Abstract
Previous studies indicate that physical exercise improves contextual fear memory as evidenced by increased freezing behavior when rats are returned to a training environment that was initially paired with foot shock. However, freezing behavior could also be affected by fatigue, especially since rats were tested shortly after the end of the dark cycle, which is when most wheel running was likely to occur. In addition, exercise has been shown to have anxiolytic effects, further confounding interpretation of the effects of exercise on cognition when using aversive conditioning tasks. These factors were examined in the present study by comparing freezing behavior in exercising and non-exercising rats that were tested at different times in the light cycle. In addition, all rats were tested on an elevated plus maze to assess anxiety-like behavior and in an open field apparatus to measure locomotor activity in order to directly examine interactions between freezing, anxiety-like behavior, and locomotion. Consistent with prior studies, exercising rats exhibited more context freezing than sedentary rats when tested early in the light cycle. However, the opposite pattern of results was obtained when testing occurred late in the light cycle, an effect driven by a difference in the amount of freezing exhibited by the sedentary control groups. Indeed, the levels of context freezing exhibited by exercising rats were comparable regardless of when the rats were tested during the light cycle. These data have implications for interpreting the effects of exercise on aversive conditioning.
Keywords: context, rat, hippocampus, running
Introduction
Physical exercise has been shown to improve learning and memory in humans and rodents in a variety of behavioral paradigms. One commonly used task for investigating the mechanisms underlying the benefits of exercise on cognition is hippocampal-dependent contextual fear conditioning. Several studies have reported that voluntary physical exercise increases contextual learning and memory as evidenced by enhanced levels of freezing behavior when rats are returned to the training chamber for a context test session (Baruch, Swain, & Helmstetter, 2004; Burghardt, Pasumarthi, Wilson, & Fadel, 2006; Greenwood, Strong, Foley, & Felshner, 2009; VanHoomissen, Holmes, & Zelner, 2004). In contrast, these same studies report no effect of exercise on fear conditioning to a discrete cue such as a tone (but see Falls, Fox & MacAulay, 2010), which is more reliant on the amygdala (Phillips & LeDoux, 1992).
In addition to its effects on learning and memory, exercise is also known to be anxiolytic (Binder, Droste, Ohl, & Reul, 2004; Fulk, Stock, Lynn, Marshall et al., 2004; Salam, Fox, Detroy, Guignon et al., 2009). Interactions between exercise-induced changes in anxiety and cognitive function are particularly relevant since the procedures used to assess the effects of exercise on memory have typically involved potent stressors (footshock in the fear conditioning paradigm, swim stress in the water maze; Kant, Mougey, Pennington, & Meyerhoff, 1983; Sandi, Loscertales, & Guaza, 1997). Because of these interactions, the underlying mechanisms mediating exercise-induced cognitive improvement are not always clear. Indeed, it has been suggested that the effects of exercise on cognitive function may be mediated in part through its anxiolytic effects (Collins, Hill, Chandramohan, Whitcomb et al., 2009). To investigate this, we examined the effects of exercise on fear conditioning and anxiety-related behavior in the same set of rats and tested for relationships between these two dependent variables.
In addition, prior studies that examined the effects of exercise on contextual fear conditioning have tested rats shortly after the dark cycle ended. Since rats are nocturnal and do almost all of their running during the dark (active) cycle, this approach raises the possibility that the increased freezing observed in these studies could be related to fatigue. A second goal of this study was to determine whether the time of day during which rats are trained and tested influences the effects of exercise on fear conditioning. One set of exercising and non-exercising rats underwent fear conditioning early in the light cycle to replicate the finding that exercise enhances freezing to the context. A second set of rats were trained and tested at the end of the light cycle. All rats were also tested on an elevated plus maze and open field apparatus, allowing for a direct assessment of relationships between the effects of exercise on memory, anxiety, and locomotor activity.
