Abstract
Although there is a sizable amount of research focusing on adult neural progenitor cells (NPCs) as a therapeutic approach for many neurodegenerative diseases, including multiple sclerosis, little is known about the pathways that govern NPC survival and apoptosis. Fas, a member of the death receptor superfamily, plays a well-characterized role in the immune system, but its function in neural stem cells remains uncertain. Our study focuses on the effects of Fas on NPC survival in vitro. Activation of Fas by recombinant Fas ligand (FasL) did not induce apoptosis in murine NPCs in culture. In fact, both an increase in the amount of viable cells and a decrease in apoptotic and dying cells were observed with FasL treatment. Our data indicate that FasL-mediated adult NPC neuroprotection is characterized by a reduction in apoptosis, but not increased proliferation. Further investigation of this effect revealed that the antiapoptotic effects of FasL are mediated by the up-regulation of Birc3, an inhibitor of apoptosis protein (IAP). Conversely, the observed effect is not the result of altered caspase activation or FLIP (Fas-associated death domain-like interleukin-1beta-converting enzyme inhibitory protein) up-regulation, which is known to inhibit caspase-8-mediated cell death in T cells. Our data indicate that murine adult NPCs are resistant to FasL-induced cell death. Activation of Fas increased cell survival by decreasing apoptosis through Birc3 up-regulation. These results describe a novel pathway involved in NPC survival.
Keywords: adult progenitor cells, neurodegenerative disease, neural stem cell therapy
Neural progenitor cell (NPC) apoptosis is significant in a variety of central nervous system (CNS) diseases ranging from Alzheimer’s disease to multiple sclerosis (MS; Watanabe et al., 1995; Haughey et al., 2002; Sohur et al., 2006; Camins et al., 2008). Fas [a member of the tumor nectrosis factor (TNF) death receptor superfamily] is known to contribute to the maintenance of lymphocyte homeostasis and the deletion of autoreactive T cells (Pluchino et al., 2005). The clinical effects of stem cell therapy in experimental autoimmune encephalomyelitis (EAE; the animal model of MS) are mediated by the induction of proinflammatory Th1 apoptosis via the Fas pathway by NPCs (Pluchino et al., 2005). However, the role of Fas/FasL in adult NPC physiology remains unknown.
Fas is believed to play a critical role in regulating programmed cell death during embryonic development of the nervous system because of its expression during critical periods of neuronal apoptosis and differentiation (Park et al., 1998; Cheema et al., 1999; Raoul et al.,1999). Fas is involved in developmental neuronal death in both motor neurons (Nat et al., 2001) and cortical neurons (van Landeghem et al., 2002). Recent studies have also implicated Fas involvement in cell processes other than cell death. A study investigating apoptosis in adult neural stem cells and a neural stem cell line (C17.2) found that the mitochondrial pathway was active, but Fas-dependent cell death was not operational (Ceccatelli et al., 2004). Desbarats and colleagues (2003) have demonstrated that Fas can mediate proliferation and regeneration via the ERK pathway in neurons. Further characterizing the role of this system in neuronal development, Fas appears to regulate neuronal branching in hippocampal neurons (Zuliani et al., 2006). Additionally, human brain endothelial cells are resistant to FasL-mediated death, and triggering Fas induces release of matrix metalloproteinase 9 (Wosik et al., 2007). Thus, it appears that the consequence of Fas activation is varied and cell-type specific. Although Fas is a ubiquitous receptor found on cells throughout the body, its specific role in various cell types is not known.
We investigated the effects on murine NPC survival, proliferation, and differentiation in vitro during Fas activation by its cognant ligand (FasL). Our results indicate that cells grown in minimal medium cell culture (deprived of growth factors) have an increased cell survival following Fas activation. This apparent neuroprotection arises from decreased apoptosis as opposed to augmented cellular proliferation. Furthermore, we found that this is not a result of altered FLIP expression or alterations of the caspase pathway. Rather, our data show the up-regulation of mRNA levels and protein expression of Birc3, a largely undescribed member of the inhibitor of apoptosis protein (IAP) family of proteins, thus providing a molecular mechanism for the protective effect of FasL
MATERIALS AND METHODS
Isolation of Murine NPCs
Mouse NPCs were obtained from Dr. Jeffrey Spees, University of Vermont Stem Cell Core. NPCs were isolated from whole brains of individual 4-day postnatal C57/BL6 mice using NeuroCult Enzymatic Dissociation Kit (Stem Cell Technologies). NPCs were identified based on neurosphere formation, then enzymatically dissociated before culturing in a single-cell monolayer.
In the same manner, NPCs were isolated from lpr (Fas- deficient) mouse pups for specificity experiments. lpr Mice have a retroviral insertion of poly-(A) adenylation signal repeats in the gene for Fas (Nagata and Golstein, 1995). This large insertion results in improper splicing and truncation of Fas mRNA so that the cells are devoid of protein expression.
Cell Culture
The undifferentiated murine neural progenitor cells and neurospheres were cultured in Nunclon delta-coated 75-cm2 filter flasks (Nunc) in mouse NPC complete medium [1 × Neurobasal-A (Invitrogen, Carlsbad, CA), 1 × B27 supplement (Invitrogen), 20 ng/ml EGF (BD Biosciences, San Jose, CA), 10 ng/ml basic FGF (Peprotech), 2 mM L-glutamine (Mediatech Inc.), 100 units/ml penicillin, and 100 µg/ml streptomycin (Mediatech Inc.)].
Statistical Analysis
One-way ANOVA was performed to determine statistical significance of the data, followed by Tukey’s test to compare individual samples. Analyses were run in Graph Pad Prism 5 software.
FasL Treatment
To assess the effect of FasL on NPCs, the cells within four or five passages were cultured in poly-D-lysine- and laminin-coated plates; upon reaching 80–90% confluence (approximately 3 days), epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) were withdrawn, cells were rinsed, and cells were treated with recombinant FasL (Alexis Biochemicals, Columbus, OH) along with an enhancer (anti-flag M2 monoclonal antibody; Sigma, St. Louis, MO) in minimal media [1 × Neurobasal-A (Invitrogen), 1 × B27 supplement (Invitrogen), 2 mM L-glutamine (Mediatech Inc.),100 U/ml penicillin, and 100 µg/ml streptomycin (Mediatech Inc.)]. Concentration of FasL was 200 ng/ml for all experiments, except for the 700 ng/ml calcein AM experiments. Similarly, an enhancer (anti-Flag; Invitrogen) was used at the concentration of 14 µg/ml except for the higher concentration calcien AM experiment, in which 14 µg/ml was used. Some treatments were done in complete medium (as described in Results).
Flow Cytometry
NPCs were left in a suspension of complete medium in a 5-ml Falcon tube (12 × 75 mm) for 2–3 hrs before pelleting, rinsing, and FasL addition. FasL treatment was performed in the presence or absence of growth factors (i.e., in complete media or minimal media, respectively). Flow cytometric evaluation of apoptotic and dead cells was performed with annexin V Alexa fluor 647 conjugate assay and Live/ Dead blue-fluorescent (UV) reactive dye kit, respectively (both Invitrogen), according to the manufacturer’s instructions. UV Live/Dead dye permeates into and reacts throughout the volume of cells that have lost their membrane integrity, thus labeling necrotic cells. Annexin labels phospholipid phosphatidylserine (Xu et al., 1997) residues on the exterior surface of apoptotic cells (translocation of PS is one of the earliest indicators of apoptosis). Flow cytometry analysis was performed with a BD LSRII flow cytometer equipped with three lasers (488, helium neon, and UV), and data were analyzed with Flow Jo software.
Cell Viability Assay
Calcein AM (acetomethoxy derivate of calcein) assay is a commonly used method to measure cell viability. Calcein AM enters living cells and is hydrolyzed by intracellular esterases to produce calcein, a strongly fluorescent compound that remains in the cytoplasm. Calcein AM was added directly to cells 24 and 48 hr posttreatment. Murine NPCs were cultured in 48-well plates, and FasL treatments performed in minimal media were compared with untreated, minimal media controls. Both FasL and minimal media control NPCs were treated with 2 µg/ml calcein AM (1 mg/ml stock diluted 1:500) and incubated at 37°C, 5% CO2, for approximately 30 min. Viability measurements were completed in Fluostar Galaxy spectrophotometry software. The data are described as relative absorbance (n = 8 per condition).
