Abstract
Background and Purpose
In the IMS III trial, we sought to demonstrate evidence of a differential treatment effect of endovascular treatment of acute ischemic stroke compared to intravenous tPA, according to baseline collateral status measured using CT angiography.
Methods
Of 656 patients enrolled in IMS III, 306 had baseline CTA. Of these, 185 patients had M1 MCA ± intracranial ICA occlusion where baseline collateral status could be measured. Collateral status was assessed by consensus using 3 different ordinal scales and categorized as good, intermediate and poor. Multivariable modeling was used to assess the effect of collateral status and treatment type on clinical outcome (mRS 0–2, mRS 0–1 and the ordinal mRS scale).
Results
Of 185 patients, 126 randomized to endovascular therapy (87.6% recanalized, 41.3% 90-day mRS 0–2) and 59 to IV tPA only (60.5% recanalized, 30.5% 90-day mRS 0–2). In multivariable modeling, collateral status was a significant predictor of all clinical outcomes (p<0.05). Maximal benefit with endovascular treatment across all clinical outcomes was seen in patients with intermediate collaterals, some benefit in patients with good collaterals and none in patients with poor collaterals) although small sample size limited the power of the analysis to show a statistically significant interaction between collateral status and treatment type (p>0.05).
Conclusion
Using data from a large RCT (IMS III), we show that baseline CTA collaterals are a robust determinant of final clinical outcome and could be used to select patients for endovascular therapy.
Clinical Trials Registration Information
Keywords: Collateral circulation, Endovascular therapy, Acute ischemic stroke
INTRODUCTION
Leptomeningeal collaterals are native (pre-existing) anastomoses that cross-connect a small number of distal-most arterioles within the crowns of the cerebral artery trees.1, 2 Collaterals maintain blood flow to the brain that would otherwise rapidly die during an acute ischemic stroke. Collateral status can now be scored non-invasively using CT angiography (CTA). Collateral status at baseline is an independent determinant of clinical outcome among patients with acute ischemic stroke.3–7 This beneficial effect of good collaterals on clinical outcome could be due to reduced severity of ischemia, resulting in potentially longer time windows for tissue salvage. Good collateral status may also be associated with shorter intracranial thrombi that are more amenable to thrombolysis.8
In this analysis we seek to demonstrate if the relationship between treatment type and clinical outcome in the cohort of patients with baseline CTA in IMS III differs by collateral status.9, 10 We hypothesize that patients with poor collaterals on baseline CTA will not achieve good clinical outcome with endovascular therapy or intravenous (IV) tissue Plasminogen Activator (tPA), patients with moderate collaterals will likely do well with additional endovascular therapy while patients with good collaterals at baseline may do equally well with both therapies. We base our hypothesis on the assumption that patients with good collateral status (moderate ischemia) at baseline may have longer time windows for tissue salvage, obviating the importance of faster recanalization with endovascular treatment. Patients with poor collaterals at baseline may not have any salvageable brain even if early recanalization is achieved, while patients with intermediate collaterals may have severe ischemia and are therefore most likely to benefit from early recanalization offered by additional endovascular therapy.
