Abstract
We describe a physical-organic study of two fluoropolymers bearing a photoreleasable PEGylated photosensitizer which generates 1O2(1Δg) [chlorin e6 methoxy tri(ethylene glycol) triester]. The surfaces are Teflon/polyvinylalcohol (PVA) nanocomposite and fluorinated silica. The relative efficiency of these surfaces to photorelease the PEGylated sensitizer [shown previously to be phototoxic to ovarian cancer cells (Kimani, S. et al J. Org. Chem 2012, 77, 10638)] was slightly higher for the nanocomposite. In the presence of red light and O2, 1O2 is formed, which cleaves an ethene linkage to liberate the sensitizer in 68–92% yields. The fluoropolymers were designed to deal with multiple problems. Namely, their success relied not only high O2 solubility and drug repellency, but that the C−F bonds physically quench little 1O2 for its productive use away from the surface. The results obtained here indicate that Teflon-like surfaces have potential uses of delivering sensitizer and singlet oxygen for applications in tissue repair and photodynamic therapy (PDT).
Keywords: Polymer photooxidation, singlet oxygen, fluorination, PEGylation, vibrational quenching, drug delivery
INTRODUCTION
Solid supports have been used as platforms for the photorelease of drug molecules.1,2 However, there are gaps in data on whether Teflon-like3,4 or superhydrophobic surfaces5 can efficiently photorelease drugs. To address this issue, two photoactive fluoropolymers have been synthesized and compared (Figure 1). One is made of fluorinated silica [glass coated with (CH3O)3SiCH2CH2CF2CF2CF2CF3] and the other is a Teflon/polyvinyl alcohol (PVA) nanocomposite which has –[CF2–CF2]m– and –[CH2–CH(OH)]n– chains. Figure 1 also shows the sensitizer drug to be photoreleased is PEGylated.
Figure 1.
Design of fluoropolymer surfaces tailored for 1O2-initiated sensitizer drug photorelease reactions. The sensitizer, a chlorin derivative PEGylated with triethylene glycol, was bound to surface OH groups of (A) fluorinated silica (Vycor glass monolith coated with nonafluorosilane), and (B) a Teflon/polyvinyl alcohol nanocomposite. Red light irradiation leads to the reaction of 1O2 with the ethene, resulting in the formation of a dioxetane. A second step follows with cleavage to release the sensitizer.
While the delivery of PEGylated compounds is an active area of research,6–8 they tend to adhere to surfaces.9–11 Even though solid state sensitizers have been established,12–14 few have been designed to release PEGylated compounds,15 and none have capitalized on fluoropolymers’ nonstick repellent properties16 for better molecule discharge from the surface. Thus, we anticipated that fluoropolymer sensitizer release systems with repellent properties and visible light activation could be established.
Visible light and NIR photocleavage reactions are known17–24 and actually represent a burgeoning area of research.25,26 For example, the sensitized generation of 1O2(1Δg) has been used with labile ethene linkers for photorelease reactions. We27,28 and others29–31 have published papers devoted to 1O2-based drug release and a book chapter has also appeared.32 Singlet oxygen is a potentially therapeutic species and is photogenerated by PAHs,33,34 chlorins,35,36 porphyrins and phthalocyanines, and their fluorinated analogs.37–41 In 2011, Röder et al. reported the deposition of perfluorinated phthalocyanines on silica gel as a composite material for generating 1O2 for sterilization.42 A Teflon ponytail fullerene (i.e. C60 adduct with CH2[CO2(CH2)3(CF2)7CF3]2) has also been prepared for 1O2 generation.43 Favorable properties of surface fluorination for 1O2 and drug potency through PEGylation would make such a combination be desirable. However, solid materials that are both fluorinated and PEGylated are rather uncommon.44 Taken together, the above topics reveal the potential utility of a Teflon-supported PEGylated-drug release system and point to the need for new studies.
Our hypothesis was that a Teflon/PVA nanocomposite will photorelease the PEGylated sensitizer more efficiently than fluorinated silica because of the higher number of C–F bonds in the former. Thus, we report here the synthesis and study of two fluoropolymers illustrated in Figure 1 to give us insight into the photosensitizer release mechanism. We show that these surfaces have (i) high solubility of ground-state oxygen, which is advantageous for photooxidation chemistry; (ii) relatively low adsorptivity for the PEGylated sensitizer, for good turnout; (iii) facile breakage of the ethene linker bond; and (iv) longer singlet oxygen lifetimes (τΔ) because the C–F bonds sap the polymer’s ability to physically quench 1O2 (i.e. paucity of C–H and O–H bonds limit unwanted vibrational relaxation of 1O2 to 3O2). The fluoropolymers we describe are biocompatible (e.g., for surgery)45,46 and our mechanistic study provides results which may be useful for applications in localized delivery of sensitizer to desired surfaces (e.g. wounds or diseased tissue) where the released photosensitizer is active upon subsequent illumination.
EXPERIMENTAL
General Aspects
Reagent grade solvents methanol, hexane, toluene, DMF, THF, CH2Cl2, CHCl3, CDCl3, CCl4, and n-butanol were used. Hydrofluoric acid, sodium sulfate, sodium bicarbonate, sodium periodate, sodium borohydride, osmium tetroxide, acetic acid, succinic acid, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N,N-dimethyl-4-aminopyridine (DMAP), (3-iodopropyl)trimethoxysilane, 3,3,4,4,5,5,6,6,6-nonafluorohexyltrimethoxysilane, tri(ethylene glycol) monomethyl ether, chlorin e6, rose bengal, polyvinyl alcohol (MW 89,000–98,000, and 99% hydrolyzed), poly(tetrafluoroethylene) (PTFE: 60 wt % solid content, 5.9 wt % nonionic surfactant, 22.3 nm average particle size, and 2.20 g/mL density) were used as received from commercial suppliers. Solid samples were cleaned with refluxing methanol in a Soxhlet extractor. Proton and carbon NMR data were recorded at 400 MHz and 100.6 MHz, respectively. UV−vis and IR spectrophotometers, a GC/MS instrument, a muffle furnace, and a portable pO2 oxygen sensor were also used. HRMS data were collected at the mass spectrometry facility in University of California, Riverside.
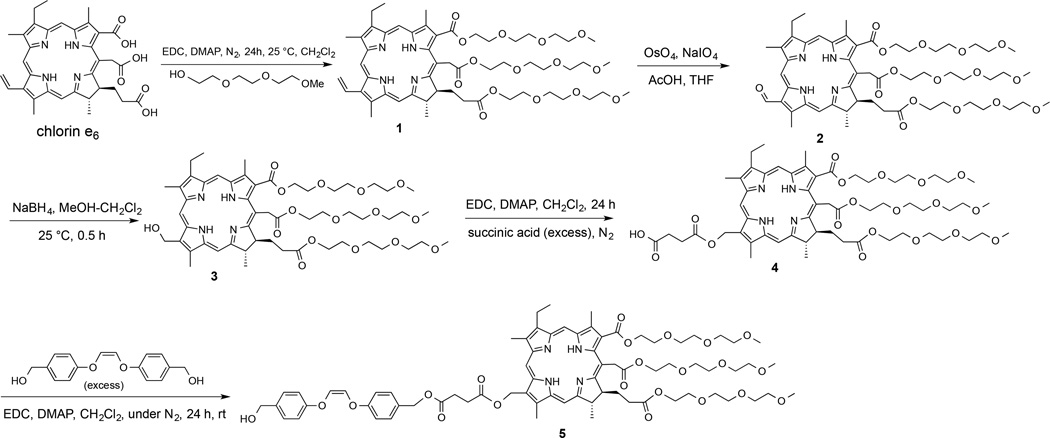
Synthesis of 3-Formyl-173,152,131-Chlorin e6 Methoxy Tri(ethylene glycol) Triester (2) (Figure 2)
Figure 2.
Synthesis of spacer triPEG chlorin 5.