Mouse NPC BrdU Proliferation Assay
Cell proliferation in the presence of FasL was characterized by bromodeoxyuridine assay. Upon reaching approximately 80% confluence, mNPCs were pulsed for 90 min with 100 µM bromodeoxyuridine (a thymidine analog used during DNA synthesis) and fixed with 4% paraformaldehyde and picric acid (Zamboni’s). DNA was denatured by using 2 M HCl and blocked (10% horse serum, 0.2% azide). The primary antibody mouse anti-BrdU IgG (Roche) was applied (1:100) and allowed to react overnight. A Cy3 anti-mouse secondary antibody (1:500) was applied and reacted for 3 hr. Cell counts were conducted by selecting random fields at a power of 20 × and counted by hand. Total cell count was compared against BrdU-positive cell nuclei to characterize mNPC proliferation. Percentage of positive cells was extrapolated in Neurolucida Stereoinvestigator Software (n = 6 per condition).
TUNEL Assay
Terminal transferase dUTP nick-end labeling (TUNEL) assay was performed to assess cell death from apoptotic signal cascades. Biotinylated dUTP (Roche) was incorporated into late-stage apoptotic DNA using terminal deoxynucleotidyl transferase (Promega, Madison, WI). Cells were stained with streptavidin fluorescein isothiocyanate (1:1,000; Jackson Immunoresearch, Wesr Grove, PA), a biotin ligand. Cell nuclei were labeled with Hoechst dye (1:2,000) and visualized with UV microscopy. Cell counts were conducted by selecting random fields at a magnification of X20 and counted by hand, comparing total Hoechst signal (491 nm) vs. apoptotic nuclei positive for strep-FITC (515–565 nm). The percentage of positive cells was extrapolated in Neurolucida Stereoinvestigator (n = 4 per condition).
RT-qPCR
Total RNA was isolated from both triplicate FasL- treated and minimal media controls using RNeasy Mini Kit (Qiagen, Valencia, CA). NPC RNA was quantified by Nanodrop, and synthesis of cDNA was completed with Superscript III First-Strand Synthesis Supermix (Invitrogen). mNPC cell fate and FasL expression analysis was completed using Applied Biosystems 7500 Fast Software real-time quantitative polymerase chain reaction (RT-qPCR). Amplification consisted of 50 cycles (95°C for 15 sec and 60°C for 1 min) with approximately 15 ng/µl cDNA; specific primer pairs for β-tubulin III, PDGFR-α, GFAP, and GLAST; and TaqMan Master Mix (all Applied Biosystems, Foster City, CA). Neuron (β-tubulin III), astrocyte (GFAP and GLAST), and oligodendrocyte (PDGFR α) mRNA transcript expressions were analyzed. Cycle threshold amplifications for mNPC cDNA were normalized against endogenous control marker β-actin and subsequently expressed as -fold differences relative to lineage marker and treatment condition. The 2−ΔΔCt method was used to calculate the relative expression of genes. The same methods were used with Birc3/c-IAP2 primer (also from Applied Biosystems; Assay on Demand). For each primer, the experiment was replicated three times (n = 3). RT-qPCR experiments were replicated six times (n = 6)
Immunocytochemistry
Cells were plated on coated (poly-D-lysine and laminin) coverslips in 12-well plates. After the treatment period, cells/ coverlips were fixed in Zamboni’s fixative (4% paraformaldehyde; 15% picric acid). Cells were incubated overnight at 4°C in the following anti-mouse antibodies: nestin (Novus Biologicals; 1:250), β-tubulin III (Abcam, Cambridge, MA; 1:100) GFAP (Eucor Biotech; 1:500), and NG2 (R&D Systems, Minneapolis, MN; 1:500). Nestin was made in chicken; all the rest were made in rabbit. After rinsing twice in phosphate-buffered saline, cells were incubated for 2 hr at room temperature in both anti-chicken Cy2 (1:250) and anti-rabbit Cy3 (1:500) secondary antibodies (both Jackson Immunore- search).
Western Blot Analyses
For Western blot analyses, the mNPC cultures were lysed in 2× lysis buffer with 0.5% NP-40 [20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM sodium orthovanadate 10% glycerol, and1 tablet/25 ml complete protease inhibitor (Roche Diagnostics), SDS]. The proteins (32 µg) were fractionated on 4–12%SDS-PAGE gels and transferred onto Immobilon-P PVDF membranes (Millipore) for Western analyses using primary anti- bodies to FLIP (ProSci Incorporated), caspase-8 (Apotech), caspase-3 (not commercially available; a gift from Dr. Ralph Budd), β-tubulin III (Sigma), GFAP (Applied Biological Material), myelin oligodendrocyte glycoprotein (MOG; R&D Systems), and actin (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were stripped in 62.5 mM Tris-HCl, pH 6.7, containing 2% SDS and 100 mM β-mercaptoethanol at 50°C for 90 min before each reprobing procedure. Horseradish peroxidase-conjugated secondary antibodies were used. The blots were processed for enhanced electrochemiluminescence detection (GE Healthcare); all blots were exposed to autoradiographic film for signal detection. Two separate Western blot experiments were performed for the FLIP/caspase-8/caspase-3 probing. Blots using c-IAP2/Birc3 (Santa Cruz Biotechnology) as a primary antibody were processed similarly, except secondary antibody (goat anti-rabbit Alexa fluor 680-conjugated; Molecular Probes, Eugene, OR) was incubated in Odyssey buffer, and signal detection was performed with an Odyssey 2.0 infrared imaging system. The Birc3 Western blot experiment was replicated three times (n = 3).
RESULTS
Murine NPCs Express Fas and FasL
To confirm the presence of Fas on murine NPCs, both surface receptor and mRNA expression were investigated. Surface expression was confirmed by using flow cytometry. Mouse CD4+ cells were used as a positive control, because T cells are known to be high expressors of Fas. mNPCs and T cells were stained with a Fas-specific antibody conjugated to PE. The intensity of fluorescence revealed that mNPCs are positive for Fas, and levels are comparable to those seen in T cells (see Fig. 1).
Fig. 1.
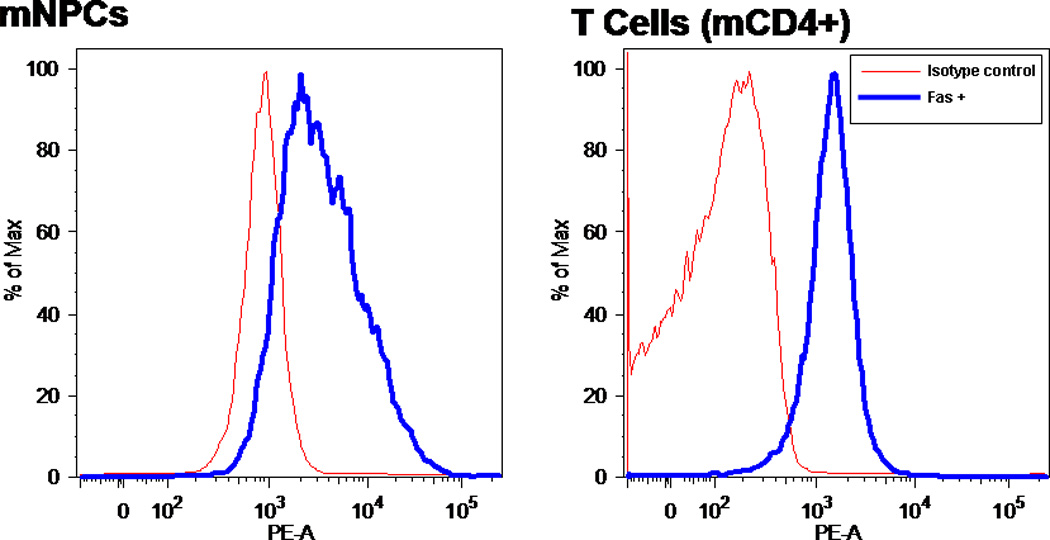
NPCs express significant amounts of FasR. PE-conjugated monoclonal antibody to FasR was detected by flow cytometry. Fluorescence represents percentage of maximum intensity; some cells have higher density of the receptor than others. Cells were stained with PE-conjugated FasR Ab for 30 min after blocking with isotype control antibody. PE fluorescence (thick line) is overlayed with baseline fluorescence (thin line) obtained from cells that were stained only with isotype control antibody
Expression of FasL in mNPC minimal and complete media cultures and unstimulated mouse CD4+ T cells was determined by using RT-qPCR. Ct values (from both mouse apoptosis Superarray plate analysis and RT-qPCR) indicated low levels of FasL mRNA expression in NPCs grown in minimal and complete media (average Ct values of 38.2 and 36.8, respectively). These expression levels are very low compared with average T-cell expression levels (average Ct value = 27.7). These data indicate that adult NPCs express high levels of the Fas receptor but minimal amounts of FasL in primary culture.
