a potential therapeutic target for inflammatory diseases.
The central nervous system (CNS) can markedly affect peripheral inflammatory responses. For example, intrathecal administration of adenosine agonists suppresses neutrophil trafficking in the skin as well as inflammation and destruction of the joints (1,2). MAP kinase signaling pathways within the CNS are also involved, as shown by the suppressive effect of intrathecal administration of a p38 inhibitor on adjuvant arthritis (3). While the spinal mechanisms have been well delineated (1–4), the effector arms of the CNS that mediate peripheral antiinflammatory effects remain poorly defined. Other components of the peripheral nervous system, such as sympathetic and sensory nerve fibers, can also influence inflammation during arthritis (5). Another possible neuroimmune mechanism could engage the cholinergic antiinflammatory pathway (6). This physiologic reflex activates parasympathetic nerve terminals to release acetylcholine (ACh), which then binds to the α7 cholinergic receptor (α7R) on immune cells and reduces the production of cytokines (7).
Various cells that shape inflammatory responses express the α7R, including macrophages, dendritic cells, and T and B lymphocytes (8). The role of this receptor has been best studied in macrophages, where α7R agonists suppress the production of interleukin-1 (IL-1), tumor necrosis factor (TNF), IL-6, and IL-18 after challenge with lipopolysaccharide (LPS) (7,9). Mice rendered deficient in α7R are resistant to the therapeutic benefit of vagal stimulation in septic shock and to the administration of cholinergic agonists (7). In contrast with the increased inflammation caused by the loss of the receptor on immune cells, brain development is normal in α7R-knockout mice, suggesting that it is redundant within the nervous system (10). Thus, the use of α7R agonists as antiinflammatory agents could potentially avoid many nonspecific side effects of vagal activation.
The potential for manipulating vagal outflow was confirmed in recent studies demonstrating that pharmacologic or electrical stimulation of the vagus nerve decreases inflammation in the rat paw (11). ACh agonists reproduced the effects of vagal stimulation in this model. In rheumatoid arthritis (RA), the cholinergic antiinflammatory pathway appears to be suppressed (12). RA patients exhibit decreased cardiac vagal activity compared with normal controls. It is not known whether more distal targets of the pathway are also affected, which could be important to the design of therapeutic interventions. For example, if ACh release mechanisms are compromised in RA, then pharmacologic or electrical stimulation of the vagus nerve, which is useful in animal models, could be inefficient. Agonists of α7R might be effective even in the absence of vagal activity.
In this study, we showed that the α7R subunit is expressed in the intimal lining of RA and osteoarthritis (OA) synovium and in cultured fibroblast-like synoviocytes (FLS). Stimulation of this receptor on FLS suppresses the expression of IL-6 and several chemokines, including IL-8. Mechanistic studies demonstrated that this action likely occurs through decreased messenger RNA (mRNA) stability. Our data suggest that targeting the α7R is potentially useful for the treatment of RA.
MATERIALS AND METHODS
Synovial tissue and FLS
FLS were isolated from synovial tissues obtained from RA and OA patients at the time of joint replacement surgery, as described previously (13). RA patients had stopped taking disease-modifying antirheumatic drugs (DMARDs) for at least 4 weeks before surgery. RA FLS were obtained from synovial tissue from the elbow (2 patients), knee (2 patients), and hip (2 patients). The ages of the patients ranged from 44 to 70 years (median 67 years). The diagnosis of RA conformed to the American College of Rheumatology (formerly, the American Rheumatism Association) 1987 revised criteria (14). OA FLS were obtained from synovial tissues from the knee. The median age of the OA patients was 67 years (range 53–84 years). All OA and RA patients were women. The protocol was approved by the Human Subjects Research Protection Program of the University of California, San Diego.
A portion of the synovial tissue samples were snap-frozen and processed for immunohistochemical or quantitative polymerase chain reaction (PCR) analysis. The remaining tissue was used to derive FLS.
For preparation of FLS, synovial tissues were minced and incubated for 1.5 hours at 37°C with 0.5 mg/ml of type VIII collagenase (Sigma, St. Louis, MO) in serum-free RPMI 1640 (Mediatech, Herndon, VA). Tissues were then filtered through a 0.22-μm cell strainer, washed extensively, and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) (endotoxin content <0.006 ng/ml; Gemini Biosciences, Calabasas, CA), penicillin, streptomycin, gentamicin, and L-glutamine in a humidified chamber containing an atmosphere of 5% CO2. After overnight culture, nonadherent cells were removed, and adherent cells were trypsinized, split at a 1:3 ratio, and cultured. Synoviocytes were used from passage 4 through 9, when FLS were a homogeneous population with <1% CD11b cells, <1% phagocytic cells, and <1% Fc receptor γ II–positive cells.
Immunohistochemistry
Cryosections (5 μm) of synovial tissue from RA and OA patients or FLS cultured in chamber slides in DMEM with 10% FCS were used for immunohistochemistry. Fixation with 4% formalin was performed for 10 minutes. Endogenous peroxidase was depleted with 0.1% H2O2. Blocking was performed for 1 hour in phosphate buffered saline (PBS) containing 20% normal goat serum, followed by overnight incubation with α7R monoclonal antibody at 4°C (clone 319; Abcam, Cambridge, MA). Rat IgG served as the control.