METHODS
Data are from the IMS III study. Of 656 patients enrolled in the study, 306 patients had CTA at baseline. Details of the IMS III study protocol have been described previously.11–13
Imaging Analyses
Baseline and follow-up images were analyzed at the imaging core lab using OsiriX version 3.5 (http://www.osirix-viewer.com) to reconstruct 2D multi-planar reconstruction images of baseline CTA in axial, coronal, and sagittal planes using 3 mm and 24 mm thick slabs. Collateral status was measured on baseline CTA indirectly by measuring the extent of backfilling pial arteries beyond an intracranial occlusion.1, 3 The size of these backfilling arteries and the extent to which they fill the ischemic territory were compared to the opposite normal hemisphere. Since assessment of backfilling pial arteries in more distal occlusions is technically difficult, collateral status was only measured in patients with proximal occlusions (i.e. trunk of MCA (M1) ± intracranial ICA occlusion). 3, 4
We chose three different scoring systems to assess collateral status on baseline CTA. The regional collateral scoring system (score 1) is derived from two previously published scores and distinguishes the extent and prominence of backfilling pial arteries in the ACA-MCA and PCA-MCA region separately.14 ACA-MCA and PCA-MCA pial arterial backfilling are each scored from 0–5 (0-absent, 1-minimal, 2-significantly decreased prominence and extent of pial arteries with regions of no vessels, 3-moderately decreased prominence and extent, 4-mildly decreased prominence and extent, 5-normal or increased prominence and extent) when compared to the opposite normal hemisphere; the total score combines scores from these two regions to give an ordinal score ranging from 0–10.14 Score 2 (Maas et al) is based on a previously published ordinal scoring system that rates pial arterial backfilling primarily in the sylvian sulcus (0-absent, 1-poor, 2-less, 3-equal, 4-greater and 5-exuberant) when compared to the opposite hemisphere;5 Score 3 (Tan et al) is a previously published score that assesses pial arterial backfilling in the whole MCA ischemic territory (0-minimal, 1- less than 50%, 2-more than 50%, 3-pial arteries filling 100% of ischemic territory).7 These three scoring systems represent the entire spectrum of scoring systems for collaterals on CTA Head examinations.
Since all CTAs in IMS III were single-phase examinations and collateral status can be significantly under-estimated if scan acquisition is triggered early, data on CTA image acquisition timing was collected [Hounsfield Units (HU) of the contralateral ICA bifurcation and proximal M1 MCA on the arterial side and HU of the superior sagittal sinus, torcula and contra-lesional transverse/sigmoid junction on the venous side].14, 15 We defined a scan as very early arterial-weighted if the mean HU in the arterial side was < 150 HU and greater than the mean venous HU.8 In addition, relative prominence of pial arteries vs. veins was measured on the ischemic side and the contralateral normal side, and a scan was considered to be early arterial weighted if pial arteries were more prominent than the veins on the contralateral side. All scans were scored for image quality. Scans were considered poor quality if collateral assessment was not possible due to incomplete brain coverage or severe motion. Two readers (BKM, VN) read all scans by consensus; the readers were blinded to all clinical and follow-up data at the time of reading the scans.
Statistical Analyses
Data were summarized using standard descriptive statistics. Collateral status on CTA using the three scores described above were trichotomized into 3 groups. Using categories good, intermediate and poor, the 3 scores were as follows; Score 1(Good 8–10, Intermediate 6–7, Poor 0–5), Score 2 (Good 3–5, Intermediate 2, Poor 0–1) and Score 3 (Good 3, Intermediate 2, Poor 0–1) (Table 1). Different score cut-points (Score 1: 0–2/3–7/8–10 and Score 2: 0–1/2–3/4–5) and alternate collateral categorization (good/intermediate vs. poor) were used for sensitivity analyses. For the primary outcome (mRS 0–2 at 90 days), we used a generalized linear model with log link and allowed for a possible interaction between treatment and collateral status. Because the available sample size limited statistical power to detect an interaction, we additionally examined models within each collateral group to assess if treatment type was associated with good clinical outcome. If we were unable to demonstrate heterogeneity of treatment effect by collateral status, we presented models with treatment type and collateral status, adjusted for age (continuous), National Institutes of Health Stroke Scale (NIHSS) score (≤19 vs. ≥20), and time from stroke symptom onset to IV tPA initiation (≤ 2 hours vs. > 2 hours). Both collateral status and baseline NIHSS were included in the model only after consideration of the variance inflation factor suggested that collinearity was not a concern. 24-hour recanalization as measured by CTA was not included in the adjusted model, since it is not a baseline variable and may be associated with treatment type. Baseline ASPECTS was also not included in the model given our a priori hypothesis and previous literature suggesting reduced inter-rater reliability in the early presenters. Similar model building exercises were repeated with 90 day mRS 0–1 as outcome.