Materials and Methods
Subjects
Sixty-four male Long Evans rats were obtained at 8 weeks of age from Harlan Laboratories (Indianapolis, IN). Rats were housed in groups of 4 and allowed 7 days to acclimate to the vivarium with food available ad libitum. Throughout the study, rats were maintained on a 14:10 light-dark cycle (lights on at 7am, off at 9pm) and monitored and cared for in compliance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Apparatus
Rats in the exercise groups had access to an exercise wheel (Med Associates, St. Albans, VT) that was attached to the side of the home cage and accessible through an opening in the wall of the cage. The wheels were 35.6cm in diameter and consisted of stainless steel rods (4.8 mm in length) spaced 1.6cm apart. Every 1/4 revolution of the wheel was recorded by an automatic counter mounted on the side of the apparatus.
Elevated plus maze testing was conducted in a small dimly-lit room using a black plexiglas platform with two open and two closed arms extending out from the center (10cm × 75cm). The maze arms were 40cm above the floor, and the walls of the closed arms were 35cm tall. A video camera was mounted above the maze to record behavior.
The fear conditioning apparatus (Med Associates Inc., St. Albans, VT) consisted of standard conditioning chambers (24cm × 30.5cm × 29cm) connected to a computer and enclosed in sound-attenuating chambers (62cm × 56cm × 56cm) outfitted with an exhaust fan to provide airflow and background noise (~68dB). The chambers consisted of aluminum front and back walls, clear acrylic sides and top, and grid floors. A house-light providing background illumination and a speaker was used to present the auditory stimulus (1500Hz, 78dB). In addition, a 1sec delivery of a 0.75-mA constant current shock through the grid floor of the operant chamber served as the unconditioned stimulus. Surveillance cameras located inside the surrounding shell were used to videotape the rats’ behavior.
The open field chamber used to assess locomotor activity was 43.2cm × 43.2cm and composed of plexiglass walls. The chamber was equipped with 16 photobeams mounted on the sides to monitor locomotor activity and was connected to a computer running Open Field Activity Software (Med Associates).
Behavioral Procedures
Thirty-two rats were provided with 24-hr access to running wheels for 2 weeks prior to the start of any behavioral training and throughout the study. The counter that recorded wheel rotations was monitored daily and reset at the same time each afternoon. Another set of 32 rats (sedentary controls) were housed in identical cages but did not have access to a wheel. After 2 weeks of access to the wheels, rats were randomly split into two groups of 16 (“EX” groups). Likewise, the sedentary rats were randomly divided into 2 groups of 16 (“NX groups”). One set of exercising rats and one set of control rats underwent the following behavioral training between 10am-12pm (“AM” groups) while the other two EX and NX groups underwent the same behavioral testing between 7pm-9pm (“PM” groups).
Timeline of Exercise and Behavioral Testing
Elevated plus maze testing took place on day 1 of behavioral testing. On day 2, subjects were exposed to fear conditioning training, followed by context testing and tone testing on days 3 and 4, respectively. Open field testing took place on day 5. Subjects in the exercise condition had access to running wheels over the five days of behavioral testing, however the wheels were locked 2hr prior to testing to rule out any potential acute effects of exercise.
Elevated plus maze
Rats were individually placed in the center of the maze and allowed to explore freely for 5 min. The amount of time spent in the open arms was recorded as a measure anxiety-like behavior, whereby increased open arm exploration is thought to reflect lower levels of anxiety-like behavior (Carobrez & Bertoglio, 2005).
Fear conditioning
Rats were trained in a standard fear conditioning task described previously (Arenos, Musty, & Bucci, 2006; Bucci, Phillips, & Burwell, 2000; Maren, Aharonov, & Fanselow, 1997). Briefly, the training session consisted of two 10-sec presentations of the tone followed immediately by a 1-sec, 0.75mA foot shock (intertrial interval of 64sec). The first trial began three minutes after the rat was placed in the chamber. Twenty-four hours after the initial training session, rats were re-exposed to the original training chamber for a 10 min-40sec context test session during which no tones or shocks were presented. Twenty-four hours later, the tone test session was carried out by placing the rats in a novel context and presenting the tone 20 times (10sec each, 30-sec intertrial interval; no shock).