Staining was performed by incubation with biotinylated secondary Fab fragment from goat (Abcam), followed by streptavidin–horseradish peroxidase (HRP) and aminoethyl-carbazole (AEC) substrate (both from DakoCytomation, Carpinteria, CA). The immunostained samples were counter-stained with hematoxylin. For bungarotoxin staining, cryosections were fixed with 0.2% formalin in PBS for 10 minutes; 0.1% H2O2 was then applied for 5 minutes. Sections were blocked in PBS with 1% bovine serum albumin and 0.1% Triton X-100 (Sigma) for 1 hour at 37°C, in the presence or absence of PNU-282,987 (1 mM; Biomol International, Ply-mouth Meeting, PA). Biotin-labeled bungarotoxin (5 μM; Invitrogen) was added for 1 hour at 37°C. Sections were washed 3 times for 5 minutes each in PBS and then incubated for 1 hour at room temperature in streptavidin–HRP solution, using AEC substrate. Sections were counterstained with hematoxylin.
Western blot analysis
Cell lysates were obtained from FLS cultured in DMEM with 10% FCS, as described previously (15). PC12 cell lysate control was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Whole cell lysates (50 μg) were heated for 5 minutes at 90°C in Laemmli buffer, fractionated by electrophoresis on Tris–glycine buffered 12% sodium dodecyl sulfate–polyacrylamide gels, and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). The membranes were blocked for 1 hour at room temperature with 5% nonfat milk in 0.05% Tween 20/Tris buffered saline, followed by overnight incubation at 4°C with α7R monoclonal antibody (Abcam clone 319). The blots were then incubated in the secondary HRP-conjugated antibody for 2 hours at room temperature. Immunoreactive protein was detected with enhanced chemiluminescence (PerkinElmer, Waltham, MA) and autoradiographic techniques.
Cytokine and chemokine protein assays
For immuno-assays, FLS were seeded in 24-well plates and cultured for 4 days in DMEM with 10% FCS. The supernatants were aspirated, replaced with fresh medium containing 0.1% FCS, and cultured for 48 hours. ACh (Sigma) was prepared fresh at the appropriate concentrations in cold PBS containing 1 mM pyridostigmine (Sigma) to inhibit endogenous cholinesterases, as previously described (9). FLS were then treated with medium or ACh solution, and recombinant human IL-1β (1 ng/ml; Calbiochem, La Jolla, CA) was added 1 hour later, except where indicated otherwise. To determine IL-6 protein levels, the supernatants were harvested 24 hours later and examined by enzyme-linked immunosorbent assay (ELISA; eBioscience, San Diego, CA). For multiplex analysis, supernatants were assayed for CXCL8 (IL-8), CCL2 (monocyte chemotactic protein 1 [MCP-1]), CCL3 (macrophage inflammatory protein 1α [MIP-1α]), CCL5 (RANTES), and granulocyte colony-stimulating factor (G-CSF) with an x-Plex panel on a Bio-Plex system (Bio-Rad, Hercules, CA).
Small interfering RNA (siRNA) transfection and lucif-erase and β-galactosidase assays
Using the Amaxa Human Dermal Fibroblast Nucleofector kit (NHDF-adult; Amaxa, Gaithersburg, MD) with program U-23, 5 × 105 cells (passages 4–6) were transfected with 3 μg of α7R or with scrambled negative control SMART pool siRNA (Dharmacon, Lafayette, CO), according to the manufacturer’s protocol (Amaxa). The 4 duplex oligonucleotides in the SMART pool had the following sequences (5′ to 3′): sense, GGGUGAAGACUGUU-CGUUUU and antisense, AAACGAACAGUCUUCAC-CCUU; sense, GCGCGUGGUUCCUGCGAAUUU and an-tisense, AUUCGCAGGAACCACGCGCUU; sense, GAGG-AGGUCCGCUACAUUGUU and antisense, CAAUGUA-GCGGACCUCCUCUU; and sense, GAUAACAGUC-UUACUCUCUUU and antisense, AGAGAGUAAGA-CUGUUAUCUU. After transfection, FLS were cultured for 6 days in DMEM with 10% FCS and were then stimulated with recombinant human IL-1β and ACh as described above.
We used the same conditions as described for siRNA to transfect reporter constructs. FLS (5 × 105/well) were transfected with 4 μg of p1168huIL-6P-Luc (the first 1,168 bp of the human IL-6 promoter cloned in front of the luciferase gene) or 4 μg of p(IL-6κB)3-50huIL-6P-Luc (3 copies of synthetic human NF-κB site and 50 bp of the IL-6 minimal promoter) and 2 μg of CMV-β-gal (the cytomegalovirus promoter for the β-galactosidase [β-gal] gene), the latter of which was used to normalize for the transduction efficiency. Plasmids p1168huIL-6P-Luc (LMBP 4495) and p(IL-6κB)3-50huIL-6P-Luc (LMBP 4735) were obtained from the plasmid library collection of Ghent University (Ghent, Belgium). After transfection, FLS were cultured in DMEM with 10% FCS in 12-well plates for 2 days and were synchronized in DMEM with 0.1% FCS for 24 hours. FLS were then treated for 24 hours with medium or recombinant human IL-1β (1 ng/ml), with or without ACh. The luciferase and β-gal enzyme activities were assayed with a luminometer, using a highly sensitive luciferase assay kit (Roche, Indianapolis, IN) and a β-gal assay kit (Stratagene, La Jolla, CA), respectively. Relative luciferase activity was determined by normalizing to β-gal activity.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were obtained from 1–1.5 × 106 cells with a nuclear extraction kit (Panomics, Fremont, CA). Five micrograms of extract was incubated for 1 hour at room temperature with a biotinylated NF-κB probe (5′-AGTTGAGGGGACTTTC-CCAGGC-3′; Panomics). The samples were resolved by electrophoresis at 4°C on a 6% nondenaturing gel, transferred to a nylon membrane, crosslinked by baking at 80°C for 1 hour, and then detected by chemiluminescence according to the manufacturer’s instructions (Panomics).