FasL Induces Cell Death in T-Cells but Not NPCs
To elucidate the physiological role of Fas/FasL in adult NPCs, we tested the ability of recombinant FasL to induce cell death. Induction of apoptosis and quantity of dead cells were assessed at two time points (3 and 15 hr) using UV Live/Dead Dye and annexin staining. UV Live/Dead dye permeates and reacts throughout the volume of cells that have lost their membrane integrity, thus labeling necrotic cells. Annexin labels phospholipid phosphatidylserine (PS) residues on the exterior surface of apoptotic cells (translocation of PS is one of the earliest indicators of apoptosis). Mouse T cells (isolated from lymph nodes of wild-type C57/BL6 mice) were used as a positive control because they are known to be sensitive to FasL-induced cell death. At both 3-hr and 15-hr time points, a substantial amount of FasL-induced cell death was observed in the T cells compared with untreated controls. There was a fourfold increase in the percentage of dead and dying T cells at 3 hr with FasL treatment, whereas FasL treatment increased the percentage of dead and dying T cells from 34.2% to 85.8% at the 15-hr time point (Fig. 2a, top row). Similar results were obtained when using mouse human Jurkat T cells (data not shown).
Fig. 2.
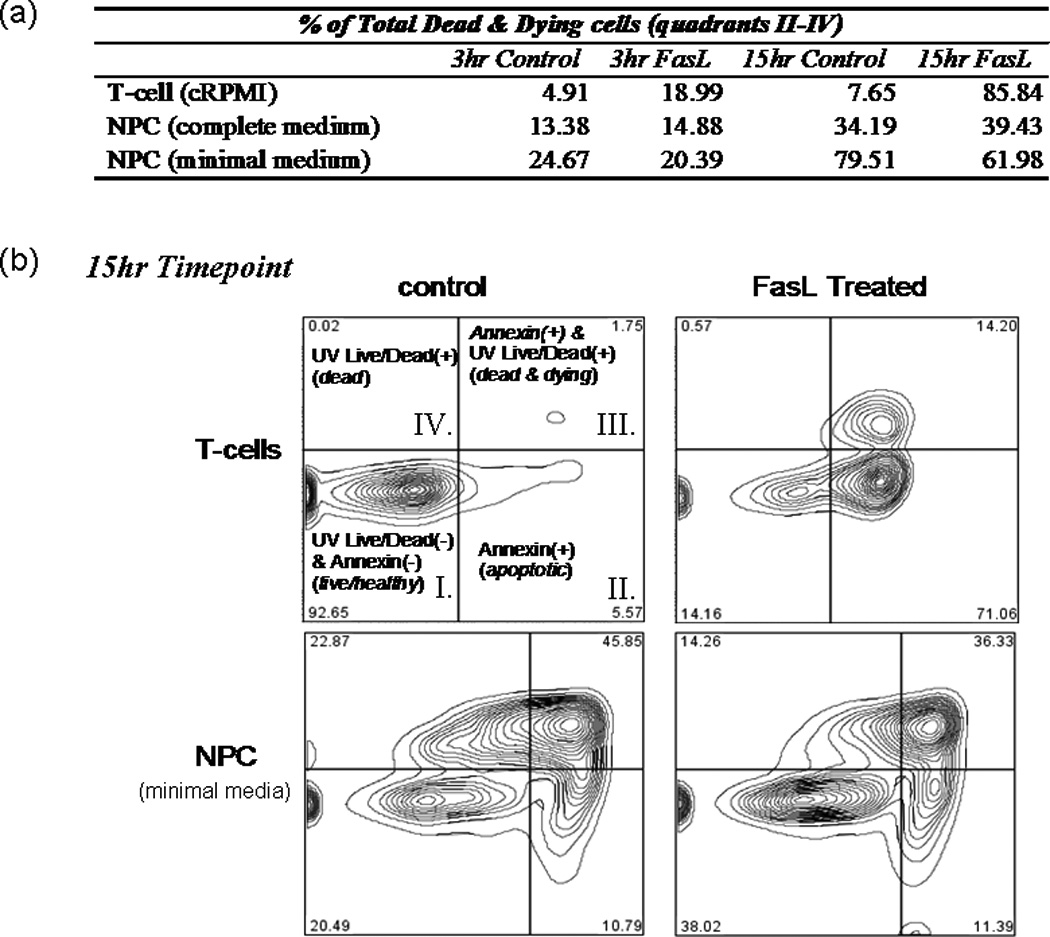
FasL does not induce cell death in NPCs. T cells and NPCs were treated with FasL (200 ng/ml) plus an enhancer (4 g/ml) for 3 and 15 hr at 37°C before UV blue Live/Dead dye and annexin Alexafluor 647 staining. Cells were subsequently analyzed by flow cytometry. a: Summary of results, where T cells or NPCs were placed in either plain media or media plus FasL for 3 or 15 hr. b: Readout from the 15-hr treatment as gated using Flow Jo software. As illustrated in b, quadrant I represents cells living healthy cells, which stain negative for either dye; quadrant II represents early apoptotic cells, which are positive for annexin; quadrant III represents dead and dying cells, which stain positively for both annexin and UV Live/Dead dye; and quadrant IV includes only necrotic cells (i.e., positive for only UV Live/Dead dye). A dramatic increase in the amount of apoptotic and/or necrotic cells (i.e., UV and/or annexin+ cells) was observed in T cells treated with FasL for 3 or 15 hr (top row of a and top plates in b). Compared with controls, no effect on NPCs was observed after 3 or 15 hr of FasL treatment in complete neurobasal medium supplemented with EGF and FGF (middle row of a). No induction of death was observed in NPCs treated for 3 hr with FasL in minimal medium compared with control cells (last row of a). A slight decrease (approximately 18%) in the total dead and dying cells was observed in NPCs treated with FasL for 15 hr compared with controls (bottom plates in b).
We did not observe any FasL-induced cell death in the NPCs at the early or late time point in either complete or minimal media (middle and last rows in Fig. 2a). Interestingly, for cells incubated in minimal media, we observed a decreased percentage of dead and dying cells in the FasL-treated NPC at 15 hr (Fig. 2b, bottom panels). Eighty percent of control NPCs stained positively for UV Live/Dead and/or annexin dyes, whereas only 62% of FasL-treated cells were positive. Therefore, it appeared FasL may exhibit a protective effect against apoptosis
FasL Increases NPC Survival Upon Growth Factor Withdrawal
When NPCs are deprived of EGF and FGF (i.e., when cells are placed in minimal medium), large numbers of cells die (Kuhn et al., 1997; Slinskey et al., 1999). To determine whether FasL exposure increased cell survival under growth-factor-deprivation conditions, we assessed cell viability via calcein AM assay. FasL- treated NPCs were compared with control NPCs at 24 and 48 hr. No change was observed in treated (at either 200 ng/ml or 700 ng/ml concentrations) vs. control at 24 hr (Fig. 3a). However, at 48 hr (see Fig. 3b) there was a dose-dependent response, with FasL-treated cells having increased survival. These results are consistent with the flow cytometry data discussed above. Therefore, two different methods of assessing cell viability demonstrate that more NPCs survive under conditions of growth factor starvation when Fas is activated.
Fig. 3.