Table 1.
Collateral assessment on CTA Head Examination using three different ordinal scoring systems.
| Regional Collateral Score (Score 1)14 | Sylvian Sulcus + Cerebral Convexity Collateral Score (Score 2)5 | MCA Territory Collateral Score (Score 3)7 | ||
|---|---|---|---|---|
| Description | ACA-MCA region | PCA-MCA region | Description | Description |
| Compared to asymptomatic contralateral hemisphere, there is increased or normal prominence and extent of pial vessels beyond the occluded artery within the symptomatic hemisphere. | Excellent (5) | Excellent (5) | Exuberant (5) | Pial arterial filling 100% of ischemic territory (3) |
| Compared to asymptomatic contralateral hemisphere, there is slightly reduced prominence and extent of pial vessels beyond the occluded artery within the symptomatic hemisphere. | Good (4) | Good (4) | Greater than contralateral normal side (4) | |
| Compared to asymptomatic contralateral hemisphere, there is moderately reduced prominence and extent of pial vessels beyond the occluded artery within the symptomatic hemisphere. | Fair (3) | Fair (3) | Equal to contralateral normal side (3) | Pial arterial filling > 50% but <100% of ischemic territory (2) |
| Compared to asymptomatic contralateral hemisphere, there is decreased prominence and extent and regions with no vessels in some part of the territory occluded. | Poor (2) | Poor (2) | Less than contralateral normal side (2) | Pial arterial filling > 0% but <= 50% of ischemic territory (1) |
| Compared to asymptomatic contralateral hemisphere there are just a few vessels visible in the occluded vascular territory. | Minimal (1) | Minimal (1) | Minimal vessels (1) | |
| Compared to asymptomatic contralateral hemisphere there are no vessels visible within the occluded vascular territory. | None (0) | None (0) | Absent (0) | Absent (0) |
Finally, we assessed 90 day mRS as an ordinal outcome. The generalized Wilcoxon test was used to test for the presence of a shift in 90-day mRS distributions by treatment group (IV tPA vs. additional endovascular treatment) stratified by collateral status (good, intermediate or poor). We used an ordinal logistic regression model with mRS as the dependent variable to test for an interaction between treatment type and collateral status. The proportional odds assumption was tested. The final reported models were adjusted for age, baseline NIHSS, and time from stroke symptom onset to IV tPA initiation.
RESULTS
Of 656 patients enrolled in the IMS III trial, 306 patients had CTA Head at baseline, of which 204 had intracranial M1 MCA ± intracranial ICA occlusion.12 After excluding 15 patients with either incomplete CTA coverage or unavailable scans and 4 patients with poor image quality, 185 patients were included in the final analysis [59 patients in the IV tPA only arm (60.5% recanalized at 24h, 30.5% achieved 90-day mRS 0–2) and 126 patients in the endovascular therapy arm (87.6% recanalized at 24h, 41.3% achieved 90-day mRS 0–2)]. Baseline clinical and imaging characteristics, including collateral status, are described in Table 2; treatment arms were well matched.
Table 2.
Baseline characteristics, CTA image quality and baseline collateral status on CTA stratified by treatment type in the IMS 3 trial.
