Abstract
Reduced shear stress and augmented oscillatory shear rate are associated with the proatherogenic phenotype observed with aging. To date, mechanisms contributing to the age-related alterations in shear rate in humans have only been examined in the conduit vessels of the arm. Therefore, this study sought to examine the contribution of nitric oxide (NO) bioavailability to age-related alterations in shear rate and the impact of common body positions (supine and seated) in the atherosclerotic-prone conduit artery of the leg. Inhibition of NO synthase (NOS) was accomplished by intra-arterial infusion of NG-Monomethyl-L-arginine (L-NMMA) and common femoral artery diameter and blood velocity were measured by Doppler ultrasound in healthy young (n=8, 24±1 yr) and old (n=8, 75±3 yr) men. Old subjects exhibited reduced mean shear rate in the supine (18±3 sec−1) and seated positions (17±3 sec−1) compared with young subjects (supine: 42±6 sec−1, seated: 32±4 sec−1). This reduced mean shear in the old was driven by attenuated antegrade shear as there were no differences in retrograde shear. Inhibition of NOS reduced antegrade shear in the young such that age-related differences were abolished. In contrast, NOS-induced reductions in retrograde shear rate were similar between groups. The seated position reduced mean shear rate in the young to that normally observed in old. Overall, this study reveals that age-related reductions in mean shear rate, assessed in the atherosclerotic-prone vasculature of the leg, are largely explained by reductions in antegrade shear as a result of reduced NO bioavailability in the elderly.
Keywords: aging, atherosclerosis, nitric oxide, shear stress, blood flow
Introduction
Peripheral conduit arteries exhibit an oscillatory pattern of blood flow, consisting of large forward (antegrade) flow during systole followed by smaller back-flow (retrograde) and subsequent forward flow during diastole1, 2. Both the magnitude and direction of blood flow and subsequent shear stress alter endothelial cell phenotype and function, influencing the development of atherosclerosis3, 4. In cell culture and isolated blood vessels, elevated mean shear rate elicits an anti-atherogenic phenotype while a pro-atherogenic phenotype is characterized by augmented retrograde shear and/or low mean shear stress3–14. In humans, enhanced antegrade shear stress improved endothelial function15 while attenuation of antegrade shear induced endothelial dysfunction16. Conversely, elevations in retrograde shear stress evoked endothelial cell apoptosis17 and endothelial dysfunction18. This array of findings highlight the profound and highly specific effects that disturbances in shear stress can have on endothelial cell phenotype, endothelial function, and the propensity for atherosclerosis.
Aging augments the development and progression of atherosclerosis and is associated with reduced NO bioavailability, impaired endothelial function19–22, and altered shear rate23–25. Interestingly, despite systemic reductions in NO bioavailability and endothelial function with aging the distribution of atherosclerosis varies within the vasculature such that the conduit arteries of the legs display a greater predisposition for atherosclerotic lesions than such vessels in the arms26–28. The underlying mechanism(s) responsible for this limb difference in the propensity for atherosclerosis is not entirely clear, but may be a consequence of low mean shear rate coupled with elevated blood pressure in the legs during periods of upright posture. The impact of these differences may not be trivial as mean shear rate in the legs is 2 to 3 fold lower than the arms23–25, 29, 30 and the average American spends nearly two-thirds of the day in an upright posture31, 32.
Recent mechanistic investigations, in the arm, focusing primarily on retrograde shear rate as the culprit of age-related increases in atherosclerosis and vascular dysfunction, revealed important roles of NO bioavailability24 and α-adrenergic vasoconstriction23. Importantly, the occurrence and severity of atherosclerosis is greater in the legs than the arms, thus limiting the ability to generalize these previous findings23, 24. Beyond a single, descriptive, report of reduced mean shear rate in the femoral artery of healthy older men25, a mechanistic examination of the factors contributing to the age-associated alterations in shear rate in this atherosclerotic-prone conduit artery of the leg is lacking.
Given the established associations between endothelial dysfunction, shear rate, and atherosclerosis33–36 this study sought to determine if reduced NO bioavailability accounts for the age-related proatherogenic shear rate pattern in the leg. We directly tested the following hypotheses; 1) inhibition of NOS would abolish differences in shear rate between young and old, revealing a critical role of NO in the recognized age-related differences in shear rate, and 2) NOS inhibition would not alter shear rate patterns in the old subjects, due to pre-existing age-associated reductions in NO bioavailability. Additionally, to evaluate the effect of posture on shear rate patterns and to provide a thorough understanding of the potential role of shear stress and NO in the preferential development of atherosclerosis in the leg we studied the impact of two common body positions (supine and seated) on femoral artery shear rate in these healthy young and old subjects.