Freezing served as the index of conditioned fear and was operationally defined as motor immobility except for breathing (Blanchard & Blanchard, 1969; Fanselow, 1980). On the training day, the incidence of freezing behavior was recorded during the 64-sec period prior to the first trial (baseline freezing) and during the 64-sec period following each trial (post-shock freezing). During each of the 64-sec periods, the rat’s behavior was observed and recorded every eight seconds, resulting in 8 observations per period. The frequency of freezing behavior was calculated by dividing the number of instances of freezing by 8 and multiplying by 100 to convert the data into a percentage of total observations. During the context test session, the 12min-48sec session was divided into 64-s bins and freezing was observed every 8 sec as described above. For the tone test session, freezing was recorded every two seconds during each 10-sec presentation of the tone.
All rats received the context test session first, followed by the cue test session since this has previously been shown to be the optimal method for obtaining the most independent assessment of both auditory and contextual fear conditioning in the same rats (Maren et al., 1997). We have previously examined whether the order of testing influences levels of freezing to the context and tone during the test sessions and we have found identical results when the cue test session was conducted prior to the context test session (Arenos et al., 2006; Chess, Landers, & Bucci, 2009).
Open field exploration
Locomotor activity was assessed by allowing each rat to freely explore an open field for 15min, during which time the total distance traveled was monitored by the computer. The chamber was cleaned with Quatricide between testing each animal.
Data Analysis
A single primary observer scored all of the behavioral data, while a second observer scored a subset of the data to assess objectivity. Both observers were blind to treatment condition and their observations were highly correlated (r = 0.9; p < 0.0001).
Analyses of freezing behavior during training and the tone test session were conducted using repeated measures analysis of variance (ANOVA) with Group (EX, NX) and Time of Day (AM, PM) as the between-subjects variable and Trial as the within-subjects variable. For the context test session, a repeated measures ANOVA was conducted using Group and Time of Day as the between-subjects variable and Epoch (64-sec period) as the within-subject variable. Significant interactions were followed up using pair-wise t-tests.
Group differences in the total distance traveled in the open field or time spent in open arms of the plus maze were analyzed using a two-way ANOVA. In both cases, Group and Time of Day were used as the between subjects variables. In addition, relationships between the behavioral measures were assessed using Pearson correlation coefficients. Correlations were calculated for each of the 4 treatment groups independently and also by combining groups across time of day or across exercise condition. An α of 0.05 was used for all analyses.
Results
Wheel running
The average number of daily wheel rotations was 6.8 ± 0.5km per cage of rats. Since 4 rats shared each wheel, it was not possible to know the exact distance each rat ran, precluding statistical analysis. However, other studies have revealed that group-housed rodents spend approximately equal amounts of time on the wheel when it is freely accessible as in the present study (Fox, Hammack, & Falls, 2008).
Fear conditioning
Four rats in the AM/EX group and one rat in the PM/NX group exhibited abnormally low levels of freezing during training (>2 standard deviations from the mean) and were excluded from all analyses. For the remaining rats, the amount of post-shock freezing increased across training trials and was comparable between exercising rats and the sedentary rats in both the AM and PM groups. This was confirmed by a repeated measures ANOVA that revealed a significant main effect of Trial [F(3,165)=305.6, p<0.0001], but no significant main effect of Group (EX, NX; p>0.2) and no significant interactions between Group and any other variable (Ps>0.4). However, there was a significant main effect of Time of Day (AM, PM) indicating that in general, rats trained in the PM exhibited more post-shock freezing that rats trained in the AM [F(1,55)=5.7, p<0.02]. The mean amount of freezing behavior exhibited by rats trained in the AM was 69.5 ± 3.6%; for rats trained in the PM, the mean was 77.6 ± 2.2%.