| IV tPA Only N = 59 |
Endovascular Treatment N = 126 |
||
|---|---|---|---|
| Age (yrs), median (range) | 70 (38 – 82) | 71 (28 – 83) | |
| Sex | Female | 25 (42.4) | 68 (54.0) |
| Baseline ASPECTS1 | 0–7 | 29 (49.2) | 65 (52.0) |
| 8–10 | 30 (50.8) | 60 (48.0) | |
| Site of occlusion | ICA +/− M1 MCA | 22 (37.3) | 37 (29.4) |
| M1 MCA alone | 37 (62.7) | 89 (70.6) | |
| Early arterial weighted CTA | No | 59 (100) | 125 (99.2) |
| Yes | 0 (0) | 1 (0.8) | |
| Collateral score 1 | 0–2 | 4 (6.8) | 14 (11.1) |
| 3–5 | 13 (22) | 24 (19) | |
| 6–7 | 18 (30.5) | 42 (33.3) | |
| 8–9 | 22 (37.3) | 40 (31.7) | |
| 10 | 2 (3.4) | 6 (4.8) | |
| Collateral Score 2 | 0 | 1 (1.7) | 7 (5.6) |
| 1 | 6 (10.2) | 12 (9.5) | |
| 2 | 26 (44.1) | 60 (47.6) | |
| 3 | 20 (33.9) | 31 (24.6) | |
| 4 | 4 (6.8) | 11 (8.7) | |
| 5 | 2 (3.4) | 5 (4) | |
| Collateral Score 3 | 0 | 2 (3.4) | 10 (7.9) |
| 1 | 14 (23.7) | 24 (19) | |
| 2 | 17 (28.8) | 30 (23.8) | |
| 3 | 26 (44.1) | 62 (49.2) | |
Percentages based on subjects with available baseline CT (IV tPA Only n=59, Endovascular Treatment n=125)
Primary Clinical Outcome (90 day mRS 0–2)
90 day good clinical outcome (mRS 0–2) within each treatment type (IV tPA alone vs. endovascular therapy) stratified by collateral score categories (good, intermediate and poor) across all 3 collateral scores is shown in Table 3. The point estimate difference between treatment types for good clinical outcome rate (mRS 0–2) seems to differ by collateral status; patients with intermediate collaterals do best with endovascular therapy, those with good collaterals tend to do better whereas patients with poor collaterals tend to do marginally worse with endovascular therapy (However, all confidence intervals of all point estimates include zero). In multivariable modeling, the three collateral scores were each significant predictors of 90 day mRS 0–2 within their respective models (p-values <0.01 for all scores) while the effect of type of treatment (IV tPA alone vs. endovascular therapy) was not significant (all p values > 0.05). The interaction term between collateral score and type of treatment was non-significant in all models (p > 0.1). Treatment was not a significant predictor of 90 day mRS 0–2 in similar models constructed for each collateral category (good, intermediate and poor) across all 3 scores. In the final adjusted models, collateral score (all p values < 0.05) and baseline NIHSS (all p-values <0.01) are significant predictors of 90 day mRS 0–2.
Table 3.
Total number of patients in each collateral status by treatment type, percentage of patients with 90-day good clinical outcome (mRS 0–2), difference in rate of good outcome between treatment types with 95% confidence interval using the three collateral scores.
| Collateral Score | Endovascular Treatment | IV tPA Only | Treatment Difference (%) | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| N | % mRS 0–2 | N | % mRS 0–2 | |||||
| Score 1 | Good (8–10) | 46 | 56.5 | 24 | 41.7 | 14.9 | −9.5 | 39.2 |
| Intermediate (6–7) | 42 | 45.2 | 18 | 22.2 | 23 | −1.4 | 47.4 | |
| Poor (0–5) | 38 | 18.4 | 17 | 23.5 | −5.1 | −28.7 | 18.5 | |
| Score 2 | Good (3–5) | 47 | 57.4 | 26 | 42.3 | 15.1 | −8.5 | 38.8 |
| Intermediate (2) | 60 | 40 | 26 | 23.1 | 16.9 | −3.5 | 37.3 | |
| Poor (0–1) | 19 | 5.3 | 7 | 14.3 | −9 | −36.8 | 18.8 | |
| Score 3 | Good (3) | 62 | 51.6 | 26 | 42.3 | 9.3 | −13.4 | 32 |
| Intermediate (2) | 30 | 43.3 | 17 | 23.5 | 19.8 | −7 | 46.7 | |
| Poor (0–1) | 34 | 20.6 | 16 | 18.8 | 1.8 | −21.6 | 25.3 | |
Secondary clinical outcome (90 day mRS 0–1)
For 90 day mRS 0–1, statistically significant difference in rate of good clinical outcome between treatment types, favoring endovascular therapy, is noted in patients with intermediate collateral status on all 3 scores (p<0.05) (Supplementary Table I). In multivariable modeling, the three collateral scores were each significant predictors within their respective models (p<0.05 for all 3 models), while the effect of type of treatment (IV tPA alone vs. endovascular therapy) was significant using scores 1 and 2 (p < 0.05). The interaction term between collateral score and type of treatment was non-significant in score 2 and score 3 models (p > 0.1). The model for score 1 is not reported due to a complete separation of data points.