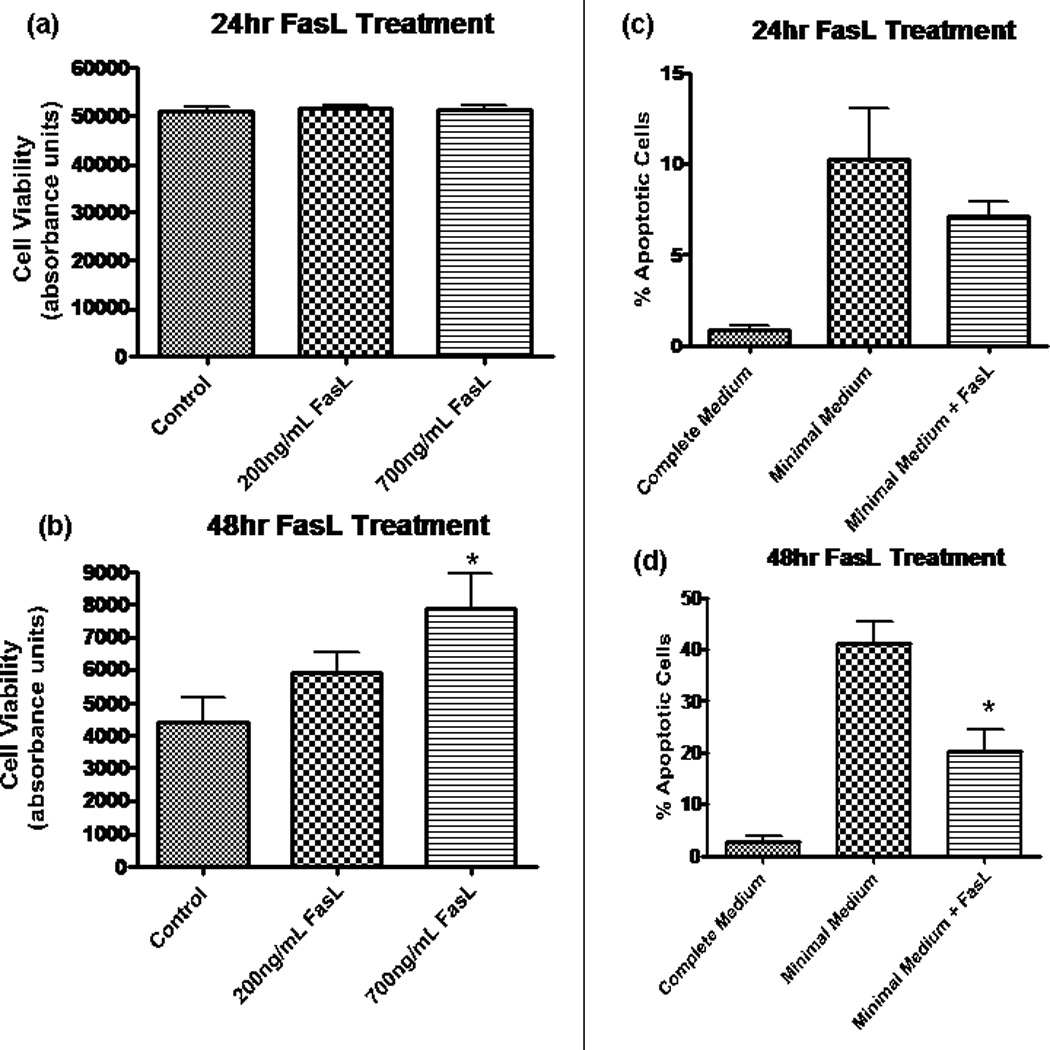
Fas activation promotes NPC survival by decreasing apoptosis. In a and b, fluorescence was measured by using Fluostar Galaxy spectrophotometry software, and the y-axis (absorbance) is in arbitrary units and is equivalent to cell viability. a: Calcien AM assay revealed no change in cell viability after 24 hr in minimal media at two different concentrations of FasL. b: At 48 hr, there was a dose-dependent increase in cell viability for treated NPCs. The difference was statistically significant at 700 ng/ml (*P < 0.05, n = 8) by ANOVA. y-Axis (absorbance) is equivalent to cell viability. c, d: Results from TUNEL assay experiments, which allow for quantification of apoptotic cells by labeling broken strands of DNA. Cells treated with FasL (200 ng/ml) in minimal medium are compared with control cells grown in minimal medium. Healthy NPCs grown in complete medium (includes EGF and FGF) provide a negative control (leftmost column). c: At 24 hr following growth factor removal, there is a trend toward a decreased percentage of apoptosis in NPCs treated with FasL. d: A significantly decreased amount of apoptosis (P < 0.01, n = 4) was observed in NPCs treated with FasL treatment for 48 hr in the absence of growth factor.
Increased Murine NPC Survival With FasL Treatment
The results from annexin/UV Live/Dead dye and calcein AM assays prompted us to investigate whether the protective effects of FasL in NPCs are due to a decrease in apoptosis, an increase in proliferation, or both. Results from the TUNEL assay showed a trend in the direction of decreased apoptosis for FasL-treated cells at 24 hr (see Fig. 3c), although this did not differ significantly from untreated controls. However, FasL-treated cells had a significantly lower percentage of apoptotic cells (see Fig. 3d) after 48 hr lacking growth factors.
FasL Does Not Influence NPC Proliferation or Differentiation
To assess whether FasL possesses proliferative properties in NPC, a series of BrdU-labeling studies was performed. In EGF- and FGF-supplemented medium, more than 75% of NPCs were labeled with BrdU to demonstrate the high proliferative state of the cells under optimal culture conditions. Growth factor withdrawal diminished NPC proliferation precipitously to less than 7% of the population. FasL-treated cells showed a similar percentage of BrdU+ cells (Fig. 4a). Therefore, these data show that the increase in viability of NPCs following Fas activation is the result of decreased apoptosis.
Fig. 4.
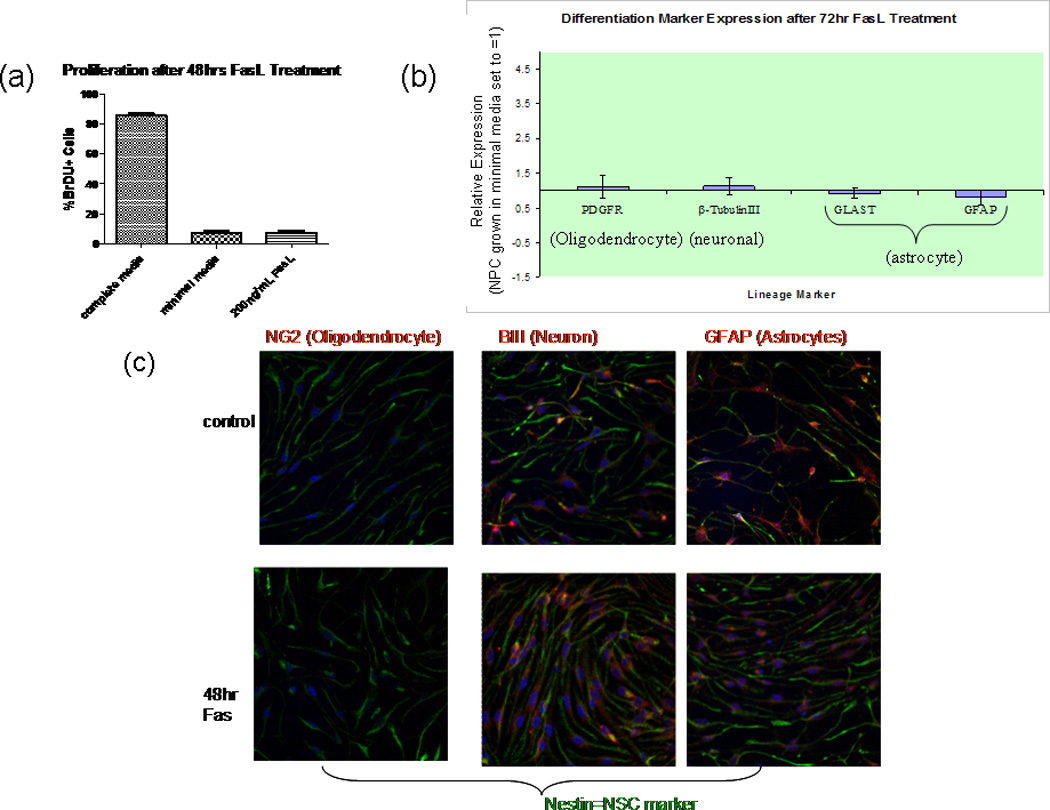
Fas activation does not influence NPC differentiation or proliferation. a: Cells treated with 200 ng/ml FasL were fixed, and proliferation was subsequently assesed using BrDU assay. No significant difference was detected between FasL-treated cells and controls (minimal media). Results include proliferation of cells in complete media (with growth factors) for comparison. Percentage of BrDU-positive cells was determined by comparing with total number of cells (stained with Hoescht nuclear dye). Random fields were counted at 20× in Neurolucida Steroinvestigator software; n = 4 (No. of slides per condition). b: RNA extracted from NPC treated for 72 hr with FasL in minimal media was subjected to cDNA reverse transcription, and the resulting cDNA was analyzed by using RT-qPCR. NPC cDNA were normalized against βactin (endogenous control marker) and subsequently expressed as -fold differences relative to NPC grown in minimal media. Specific primer pairs for β-tubulin III, platelet-derived growth factor receptor (PDGFR)-α, glial fibrillary acidic protein GFAP), and astrocyte-specific glutamate transporter (GLAST) were utilized to determine mRNA expression. The 2−ΔΔCt method was used to calculate the relative expression of genes. FasL treatment had no effect on oligodendrocyte (PDGFR), neuronal (β- tubulin III), or astrocyte GLAST and GFAP) lineage differentiation. Data represent the mean from five different cultures ±SEM (n = 4, P > 0.05). c: Cells were grown to confluence on poly-D-lysine- and laminin-coated coverslips. Spontaneous differentiation, upon withdrawal of growth factors for 48 hr (top), was compared with 48 hr FasL-treated cells (bottom). Cells were fixed with Zamboni’s fixative and then costained with anti-mouse nestin (chicken) and NG2, β - tubulin III, or GFAP (all rabbit anti-mouse) primary antibodies. All coverslips were then stained with both anti-chicken Cy2 and anti- rabbit Cy3 secondary antibodies. Mounted slides were visualized with a Nikon Eclipse E800 fluorescent microscope, and photos were taken with Photometrics CoolSNAP cf software. No significant difference in any lineage marker was observed upon FasL treatment (n = 3).