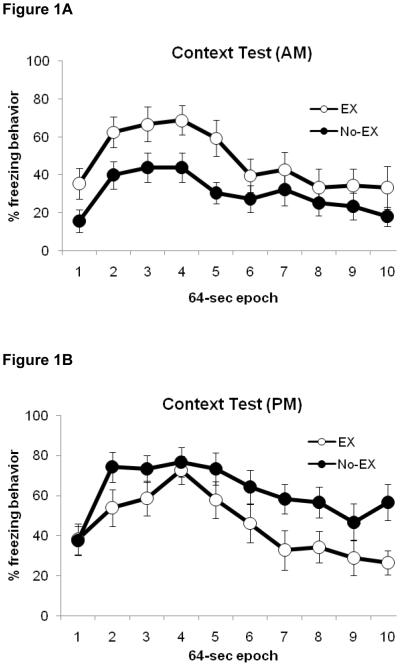
Freezing observed during the context test session is illustrated in Figure 1. There was a significant main effect of Epoch [F(9,495)=15.7, p<0.0001] indicating that the frequency of freezing behavior decreased over the context test session, as expected since the context was no longer paired with shock (i.e., extinction). As was observed during training, there was a significant main effect of Time of Day in that rats tested in the context in the AM froze less than rats tested in the PM [F(1,55)=7.2, p<001]. Importantly, there was also a significant Group × Time-of-Day interaction [F(1,55)=10, p<0.003]. Post-hoc analyses revealed that rats in the AM/EX group froze more than rats in the AM/NX group (46.4 ± 5.2% vs 29.3 ± 5.2%, respectively; t(26)=2.3, p<0.03). Conversely, rats in the PM/EX group froze less than rats in the PM/NX group (43.5 ± 5.8% vs 60.4 ± 5.2%, respectively; t(26)= −2.2, p<0.04). Further comparisons revealed that the two NX groups significantly differed from each other [t(29)= −4.2, p<0.01] but that the two EX groups exhibited comparable amounts of freezing (p>0.7).
Figure 1.
Freezing behavior during the context test session in rats tested in the A) morning and B) evening. Data are mean ± S.E.M. Abbreviations: EX, rats with access to the running wheel; no-EX, sedentary control rats; AM, rats tested in morning; PM, rats tested at evening. Rats in the AM/EX group froze more than rats in the AM/NX group (p<0.03). Conversely, rats in the PM/EX group froze less than rats in the PM/NX group (p<0.04). The two NX groups also significantly differed from each other (p<0.01)
During the tone test session, rats in all groups exhibited initially high levels of freezing to the tone that gradually diminished over the course of the extinction session [F(19,1026)=14.5, p<0.0001]. There were no main effects of Group (p>0.3) or Time of Day (p>0.5) and no significant interactions between any variables (ps>5), indicating that freezing to the tone was comparable across all treatment groups. The mean amount of freezing behavior exhibited by each group during the tone test session was: AM/NX, 33.3 ± 4.1; AM/EX, 34.2 ± 4; PM/NX, 37.7 ± 4; PM/X 35.2 ± 3.8.
Elevated plus maze
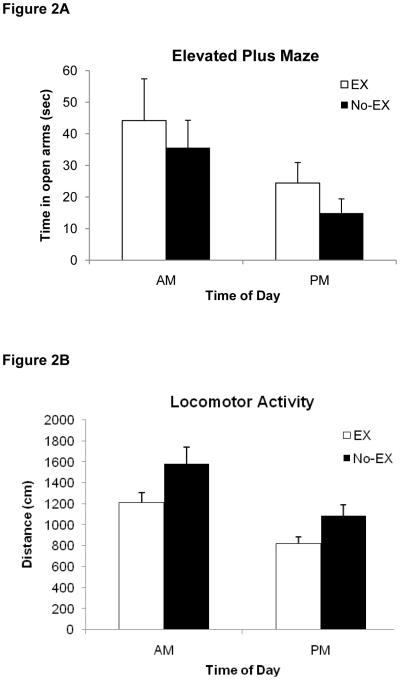
The average amount of time rats in each group spent in the open arms of the plus maze is illustrated in Figure 2A. There was a significant main effect of Time of Day indicating that rats tested in the plus maze in the AM spent more time in the open arms than rats tested in the PM [F(1,55)=5.8, p<0.02]. There were no significant differences between exercising and non-exercising rats (p>0.3) and no Group × Time of Day interaction (p>0.9). Furthermore, there were no significant correlations between time spent in the open arms and freezing during the context test session for any of the treatment conditions (ps>0.3).