Yield 12.0 mg (60%); purity: 98%. Chlorin e6 was converted to triPEG chlorin 1 (i.e. methoxy triethylene glycol attached at the ester bonds for a 3:1 conjugate) by a previously described procedure.47 To the 20.0 mg (0.019 mmol) of 1 in 15 mL THF, 7.68 mg (0.03 mmol) of OsO4 in 20 µL CCl4 was added at 0 °C under N2 atmosphere. Reaction mixture was stirred under 0–5 °C temperature for 25 min. 82.8 mg (0.38 mmol) of NaIO4 was dissolved in 1% AcOH solution and added to the reaction mixture. Reaction was stirred overnight at room temperature. A drop of saturated sodium bicarbonate solution was added to neutralize the acid. The reaction mixture was extracted with 50 mL of CH2Cl2 and washed with water. The organic layer was dried over sodium sulfate. After evaporating the organic solvent, the residue was purified by column chromatography using 1.1% methanol−CH2Cl2. Rf = 0.69 in 3% methanol−CH2Cl2. HPLC: tR = 12.2 min in gradient mixture of methanol and H2O. 1H NMR (400.0 MHz, CDCl3) δ 11.56 (s, 1H), 10.28 (s, 1H), 9.70 (s, 1H), 8.97 (s, 1H), 5.48 (d, J = 18.4Hz, 1H), 5.30 (d, J = 18.4 Hz, 1H), 4.96 (m, 1H), 4.89 (m, 1H), 4.51 (m, 2H), 4.35 (m, 2H), 4.16 (m, 4H), 3.86 (m, 3H), 3.84 (s, 3H), 3.82 (m, 3H), 3.71 (t, J = 4.8 Hz, 3H), 3.65 (m, 5H), 3.56 (m, 10H), 3.44 (t, J = 4.8 Hz, 3H), 3.37 (s, 3H), 3.34 (m, 4H), 3.30 (s, 3H), 3.23 (s, 3H), 3.22 (s, 3H), 3.18 (t, J = 4.8 Hz, 2H), 2.60 (m, 1H), 2.25 (m, 2H), 1.77 (m, 8H), −1.24 (br s, 1H), 1.76 (br s, 1H); 13C NMR (100.6 MHz, CDCl3) δ 188.3, 173.0, 172.2, 168.9, 168.5, 167.6, 155.1, 151.5, 144.9, 138.3, 138.1, 137.9, 136.6, 136.0, 134.0, 131.9, 128.6, 125.8, 103.4, 101.3, 100.7, 95.5, 71.9, 71.8, 71.6, 70.7, 70.6, 70.5, 70.4, 70.2, 70.1, 69.2, 69.0, 68.9, 65.2, 64.3, 63.6, 59.0, 58.9, 58.8, 53.4, 48.7, 38.5, 31.0, 29.7, 29.5, 23.3, 19.6, 17.5, 12.4, 11.4, 11.3. HRMS (+ESI) m/z calculated for C54H77N4O16 [M+H]+ 1037.5335, found: 1037.5357. UV−vis (CHCl3) λmax 418 and 694 nm.
Synthesis of 31-Hydroxyl-173,152,131-Chlorin e6 Methoxy Tri(ethylene glycol) Triester (3)
Yield 10.0 mg (77%); purity: 92%. To 13.0 mg (0.012 mmol) of 2 in 4:1 mL methanol–CH2Cl2 at 5–10 °C, 1.0 mg (0.025 mmol) of NaBH4 was added. A sudden color change from brown to green was observed. The reaction was monitored by TLC. It was completed in 0.5 h. The reaction mixture was quenched with water and extracted by CH2Cl2. The organic layer was dried over sodium sulfate and evaporated by a rotavapor system. Rf = 0.50 in 3% methanol−CH2Cl2. HPLC: tR = 7.79 min in gradient mixture of methanol and H2O. 1H NMR (400.0 MHz, CDCl3) δ 9.72 (s, 1H), 9.55 (s, 1H), 8.76 (s, 1H), 5.87 (s, 2H), 5.44 (d, J = 18.8 Hz, 1H), 5.29 (d, J = 19.6 Hz, 1H), 4.93 (m, 1H), 4.88 (m, 1H), 4.47 (m, 2H), 4.32 (m, 2H), 4.11 (m, 4H), 3.85 (m, 3H), 3.79 (m, 3H), 3.70 (t, J = 4.8 Hz, 2H), 3.60 (m, 5H), 3.55 (t, J = 4.8 Hz, 3H), 3.48 (m, 11H), 3.40 (m, 2H), 3.36 (s, 3H), 3.30 (s, 3H), 3.28 (s, 3H), 3.20 (m, 4H), 3.16 (m, 3H), 3.11 (m, 2H), 3.02 (t, J = 4.8 Hz, 2H), 2.27 (m, 1H), 2.16 (m, 1H), 2.09 (m, 2H), 1.86 (m, 2H), 1.75 (m, 7H), −1.58 (br s, 1H); 13C NMR (100.6 MHz, CDCl3) δ 173.0, 172.4, 169.7, 168.8, 166.8, 154.5, 148.9, 145.0, 139.1, 136.6, 136.0, 135.7, 135.5, 135.2, 132.5, 129.4, 123.7, 102.6, 102.0, 98.2, 93.7, 71.9, 71.7, 71.5, 70.7, 70.6, 70.3, 70.1, 70.0, 69.2, 68.8, 65.0, 64.3, 63.5, 59.0, 58.9, 58.7, 56.3, 53.4, 52.9, 49.3, 38.6, 30.9, 29.7, 29.6, 23.0, 19.6, 17.6, 14.1, 12.4, 11.3, 11.1; HRMS (+ESI) m/z calculated for C54H79N4O16 [M+H]+ 1039.5491, found: 1039.5490. UV−vis (CHCl3) λmax 404 and 661 nm.
Synthesis of 31-Succinate-173,152,131-Chlorin e6 Methoxy Tri(ethylene glycol) Triester (4)
Yield 8.0 mg (73.4%); purity: 90%. To the 10.0 mg (0.0096 mmol) of 3 in 10 mL of dry CH2Cl2 under nitrogen atmosphere, 5.65 mg (0.048 mmol) of succinic acid, 4.57 mg (0.024 mmol) of EDC and 2.9 mg (0.024 mmol) of DMAP were added. Reaction was stirred for 36 h under N2 at room temperature after which 15 mL CH2Cl2 was added. The organic layer was washed with water and dried over sodium sulfate. The organic layer was evaporated and 3 was purified by 2% methanol−CH2Cl2. Rf = 0.37 in 3% methanol−CH2Cl2. HPLC: tR = 7.79 min in gradient mixture of methanol and H2O. 1H NMR (400.0 MHz, CDCl3) δ 9.73 (s, 1H), 9.58 (s, 1H), 8.80 (s, 1H), 6.46 (s, 2H), 5.44 (d, J = 18.4 Hz, 1H), 5.29 (d, J = 19.6 Hz, 1H), 4.92 (m, 2H), 4.48 (m, 2H), 4.30 (t, J = 4.8 Hz, 2H), 3.80 (m, 6H), 3.70 (t, J = 4.8 Hz, 2H), 3.62 (s, 3H), 3.55 (m, 4H), 3.51 (s, 3H), 3.47 (s, 3H), 3.45 (m, 4H), 3.36 (m, 8H), 3.23 (s, 3H), 3.11 (s, 3H), 3.07 (m, 2H), 2.99 (t, J = 4.8 Hz, 2H), 2.88 (m, 2H), 2.82 (m, 2H), 2.75 (m, 2H), 2.69 (m, 2H), 2.48 (m, 2H), 2.29 (m, 4H), 1.86 (m, 2H), 1.74 (dd, J = 16.4 Hz, 11.6 Hz, 6H), −1.67 (br s, 1H). HRMS (+ESI) m/z calculated for C58H83N4O19 [M+H]+ 1139.5652, found: 1139.5634.