Methods
Eight healthy young (24 ± 1 yrs) and 8 healthy older men (75 ± 3 yrs) volunteered to participate in this research study. Subjects were not taking any prescription medications and were free of overt cardiovascular disease. Protocol approval and written informed consent were obtained according to the University of Utah and Salt Lake City Veterans Affairs Medical Center (VAMC) Institutional Review Board, in accordance with the principles outlined in the Declaration of Helsinki. All data collection took place at the Salt Lake City VAMC’s Geriatric Research, Education, and Clinical Center in the Utah Vascular Research Laboratory.
Experimental Protocol
Subjects reported to the laboratory between 0700 and 0800 after an overnight fast. Upon arrival at the laboratory, body mass and height were recorded and the right femoral artery was catheterized (18-gauge central venous catheter, Arrow International, Reading, PA) using the Seldinger technique. Following a 30 min rest period subjects were positioned in either the seated or supine position. Subjects then rested in either the seated or supine position for an additional 20 min while instrumentation was completed and to ensure stable blood flow and central hemodynamics. Body position was counter-balanced. Due to lasting effects of L-NMMA, the control (saline) trials were always performed prior to L-NMMA infusion.
L-NMMA infusion
Thigh volume was determined anthropometrically and then used for the calculation of drug dosing. L-NMMA (Bachem, Switzerland) was diluted from 250 mg lyophilized powder in normal saline to a concentration of 5 mg/ml. L-NMMA was infused at a priming dose of 0.48 mg/dl thigh volume for 5 minutes prior to measurements. Following the loading dose L-NMMA was infused at a maintenance dose of 0.24 mg/dl thigh volume which was then maintained for the duration of the study. All measures were obtained 2 min after starting the maintenance dose and were collected over 60 sec. During control trials normal saline was infused intra-arterially at the same rate and duration, as described for L-NMMA.
Measurements
Femoral artery blood velocity, vessel diameter, and intima-media thickening were measured by Doppler ultrasound (Logic 7, General Electric Medical Systems, Milwaukee, WI). Please refer to the online supplement for complete description of the measurements.
Data and Statistical Analysis
Ultrasound images and Doppler velocity spectra were recorded continuously during infusions. During each 60 s ultrasound Doppler segment, Vmean was averaged across five 12 s intervals, which were matched with intima-to-intima femoral artery diameter measurements evaluated during diastole. Statistics were performed with the use of commercially available software (SigmaPlot 11.0, Systat Software, Point Richmond, CA). A two-way repeated-measures analysis of variance (ANOVA) was used to identify significant changes in measured variables within and between conditions and groups. Following a significant main effect or interaction pairwise comparisons were made using Fischer’s LSD. Significance was set at an α level of 0.05, and data are presented as means ± SE.
Results
Subject Characteristics, Intima-media Thickening, and Mean Arterial Pressure
Young and old subjects were well-matched for height, weight, BMI, thigh volume, femoral artery diameter, and blood characteristics (Table 1). Absolute IMT and IMT normalized for femoral artery diameter were both greater in the old than the young (P < 0.01) (Table 1). Resting MAP was not different between young and old in either the supine or seated positions (Table 2). MAP was increased in the seated compared to supine position in both groups, reflecting an increase in hydrostatic pressure. NOS inhibition evoked a small, but significant elevation in MAP in all conditions (Table 2) except in the young in the seated position (P = 0.12).
Table 1.
Subject stature, IMT, and blood characteristics
| Variables
|
YOUNG
|
OLD
|
|---|---|---|
| Age, yr | 24 ± 1 | 75 ± 3* |
| Height, cm | 177 ± 2 | 177 ± 2 |
| Weight, kg | 76 ± 4 | 78 ± 4 |
| Body mass index, kg/m2 | 24 ± 1 | 25 ± 1 |
| Thigh volume, dl | 72 ± 4 | 64 ± 4 |
| Femoral artery diameter, cm | 0.90 ± 0.03 | 1.05 ± 0.07 |
| Intima-media thickening, cm | 0.052 ± 0.002 | 0.093 ± 0.006* |
| Intima-media thickening normalized for Femoral artery diameter | 0.058 ± 0.003 | 0.093 ± 0.010* |
| Glucose, mg/dl | 71 ± 5 | 75 ± 4 |
| Cholesterol, mg/dl | 164 ± 16 | 179 ± 12 |
| Triglycerides, mg/dl | 97 ± 16 | 99 ± 16 |
| HDL, mg/dl | 48 ± 4 | 49 ± 4 |
| LDL, mg/dl | 101 ± 13 | 118 ± 9 |
Values are means ± SEM.
p < 0.05, significant difference between young and old.