Quantitative real-time PCR
Messenger RNA derived from cultured FLS and frozen tissue samples was isolated using RNA Stat (Tel-Test, Friendswood, TX), as described previously (16), and complementary DNA was prepared according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed with Assays-on-Demand gene expression products (Applied Biosys-tems) to determine relative mRNA levels, using the GeneAmp 7300 Sequence Detection system (Applied Biosystems), as described previously (16). The α7R 5′ primer is located in exon 4 (RefSeq NM_000746.3) and the 3′ primer in exon 5 (catalog no. Hs01063373_m1; Applied Biosystems). IL-6 primers (catalog no. Hs00174131_m1; Applied Biosystems) were designed across exons 3 and 4 (RefSeq NM_000600.2). Sample threshold cycle (Ct) values were used to calculate the number of cell equivalents in the test samples. The data were then normalized to GAPDH expression (determined with GAPDH control reagents) (catalog no. 402869; Applied Biosystems) to obtain relative cell equivalents (CE).
Statistical analysis
Statistical analysis was performed with the Mann-Whitney U test, except where indicated otherwise. P values less than 0.05 were considered significant.
RESULTS
Expression of α7 receptor in synovium
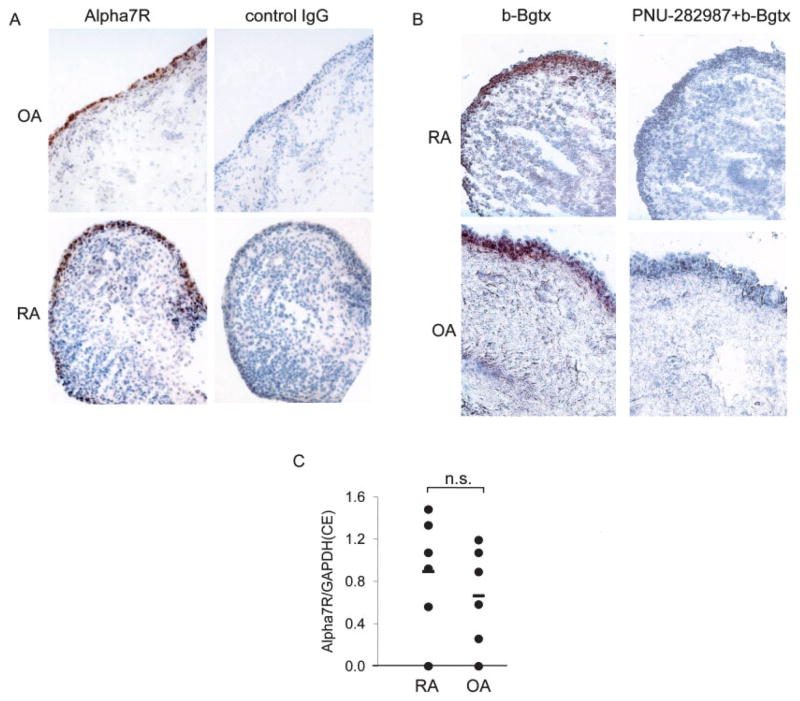
In systemic models of inflammation, the antiinflammatory effect of ACh is mediated by the α7R on macrophages (7). The ability of the cholinergic antiinflammatory pathway to suppress peripheral inflammation in the carrageenan-induced rat paw edema model (11) prompted us to determine if the same receptor is expressed in human synovium. We performed immuno-histochemical analyses of tissue samples from 3 RA and 3 OA patients, using an α7R-specific monoclonal antibody or biotin-labeled bungarotoxin (17). Using either method, the receptor was found to be abundantly expressed mainly in the synovial intimal lining (Figure 1A), with a similar staining pattern and intensity in OA and RA samples. We then measured levels of mRNA for the α7R gene by quantitative PCR in both RA and OA samples (n = 6 each). The levels were similar in samples from both groups of patients (Figure 1B).
Figure 1.
Expression of the α7 receptor subunit (α7R) in synovial tissue from rheumatoid arthritis (RA) and osteoarthritis (OA) patients. A and B, Immunohistochemistry of α7R in RA and OA synovial tissue was performed with an anti-α7R monoclonal antibody or control IgG (A) or with biotin-labeled bungarotoxin (b-Bgtx), with or without PNU-282,987, a selective agonist of α7R (B), as described in Materials and Methods. Staining for the receptor was highest in the intimal lining. Representative serial sections from an RA and an OA patient (of 3 RA and 3 OA samples examined) are shown. C, Expression of α7R mRNA in 6 RA and 6 OA synovial tissue samples was not significantly different (NS). Values are the ratio of α7R expression to GAPDH expression. Horizontal bars show the mean. CE = relative cell equivalents (see Materials and Methods for details).