Synthesis of Spacer Alkene-31-Succinate-173,152,131-Chlorin e6 Methoxy Tri(ethylene glycol) Triester (5)
Yield 1.9 mg (52%); purity: 91%. To the 3.0 mg (0.0026 mmol) of 4 in dry CH2Cl2 in nitrogen atmosphere, 2.0 mg (0.0073 mmol) of spacer alkene alcohol, 1.06 mg (0.0052 mmol) of EDC and 1.0 mg (0.0078 mmol) of DMAP were added. Reaction was stirred for 36 h under N2 at room temperature after which 15 mL CH2Cl2 was added. Organic layer was washed with water and dried over sodium sulfate. The organic layer was evaporated and 5 was purified by 1.5% methanol−CH2Cl2. Rf = 0.64 in 3% methanol-CH2Cl2. HPLC: tR = 9.24 min in gradient mixture of methanol and H2O. 1H NMR (400.0 MHz, CDCl3) δ 9.73 (s, 1H), 9.58 (s, 1H), 8.80 (s, 1H), 7.32 (d, J = 8.4 Hz, 2H), 7.01 (d, J = 8.4 Hz, 2H), 6.97 (d, J = 8.4 Hz, 2H), 6.71 (d, J = 8.4 Hz, 2H), 6.45 (s, 2H), 5.91 (d, J = 3.2 Hz, 1H), 5.71 (d, J = 3.2 Hz, 1H), 5.44 (d, J = 18.8 Hz, 1H), 5.27 (d, J = 18.4 Hz, 1H), 4.89 (m, 4H), 4.61 (s, 2H), 4.47 (m, 2H), 4.33 (m, 2H), 4.14 (m, 5H), 3.83 (m, 3H), 3.77 (m, 3H), 3.70 (t, J = 4.8, 4H), 3.62 (m, 5H), 3.56 (m, 10H), 3.50 (s, 3H), 3.43 (m, 3H), 3.36 (s, 3H), 3.33 (s, 3H), 3.29 (s, 3H), 3.21 (m, 7H), 3.14 (t, J = 4.8 Hz, 2H), 2.80 (m, 2H), 2.74 (m, 2H), 2.55 (m, 1H), 2.19 (m, 3H), 1.73 (m, 7H), −1.40 (br s, 1H), −1.65 (br s, 1H). 13C NMR (100.6 MHz, CDCl3) δ 173.5, 173.0, 172.4, 172.2, 172.0, 169.5, 168.7, 167.1, 157.0, 156.7, 145.0, 138.6, 136.7, 136.2, 135.6, 135.5, 135.2, 134.0, 130.7, 129.8, 129.7, 129.6, 128.4, 127.6, 123.9, 116.1, 115.8, 101.9, 98.3, 94.0, 71.9, 71.8, 71.5, 70.7, 70.6, 70.5, 70.4, 70.1, 69.2, 68.9, 66.8, 66.0, 65.0, 64.7, 64.3, 63.5, 59.0, 58.9, 58.8, 57.6, 53.4, 53.0, 49.2, 38.7, 38.6, 34.0, 31.0, 30.4, 29.7, 29.6, 29.3, 29.2, 28.9, 24.4, 23.8, 23.0, 22.9, 19.6, 17.7, 14.1, 14.0, 12.4, 11.3, 11.0. HRMS (+ESI) m/z calculated for C74H97N4O22 [M+H]+ 1393.6594, found: 1393.6592. UV−vis (CHCl3) λmax (ε/M−1cm−1) 400 nm (338680), 661 nm (92760).
TriPEG Chlorin-Modified Fluorinated Silica (7) (Figure 3)
Figure 3.
Synthesis of sensitizer-conjugated organically to fluorinated silica 7.
Fluorinated glass 6 was prepared based on the methods established from our previous work.48 Briefly, Vycor pieces were added to the nonafluorotrimethoxysilane in 0.07 wt/wt % in toluene and refluxed for 24 h under N2. Unreacted nonafluorotrimethoxysilane was washed off of the silica by Soxhlet extraction in methanol for 24 h. TriPEG chlorin 5 reacted with (3-iodopropyl)trimethoxysilane and was added to 0.33 g fluorinated silica 6 [shaped into pieces sized ∼5 mm × ∼8 mm (d × l)] in refluxing toluene for 24 h to reach 7. Washings with CH2Cl2, THF, methanol and hexane were followed by a Soxhlet extraction in methanol for 24 h to remove any adsorbed sensitizer from the silica. No sensitizer leaching from the surface was observed in the dark or under subdued light. UV−vis (λmax, air) 400 and 661 nm. After dissolution of silica 7 by HF treatment and extraction with CHCl3, evidence suggested the liberation of sensitizer (Soret band observed at 400 nm), HOCH2C6H4OCH=CHOC6H4CH2OCH2CH2CH2SiF3 (+ESI) m/z calculated for C19SiH20O4F3 = 398, found = 399, and HOCH2C6H4OCH=CHOC6H4CH2OCH2CH2CH2Si(OH)3 (+ESI) m/z calculated for C19H24O7Si = 392, found = 391. The amount of sensitizer that was loaded into 7 was 90 nmol/g of silica.
TriPEG Chlorin-Modified Teflon/PVA (10) (Figure 4)
Figure 4.
Synthesis of sensitizer-conjugated organically to Teflon/PVA nanocomposite 10 and by-product formation of surface diester sites.
Teflon/PVA 8 was prepared from a literature procedure and is a polymeric nanocomposite material of a PVA matrix filled with Teflon nanospheres.49 Teflon suspension was added to a PVA solution (PVA dissolved in boiling H2O with stirring for 5 h) in a 6:1 Teflon:PVA mass ratio. The mixture was stirred at room temperature and atmospheric pressure for 2.5 h using a mechanical agitator. Samples were placed in a mold and dried at room temperature and atmospheric pressure for 1 week. FT-IR (cm−1): 3600–3100, 2950–2850, 1419–1325, 1203, 1147. UV−vis (λ, air): transparent. Succinic acid (0.100 g, 8.5 mmol) was dissolved in 1 mL anhydrous DMF and added to five pieces (ea. 0.22 g) of Teflon/PVA in 15 mL anhydrous CH2Cl2. EDC (0.081 g, 0.425 mmol), and DMAP (0.052 g, 0.425 mmol) were added and the mixture was stirred under nitrogen atmosphere for 5 days. The samples were washed with CH2Cl2, THF, methanol, toluene, and hexane, and then Soxhlet extracted with methanol for 24 h to reach Teflon/PVA 9. FT-IR (cm−1): 1723. UV−vis (λ, air): transparent. TriPEG chlorin 5 (6 mg, 4.3 µmol), EDC (0.010 g, 0.052 mmol), and DMAP (0.010 g, 0.082 mmol) were added to 0.21 g Teflon/PVA 9 [sized ∼4 mm × ∼7 mm (d × l)] in 10 mL CH2Cl2 in N2 atmosphere. The reaction mixture was stirred for 5 days. After reaction the samples were washed with several solvents (CH2Cl2, THF, methanol, toluene, hexane) and Soxhlet extracted using methanol for 24 h. FT-IR (cm−1): 1731. UV−vis (λ, air): 396 and 660 nm. The amount of sensitizer attached onto the succinic acid sites of Teflon/PVA was determined to be 23.4 nmol/g by UV−vis spectroscopy. IR data were unable to distinguish whether the excess EDC used converted many of the surface acid sites to diesters (Figure S22, Supporting Information). Teflon/PVA 10 was stable in the dark; the sensitizer did not leach off to any measurable extent after CH2Cl2, methanol, THF and hexane solvent washings or Soxhlet extraction with methanol.
Photorelease of Sensitizer 13 from Surfaces 7 and 10 (Figure 5)
Figure 5.
The photooxidation of fluorinated silica 7 and Teflon/PVA nanocomposite 10 leads to the photorelease of sensitizer 13.
The photolysis setup included a continuous wave diode laser (669 nm output) where the light was passed through an SMA port, and out of the end of a borosilicate optical fiber with SMA optical fiber coupling as has been described in our previous work.48 Under subdued light, n-butanol solutions were presaturated with O2 for 20 min and then illuminated with red light for 1.5 h, upon which 1O2 was generated and trapped by the alkene sites on the solid supports. These heterogeneous photolysis reactions contained 0.328 and 0.214 g of solids 7 and 10, respectively. Concentrations of 13 were measured based on calibration curves, which followed its Q−band absorption at 665 nm. The number of broken alkene bond was quantified by the amount of sensitizer detected in after Soxhlet extraction in methanol. Control experiments demonstrated that the photorelease does not deviate from Beer’s Law in the UV−vis detection of sensitizer. Compound 13: HRMS (+ESI) m/z calculated for C66H89N4O21 [M+H]+ 1273.6014, found: 1273.6018. UV−vis (CHCl3) λmax 399 and 660 nm. No other products were found in solution based on GC/MS and NMR spectroscopy.
Lifetime Measurements
The singlet oxygen lifetime was determined using the output of a Continuum Surelite I-20 Nd:YAG pumped Surelite OPO Plus (type I BBO broadband) laser producing 5-ns pulses of 460 nm light at ∼0.2 mJ/pulse and a Hamamatsu H10330B-45 photomultiplier tube at an operating voltage of 650 V. Four-milliliter O2-saturated solutions of acetone-h6 were used containing 2.5×10−4 M rose bengal and 75–150 µm sized silica and ∼300–400 µm sized Teflon particles. The 1O2 luminescence intensity was monitored at a right angle through an interference filter centered at 1270 nm. The 1O2 signal was recorded on a 600 MHz oscilloscope (LeCroy WaveSurfer) and processed with OriginPro software.
RESULTS AND DISCUSION
O2 Solubility Enhancements (Table 1)
Table 1.