Figure 2.
A) Time spent in the open arms of the elevated plus maze and B) Distance traveled in the open field. Data are mean ± S.E.M; abbreviations as in Figure 1. There was a significant main effect of Time of Day for open arm time (p<0.02) and distance travelled in the open field (p<0.001). In addition, there was a main effect of Group for distance travelled (p<0.01).
Open field locomotor activity
The distance traveled during the open field exploration period is depicted in Figure 2B. Rats tested in the open field in the AM exhibited more activity than rats tested in the PM [main effect of Time of Day, F(1,55)=14.7, P<0.001]. There was also a main effect of Group in that exercising rats traveled a shorter total distance than non-exercising rats [F(1,55)=7.7, P>0.01]. The interaction between Group and Time of Day was not statistically significant (P>0.6). In addition, there were no significant correlations between locomotor activity and freezing during the context test session for any of the treatment conditions (Ps>0.5).
Discussion
A goal of the present study was to determine whether the effect of exercise on contextual fear conditioning depends on the time of day during which rats are trained and tested. Indeed, prior reports of exercise-induced increases in the frequency of freezing behavior could have been confounded by fatigue since rats were always tested shortly after the end of the dark cycle (when rats in the exercise group were most likely to be wheel running; Armstrong, 1980). Consistent with prior studies (Baruch et al., 2004; Burghardt et al., 2006; Greenwood et al., 2009; VanHoomissen et al., 2004), we found that when the behavioral procedures were carried out in the early part of the light cycle, rats in the exercise group exhibited more freezing during the context test session that non-exercising rats. However, we observed the opposite effect when training and testing occurred at the end of the light cycle in that exercising rats froze significantly less than non-exercising rats in the training context. Of particular importance is that this time-of-day effect was not due to a difference in freezing between the two exercise groups, which exhibited very similar levels of freezing behavior during the context test session. The effect was driven instead by a significant difference between the two non-exercising groups in that sedentary rats trained and tested later in the light cycle exhibited more freezing behavior that those trained and tested early in the light cycle.
These findings indicate that the amount of contextual freezing exhibited by exercising rats does not depend on when rats are tested during the light cycle and argues against the possibility that prior reports of higher freezing levels in exercising rats compared to sedentary rats were simply due to fatigue. Indeed, freezing levels were comparable in the two exercising groups despite the fact that exercising rats exhibited less locomotor activity in the open field overall compared to sedentary rats. There was also no correlation between freezing levels and locomotor activity in exercising rats. Moreover, the lack of group differences in freezing to the tone provides further evidence that the exercise effects are not due to fatigue, and suggests that the interaction between exercise and time of day effects converge on context-specific brain regions, such as the hippocampus, rather than regions associated with cue-specific freezing, such as the amygdala (Phillips & LeDoux, 1992). Of particular note is the substantial literature indicating that exercise induces neurogenesis and other plastic changes in the hippocampus (Olson, Eadie, Ernst, & Christie, 2006).
Nonetheless, the interpretation of the effects of exercise on context fear conditioning must consider that sedentary rats exhibit different levels of freezing during the light cycle. Interestingly, regardless of exercise condition, rats exhibited more locomotor behavior in the open field apparatus when they were tested early in the light cycle. Similarly, rats tested early in the light cycle spent more time in the open arms of the elevated plus maze (indicative of less anxiety-like behavior) compared to rats tested later in the light cycle. These time of day dependent changes in anxiety-like behavior and context freezing may be related to circadian fluctuations of circulating stress hormones. Corticosterone levels are lowest early in the light cycle and peak just before the start of the dark cycle (Szafarczyk, Alonso, Ixart, Malaval, et al., 1980), and it has been shown that elevated corticosterone can increase anxiety-like behavior (Calvo, Martijena, Molina, & Volosin, 1998; Calvo & Volosin, 2001). Moreover, exercise can induce changes in circulating glucocorticoid levels and HPA stress-reactivity (Campbell, Rakhshani, Fediuc, Bruni, et al., 2008; Droste, Chandramohan, Hill, Linthorst, et al., 2007). In addition, naturally cycling (circadian) variations in corticosterone levels can alter cognitive performance and there is evidence that hippocampal-dependent memory may be particularly sensitive to these circadian effects (Winocour & Hasher, 2004). Thus, one interpretation of the present findings is that exercising rats are less prone to circadian variations in anxiety-like behavior and cognitive function, which may be reflected by the consistent level of freezing behavior observed early and late in the light cycle. Future studies are needed to investigate this further and to more completely understand how exercise affects conditioned fear.