In addition to attenuating apoptosis in NPCs, Fas could play a role in differentiation. Effects of FasL exposure on mNPC differentiation were assessed by using both RT-qPCR and immunocytochemistry. RT-qPCR revealed that the mRNA expression levels of GFAP and GLAST (astrocyte markers), β-tubulin III (neuron marker), and PDGFR-α (oligodendrocyte marker) were not significantly different when comparing FasL-treated NPCs with control NPCs in minimal media (Fig. 4b). Control cells were used as the calibrator sample and normalized to β-actin. Thus, Fas activation does not facilitate differentiation into astrocytes, neurons, or oligodendrocytes. The RT-qPCR data are consistent with immunocytochemistry results (Fig. 4c).
FasL Treatment Does Not Affect the Levels of FLIP, Caspase-8, or Caspase-3 in NPCs
In light of our data supporting decreased apoptosis after Fas activation, we sought to investigate the intracellular pathways that may be responsible for this effect. Fas mediates cell death by way of the extrinsic pathway, wherein activation of the receptor causes receptors to oligomerize, subsequently inducing the formation of death-inducing signaling complex (DISC), which includes Fas-associated death domain (FADD) and caspase-8 (Wajant, 2002). Proteolytic activity of the DISC complex results in the activation of caspase-8, which consequently activates (either directly or indirectly, depending on the type of cell) caspase-3, the effector caspase (Wajant, 2002). FLIP, an inhibitor of Fas-mediated cell death, acts by inhibiting the activation of caspase-8 (Nagata and Golstein, 1995). It is known that high levels of FLIP expression correlates with resistance to Fas-mediated cell death, whereas decreased expression of FLIP renders cells sensitive to apoptosis (Tschopp et al., 1998). Thus, we investigated whether altered FLIP expression could account for the antiapoptotic effects of Fas activation that we observed. Western blot revealed an increase in the p43 DISC-associated cleavage length form in T-cell controls treated with FasL (Fig. 5b). This pattern was not seen in treated NPCs, however, and there was no change in the FLIP expression pattern upon Fas activation. Thus, FLIP up-regulation observed with Fas activation in NPC.
Fig. 5.
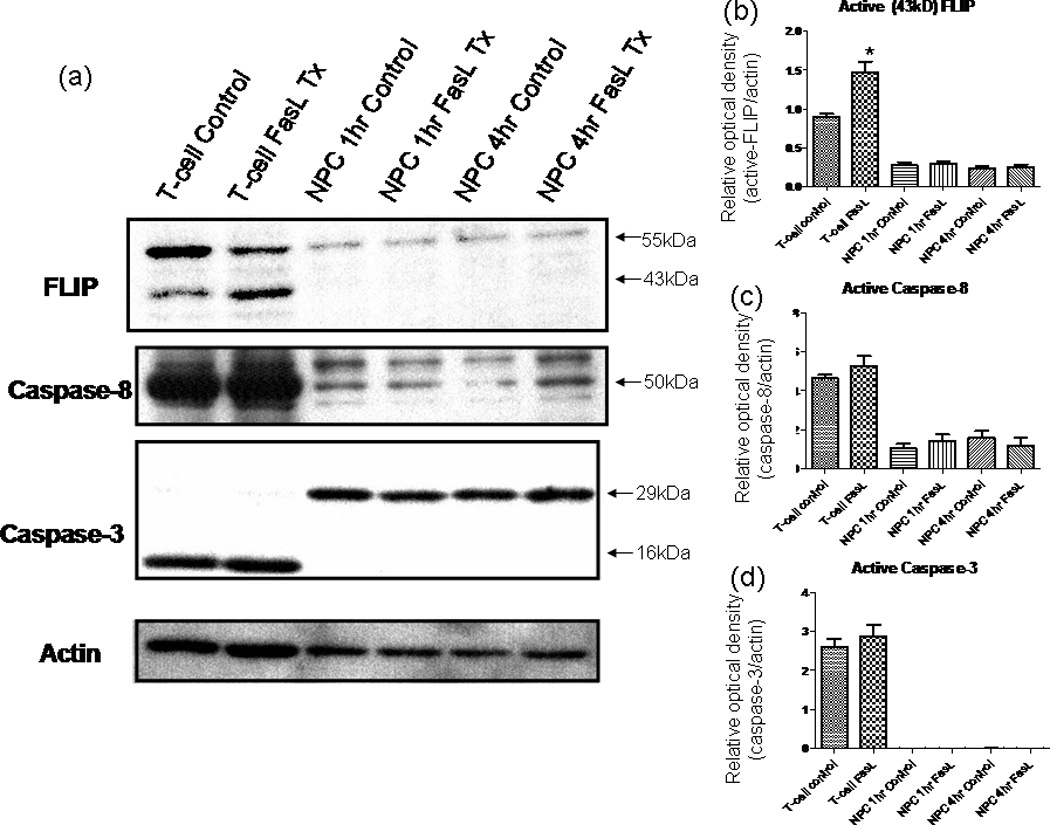
a–d: FasL does not influence FLIP or caspase levels in NPCs. Western blot results for probing the signaling cascades involved in Fas activation. Cell lysates obtained using NP-40 after various treatment periods of 200 ng/ml FasL + 4 µg/ml ą-flag (enhancer). The blot was first probed using an anti-FLIP antibody; then, the blot was consecutively stripped and reprobed for caspase-8, caspase-3, and finally β-actin. Secondary antibodies were HRP conjugated and visualized using ECL development reagents. FLIP levels remain unchanged in NPCs after FasL treatment; NPCs express low levels of caspase-8, and expression is not changed with FasL treatment at various time points; the levels of procaspase-3 (~29 kDa) remain unchanged. In contrast to the casde for T cells, the activated (cleaved) form of caspase-3 (~16 kDa) is not produced in NPCs after FasL treatment at various time points. *P < 0.05.
Furthermore, we were interested in caspase-8 and caspase-3 protein expression, which are indicators of up and downstream stages of caspase-mediated apoptosis respectively. Levels of both caspase-3 and caspase-8 remain steady in T cells (Fig. 5c,d). This likely is due to active FLIP inhibiting the activation of caspase-8 (Tschopp et al., 1998). There was no difference in the expression of caspase-8 in FasL-treated NPCs vs. controls (Fig. 5c). Furthermore, we found no change in procaspase-3 levels and no detectable induction of cleavage into the active form (see Fig. 5a). Consequently, our findings show that the protective effects against apoptosis resulting from Fas activation are not the result of FLIP up-regulation or of the inhibition of caspase-8 or caspase-3 activity in the extrinsic cell death pathway. Additionally, our Western results on the levels of these caspases are consistent with our initial experiments that show no induction of cell death
The Antiapoptotic Gene Birc3 Is Responsible for FasL Antiapoptotic Effects
The novel finding of increased cell survival, without involvement of FLIP, led us to search for possible genes that could be involved in this process. We used a mouse apoptosis PCR microarray (SuperArray) to screen for genes involved in the FasL antiapoptotic effects on NPC. We found that the IAP Birc3 (also known as cIAP-2) was up-regulated by a factor of 7.19 (P <0.000001) in FasL-treated cells compared with controls (averaged from three samples each of FasL-treated and nontreated NPCs in minimal media). Birc3 mRNAup-regulation appeared to be specific, insofar as other antiapoptotic genes were not affected by FasL treatment (Table I). This finding was confirmed by using the Applied Biosystems Assay on Demand primer for Birc3/c-IAP2 (Ref. Seq.: NM_007464.3; Assay ID:Mm00431800_m1), results showed an increased Birc3mRNA expression of approximately 7.63-fold (SD =1.53, n = 4) in FasL-treated cells compared with controls (Fig. 6a). Western blot analysis was consistent with the mRNA data, showing a statistically significant increase in Birc3 protein expression in the FasL-treated sample (Fig. 6b). Birc3 is one of several genes in the IAP family that were originally identified in baculoviruses, where they inhibited infected cells from death (Schimmer, 2004)
TABLE I.