Oxygen Solubility Enhanced by Fluorinated Materials in Solutiona
| Solid | ppm | mM |
|---|---|---|
| Native silica | 14.9±0.4 | 0.47±0.02 |
| Fluorinated silica 6 | 16.7±0.4 | 0.522±0.01 |
| Native PVA | 14.9±0.03 | 0.466±0.001 |
| Teflon/PVA 8 | 17.9±0.07 | 0.559±0.003 |
Pyrex test tubes contained 5.0 mL of O2–saturated n-butanol with 0.58 g of solid at 25 °C. A pO2 electrode was used to measure the O2 solubility. After removing the solids from solution, the O2 solubility returns to 14.9 ppm (0.466 mM).
Table 1 shows that the O2 solubility increases in the presence of the fluorinated materials (fluorinated silica 6 and Teflon/PVA nanocomposite 8) compared to native PVA and silica. Table 1 also shows an O2 solubility increase in Teflon/PVA 8 compared to fluorinated silica 6, where a 1.2 ppm increase is shown. O2 solubility increases in fluorous media has been observed previously in fluorinated artificial bloods,50 ionic liquids,51 solvents52–54 and biological systems.55,56 As we will see the fluorinated solids provide an opportunity to enhance the production of 1O2 due to higher local O2 concentrations.
PEGylated Sensitizer Photorelease from the Fluoropolymers (Figure 6 and Table 2 and Table 3)
Figure 6.
Evolution of the sensitizer 13 which photo-departed away from the fluorinated silica 7 (●) and Teflon/PVA 10 (■) into n-butanol solution at 25 °C. Error bars represent the standard deviation obtained from 3 measurements.
Table 2.
| solid support | Sens Loading (nmol) |
sens 13 photocleaved (nmol) |
% sens photocleaved |
Sens Adsorbed (nmol) |
ethene linker bonds broken (nmol) |
|---|---|---|---|---|---|
| Fluorinated silica 7 |
89.4 | 12.7 | 68.3 | 48.4 | 61.1 |
| Teflon/PVA 10 | 21.7 | 6.5 | 91.7 | 13.4 | 19.9 |
Red light from a diode laser was used to photocleave the sensitizer. Absorption spectroscopy was used to quantitate the amount of sensitizer 13 in the surrounding n-butanol solution.
The data show a weights for 7 and 10 normalized to 1.0 g.
Table 3.
Absolute Number of Bonds or Functional Groups on the Fabricated Surfaces per Grama
| solid support |
Si−OH | C−OH | C−H | C−F | sens | C−OH: C−F:sens |
Si−OH: C−F:sens |
|---|---|---|---|---|---|---|---|
| fluorinated silica 7 |
1.3×1020 | - | 3.5×1021 | 7.8×1021 | 5.5×1016 | - | 2400:142000:1 |
| Teflon/PVA 10 | - | 6.2×1020 | 1.9×1021 | 9.6×1021 | 1.3×1016 | 48000:750000:1 | - |
Fluorinated silica 7 contains pendent nonafluorosilane groups. Teflon/PVA 10 contains repeating –[CF2-CF2]m– and –[CH2-CH(OH)]n– units. The penetration depth of sensitizer into solid 7 was 0.08 mm and into solid 10 was 1.0 mm.
Figure 5 and Table 2 show that the percent of PEGylated sensitizer 13 photoreleased into n-butanol was higher for Teflon/PVA 10 than for fluorinated silica 7. The emergence of sensitizer 13 in n-butanol was quantified by monitoring its Q−band absorption by UV−vis. Control experiments show that the red light and O2 were needed to cause the photorelease of 13. Control experiments also showed that the ester groups of 7, 10, and 13 remained intact under dark reaction conditions. We did not find any evidence for ester bond hydrolysis under the conditions wherein all sensitizer release was due to reaction of 1O2 with the ethene sites. In terms of loading, the quantity of sensitizer loaded onto 7 was greater than 10. “High” loading of sensitizer molecules is undesirable especially when porphyrin sites are within the ∼15 Å Förster radius producing self-quenching for a photothermal57 rather than a photosensitizer polymer. This led us to examine the number and type of bonds on our fabricated surfaces. Table 3 shows the absolute number of Si−OH, C−OH C−F, C−H groups and sensitizer sites in fluorinated silica 7 and Teflon/PVA 10. The number of O−H bonds for 10 is 4.8 fold greater than in 7. For 10 compared to 7, there are also 1.2-fold more C−F bonds and 1.8-fold less C−H bonds. A driving force in sensitizer adsorption may be the higher number of H-bonding sites in 10 (C−OH 6.2×1020) compared to 7 (Si−OH 1.3×1020). There is a greater number of O−H sites on 10 than 7, which led us to study the adsorptive affinity of sensitizer 13.
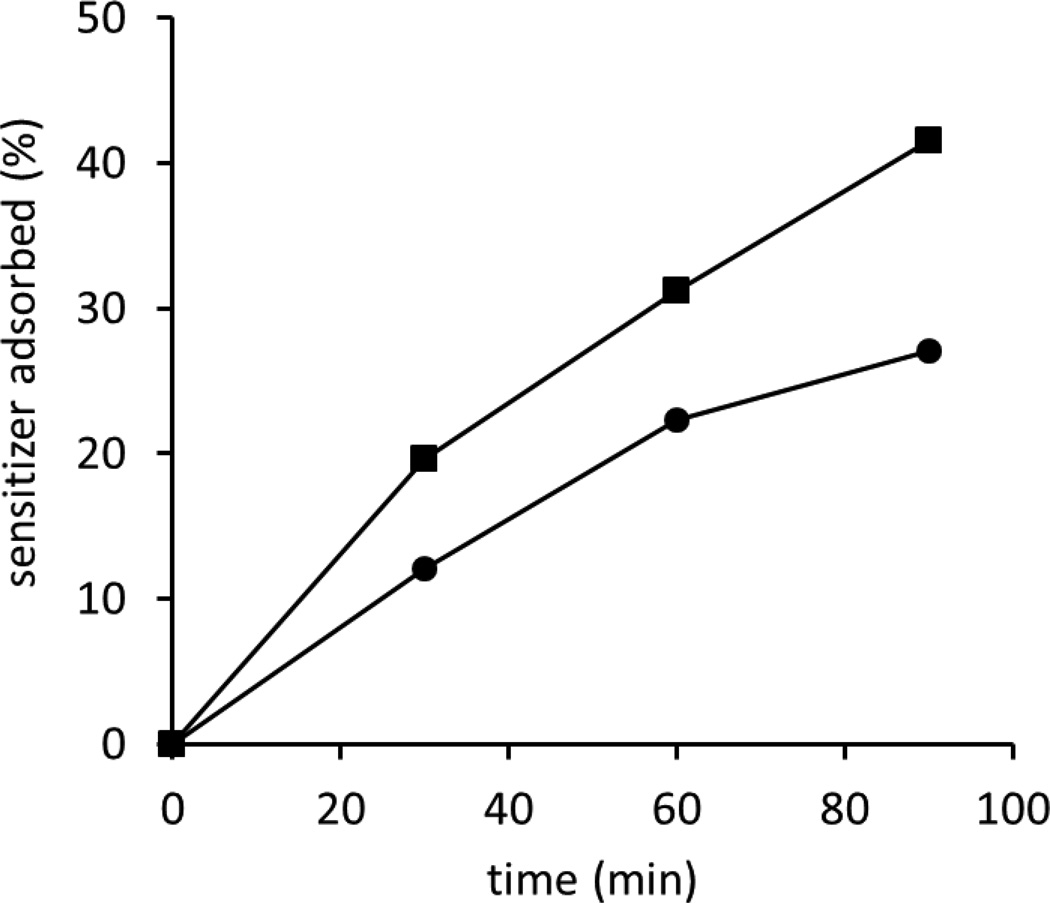
Testing the Fluorinated Surfaces for Sensitizer Adsorption (Figure 7)
Figure 7.
Percent of PEGylated sensitizer 13 adsorbed onto fluorinated surfaces [fluorinated silica 6 (●) and Teflon-PVA 9 (■)] in n-butanol. The samples were immersed in n-butanol, which contained 10 µM sensitizer 13 and then samples were removed at the indicated times.
We wondered what levels of unwanted adhering of the PEG sensitizer 13 would occur with our fluoropolymer supports. Figure 7 shows the amount of sensitizer that adsorbs onto fluoropolymers (here “sensitizer-less” 6 and 9 were examined) placed in 10-µM n-butanol solution of 13. The sensitizer’s ability to adsorb to the surfaces was increased for 9 compared to 6. This can be understood by the tendency of PEGylated compounds to adhere to surfaces with O−H groups. This result is similar with our recent work48 where a pheophorbide sensitizer increased its adsorptive affinity for silica with higher quantities of O−H groups. The number of O−H bonds was important to tabulate because they can serve as adsorption sites. Next, we examined the physical quenching of 1O2 by the fluoropolymers, where less would be better.