Acknowledgements
Research supported by a Rockefeller Center Grant and NIH Grant MH082893. The authors thank Farshad Chowdhury for assistance in condcuting preliminary experiments leading up to this study.
References
- Arenos JD, Musty RE, Bucci DJ. Blockade of cannabinoid CB1 receptors alters contextuallearning and memory. European Journal of Pharmacology. 2006;539:177–183. doi: 10.1016/j.ejphar.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Armstrong S. A chronometric approach to the study of feeding behavior. Neuroscience & Biobehavioral Reviews. 1980;4(1):27–53. doi: 10.1016/0149-7634(80)90024-x. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behavioral Neuroscience. 2004;118(5):1123–7. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. Journal of Comparative Physiology and Psychology. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Binder E, Droste S, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behavior and impulsiveness in mice. Behavioral Brain Research. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behavioral Neuroscience. 2000;114:882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacology Biochemistry and Behavior. 2006;84:306–312. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Calvo N, Martijena ID, Molina VA, Volosin M. Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Research. 1998;800(2):227–35. doi: 10.1016/s0006-8993(98)00515-0. [DOI] [PubMed] [Google Scholar]
- Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology. 2001;73(4):261–271. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Rakhshani N, Fediuc S, Bruni S, Riddell MC. Voluntary wheel running initially increases adrenal sensitivity to adrenocorticotrophic hormone, which is attenuated with long-term training. Journal of Applied Physiology. 2008;106(1):66–72. doi: 10.1152/japplphysiol.91128.2008. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neuroscience and Biobehavioral Reviews. 2005;29(8):1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behavioural Brain Research. 2009;201:325–331. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Collins A, Hill LE, Chandramohan Y, Whitcomb D, Droste SK, Reul JM. xercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009;4(1):e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86(1):26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behavioral Brain Research. 2010;207(2):321–31. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlovian Journal of Biological Science. 1980;15(4):177–82. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behavioral Neuroscience. 2008;122(4):943–8. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. International Journal of Sports Medicine. 2004;25:78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A Behavioral Analysis of the Impact of Voluntary Physical Activity on Hippocampus-Dependent Contextual Conditioning Hippocampus. Hippocampus. 2009;19(10):988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant GJ, Mougey EH, Pennington LL, Meyerhoff JL. Graded footshock stress elevates pituitary cyclic AMP and plasma beta-endorphin, beta-LPH corticosterone and prolactin. Life Sciences. 1983;33(26):2657–2663. doi: 10.1016/0024-3205(83)90350-8. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioral Brain Research. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. oluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behavioral Brain Research. 2009;197(1):31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. European Journal of Neuroscience. 1997;9(4):637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, Alonso G, Ixart G, Malaval F, Nouguier-Soule J, Assenmacher I. Serotoninergic system and circadian rhythms of ACTH and corticosterone in rats. American Journal of Physiology. 1980;239(6):E482–489. doi: 10.1152/ajpendo.1980.239.6.E482. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Holmes PV, Zellner AS. Effects of B-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophoc factor. Behavioral Neuroscience. 2004;118(6):1378–1390. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- Winocur G, Hasher L. Age and time-of-day effects on learning and memory in a non-matching-to-sample test. Neurobiology of Aging. 2004;25(8):1107–1115. doi: 10.1016/j.neurobiolaging.2003.10.005. [DOI] [PubMed] [Google Scholar]