Table 2.
Hemodynamics in the supine and seated position under control and L-NMMA conditions in young and old subjects.
| Variable | Supine | Seated | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Young | Old | Young | Old | |||||
|
| ||||||||
| Control | L-NMMA | Control | L-NMMA | Control | L-NMMA | Control | L-NMMA | |
| Antegrade BF (ml/min) | 436 ± 28 | 341 ± 39* | 398 ± 36 | 355 ± 25 | 336 ± 36# | 274 ± 27* | 320 ± 18# | 322 ± 23 |
| Retrograde BF (ml/min) | 93 ± 7 | 159 ± 17* | 131 ± 18 | 234 ± 34* | 54 ± 6# | 87 ± 11* | 103 ± 17† | 173 ± 27* |
| MAP (mmHg) | 90 ± 3 | 97 ± 3* | 93 ± 2 | 100 ± 2* | 114 ± 2# | 118 ± 4# | 111 ± 2# | 115 ± 3*# |
| LVC (ml/min/mmHg) | 3.8 ± 0.3 | 1.9 ± 0.2* | 2.9 ± 0.6 | 1.2 ± 0.4* | 2.5 ± 0.3# | 1.5 ± 0.2* | 2.0 ± 0.2 | 1.3 ± 0.2* |
Values are means ± SEM. Mean Arterial Pressure (MAP), LVC (leg vascular conductance).
P < 0.05, significant difference from control.
P < 0.05, significant difference from young.
P < 0.05, significant difference from supine.
Leg Blood Flow and Leg Vascular Conductance
Resting LBF and LVC were, on average, ~ 20 to 25% lower in the old compared to the young, independent of body position, however, none of these differences reached statistical significance (Figure 1 and Table 2). Although LBF was not significantly lower in the old, this group demonstrated augmented retrograde LBF while seated (p = 0.02) and tended to exhibit elevated retrograde LBF while supine (p = 0.06) (Table 2). NOS inhibition reduced LBF in both groups (Figure 1) and the magnitude of this reduction was greater in the supine compared to the seated position (Young; supine: −162 ± 21, seated, −94 ± 10 Δ ml • min−1, p = 0.01: Old; supine: −146 ± 29, seated: 68 ± 13 Δ ml • min−1, p ≤ 0.05).
Figure 1.
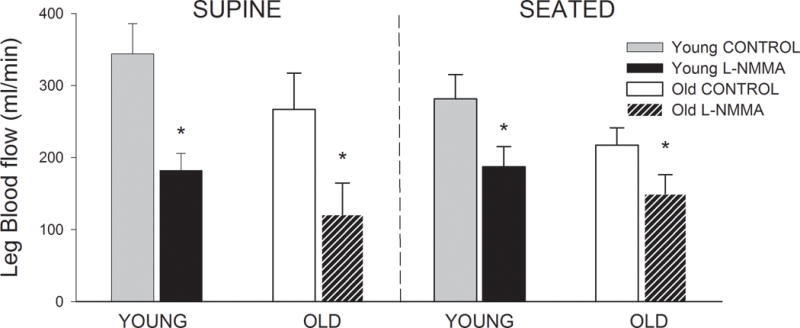
Leg Blood Flow (ml•min−1) during control and L-NMMA in the supine and seated position in young and old subjects. *P < 0.05, significant difference from control.
Shear Rate: Impact of Aging, NOS Inhibition, and Body Position
Mean shear rate
As illustrated in Figure 2A, old subjects exhibited a lower mean shear rate compared to the young subjects and this was evident in both the supine and seated positions. The magnitude of reduction in mean shear rate due to NOS inhibition was not different between young and old subjects (Table 3). The seated position resulted in a lower mean shear rate in the young, but not the old subjects. The NOS-induced reduction in mean shear rate in the young was less when seated than when supine (Table 3).
Figure 2.