FasL Effects on Anti-apoptotic Gene ExpressionϮ
| Anti-apoptosis gene | Fold up- or down-regulation (FasL NPC/control NPC) |
|---|---|
| Akt1 | 1.07 |
| Api5 | 1.05 |
| Ataf | −1.19 |
| Bag1 | −1.15 |
| Bag3 | −1 |
| Bcl2 | 1.27 |
| Bcl2l1 | −1.15 |
| Bcl2l2 | −1.08 |
| Birc1a | 1.15 |
| Birc1b | 1.07 |
| Birc2 | 1.07 |
| Birc3 | 7.19** |
| Birc4 | 1.14 |
| Birc5 | 1.03 |
| Bnip3 | 1.05 |
| Casp2 | −1.25 |
| Cflar | 1.49 |
| Dad1 | 2.06* |
| Tsc22d3 | 1.01 |
| Hells | 1.52 |
| Il01 | 1.69 |
| Lhx4 | −1.29 |
| Mcl1 | −1.02 |
| Nfkb1 | 1.02 |
| Nme5 | 1.09 |
| Pax7 | 1.21 |
| Pim2 | 1.19 |
| Polb | 1.02 |
| Prdx2 | −1.15 |
| Rnf7 | −1.02 |
| Sphk2 | 1.01 |
| Tnf | −1.03 |
| Cd40lg | 1.08 |
| Zc3hc | 1.12 |
Results from mouse apoptosis gene array analysis. RNA was extracted from NPC control and FasL (200 ng/ml)-treated cells grown in minimal medium for 48 hr. Among all the antiapoptotic genes tested, Birc3 was the only one that showed a significant change in mRNA expression with FasL treatment. -Fold differences and P values obtained from averaging three separate arrays. Positive numbers indicate up-regulation; negative numbers indicate down-regulation. -Fold difference obtained with the equation (2−ΔCt FasL)/(2−ΔCt control)
P < 0.05
P < 0.00001
Fig. 6.
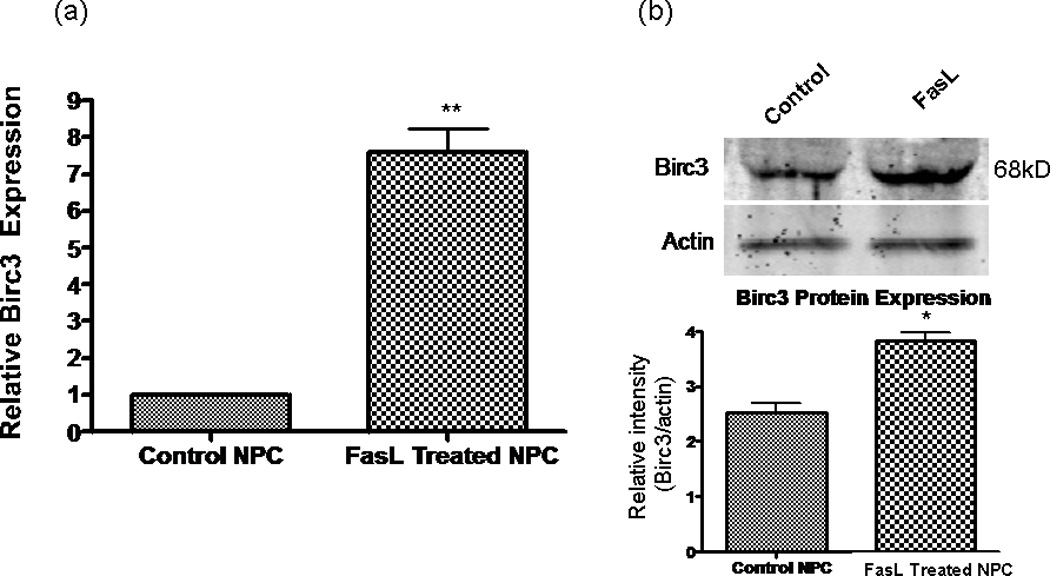
Birc3 up-regulation correlates with Fas activation. a: RT-qPCR results showing that mRNA expression of Birc3 is up-regu- lated in FasL-treated cells compared with controls after growing for 48 hr in minimal medium. Control cells were used as a calibrator sample and results normalized to β-actin mRNA expression. **P < 0.01 (n = 4). b: Birc3 protein expression is significantly up-regulated in NPCs treated with FasL. Cell lysates collected from control and FasL cells grown in minimal medium for 48 hr. Top: Western blot probed with rabbit α-Birc3/cIAP-2 antibody and then incubated with an α-rabbit secondary antibody conjugated to Alexa fluor 680. Positive band for Birc3 (~68 kDa) was visualized with an Odyssey infrared imaging system. Bottom: Semiquantitation of bands by densitometric analysis (n = 3, P < 0.05 using Students t-test); protein levels were normalized to β-actin
FasL Effects on NPCs Are Specific
It is possible that the observed effects on NPC could work through some Fas-independent/nonspecific mechanism. To explore this possibility, we compared the effects of FasL on wild-type and Fas-deficient (lpr) NPCs. Both lpr and wild-type NPCs were treated with FasL or control (minimal media) for 48 hr, as in previous experiments. Afterward, RNA was extracted, and the expression level of Birc3 mRNA was analyzed by using RT-qPCR. In contrast to wild-type NPCs, there was no difference in Birc3 levels for FasL and control lpr samples (Fig. 7). This is indicative of a specific interaction between FasL and its cognate receptor that leads to a decrease in apoptosis.
Fig. 7.