Shift analysis across the mRS scale
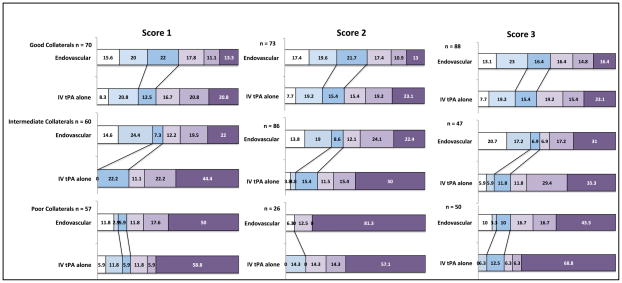
In univariate analysis, a statistically significant shift in 90 day mRS distribution between IV tPA only and endovascular therapy arms was noted only for subjects with intermediate collateral status as defined by score 1 (p=0.01) and score 2 (p=0.01). Shifts in mRS distribution for patients with good or poor collaterals across all 3 scores were not significant (p>0.05). (Figure 1 and Supplementary Table II) Using multivariable modeling (ordinal logistic regression), collateral status by score 1 and treatment type were significant predictors of 90 day mRS (p<0.05), but there was no significant interaction between them (p=0.34). Collateral status and type of treatment remained significant when adjusted for age, baseline NIHSS and time from stroke onset to IV tPA initiation. Similar results were obtained for score 3; we could not test score 2 since the proportional odds assumption was not met in this case (score test p <0.0001).
Figure 1.
90-day mRS distribution for endovascular therapy vs. IV tPA in the IMS 3 trial stratified by good, intermediate and poor collateral status as per the three collateral scores. Black lines indicate shifts in mRS 0–1 and mRS 0–2 across treatment types.
Sensitivity analyses
In sensitivity analyses with the primary outcome (mRS 0–2 at 90 days), results remained consistent when using alternate trichotomization cut-points for collateral status (Score 1: 0–2/3–7/8–10 and Score 2: 0–1/2–3/4–5). In similar sensitivity analyses correcting for possible mislabeling of collateral status on single phase CTA due to bolus timing delay (collateral status was changed from good to intermediate if contralateral pial veins > pial arteries), results again were consistent with those from primary analysis. Similar results were obtained when collateral status was categorized as good/intermediate vs. poor across all 3 scores. Patients with good/intermediate collaterals showed a trend towards better clinical outcome with endovascular therapy while patients with poor collaterals did not benefit additionally with this therapy. (Supplementary Table III)
DISCUSSION
Our results show that collateral status measured on baseline single-phase CTA of the head is a robust independent predictor of clinical outcome in patients enrolled in the IMS III trial. We are also able to show some evidence that additional endovascular treatment in the treatment time window of IMS III are likely to benefit patients with good and intermediate collateral status, with maximal benefit seen in patients with intermediate collaterals. Our results are consistent across different methods of CTA collateral assessment.5, 7, 14 Small number of patients limits the power of the current analysis and so effect modification of the relationship between treatment type and clinical outcome by collateral status was not observable. Larger data sets may be useful to sort out this question.