Lifetime Measurements (Table 4)
Table 4.
Rates of Reaction with Singlet Oxygen by Native Silica, Fluorinated Silica 6, Teflon/PVA 8, and Teflon in Acetonea
| Entry | Sample | kT (L g−1 s−1) | R square |
| 1 | Native silicaa | 71±6 | 0.09 |
| 2 | Fluorinated silica 6a | 27±3 | 0.99 |
| 3 | Teflon/PVA 8c | 29±9 | 0.84 |
| 4 | Teflond | 10±8 | 0.60 |
Heterogeneous mixture of particles [6 (75–150 µm) and 8 (~300–400 µm)] in acetone-h6 at 25 °C. Average of 2–3 experiments.
Table 4 shows that the total rate constants (kT) of 1O2 decreased in the presence of fluorinated silica 6, Teflon/PVA 8, and Teflon particles compared to native silica. In these experiments, the 1270 nm signal of 1O2 was followed with added quantities of the solid particles in acetone-h6 at 25 °C. Native silica, 6, 8, and Teflon were used because they lacked the sensitizer heads of 7 and 10, where a constant concentration of rose bengal was used in the heterogeneous mixtures. Teflon, 6 and 8 contain a high number of C−F oscillators which do not efficiently physically quench 1O2 compared to C−H or O−H oscillators due to electronic to vibronic overlap.59,60 Consistent with this notion, for native silica the kT value increased by 2–3−fold because of greater numbers of O−H bonds. The “poor” R square values in entries 3 and 4 (Table 4) are not a cause for concern where Teflon/PVA 8 and Teflon were ground to flakes that were larger and harder to stir as a slurry than the silica particles. Clearly, the data show success in using fluorinated surfaces due to their reduced physical quenching of 1O2. The low kT values we observe are encouraging since less wasted 1O2 by the fluorinated surface is vital to its success for drug photorelease as is described next.
Mechanism of Sensitizer Photorelease from the Teflon-like Surfaces (Figure 8)
Figure 8.
Mechanism of photorelease of sensitizer drug bound to (A) fluorinated silica 7, and (B) Teflon/PVA 10. The green spheres are fluorine atoms.
A proposed mechanistic explanation of the results is as follows:
(i) O2 solubility
There is enhanced O2 solubility with our fluoropolymers than non-fluorinated media so more 1O2 can be generated. Fluoropolymer surface topology likely relates to O2 solubility increases based on previous NMR studies61 where shapes of perfluoro sites and the existence of cavities in the liquid phase dominate over any direct interactions of O2 with the fluorine atoms, such as charge-transfer interactions.
(ii) Steric Effects
Singlet oxygen is generated by surfaces 7 and 10, mainly from surface 3sensitizer* quenching by O2 that leads to a dioxetane. We know that the dioxetane is not stable62,63 and spontaneously breaks apart at room temperature so the covalent bond between the sensitizer and the surface is lost. As yet increased kinetic persistence of dioxetanes through sterics, as has been noted in adamantaneadamantylidene dioxetane which is stable at room temperature64 cannot be attributed to our fluoropolymers. But compare parts A and B of Figure 8 where the fluorine groups are oriented differently. Because the fluorinated silica 7 consists of branched fluorosilane (high “aspect ratio”) groups they are more subject to dynamical motion.65 Whereas, Teflon/PVA 10 consists of continuous end-on carbon-fluorine chains in which Teflon –[CF2-CF2]m– may also coil into a helix due to repulsion between vicinal fluorine atoms.66 The importance of steric interactions of the fluorosilane is consistent with our observation of a reduced photocleavage efficiency in 7 compared to 10, but this is confounded by factors such as repelling and quenching (described below). Although, it seems obvious, crowding by branched fluorosilanes can prevent 1O2 accessibility to the ethene site. Indeed, dioxetane experiments come to mind probing mechanical,67 cage and chemiluminescence,68–70 and dynamical features of the ∼1.2-nm length fluorosilane, i.e. nano-heterogeneity, of 7 and 10 but are beyond the scope of the present study.
(iii) Resistance to Adsorption
After the ethene is photooxidized and broken, there is a partition between surface release and adsorption channels. The shuttling71 or mobility of the sensitizer on the surface prior to departure was not scrutinized with our fluoropolymers. However, the small decrease in the adsorptive affinity of 13 for 10 than 7 is attributed to the higher number of surface O−H groups in 10 than 7. But does the adsorptive affinity interfere with further ethene photooxidation? Tying up some of sensitizer 13 in an adsorbed state means that autocatalytic-assisted release kinetics are unavailable unlike more repellent hydrophobic sensitizers.72
(iv) Reactive Ethene Sites; Inert Support
For our fluoropolymers 7 and 10, the ethene sites are photooxidized. The data point to fluorinated media and an increase in τΔ due to inefficient radiationless deactivation of 1O2 by C–F bonds compared to O–H and C–H bonds.73–75 Unlike polyfunctionalized compounds or proteins,76,77 the reaction center for 7 and 10 is the ethene site. Other than the ethene site, the surfaces do not chemically react with 1O2, as would be expected of Teflon-like materials that are known to resist oxidation.78–81 We find the PEGs groups of 7 and 10 were not susceptible to photooxidation. This is not the case of oxygen radicals, e.g., via autoxidation, where PEG hydroperoxides can form and degrade.82
In summary, we find that the presence of C−F bonds in these materials lead to efficient photocleavage of the PEGylated sensitizer. The Teflon/PVA 10 provides a slight advantage with respect to the sensitizer photorelease application compared to fluorinated silica 7.
CONCLUSION
We have developed two fluoropolymers and examined their ability to photorelease a PEGylated sensitizer drug. The presence of C−F bonds in the polymers is beneficial for high O2 solubility, repelling action, and low physical quenching of 1O2. Designing photoactive repellent surfaces so that drugs are released rather than retained is largely an empirical endeavor and remains a challenge. However, opportunities exist by integrating methodologies from the photosciences to engineering to address the problem.
Future Directions
We have synthesized surfaces that are Teflon-like so a PEGylated sensitizer drug that is otherwise quite sticky can be photoreleased into the surrounding solution, however further advancements could be made. The fluoropolymers could be shaped into device tips to discharge controlled sensitizer and 1O2 quantities for tissue repair83,84 or pointsource photodynamic therapy85 in vivo. The fluorinated materials provide intriguing possibilities for generating new types of device tips for manual precision in singlet oxygen delivery. Next, we will turn to in vivo experiments to examine the fluoropolymers’ functional capacity in a mouse tumor model for sensitizer delivery and tumor killing but not biofouling.
Supplementary Material
ACKNOWLEDGMENTS
G.G, M.M., A.A.G., I.A. and A.G. acknowledge support from the National Institute of General Medical Sciences (NIH SC1GM093830). K.C. and T.M.B. acknowledge support from the NIH (P01 CA087971). A.G. acknowledges support from the George and Beatrice Schwartzman Professorship in Chemistry at Brooklyn College. We thank Leda Lee for the graphic arts work.