Expression of α7R in cultured FLS
Because of strong positive staining in the intimal lining layer of the synovium, we examined whether cultured FLS express the α7R. Indeed, immunohistochemical analysis showed that both OA and RA FLS lines (n = 3–4 each) expressed the receptor (Figure 2A). These results were confirmed by Western blot analysis, which revealed a band migrating at the predicted molecular size (7) (Figure 2B). The α7R band comigrated with α7R expressed by PC12 cells and was knocked down in FLS transfected with α7R-specific siRNA, but not with control siRNA (Figure 2C). Alpha-7 receptor mRNA was expressed at similar levels in the OA and RA FLS lines, as determined by quantitative PCR (Figure 2D).
Figure 2.
Expression of the α7 receptor subunit (α7R) in fibroblast-like synoviocytes (FLS) from rheumatoid arthritis (RA) and osteoarthritis (OA) patients. A, FLS cultured in chamber slides show positive staining for α7R, but not control IgG, by immunohistochemistry. A representative section (of 6 FLS lines examined) is shown. No difference between RA and OA FLS was observed. B, Western blot analysis shows a band migrating at ~55 kd (similar to the molecular mass of the α7R subunit) in 3 OA and 3 RA FLS extracts. C, FLS transfected with α7R-specific small interfering RNA (siRNA) to knock down α7R protein expression show lower α7R protein expression in 2 RA and 1 OA samples compared with FLS transfected with the scrambled (sc) control siRNA. The 55-kd band comigrates with α7R expressed by PC12 cells. The blot was stripped and reprobed with an anti–β-actin antibody to verify equal loading. D, Expression of α7R mRNA in 7 RA and 7 OA FLS lines shows no significant difference (NS). Values are the ratio of α7R expression to GAPDH expression. Horizontal bars show the mean. CE = relative cell equivalents (see Materials and Methods for details). Color figure can be viewed in the online issue, which is available at http://www.arthritisrheum.org.
Regulation of proinflammatory mediators by ACh
Activated FLS produce several proinflammatory cytokines and chemokines that contribute to arthritis, notably, IL-6 (18). The expression of the α7R by FLS suggested that ACh could have direct antiinflammatory effects on this cell lineage. Resting FLS produced little IL-6 (<40 pg/ml after 24 hours), and the addition of ACh had no effect (data not shown). In comparison, IL-1–activated FLS released >20 ng/ml of IL-6 after 24 hours of stimulation. As shown in Figure 3A, ACh in the presence of pyridostigmine decreased the release of IL-6 by IL-1–treated FLS in a dose-dependent manner. Pyri-dostigmine alone had no significant effect (data not shown). The effective ACh concentration range was consistent with dose-response curves reported for α7R on neuronal cell types (19). No significant differences were noted between RA and OA FLS lines. Interestingly, adding ACh after IL-1 still caused a significant reduction in the release of IL-6 (Figure 3B).
Figure 3.
Acetylcholine (ACh)–induced decrease in the production of interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF), and the chemokines IL-8, monocyte chemotactic protein 1 (MCP-1), RANTES, and macrophage inflammatory protein 1α (MIP-1α) by IL-1–activated fibroblast-like synoviocytes (FLS) from rheumatoid arthritis (RA) and osteoarthritis (OA) patients. A, ACh dose-dependently decreased IL-6 release following IL-1 activation. FLS from 7 RA and 7 OA patients were pretreated with the indicated concentrations of ACh (see Materials and Methods for details) and stimulated with IL-1. No significant difference between OA and RA lines was noted. B, ACh (1 mM) decreased the release of IL-6 even when applied hours after IL-1 stimulation, as measured in 24-hour supernatants from 7 independent FLS lines. C, ACh reduced the release of G-CSF and chemokines in 3 independent FLS lines stimulated for 24 hours with IL-1 in the presence and absence of 1 mM ACh, as determined by multiplex assay. D, The selective α7R agonist PNU-282,987 dose-dependently inhibited IL-6 production in 24-hour supernatants from 7 independent FLS lines. PNU-282,987 (PNU) was added to the culture 1 hour before IL-1 stimulation. Values are the median, with first and third quartiles (lower and upper ends of error bars, respectively). ‡ = P < 0.001; * = P < 0.05 versus controls without ACh, by unpaired t-test.
Multiplex analysis of chemokines released by IL-1–stimulated FLS was then performed (Luminex, Austin, TX). ACh reduced the release of CXCL8 (IL-8), CCL2 (MCP-1), CCL3 (MIP-1α), and CCL5 (RANTES) from FLS (Figure 3C). ACh-treated FLS also released less G-CSF (Figure 3C). As shown in Figure 3D, the selective α7R agonist PNU-282,987 also reduced IL-6 production in IL-1–stimulated FLS. The dose-response range was comparable with that in previous experiments describing its effect, in which primary tissue culture of neurons was used (20).