Multiple studies have shown that baseline CTA collaterals determine clinical outcome in patients with acute ischemic stroke.3–6 Some previous studies have also shown effect modification of the relationship between recanalization status and clinical outcome by collateral status.4, 9, 16 Our study demonstrates the effect of CTA collateral status on clinical outcome within a large randomized controlled trial (IMS III).11 Similar results have been demonstrated using collaterals on conventional angio in IMS III.13 Although our results do not convincingly demonstrate effect modification by collateral status, these results could be used to support the use of CTA collateral status as an imaging selection tool to select patients for endovascular therapy in clinical trials and potentially in clinical practice. The ESCAPE trial (testing benefit of additional endovascular therapy over current standard of care in patients with acute ischemic stroke and proximal intracranial occlusions) has incorporated baseline CTA collateral status (good and intermediate) as an important selection criteria for trial enrollment.17
Our results show that the differential effect of additional endovascular therapy vs. IV tPA alone is likely maximal in patients with intermediate CTA collaterals. These patients are more likely to have severe ischemia and consequently grow their baseline infarct at a faster rate.9 They are also more likely to have longer intracranial thrombus (due to poorer backfilling pial arteries resulting in stasis distal to original thrombus) that is less likely to recanalize early with IV tPA alone.8 Endovascular therapy offers a higher likelihood of early recanalization in these patients vs. IV tPA alone; thus preventing significant infarct growth from happening and improving clinical outcome. In comparison, patients with good baseline CTA collaterals may grow infarcts at a slower rate and allow more time for IV t-PA to reopen the clot prior to permanent brain injury. These patients therefore may have a higher chance of doing well with IV tPA alone; endovascular therapy may only benefit additionally if recanalization is achieved very early. Patients with poor baseline CTA collaterals have a high likelihood of having severe ischemia, large baseline infarcts and large thrombus burden.9 Endovascular therapy, if successful in achieving reperfusion in such patients, could put them at risk of reperfusion injury and consequent harm. Other studies have also shown marginal harm in such patients using perfusion imaging.18–20
Our study has some limitations. CTA was only done in a proportion of patients within the IMS III trial. As such, our study was underpowered to show statistical interaction in multivariable modeling exercises. All CTAs within the IMS III trial are single-phase CTA Head examinations; this technique lacks temporal resolution unlike dynamic or multi-phase CTA and therefore runs the risk of mislabeling collateral status.14, 15, 21 To account for this limitation, we did detailed analysis of bolus timing, conducting sensitivity analysis excluding patients with very early arterial weighted scans and correcting for possible mislabeling of collateral status. These sensitivity analyses were consistent with our overall results.
In conclusion, using data from a large RCT (IMS III), we show that baseline CTA collaterals are a robust determinant of clinical outcome. Patients with good/intermediate collaterals may benefit from additional endovascular therapy while those with poor collaterals may not.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work has been funded by NIH-National Institute of Neurological Disorders and Stroke awards (NIH/NINDS) K24NS072272. Dr. Menon is funded through a Professorship in Stroke Imaging from the Heart and Stroke Foundation of Canada and has received grant support from the Canadian Institute of Health Research (CIHR). The Canadian Institute of Health Research and the Heart and Stroke Foundation of Canada fund Dr. Demchuk. Alberta Innovates Health Solutions and the Heart & Stroke Foundation of Alberta/NWT/NU fund Dr. Hill.
Footnotes
CONFLICTS OF INTEREST/DISCLOSURE
Dr. Demchuk has received speaker fees from Covidien Inc. Dr. Goyal has received honoraria from Covidien Inc. Drs. Hill, Goyal and Demchuk are PIs of the ESCAPE trial; Dr. Menon is a member of the steering and executive committee of this trial. Dr. Hill has received grant funding from Covidien Inc and Hoffman-La Roche, owns stock in Calgary Scientific Inc. and has received compensation from Merck Canada for being on a trial Safety Committee. Dr. Goyal has a licensing agreement with GE Healthcare for multiphase CTA. Dr Liebeskind is scientific consultant regarding trial design and conduct to Stryker (modest) and Covidien (modest). He was employed by the University of California (UC), which holds a patent on retriever devices for stroke, at the time of this work. Miss Foster receives significant compensation from NINDS (IMS U01 NS077304) through MUSC. Dr. Yeatts has received significant compensation from the IMS III trial as statistician and is a consultant to Genentech and the PRISMS study. Dr. Jovin is a consultant with Praxair and Silk Road Medical. Dr. Broderick has received research support from Genentech, EKOS Cerp, Concentric Inc and Cordis Neurovascular and remuneration from Boehringer Ingelheim for travel.