Footnotes
Supporting Information Available: Absorption, FT-IR, HRMS, and 1H and 13C NMR spectra for 1–5. Absorption and FT-IR spectra of solids 7 and 10. This information is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Klán P, Šolomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Givens RS, Lee J-I, Kotala M. Dynamic Studies in Biology: Phototriggers, Photoswitches, and Caged Biomolecules. Ch. 2. Wiley and Sons; 2005. Mechanistic Overview of Phototriggers and Cage Release; pp. 95–112. [Google Scholar]
- 3.Rolland JP, Van Dam RM, Schorzman DA, Quake SR, DeSimone JM. Solvent-Resistant Photocurable “Liquid Teflon” for Microfluidic Device Fabrication. J. Am. Chem. Soc. 2004;126:2322–2323. doi: 10.1021/ja031657y. [DOI] [PubMed] [Google Scholar]
- 4.Liu B, Rolland JP, DeSimone JM, Bard A. Fabrication of Ultramicroelectrodes Using A “Teflon-like” Coating Material. J.Anal. Chem. 2005;77:3013–3017. doi: 10.1021/ac0482918. [DOI] [PubMed] [Google Scholar]
- 5.Detty MR, Ciriminna R, Bright F, Pagliaro M. Environmentally Benign Sol-Gel Antifouling and Foul-Releasing Coatings. Acc. Chem. Res. 2014;47:678–687. doi: 10.1021/ar400240n. [DOI] [PubMed] [Google Scholar]
- 6.Rissanen S, Kumorek M, Martinez-Seara H, Li Y-C, Jamroz D, Bunker A, Nowakowaska M, Vattulainen I, Kepczynski M, Rog T. Effect of PEGylation on Drug Entry into Lipid Bilayer. J. Phys. Chem. B. 2014;118:144–151. doi: 10.1021/jp4105745. [DOI] [PubMed] [Google Scholar]
- 7.Li Y-C, Rissanen S, Stepniewski M, Cramariuc O, Róg T, Mirza S, Xhaard H, Wytrwal M, Kepczynski M, Bunker A. Study of Interaction Between PEG Carrier and Three Relevant Drug Molecules: Piroxicam, Paclitaxel, and Hematoporphyrin. J. Phys. Chem. B. 2012;116:7334–7341. doi: 10.1021/jp300301z. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Shi Y, Feng H, Du M, Zhang JZ, Hu J, Yang D. Preparation of Copolymer Paclitaxel Covalently Linked via a Disulfide Bond and Its Application on Controlled Drug Delivery. J. Phys. Chem. B. 2012,;116:9231–9237. doi: 10.1021/jp303260f. [DOI] [PubMed] [Google Scholar]
- 9.Pai SS, Przybycien TM. Protein PEGylation Attenuates Adsorption and Aggregation on a Negatively Charged and Moderately Hydrophobic Polymer Surface. Langmuir. 2010;26:18231–18238. doi: 10.1021/la102709y. [DOI] [PubMed] [Google Scholar]
- 10.Pai SS, Heinrich F, Canady AL, Przybycien TM, Tilton RD. Coverage-Dependent Morphology of PEGylated Lysozyme Layers Adsorbed on Silica. J. Colloid Interface Sci. 2012;370:170–175. doi: 10.1016/j.jcis.2011.12.065. [DOI] [PubMed] [Google Scholar]
- 11.Werner A, Blaschke T, Hasse H. Microcalorimetric Study of the Adsorption of PEGylated Lysozyme and PEG on a Mildly Hydrophobic Resin: Influence of Ammonium Sulfate. Langmuir. 2012;28:11376–11383. doi: 10.1021/la302239e. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Gao R, Zhou F, Selke M. Nanomaterials and Singlet Oxygen Photosensitizers: Potential Applications in Photodynamic Therapy. J. Mater. Chem. 2004;14:487–493. [Google Scholar]
- 13.Li W, Lu W, Fan Z, Zhu X, Reed A, Newton B, Zhang Y, Courtney S, Tiyyagura PT, Ratcliff RR, et al. Enhanced Photodynamic Selectivity of Nano-Silica-Attached Porphyrins Against Breast Cancer Cells. J. Mater. Chem. 2012;22:12701–12708. doi: 10.1039/C2JM30897E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arzoumanian E, Ronzani F, Trivella A, Oliveros E, Sarakha M, Richard C, Blanc S, Pigot T, Lacombe S. Transparent Organosilica Photocatalysts Activated by Visible Light: Photophysical and Oxidative Properties at the Gas–Solid Interface. ACS Appl. Mater. Interfaces. 2014;6:275–288. doi: 10.1021/am404175y. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda Y, Nagasaki Y. PEGylation Technology in Nanomedicine. Advances in Polymer Science. 2012;247:115–140. Polymers in Nanomedicine. [Google Scholar]
- 16.Chessick JH, Healey FH, Zettlemoyer AC. Adsorption and Heat of Wetting Studies of Teflon. J. Phys. Chem. 1956;60:1345–1347. [Google Scholar]
- 17.Šebej P, Wintner J, Müller P, Slanina T, Anshori JA, Antony LAP, Klán P, Wirz J. Fluorescein Analogues as Photoremovable Protecting Groups Absorbing at ∼520 nm. J. Org. Chem. 2013;78:1833–1843. doi: 10.1021/jo301455n. [DOI] [PubMed] [Google Scholar]
- 18.Doane T, Cheng Y, Sodhi N, Burda C. NIR Photocleavage of the Si–C Bond in Axial Si-Phthalocyanines. J. Phys. Chem. A. 2014;118:10587–10595. doi: 10.1021/jp505656e. [DOI] [PubMed] [Google Scholar]
- 19.Borak JB, Falvey DE. Ketocoumarin Dyes as Electron Mediators for Visible Light Induced Carboxylate Photorelease. Photochem. Photobiol. Sci. 2010;9:854–860. doi: 10.1039/c0pp00072h. [DOI] [PubMed] [Google Scholar]
- 20.Sundararajan C, Falvey DE. Photorelease of Carboxylic Acids, Amino Acids, and Phosphates from N-Alkylpicolinium Esters Using Photosensitization by High Wavelength Laser Dyes. J. Am. Chem. Soc. 2005;127:8000–8001. doi: 10.1021/ja050760f. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Johns VK, Liao Y. Controlled Release of Fragrant Molecules with Visible Light. Chem. Eur. J. 2014;20:14637–14640. doi: 10.1002/chem.201404203. [DOI] [PubMed] [Google Scholar]
- 22.Sgambellone MA, David A, Garner RN, Dunbar KR, Turro C. Cellular toxicity induced by the photorelease of a caged bioactive molecule: Design of a Potential Dual-Action Ru(II) Complex. J. Am. Chem. Soc. 2013;135:11274–11282. doi: 10.1021/ja4045604. [DOI] [PubMed] [Google Scholar]
- 23.Jana A, Ikbal M, Singh NDP. Perylen-3-ylmethyl: Fluorescent Photoremovable Protecting Group (FPRPG) for Carboxylic Acids and Alcohols. Tetrahedron. 2012;68:1128–1136. [Google Scholar]
- 24.Lin Q, Bao C, Yang Y, Liang Q, Zhang D, Cheng S, Zhu L. Highly Discriminating Photorelease of Anticancer Drugs Based on Hypoxia Activatable Phototrigger Conjugated Chitosan Nanoparticles. Adv. Mater. 2013;25:1981–1986. doi: 10.1002/adma.201204455. [DOI] [PubMed] [Google Scholar]
- 25.Shanmugam V, Selvakumar S, Yeh C-S. Near-Infrared Light-Responsive Nanomaterials in Cancer Therapeutics. Chem. Soc. Rev. 2014;43:6254–6287. doi: 10.1039/c4cs00011k. [DOI] [PubMed] [Google Scholar]
- 26.Carling C-J, Viger ML, Nguyen Huu VA, Garcia AV, Almutairi A. In Vivo Visible Light-Triggered Drug Release From an Implanted Depot. Chem. Sci. 2015;6:335–341. doi: 10.1039/c4sc02651a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamadar M, Ghosh G, Mahendran A, Minnis M, Kruft BI, Ghogare A, Aebisher D, Greer A. Photosensitizer Drug Delivery via an Optical Fiber. J. Am. Chem. Soc. 2011;133:7882–7891. doi: 10.1021/ja200840p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahendran A, Kopkalli Y, Ghosh G, Ghogare A, Minnis M, Kruft BI, Zamadar M, Aebisher D, Davenport L, Greer A. A Hand-Held Fiber-Optic Implement for the Site-Specific Delivery of Photosensitizer and Singlet Oxygen. Photochem. Photobiol. 2011;87:1330–1337. doi: 10.1111/j.1751-1097.2011.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nkepang G, Bio M, Rajaputra P, Awuah SG, You Y. Folate Receptor-Mediated Enhanced and Specific Delivery of Far-Red Light-Activatable Prodrugs of Combretastatin A-4 to FR-Positive Tumor. Bioconjugate Chem. 2014;25:2175–2188. doi: 10.1021/bc500376j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bio M, Rajaputra P, Nkepang G, Awuah SG, Hossion AMl, You Y. Site-Specific and Far-Red-Light-Activatable Prodrug of Combretastatin A-4 Using Photo-Unclick Chemistry. J. Med. Chem. 2013;56:3936–3942. doi: 10.1021/jm400139w. [DOI] [PubMed] [Google Scholar]