ACh inhibition of IL-6 release from FLS through α7R
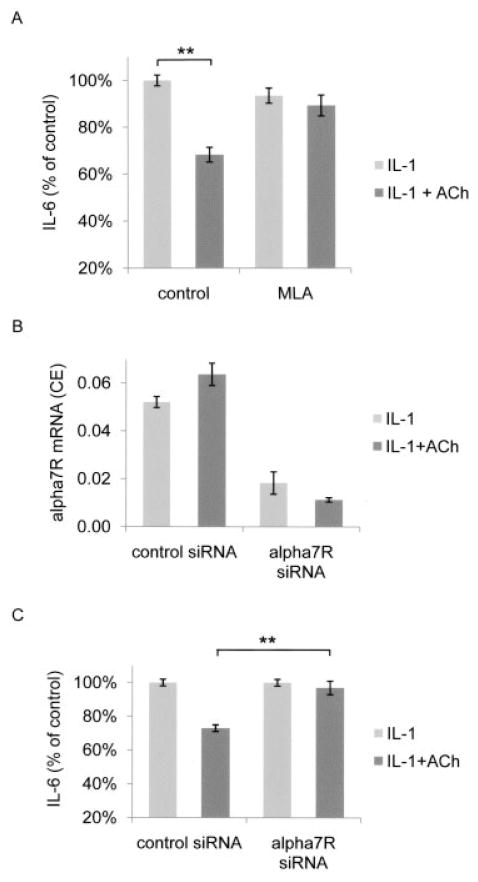
In human and mouse macrophages, ACh suppresses the release of inflammatory mediators via the α7R (7). The effect of PNU-282,987 on FLS suggested that the effect of ACh on FLS is also mediated by this receptor. We next designed experiments to confirm this hypothesis. Methyllycaconitine (MLA) is a selective antagonist of α7R. The addition of 10 nM MLA antagonized the effect of 1 mM ACh on the release of IL-6 (Figure 4A). We confirmed the role of the α7R using siRNA knockdown techniques. Alpha-7 receptor siRNA decreased α7R mRNA expression by >75%, as determined by quantitative PCR (Figure 4B). The α7R knockdown FLS were unresponsive to the addition of 1 mM ACh (n = 3 FLS lines for each α7R siRNA and control siRNA) (Figure 4C). Control siRNA, however, did not alter the effects of ACh on IL-6 production. Taken together, these findings show that chemical or genetic inactivation of α7R confirms the crucial role of this receptor in the inhibitory effect of ACh on IL-1–activated FLS.
Figure 4.
Acetylcholine (ACh)–induced decrease in the release of interleukin-6 (IL-6) from IL-1–activated fibroblast-like synoviocytes (FLS) via the α7 receptor subunit (α7R). A, The selective α7R antagonist methyllycaconitine (MLA; 10 nM) inhibited the effect of 1 mM ACh on the release of IL-6. MLA was added 1 hour before ACh. Four FLS lines were stimulated 1 hour later with IL-1, and IL-6 levels in supernatants were determined 24 hours later. B, Knockdown of α7R mRNA with small interfering RNA (siRNA), but not control siRNA, strongly reduced the levels of α7R mRNA. FLS lines were transfected with control or target-specific α7R siRNA as described in Materials and Methods, stimulated with IL-1 in the presence or absence of 1 mM ACh, and the cellular pellets were analyzed (n = 7). CE = relative cell equivalents (see Materials and Methods for details). C, Knockdown of α7R mRNA inhibited the effect of ACh on the release of IL-6. FLS lines were stimulated with IL-1 in the presence or absence of 1 mM ACh, and the supernatants were analyzed for IL-6 content by enzyme-linked immunosorbent assay. The effect of ACh was abolished when FLS were transfected with α7R-specific siRNA, but not with control siRNA (n = 3). Values are the mean ± SEM. ** = P < 0.001 by unpaired t-test.
ACh-induced decrease in IL-6 mRNA levels
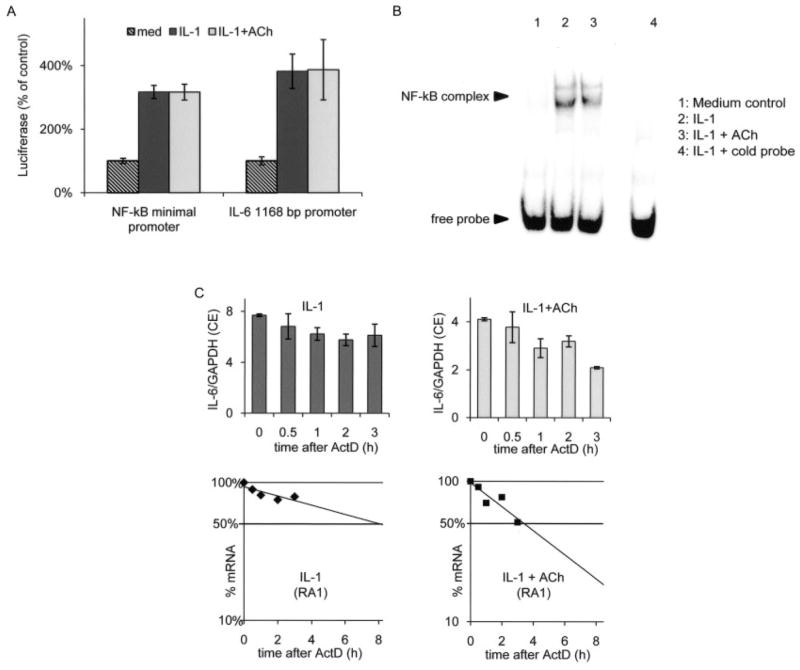
We then investigated whether the effect of ACh on IL-6 protein release was caused by pretranscriptional or posttranscriptional events. We first determined whether ACh affects IL-6 mRNA steady-state levels in IL-1–stimulated FLS. IL-6 mRNA levels were modestly, but significantly, reduced by ACh (median reduction 33% in 7 independent FLS lines; P < 0.001). In activated macrophages, ACh has been reported to decrease NF-κB activity. Since the IL-6 promoter contains functionally important NF-κB sites, we performed reporter experiments to examine the contribution of this pathway in FLS. We transfected an IL-6 minimal promoter containing the NF-κB into the FLS and evaluated the expression of luciferase. IL-1 increased the activity of this NF-κB construct, but ACh did not reduce it (n = 6 independent FLS lines) (Figure 5A). To examine the possible involvement of additional promoter elements, we then transfected a construct comprising the first 1,168 bp upstream of the IL-6 transcription start site. Again, ACh had no influence on the activity of the IL-6 promoter (n = 3 FLS lines) (Figure 5A). Finally, we confirmed by EMSA that ACh does not specifically reduce the endogenous NF-κB nuclear binding in FLS (Figure 5B).