References
- 1.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 2.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. Lancet neurology. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 3.Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, et al. Regional leptomeningeal score on ct angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR American journal of neuroradiology. 2011;32:1640–1645. doi: 10.3174/ajnr.A2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 5.Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on ct angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. The pattern of leptomeningeal collaterals on ct angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke; a journal of cerebral circulation. 2010;41:2316–2322. doi: 10.1161/STROKEAHA.110.592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-ct and ct-angiography in acute stroke patients. Ann Neurol. 2007;61:533–543. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- 8.Mishra SM, Dykeman J, Sajobi TT, Trivedi A, Almekhlafi M, Sohn SI, et al. Early reperfusion rates with iv tpa are determined by cta clot characteristics. AJNR Am J Neuroradiol. 2014;35:2265–72. doi: 10.3174/ajnr.A4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nambiar V, Sohn SI, Almekhlafi MA, Chang HW, Mishra S, Qazi E, et al. Cta collateral status and response to recanalization in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2014;35:884–890. doi: 10.3174/ajnr.A3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-pa versus t-pa alone for stroke. The New England journal of medicine. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demchuk AM, Goyal M, Yeatts SD, Carrozzella J, Foster LD, Qazi E, et al. Recanalization and clinical outcome of occlusion sites at baseline ct angiography in the interventional management of stroke iii trial. Radiology. 2014;273:202–210. doi: 10.1148/radiol.14132649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, et al. Collaterals at angiography and outcomes in the interventional management of stroke (ims) iii trial. Stroke. 2014;45:759–764. doi: 10.1161/STROKEAHA.113.004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon BK, O’Brien B, Bivard A, Spratt NJ, Demchuk AM, Miteff F, et al. Assessment of leptomeningeal collaterals using dynamic ct angiography in patients with acute ischemic stroke. J Cereb Blood Flow Metab. 2013;33:365–371. doi: 10.1038/jcbfm.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frolich AM, Wolff SL, Psychogios MN, Klotz E, Schramm R, Wasser K, et al. Time-resolved assessment of collateral flow using 4d ct angiography in large-vessel occlusion stroke. Eur Radiol. 2014;24:390–6. doi: 10.1007/s00330-013-3024-6. [DOI] [PubMed] [Google Scholar]
- 16.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 17.Demchuk AM, Goyal M, Menon BK, Eesa M, Ryckborst KJ, Kamal N, et al. Endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing ct to recanalization times (escape) trial: Methodology. [Accessed December 25, 2014];International Journal of Stroke. 2014 doi: 10.1111/ijs.12424. Published online ahead of print December 25, 2014. http://www.ncbi.nlm.nih.gov/pubmed/25546514. [DOI] [PubMed]
- 18.Albers GW. Impact of recanalization, reperfusion, and collateral flow on clinical efficacy. Stroke. 2013;44:S11–12. doi: 10.1161/STROKEAHA.111.000258. [DOI] [PubMed] [Google Scholar]
- 19.Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, et al. Refining the definition of the malignant profile: Insights from the defuse-epithet pooled data set. Stroke. 2011;42:1270–1275. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, et al. Pretreatment diffusion- and perfusion-mr lesion volumes have a crucial influence on clinical response to stroke thrombolysis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1214–1225. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon BK, dEsterre C, Qazi E, Almekhlafi MA, Hahn L, Demchuk AM, et al. Multi-phase cta: A new tool for the imaging triage of patients with acute ischemic stroke. [Accessed January 29, 2015];Radiology. 2015 doi: 10.1148/radiol.15142256. Published online ahead of print January 29, 2015. http://pubs.rsna.org/doi/full/10.1148/radiol.15142256. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.