- 31.Bio M, Rajaputra P, Nkepang G, You Y. Far-Red Light Activatable, Multifunctional Prodrug for Fluorescence Optical Imaging and Combinational Treatment. J. Med. Chem. 2014;57:3401–3409. doi: 10.1021/jm5000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nkepang G, You Y. Heteroatom-Substituted Dioxetanes and Their Emerging Biomedical Applications. In: Greer A, Liebman JF, editors. The Chemistry of Peroxides. Vol. 3. Chichester, UK,: J. Wiley & Sons, Ltd; 2014. pp. 683–712. [Google Scholar]
- 33.Flors C, Nonell S. Light and Singlet Oxygen in Plant Defense Against Pathogens: Phototoxic Phenalenone Phytoalexins. Acc. Chem. Res. 2006;39:293–300. doi: 10.1021/ar0402863. [DOI] [PubMed] [Google Scholar]
- 34.Lorente C, Arzoumanian E, Castaño C, Oliveros E, Thomas AH. A Non-Singlet Oxygen Mediated Reaction Photoinduced by Phenalenone, a Universal Reference for Singlet Oxygen Sensitization. RSC Adv. 2014;4:10718–10727. [Google Scholar]
- 35.Hargus JA, Fronczek FR, Vicente MGH, Smith KM. Mono-(L)-aspartylchlorin-e6. Photochem. Photobiol. 2007;83:1006–1015. doi: 10.1111/j.1751-1097.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 36.Jinadasa RGW, Hu X, Vicente MGH, Smith KM. Syntheses and Cellular Investigations of 173-, 152-, and 131-Amino Acid Derivatives of Chlorin e6. J. Med. Chem. 2011;54:7464–7476. doi: 10.1021/jm2005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiMagno SG, Dussault PH, Schultz JA. Fluorous Biphasic Singlet Oxygenation with a Perfluoroalkylated Photosensitizer. J. Am. Chem. Soc. 1996;118:5312–5313. [Google Scholar]
- 38.Allémann E, Brasseur N, Kudrevich SV, La Madeleine C, van Lier JE. Photodynamic Activities and Biodistribution of Fluorinated Zinc Phthalocyanine in the Murine EMT-6 Tumour Model. Int. J. Cancer. 1997;72:289–294. doi: 10.1002/(sici)1097-0215(19970717)72:2<289::aid-ijc15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Yang SI, Seth J, Strachan J-P, Gentemann S, Kim D, Holten D, Lindsey JS, Bocian DF. Ground, Excited State Electronic Properties of Halogenated Tetraarylporphyrins Tuning the Building Blocks for Porphyrin-Based Photonic Devices. J. Porphyrins Phthalocyanines. 1999;3:117–147. [Google Scholar]
- 40.Gryshuk AL, Chen Y, Potter W, Ohulchansky T, Oseroff A, Pandey RK. In Vivo Stability and Photodynamic Efficacy of Fluorinated Bacteriopurpurinimides Derived from Bacteriochlorophyll-a. J. Med. Chem. 2006;49:1874–1881. doi: 10.1021/jm050919z. [DOI] [PubMed] [Google Scholar]
- 41.Ko Y-J, Yun K-J, Kang M-S, Park J, Lee K-T, Park SB, Shin J-H. Synthesis and in vitro Photodynamic Activities of Water-Soluble Fluorinated Tetrapyridylporphyrins as Tumor Photosensitizers. Bioorg. Med. Chem. Lett. 2007;17:2789–2794. doi: 10.1016/j.bmcl.2007.02.083. [DOI] [PubMed] [Google Scholar]
- 42.Patent:Röder B, Gorun SM, Gerdes R, Litwinski C. PCT Int. 2011 Appl; WO 2011045029 A1 20110421. [Google Scholar]
- 43.Wilson SR, Yurchenko ME, Schuster DI, Yurchenko EN, Sokolova O, Braslavsky SE, Klihm G. Preparation and Photophysical Studies of a Fluorous Phase-Soluble Fullerene Derivative. J. Am. Chem. Soc. 2002;124:1977–1981. doi: 10.1021/ja0119200. [DOI] [PubMed] [Google Scholar]
- 44.Lin N-J, Yang H-S, Chang Y, Tung K-L, Chen W-H, Cheng H-W, Hsiano S-W, Aimar P, Yamamoto K, Lai J-Y. Surface Self-Assembled PEGylation of Fluoro-Based PVDF Membranes via Hydrophobic-Driven Copolymer Anchoring for Ultra-Stable Biofouling Resistance. Langmuir. 2013;29:10183–10193. doi: 10.1021/la401336y. [DOI] [PubMed] [Google Scholar]
- 45.Yashiro B, Shoda M, Tomizawa Y, Manaka T, Hagiwara N. Long-Term Results of a Cardiovascular Implantable Electronic Device Wrapped with an Expanded Polytetrafluoroethylene Sheet. J. Artif. Organs. 2012;15:244–249. doi: 10.1007/s10047-012-0634-8. [DOI] [PubMed] [Google Scholar]
- 46.Pandian RP, Meenakshisundaram G, Bratasz A, Eteshola E, Lee SC, Kuppusamy P. An Implantable Teflon Chip Holding Lithium Naphthalocyanine Microcrystals for Secure, Safe, and Repeated Measurements of pO2 in Tissues. Biomed. Microdevices. 2010;12:381–387. doi: 10.1007/s10544-009-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimani S, Ghosh G, Ghogare A, Rudshteyn B, Bartusik D, Hasan T, Greer A. Synthesis and Characterization of Mono-, Di-, and Tri-Poly(ethylene glycol) Chlorin e6 Conjugates for the Photokilling of Human Ovarian Cancer Cells. J. Org. Chem. 2012;77:10638–10647. doi: 10.1021/jo301889s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartusik D, Aebisher D, Ghosh G, Minnis M, Greer A. Fluorine End-Capped Optical Fibers for Photosensitizer Release and Singlet Oxygen Production. J. Org. Chem. 2012;77:4557–4565. doi: 10.1021/jo3006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avella M, Errico ME, Rimedio R. PVA−PTFE Nanocomposites: Thermal, Mechanical, and Barrier Properties. J. Mater. SciLett. 2004;39:6133–6136. [Google Scholar]
- 50.Lawson DD, Moacanin J, Scherer KV, Jr, Terranova TF, Ingham JD. Methods for the Estimation of Vapor Pressures and Oxygen Solubilities of Fluorochemicals for Possible Application in Artificial Blood Formulations. J. Fluorine Chem. 1978;12:221–36. [Google Scholar]
- 51.Pereiro AB, Araujo JMM, Martinho S, Alves F, Nunes S, Matias A, Duarte CMM, Rebelo LPN, Marrucho IM. Fluorinated Ionic Liquids: Properties and Applications. ACS Sustainable Chem. Eng. 2013;1:427–439. [Google Scholar]
- 52.Fraker CA, Mendez AJ, Stabler CL. Complementary Methods for the Determination of Dissolved Oxygen Content in Perfluorocarbon Emulsions and Other Solutions. J. Phys. Chem. B. 2011;115:10547–10552. doi: 10.1021/jp204146n. [DOI] [PubMed] [Google Scholar]
- 53.Dias AMA, Goncalves CMB, Legido JL, Coutinho JAP, Marrucho IM. Solubility of Oxygen in Substituted Perfluorocarbons. Fluid Phase Equilib. 2005;238:7–12. [Google Scholar]
- 54.Wesseler EP, Iltis R, Clark LC., Jr The Solubility of Oxygen in Highly Fluorinated Liquids. J. Fluorine Chem. 1977;9:137–146. [Google Scholar]
- 55.Cosco D, Fattal E, Fresta M, Tsapis N. Perfluorocarbon-Loaded Micro and Nanosystems for Medical Imaging: A State of the Art. J. Fluorine Chem. 2014 in press. [Google Scholar]
- 56.Wang J, Lu F. Oxygen-Rich Oxidase Enzyme Electrodes for Operation in Oxygen-Free Solutions. J. Am. Chem. Soc. 1998;120:1048–1050. [Google Scholar]