Figure 5.
Acetylcholine (ACh)–induced reduction in the half-life of mRNA for interleukin-6 (IL-6), but not NF-κB nuclear binding or IL-6– or NF-κB–driven promoter activity. A, FLS were transfected with a 1,168-bp IL-6 promoter construct (n = 3) or an NF-κB artificial promoter construct (n = 6) driving the luciferase gene. IL-1 increased the promoter activity of both constructs compared with medium (med) control, but the IL-1 activity was not influenced by the addition of ACh. Values are the mean ± SEM. B, NF-κB binding activity induced by IL-1 (lane 2) was not significantly reduced by preincubation with ACh in 3 separate experiments. C, ACh decreased the half-life of IL-6 mRNA. FLS from a rheumatoid arthritis patient (RA1) were stimulated with IL-1 for 24 hours in the presence or absence of 1 mM ACh. Actinomycin D (ActD) was then added to block transcription, and the IL-6 mRNA decay over time was measured by quantitative polymerase chain reaction (top). Values are the ratio of IL-6 expression to GAPDH expression. CE = relative cell equivalents (see Materials and Methods for details). Half-life values are also shown as semilogarithmic plots (bottom). See Table 1 for a summary of the results obtained in 4 independent RA FLS lines and 2 independent osteoarthritis cell lines.
ACh-induced decrease in IL-6 mRNA stability
The promoter studies suggested that the reduction in IL-6 mRNA levels might be caused by posttranscrip-tional mechanisms. IL-6 levels are regulated in part by the stability of its mRNA (21). Therefore, we measured the half-life of IL-6 mRNA after inhibiting transcription with actinomycin D. In preliminary experiments, 10 μg/ml of actinomycin D completely blocked IL-1–induced transcription of IL-6 in FLS (data not shown). For half-life measurements, FLS stimulated with IL-1 for 24 hours in the presence or absence of ACh were then treated with 10 μg/ml of actinomycin D (Figure 5C). ACh significantly decreased the half-life of IL-6 mRNA in IL-1–stimulated FLS, from 13.8 hours to 6.5 hours (mean of 6 FLS lines) (Table 1). These experiments suggest that ACh increases IL-6 mRNA instability, which reduces the steady-state levels of the mRNA.
Table 1.
ACh-induced reduction in the half-life of IL-6 mRNA in IL-1-activated FLS*
| FLS line | IL-6 mRNA half-life, hours
|
% reduction | |
|---|---|---|---|
| IL-1 treatment | IL-1 plus ACh treatment | ||
| RA1 | 8.25 | 3.5 | 58 |
| RA4 | 7.75 | 5.5 | 29 |
| RA5 | 18 | 7.5 | 58 |
| RA6 | 18 | 8.4 | 53 |
| OA2 | 21 | 7 | 67 |
| OA3 | 10 | 7.3 | 27 |
| Mean ± SEM | 13.8 ± 2.4 | 6.5 ± 0.7 | 49 ± 7† |
Fibroblast-like synoviocytes (FLS) from 4 rheumatoid arthritis (RA) and 2 osteoarthritis (OA) patients were incubated for 18 hours with interleukin-1 (IL-1) or with IL-1 plus acetylcholine (ACh). Actinomycin D was added, and the cells were harvested at various time points thereafter. IL-6 mRNA levels were determined by quantitative polymerase chain reaction analysis, and the half-life of each cell line was calculated.
P = 0.002.
DISCUSSION
Several experimental models suggest that the CNS influences somatic host inflammatory responses, including arthritis. Using intrathecal delivery of a p38 inhibitor, we previously showed that targeted inhibition of this MAP kinase in the CNS could decrease inflammation and bone destruction in the adjuvant arthritis model (3). These results imply the existence of efferent neuroimmune signaling mechanisms that could potentially involve the autonomic system. For example, direct electrical stimulation of the vagal nerve can suppress several mediators involved in systemic and local inflammation, such as TNF production by immune cells and endothelial cell activation in the skin (9,22). Activation of this cholinergic antiinflammatory pathway suppresses inflammation in the carrageenan paw edema model in the rat (11). These results suggest that ACh might be active at distal sites such as the synovium, where its influence on joint tissue was previously unexplored. We therefore evaluated the potential for cholinergic antiin-flammatory mechanisms in the joint.