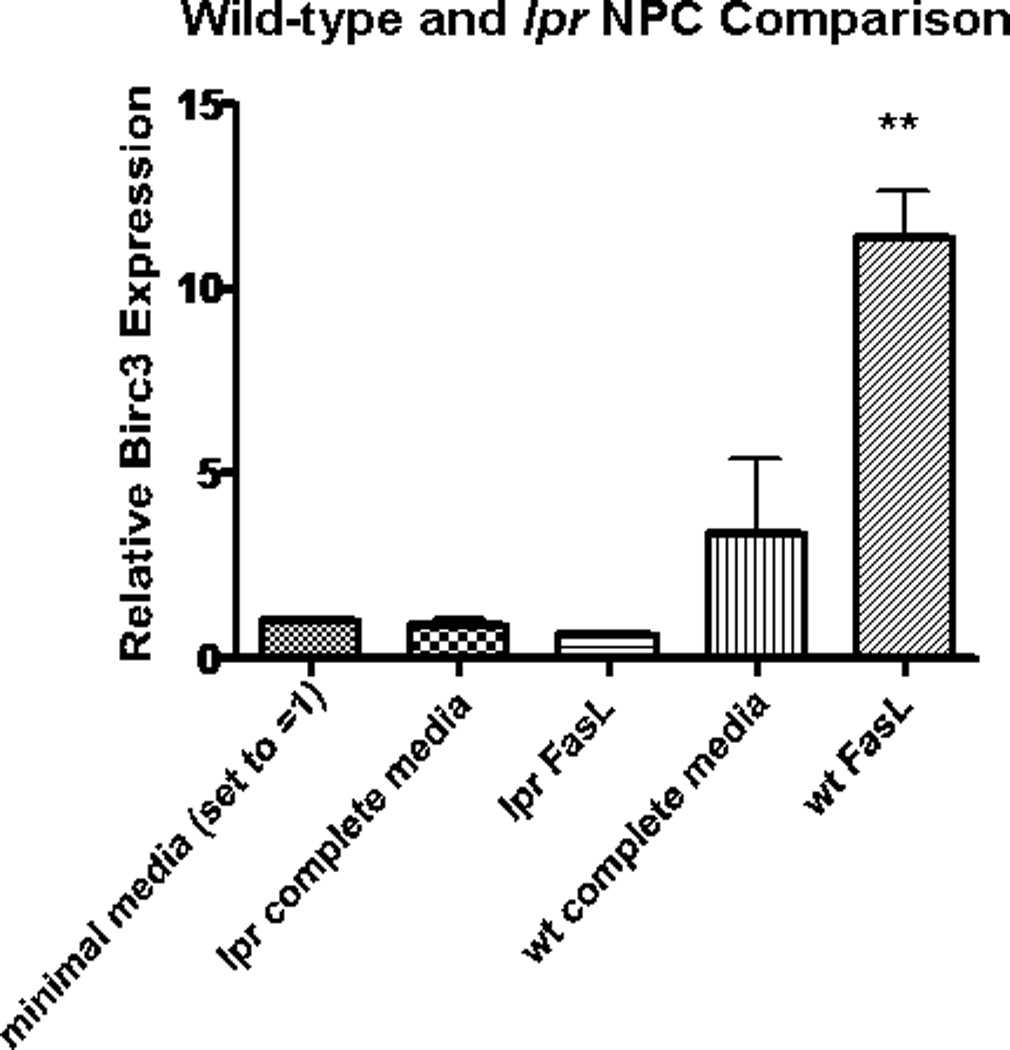
Induction of Birc3 expression is dependent on Fas expression. RT-qPCR results comparing Birc3 mRNA expression in FasL- treated wild-type (wt) vs. lpr (Fas-deficient) NPCs showed statistically significant Birc3 up-regulation in wt (**P < 0.01, n = 3) but not lpr NPCs. Both wt and lpr NPCs were isolated from 4-day postnatal BL6/C57 mouse pups according to the manufacturer’s instructions using NeuroCult Kit for CNS tissues. Cells were grown into free- floating neurospheres in complete medium before consequently disso- ciating into single cells and plating in a monolayer. These monolayer cells were grown to 80% confluence and treated with FasL for 48 hr before RNA extraction
DISCUSSION
We investigated the effects of FasL/Fas on NPC survival, proliferation, and differentiation in vitro. NPCs express high levels of FasR and relatively low levels of FasL. Our data show that, under optimal conditions (complete media with growth factors), Fas activation via FasL in NPCs does not induce apoptosis. This is in contrast to T-cell controls (known to be highly sensitive to Fas-induced cell death), which demonstrated a significant amount of cell death following Fas activation. In the absence of growth factors (under minimal medium conditions), FasL promoted NPC survival and abrogated the apoptosis response. Our findings are in line with results of other studies with adult NPCs (Ceccatelli et al., 2004), neuronally differentiated precursor cells (Brunlid et al., 2007), and a neural stem cell line (Tamm et al., 2004), all of which also showed no induction of apoptosis after Fas activation using various methodologies. Although insensitivity to Fas-induced cell death in NPCs has been previously reported (Ceccatelli et al.,2004; Tamm et al., 2004; Brunlid et al., 2007), ours is the first and only study that suggests a protective effect of Fas activation against apoptosis.
To our knowledge, this is the first study that begins to provide a detailed characterization of the intra-cellular pathways involved in Fas activation. Our results show that FLIP, an inhibitor of caspase-8 mediated cell death, is not involved in the inhibition of apoptosis. In addition, we found no activation of caspase-8 or caspase-3, further supporting findings of the lack of apoptosis induction with Fas activation in NPCs. It is possible that caspase-8 levels in NPC are not sufficient to initiate the apoptosis cascade (Ricci-Vitiani et al., 2004). Interested in finding what other genes could be involved in this process, we discovered that Birc3, an IAP, is up-regulated following Fas activation, providing a probable mechanism for the antiapoptotic effects of FasL on NPCs. Furthermore, presence of Fas is required for the observed antiapototic effects to occur. Therefore, FasL does not work through a nonspecific mechanism or alternate pathway.
The identification of factors capable of maintaining CNS NPC populations is important in a variety of contexts. Developmentally, the rates of NPC apoptosis and differentiation are determinants that define neuronal populations and cytoarchitecture. In adults, continuous NPC renewal has been implicated in learning and memory, maintaining olfactory function, mediating therapeutics for behavioral or psychiatric disorders, and providing an endogenous means of neuronal repair after injury (Gould et al., 1999; Sohur et al., 2006; Okano et al., 2007; Sahay and Hen, 2007). If accumulating cellular insults or changes in environmental niches result in decreased PC regenerative capacity to repair CNS damage from degenerative diseases or injury, then diminished NPC apoptosis may be an important contributor to the constellation of neurological defects associated with CNS repair. Several growth factors and cytokines, including EGF, bFGF, vascular endothelial growth factor (VEGF), brain-derived nerve growth factor (BDNF), prolactin, leukemia inhibitory factor (LIF), and ciliary neurotrophic factor (CNTF) have been shown to be capable of promoting stem cell renewal; the addition of FasL to the repertoire presents not only alternatives but also options for coordinated treatments to amplify and refine NPC survival. Although the role of Fas for immunoregulatory cells is fairly well characterized, the role of Fas in neural stem cell physiology is not clear.
Our results suggest a novel role for a member of the IAP family, Birc3, in promoting neural stem cell survival following activation of the Fas/FasL pathway. IAPs are a family of antiapoptotic proteins that specifically inhibit caspases-3, -8, and -9 (Schimmer, 2004). In total eight proteins have been identified in humans thus far, based on both presence of BIR (baculovirus IAP repeat) domain and antiapoptotic activity (Schimmer, 2004). Little is known about the functions of IAPs, particularly c-IAP2/Birc3, in the nervous system. Different members of the IAP family have been investigated for involvement in various neurological diseases. For example, neuronal apoptosis inhibitor protein (NAIP) is up-regulated in rat neurons in response to transient forebrain ischemia (Xu et al., 1997). At the molecular level, IAP-1 and -3 are down-regulated in microglia following treatment with transforming growth factor (TGF)-βI, indicating a role for IAPs in mediating CNS inflammatory response (Jung et al., 2003). Knowledge of these mechanisms could be translated to therapeutic technologies for diseases such as MS (Sohur et al., 2006). Furthermore, increased AP expression in the T cells of MS patients correlates with disease activity, whereas interferon-β therapy decreases IAP expression in these cells (Semra et al., 2002; Sharief et al., 2002). This implicates IAP expression as an important regulator of T-cell survival in, and thus pathology of, MS. The full involvement of IAPs and the specific pathways they affect remain to be elucidated in both healthy neuronal cells and in disease states. Further elucidation of this and other pathways regulating the apoptosis of NPCs is required before their full therapeutic potential can be realized.
It is possible that the role of Fas changes depending on the developmental stage and proliferative properties of the stem cells. Unlike the case in our observations, Fas monoclonal antibody Jo2-induced apoptosis of wild-type embryonic stem cells (ESCs) isolated from 15-day-old mouse but not the ESCs from Fas−/− (lpr) mice (Semont et al., 2004). A recent study on human ESCs found that Fas, FasL, caspase-8, and caspase-3 are all up-regulated after exposure to the toxic environmental contaminants nonylphenol and octylphenol (Kim et al., 2006). It is unclear whether the Fas up-regulation in response to toxins in this study had a net protective or death-inducing effect. These results appear contrary to our work and other work with adult NPCs (Ceccatelli et al., 2004), neuronally differentiated progenitor cells (Brunlid et al., 2007), and a neural stem cell line (Tamm et al., 2004), all of which demonstrate that a Fas-dependent apoptosis pathway is not functional in these cells. Although the reasons for these differences are unclear, they may be related to the original source of the neural stem cells or culture maintenance and treatment conditions. Taken together, our findings and others suggest an important role of Fas in regulating developmental neuronal death. Fas may play an evolving, pluripotency-dependent role in maintenance of the CNS.
Neural stem cell transplantation therapies are a promising investigative avenue for several neurodegenerative diseases. According to Pluchino et al.’s (2005) fndings, NPC transplantation results in a therapeutic effect for EAE mice, the widely used animal model of human CNS demyelinating diseases. It is thought that this observed effect could be mediated by a “bystander” process in which transplanted neural stem cells are able to inhibit destructive T cells and promote the growth and survival of existing neurons by releasing neuroprotective molecules (Martino and Pluchino, 2006; Pluchino et al., 2007). This is in contrast to the “cell replacement” hypothesis, in which transplanted NPCs repopulate a damaged CNS. To be able to harness the full therapeutic potential of neural stem cells, characterization of the biochemical mechanisms that regulate their survival is essential. Our study shows that the FasL/Fas pathway does not induce cell death in murine progenitor cells and that FasL increases the survival of NPCs by decreasing apoptosis; Birc3 is a probable candidate for modulating this process, although further studies are required before a definitive statement can be made. Modulating this pathway may affect NPC survival and cell fate commitment, which may be important for NPC in vitro expansion and in vivo therapeutic applications.