- 57.Carter KA, Shao S, Hoopes MI, Luo D, Ahsan B, Grigoryants VM, Song W, Huang H, Zhang G, Pandey RK, et al. Porphyrin-phospholipid Liposomes Permeabilized by Near-infrared Light. Nat. Comm. 2014;5:4546/1–4546/11. doi: 10.1038/ncomms4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tindale JJ, Ragogna PJ. Highly Fluorinated Phosphonium Ionic Liquids: Novel Media for the Generation of Superhydrophobic Coatings. Chem. Commun. 2009:1831–1833. doi: 10.1039/b821174d. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt R, Brauer H-D. Radiationless Deactivation of Singlet Oxygen by Solvent Molecules. J. Am. Chem. Soc. 1987;109:6976–6981. [Google Scholar]
- 60.Ogilby PR, Foote CS. Chemistry of Singlet Oxygen 42 Effect of Solvent, Solvent Isotopic Substitution, and Temperature on the Lifetime of Singlet Molecular Oxygen. J. Am. Chem. Soc. 1983;105:3423–3430. [Google Scholar]
- 61.Hamza MA, Serratrice G, Stebe MJ, Delpuech JJ. Solute-Solvent Interactions in Perfluorocarbon Solutions of Oxygen. An NMR Study. J. Am. Chem. Soc. 1981;103:3733–3738. [Google Scholar]
- 62.Baumstark AL. Thermolysis of Alkyl-1,2-Dioxetanes. In: Baumstark AL, editor. In Advances in Oxygenated Processes. Greenwich, CT: JAI Press; 1988. pp. 31–84. [Google Scholar]
- 63.Adam W, Trofimov AV. Contemporary Trends in Dioxetane Chemistry. In: Rappoport Z, editor. The Chemistry of Peroxides. Vol. 2. New York: Wiley-VCH; 2006. pp. 1171–1209. [Google Scholar]
- 64.Schuster GB, Turro NJ, Steinmetzer HC, Schaap AP, Faler G, Adam W. Adamantylideneadamantane-1,2-Dioxetane Chemiluminescence and Decomposition Kinetics of an Unusually Stable 1,2-Dioxetane. J. Am. Chem. Soc. 1975;97:7110–7118. [Google Scholar]
- 65.Chen K-H, Lii J-H, Walker GA, Xie Y, Schaefer HF, III, Allinger NL. Molecular Mechanics (MM4) Study of Fluorinated Hydrocarbons. J. Phys. Chem. A. 2006;110:7202–7227. doi: 10.1021/jp060430x. [DOI] [PubMed] [Google Scholar]
- 66.Breiby DW, Sølling TI, Bunk O, Nyberg RB, Norrman K, Nielsen MM. Surprises in Friction-Deposited Films of Poly(tetrafluoroethylene) Macromolecules. 2005;38:2383–2390. [Google Scholar]
- 67.Collins CG, Lee JM, Oliver AG, Wiest OG, Smith BD. Internal and External Stereoisomers of Squaraine Rotaxane Endoperoxide: Synthesis, Chemical Differences and Structural Revision. J. Org. Chem. 2014;79:1120–1130. doi: 10.1021/jo402564k. [DOI] [PubMed] [Google Scholar]
- 68.Baader WJ, Stevani CV, Bastos EL. In: Chemiluminescence of Organic Peroxides, in Chemistry of Peroxides. Pt. 2. Rappoport Z, editor. Vol. 2. New York: Wiley-VCH; 2006. pp. 1211–1278. [Google Scholar]
- 69.Tanimura M, Watanabe N, Ijuin HK, Matsumoto M. Base-Induced Chemiluminescent Decomposition of Bicyclic Dioxetanes Bearing a (Benzothiazol-2-yl)-3-hydroxyphenyl Group: A Radiationless Pathway Leading to Marked Decline of Chemiluminescence Efficiency. J. Org. Chem. 2012;77:4725–4731. doi: 10.1021/jo300417e. [DOI] [PubMed] [Google Scholar]
- 70.Roda A, Di Fusco M, Quintavalla A, Guardigli M, Mirasoli M, Lombardo M, Trombini C. Dioxetane-Doped Silica Nanoparticles as Ultrasensitive Reagentless Thermochemiluminescent Labels for Bioanalytics. Anal. Chem. 2012;84:9913–9919. doi: 10.1021/ac302306u. [DOI] [PubMed] [Google Scholar]
- 71.Kirkpatrick I, Worrall DR, Williams SL, Buck CJT, Meseguer RG. Probing the Interplay Between Factors Determining Reaction Rates on Silica Gel Using Termolecular Systems. Photochem. Photobiol. Sci. 2012;11:1585–1591. doi: 10.1039/c2pp25171j. [DOI] [PubMed] [Google Scholar]
- 72.Bartusik D, Minnis M, Ghosh G, Greer A. Autocatalytic-Assisted Photorelease of a Sensitizer Drug Bound to a Silica Support. J. Org. Chem. 2013;78:8537–8544. doi: 10.1021/jo401266r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pace A, Pierro P, Buscemi S, Vivona N, Clennan EL. Photooxidations of Alkenes in Fluorinated Constrained Media: Fluoro-Organically Modified NaY as Improved Reactors for Singlet Oxygen “Ene” Reaction. J. Org. Chem. 2007;72:2644–2646. doi: 10.1021/jo0625047. [DOI] [PubMed] [Google Scholar]
- 74.Hall JFB, Han X, Poliakoff M, Bourne RA, George MW. Maximizing the Efficiency of Continuous Photooxidation with Singlet Oxygen in Supercritical CO2 by Use of Fluorous Biphasic Catalysis. Chem. Commun. 2012;48:3073–3075. doi: 10.1039/c2cc17429d. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt R. Influence of Heavy Atoms on the Deactivation of Singlet Oxygen (1∆g) in Solution. J. Am. Chem. Soc. 1989;111:6983–6987. [Google Scholar]
- 76.Lemp E, Zanocco AL, Lissi EA. Linear Free Energy Relationship Analysis of Solvent Effects on Singlet Oxygen Reactions. Photochem. Photobiol. Sci. 2012;11:1585–1591. [Google Scholar]
- 77.Chin KK, Trevithick-Sutton CC, McCallum J, Jockusch S, Turro NJ, Scaiano JC, Foote CS, Garcia-Garibay MA. Quantitative Determination of Singlet Oxygen Generated by Excited State Aromatic Amino Acids, Proteins, and Immunoglobulins. J. Am. Chem. Soc. 2008;130:6912–6913. doi: 10.1021/ja800926v. [DOI] [PubMed] [Google Scholar]
- 78.Hoflund GB, Everett ML. Chemical Alteration of Poly(tetrafluoroethylene) TFE Teflon Induced by Exposure to Electrons and Inert-Gas Ions. J. Phys. Chem. 2004;108:16676–16683. doi: 10.1021/jp051430k. [DOI] [PubMed] [Google Scholar]
- 79.Bernini R, Cacchi S, Fabrizi G, Forte G, Niembro S, Petrucci F, Pleixats R, Prastaro A, Shafir A, Vallribera A. Perfluoro-Tagged Gold Nanoparticles Immobilized on Fluorous Silica Gel: A Reusable Catalyst for the Benign Oxidation and Oxidative Esterification of Alcohols. ChemSusChem. 2009;2:1036–1040. doi: 10.1002/cssc.200900211. [DOI] [PubMed] [Google Scholar]
- 80.De Vietro N, Annese C, D’Accolti L, Fanelli F, Fusco C, Fracassi F. New Synthetic Approach to Oxidation Organocatalysts Supported on Merrifield Resin Using Plasma-Enhanced Chemical Vapor Deposition. Appl. Catal., A: General. 2014;470:132–139. [Google Scholar]
- 81.Rahnamoun A, van Duin ACT. Reactive Molecular Dynamics Simulation on the Disintegration of Kapton, POSS Polyimide, Amorphous Silica, and Teflon During Atomic Oxygen Impact Using the Reaxff Reactive Force-Field Method. J. Phys. Chem. 2014;118:2780–2787. doi: 10.1021/jp4121029. [DOI] [PubMed] [Google Scholar]
- 82.Hamburger R, Azaz E, Donbrow M. Autoxidation of Polyoxyethylenic Non-Ionic Surfactants and of Polyethylene Glycols. Pharm. Acta Helv. 1975;50:10–17. [PubMed] [Google Scholar]
- 83.Johnson TS, O’Neill AC, Motarjem PM, Amann C, Nguyen T, Randolph MA, Winograd JM, Kochevar IE, Redmond RW. Photochemical Tissue Bonding: A Promising Technique for Peripheral Nerve Repair. J. Surg. Res. 2007;143:224–229. doi: 10.1016/j.jss.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 84.Yao M, Gu C, Doyle FJ, Jr, Zhu H, Redmond RW, Kochevar IE. Why is Rose Bengal More Phototoxic to Fibroblasts In Vitro than In Vivo? Photochem. Photobiol. 2014;90:297–305. doi: 10.1111/php.12215. [DOI] [PubMed] [Google Scholar]
- 85.Ghogare AA, Rizvi I, Hasan T, Greer A. Pointsource Delivery of a Photosensitizer Drug and Singlet Oxygen: Eradication of Glioma Cells in Vitro. Photochem. Photobiol. 2014;90:1119–1125. doi: 10.1111/php.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.