First, the α7 cholinergic receptor, which contributes to the antiinflammatory effects of acetylcholine in various models (6,8,23), is expressed in human syno-vium. Consistent with the staining of the intimal lining observed in synovial tissue samples, we found that cultured FLS also expressed α7R. The receptor levels remained stable even when FLS were stimulated with inflammatory cytokines or were treated with ACh, suggesting that it is expressed constitutively. Consistent with these in vitro observations, the expression of α7R was similar in OA and RA tissue samples.
The presence of the α7R on FLS allowed us to evaluate its role in vitro. FLS stimulated with IL-1 produced less IL-6 mRNA and protein in the presence of ACh. We then sought to determine whether the α7R governs the effects of acetylcholine on cytokine production in activated FLS, as has been reported for macrophages (7). Treatment with PNU-282,987, a selective agonist of α7R, mimicked the antiinflammatory effect of ACh on FLS. ACh activity was blocked by specific inhibition of α7R using the chemical antagonist MLA or using siRNA knockdown techniques, confirming the key role of this receptor in FLS. Thus, the results obtained using 3 separate analysis methods—selective agonist, selective antagonist, and siRNA knockdown of the receptor—support the hypothesis that ACh is acting through the α7 receptor.
The stimulation of α7 receptors with ACh in FLS led to additional effects, such as a decrease in the production of chemokines and growth factors. CCL2, CCL3, CCL5, and CXCL8 were abundantly produced by IL-1–stimulated FLS, and their levels were significantly reduced by ACh. G-CSF, which stimulates the function and survival of neutrophils and is released by activated FLS, was also reduced in the presence of ACh. These data suggest that α7R stimulation could diminish the number of infiltrating neutrophils and macrophages in the synovium. The effects on FLS can potentially synergize with the reported mode of action of α7R agonists during inflammation of the endothelium (22). Indeed, both the production of chemokines and the expression of adhesion molecules necessary for leukocytes to attach and migrate through the inflamed endothelium were shown to be down-regulated by cholinergic agonists.
Deciphering the mechanisms triggered by α7R agonists could help support the therapeutic potential of this approach. In the next series of experiments, we showed that α7R activation in FLS did not affect the IL-6 transcription rate, but instead, decreased the stability of its mRNA. Posttranscriptional regulation of messenger stability is a key homeostatic step that regulates the expression of several inflammatory proteins, including IL-6 and TNF (21,24). This mechanism should also be effective when transcription of the gene is driven by preexisting stimuli, as in chronic inflammation. In support of this hypothesis, the antiinflammatory effect was preserved when ACh was applied several hours after the activating stimulus.
Our observations in FLS provide the first reported example of α7R affecting cytokine levels by decreasing mRNA stability. Indeed, this receptor is able to signal via different routes depending on the cell type and the species examined. In electrically active cells such as neurons, the α7R is a calcium channel that modulates synaptic transmission. JAK-2/STAT-3 and the NF-κB pathways have been implicated in α7R signaling in mouse macrophages (25,26). Our studies show that cholinergic signaling does not influence NF-κB–driven promoter activity in FLS. In human macrophages, TNF protein is strongly suppressed by ACh but mRNA levels are not (9), thus ruling out a functional contribution of NF-κB, which mostly affects transcription. Identifying the pathways by which ACh modulates TNF protein levels in human macrophages and the IL-6 mRNA half-life in FLS could have important therapeutic implications.
Immunohistochemistry studies showed a staining pattern of α7R in the lining, which suggests that both FLS and macrophage-like cells express the receptor. Previous in vitro work has shown that LPS-stimulated macrophages released less TNF and IL-1 in the presence of an α7R agonist (9). In the RA synovium, macrophages are an important source of these cytokines, which they produce in response to various stimuli, including Toll-like receptor ligands (18). If the receptor is functional on synovial macrophages, then α7R agonists could reduce the local levels of IL-1 and TNF in addition to the levels of IL-6 and chemokines. Confirmation of this hypothesis will require in vivo studies.
Central regulation of the vagal antiinflammatory pathway uses several types of cholinergic receptors, including muscarinic receptors in the brain (27). Spinal mechanisms involving the MAP kinase pathway down-regulate adjuvant arthritis and might contribute to increase vagal activity (3). In the periphery, the finding that circulating macrophages (7), endothelial cells (22), and FLS express α7R suggests that endogenous cholin-ergic agonists can participate. In somatic territories, ACh is released by autonomic fibers that innervate sweat glands (28), as shown by the efficacy of the specific anticholinergic agent botulinum toxin in the treatment of localized hyperhidrosis (29). In addition, a nonneu-ronal cholinergic system regulates the biology of several organs, including the skin (30), endothelium (31), lung (32), and immune system (8,33). More experiments will be needed to determine the amount and cellular origin of ACh in the joints and its relationship to local inflammation.
In conclusion, α7R agonists could potentially inhibit several steps of the proinflammatory cascade that occur in the rheumatoid joint, including the production of TNF, the release of IL-6, and the recruitment of leukocytes. Centrally acting α7 agonists are under development and might help improve cognitive function in patients with various neurologic disorders, with a lower toxicity profile than currently available cholinergic agents (23). Clinical studies from this field have thus far not revealed major drug-related, neurologic adverse events (34). Selecting compounds that do not penetrate the blood–brain barrier could help increase the therapeutic index in inflammatory diseases.