CONCLUSIONS
We have investigated the effects of FasL/Fas on NPC survival, proliferation, and differentiation in vitro and found that FasL increases NPC survival upon withdrawal from growth factors without affecting cell differentiation properties. This increased cell viability appears to be the result of decreased apoptosis rather than an increase in proliferation. The apparent protective effect against apoptosis induced by FasL is not a result of increased FLIP, a molecule known to inhibit caspase-8- mediated cell death in T cells. However, a possible mechanism could be through Birc3, a member of the inhibitor of apoptosis family of proteins, which was found to be up-regulated in cells treated with FasL. These effects were negated in Fas-deficient NPC, confirming the specificity of this interaction. Therefore, we describe a novel pathway for NPC survival involving the Fas system and Birc3.
ACKNOWLEDGMENTS
We thank Dr. Rae Nishi for helpful discussion and guidance and Dr. Jeff Spees, University of Vermont Stem Cell Core, for supplies and expertise concerning NPCs; lpr mice were graciously supplied by Dr. Ralph Budd (UVM Immunology Department). We also thank Dr. Ralph Budd and his laboratory for sharing Western blot supplies and help with interpretation of results; Dr. Karen Fortner for supplying reagents and T cells as well as teaching us about flow cytometry; and Timothy Hunter and colleagues for services provided by the Vermont Cancer Center DNA Analysis Facility.
Contract grant sponsor: NIH; Contract grant number: P20 RR16435; Contract grant sponsor: COBRE Program of the National Center for Research Resources.
REFERENCES
- Brunlid G, Pruszak J, Holmes B, Isacson O, Sonntag KC. Immature and neurally differentiated mouse embryonic stem cells do not express a functional Fas/Fas ligand system. Stem Cells. 2007;25:2551–2558. doi: 10.1634/stemcells.2006-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camins A, Pallas M, Silvestre JS. Apoptotic mechanisms involved in neurodegenerative diseases: experimental and therapeutic approaches. Methods Findings Exp Clin Pharmacol. 2008;30:43–65. doi: 10.1358/mf.2008.30.1.1090962. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Tamm C, Sleeper E, Orrenius S. Neural stem cells and cell death. Toxicol Lett. 2004;149:59–66. doi: 10.1016/j.toxlet.2003.12.060. [DOI] [PubMed] [Google Scholar]
- Cheema ZF, Wade SB, Sata M, Walsh K, Sohrabji F, Miranda RC. Fas/Apo [apoptosis]-1 and associated proteins in the differentiating cerebral cortex: induction of caspase-dependent cell death and activation of NF-kappaB. J Neurosci. 1999;19:1754–1770. doi: 10.1523/JNEUROSCI.19-05-01754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK. Fas engagement induces neurite growth through ERK activation and p35 up-regulation. Nat Cell Biol. 2003;5:118–125. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neuro- Sci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Jung B, Kim MO, Yun SJ, Lee EH. Down-regulation of the expression of rat inhibitor-of-apoptosis protein-1 and -3 during transforming growth factor-beta1-mediated apoptosis in rat brain microglia. Neuroreport. 2003;14:857–860. doi: 10.1097/00001756-200305060-00016. [DOI] [PubMed] [Google Scholar]
- Kim SK, Kim BK, Shim JH, Gil JE, Yoon YD, Kim JH. Nonyl phenol and octylphenol-induced apoptosis in human embryonic stem cells is related to Fas-Fas ligand pathway. Toxicol Sci. 2006;94:310–321. doi: 10.1093/toxsci/kfl114. [DOI] [PubMed] [Google Scholar]
- Kuhn HWJ, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Nat R, Radu E, Regalia T, Popescu LM. Apoptosis in human embryo development: 3. Fas-induced apoptosis in brain primary cultures. J Cell Mol Med. 2001;5:417–428. doi: 10.1111/j.1582-4934.2001.tb00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102:1459–1465. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- Park C, Sakamaki K, Tachibana O, Yamashima T, Yamashita J, Yonehara S. Expression of fas antigen in the normal mouse brain. Biochem Biophys Res Commun. 1998;252:623–628. doi: 10.1006/bbrc.1998.9572. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Martino G. Rational for the use of neural stem/precursor stells in immune-mediated demyelinating disorders. J Neurol. 2007;224(Suppl 1):I23–I28. [Google Scholar]
- Raoul C, Henderson CE, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147:1049–1062. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Pedini F, Mollinari C, Condorelli G, Bonci D, Bez A, Colombo A, Parati E, Peschle C, De Maria R. Absence of caspase 8 and high expression of PED protect primitive neural cells from cell death. J Exp Med. 2004;200:1257–1266. doi: 10.1084/jem.20040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- Semont A, Nowak EB, Silva Lages C, Mathieu C, Mouthon MA, May E, Allemand I, Millet P, Boussin FD. Involvement of p53 and Fas/CD95 in murine neural progenitor cell response to ionizing irradiation. Oncogene. 2004;23:8497–8508. doi: 10.1038/sj.onc.1207821. [DOI] [PubMed] [Google Scholar]
- Semra YK, Seidi OA, Sharief MK. Disease activity in multiple sclerosis correlates with T lymphocyte expression of the inhibitor of apoptosis proteins. J Neuroimmunol. 2002;122:159–166. doi: 10.1016/s0165-5728(01)00464-7. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Nouri MA, Zoukos Y. Reduced expression of the inhibitor of apoptosis proteins in T cells from patients with multiple sclerosis following interferon-beta therapy. J Neuroimmunol. 2002;129:224–231. doi: 10.1016/s0165-5728(02)00185-6. [DOI] [PubMed] [Google Scholar]
- Slinskey A, Barnes D, Pipas JM. Simian virus 40 large T antigen J domain and Rb-binding motif are suffcient to block apoptosis induced by growth factor withdrawal in a neural stem cell line. J Virol. 1999;73:6791–6799. doi: 10.1128/jvi.73.8.6791-6799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohur US, Emsley JG, Mitchell BD, Macklis JD. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci. 2006;361:1477–1497. doi: 10.1098/rstb.2006.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm C, Robertson JD, Sleeper E, Enoksson M, Emgard M, Orrenius S, Ceccatelli S. Differential regulation of the mitochondrial and death receptor pathways in neural stem cells. Eur J Neurosci. 2004;19:2613–2621. doi: 10.1111/j.0953-816X.2004.03391.x. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Irmler M, Thome M. Inhibition of fas death signals by FLIPs. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- van Landeghem FK, Felderhoff-Mueser U, Moysich A, Stadelmann C, Obladen M, Bruck W, Buhrer C. Fas (CD95/Apo-1)/Fas ligand expression in neonates with pontosubicular neuron necrosis. Pediatr Res. 2002;51:129–135. doi: 10.1203/00006450-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Wajant H. The Fas signaling pathway more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Suda T, Hashimoto H, Nagata S. Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. EMBO J. 1995;14:12–18. doi: 10.1002/j.1460-2075.1995.tb06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosik K, Biernacki K, Khouzam MP, Prat A. Death receptor expression and function at the human blood brain barrier. J Neurol Sci. 2007;259:53–60. doi: 10.1016/j.jns.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Xu DG, Crocker SJ, Doucet JP, St.-Jean M, Tamai K, Hakim AM, Ikeda JE, Liston P, Thompson CS, Korneluk RG, MacKenzie A, Robertson GS. Elevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampus. Nat Med. 1997;3:997–1004. doi: 10.1038/nm0997-997. [DOI] [PubMed] [Google Scholar]
- Zuliani C, Kleber S, Klussmann S, Wenger T, Kenzelmann M, Schreglmann N, Martinez A, del Rio JA, Soriano E, Vodrazka P, Kuner R, Groene HJ, Herr I, Krammer PH, Martin-Villalba A. Control of neuronal branching by the death receptor CD95 (Fas/Apo-1) Cell Death Differ. 2006;13:31–40. doi: 10.1038/sj.cdd.4401720. [DOI] [PubMed] [Google Scholar]