Acknowledgments
Supported by grants from the Arthritis Foundation and the Within Our Reach program of the Research and Education Foundation, American College of Rheumatology. Dr. Waldburger’s work was supported by the Swiss National Science Foundation. Dr. Firestein’s work was supported by Procter & Gamble.
Footnotes
Dr. Tracey has received consulting fees, speaking fees, and/or honoraria from Innovative Metabolics and MedImmune (more than $10,000 each), owns stock or stock options in Innovative Metabolics, is cofounder of Critical Therapeutics, Inc, and holds patents related to vagus nerve stimulation and the use of cholinergic agents to treat inflammation. Dr. Firestein has received consulting fees from Procter & Gamble (less than $10,000).
AUTHOR CONTRIBUTIONS
Dr. Firestein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Waldburger, Boyle, Tracey, Firestein.
Acquisition of data. Waldburger.
Analysis and interpretation of data. Waldburger, Boyle, Firestein.
Manuscript preparation. Waldburger, Boyle, Pavlov, Tracey, Firestein.
Statistical analysis. Waldburger.
References
- 1.Bong GW, Rosengren S, Firestein GS. Spinal cord adenosine receptor stimulation in rats inhibits peripheral neutrophil accumulation: the role of N-methyl-D-aspartate receptors. J Clin Invest. 1996;98:2779–85. doi: 10.1172/JCI119104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle DL, Moore J, Yang L, Sorkin LS, Firestein GS. Spinal adenosine receptor activation inhibits inflammation and joint destruction in rat adjuvant-induced arthritis. Arthritis Rheum. 2002;46:3076–82. doi: 10.1002/art.10595. [DOI] [PubMed] [Google Scholar]
- 3.Boyle DL, Jones TL, Hammaker D, Svensson CI, Rosengren S, Albani S, et al. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 2006;3:e338. doi: 10.1371/journal.pmed.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sluka KA, Westlund KN. Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain. 1993;55:217–25. doi: 10.1016/0304-3959(93)90150-N. [DOI] [PubMed] [Google Scholar]
- 5.Cutolo M, Straub RH, Bijlsma JW. Neuroendocrine-immune interactions in synovitis. Nat Clin Pract Rheumatol. 2007;3:627–34. doi: 10.1038/ncprheum0601. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 8.De Jonge WJ, Ulloa L. The α7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–29. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 10.Yu WF, Guan ZZ, Nordberg A. Postnatal upregulation of α4 and α3 nicotinic receptor subunits in the brain of α7 nicotinic receptor-deficient mice. Neuroscience. 2007;146:1618–28. doi: 10.1016/j.neuroscience.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–7. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein RS, Bruchfeld A, Yang L, Qureshi AR, Gallowitsch-Puerta M, Patel NB, et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210–5. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-γ and tumor necrosis factor-α on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990;86:1790–8. doi: 10.1172/JCI114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of JNK by MKK-7 in fibroblast-like synoviocytes. Arthritis Rheum. 2006;54:2127–35. doi: 10.1002/art.21919. [DOI] [PubMed] [Google Scholar]
- 16.Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5:R352–60. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, et al. Prenatal nicotine increases pulmonary α7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103:637–47. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 19.Papke RL, Porter Papke JK. Comparative pharmacology of rat and human α7 nAChR conducted with net charge analysis. Br J Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, et al. Discovery and structure-activity relationship of quinuclidine benzamides as agonists of α7 nicotinic acetylcholine receptors. J Med Chem. 2005;48:905–8. doi: 10.1021/jm049363q. [DOI] [PubMed] [Google Scholar]
- 21.Paschoud S, Dogar AM, Kuntz C, Grisoni-Neupert B, Richman L, Kuhn LC. Destabilization of interleukin-6 mRNA requires a putative RNA stem-loop structure, an AU-rich element, and the RNA-binding protein AUF1. Mol Cell Biol. 2006;26:8228–41. doi: 10.1128/MCB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–84. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 24.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 25.De Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway [published erratum appears in Nat Immunol 2005;6:954] Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 27.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103:5219–23. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis SC. Development of muscarinic receptors and regulation of secretory responsiveness in rodent sweat glands. Life Sci. 1999;64:381–5. doi: 10.1016/s0024-3205(98)00578-5. [DOI] [PubMed] [Google Scholar]
- 29.Heckmann M, Ceballos-Baumann AO, Plewig G. Botulinum toxin A for axillary hyperhidrosis (excessive sweating) N Engl J Med. 2001;344:488–93. doi: 10.1056/NEJM200102153440704. [DOI] [PubMed] [Google Scholar]
- 30.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–65. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick CJ, Bittinger F, Nozadze K, Wessler I. Expression and function of the non-neuronal cholinergic system in endothelial cells. Life Sci. 2003;72:2111–6. doi: 10.1016/s0024-3205(03)00069-9. [DOI] [PubMed] [Google Scholar]
- 32.Racke K, Juergens UR, Matthiesen S. Control by cholinergic mechanisms. Eur J Pharmacol. 2006;533:57–68. doi: 10.1016/j.ejphar.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 33.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–9. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, et al. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–51. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]