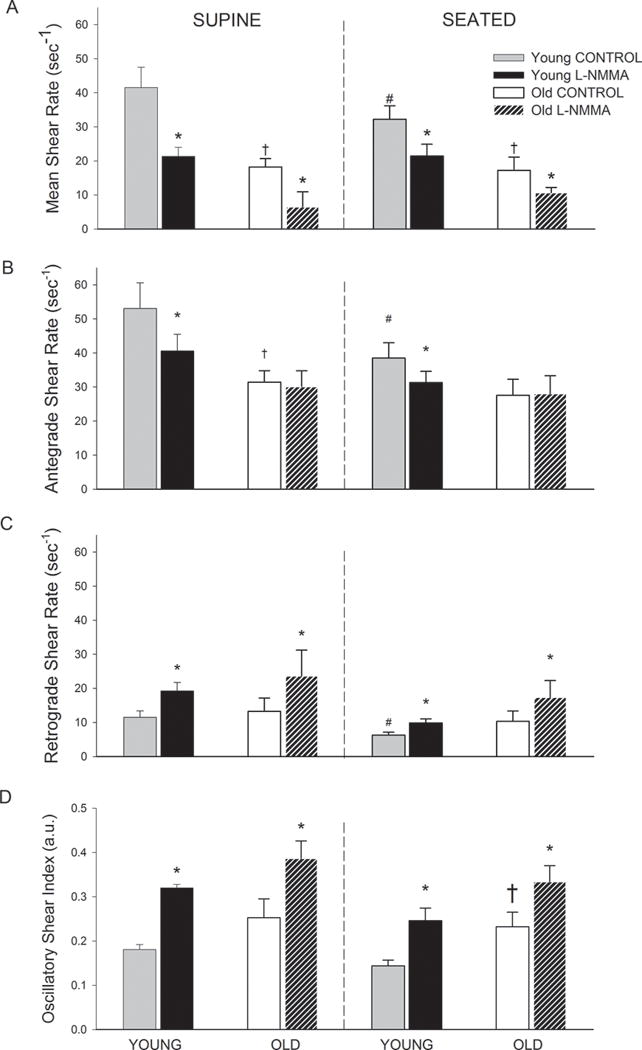
(A) Mean Shear Rate (sec−1), (B) Antegrade Shear Rate (sec−1), (C) Retrograde Shear Rate (sec−1), and (D) Oscillatory Shear Index (a.u.) during control and L-NMMA in the supine and seated position in young and old subjects. For the purpose of comparison retrograde shear rate is presented with a positive y-axis. * P < 0.05, significant difference from control. †P < 0.05, significant difference from young in same position. # P < 0.05, significant difference from supine.
Table 3.
Change in Shear Rate due to NOS-inhibition in the Supine and Seated Positions
| Supine
|
Seated
|
|||
|---|---|---|---|---|
| Variable | Young | Old | Young | Old |
|
|
|
|
||
| Mean Shear Rate, Δ sec−1 | −20.2±3.7 | −11.7±3.5 | −10.8±1.1# | −6.6±1.9 |
| Antegrade Shear Rate, sec−1 | −12.5±3.0 | −1.3±1.8† | −7.2±1.8 | 0.5±1.7† |
| Retrograde Shear Rate, sec−1 | 7.7±1.5 | 10.4±4.2 | 3.6±1.8 | 7.1±2.6 |
| Oscillatory Shear Rate, sec−1 | 0.14±0.01 | 0.13±0.02 | 0.10±0.02 | −0.10±0.02 |
Values are means ± SEM.
P < 0.05, significant difference from young.
P < 0.05, significant difference from supine.
Antegrade shear rate
As illustrated in Figure 2B, antegrade shear rate was lower in the old subjects in the supine, but not in the seated position when compared to the young. NOS inhibition attenuated antegrade shear in the young in both positions. In contrast, NOS inhibition had no impact on antegrade shear in the old subjects (Figure 2B and Table 3). The seated position resulted in a lower antegrade shear in the young, but had no effect in the old.
Retrograde shear rate
As depicted in Figure 2C, there were no age-related differences in retrograde shear rate. Moreover, the magnitude of the NOS-induced increase in retrograde shear rate was similar between the young and old subjects (Table 3). The seated position resulted in a reduction in retrograde shear rate in the young, but not old subjects.
Oscillatory shear index
As illustrated in Figure 2D, the old subjects exhibited elevated oscillatory shear in the seated position compared to the young subjects. In the supine position, oscillatory shear tended to be elevated in the old subjects (p = 0.12). NOS inhibition elicited similar increases in oscillatory shear in the young and old subjects (Table 3). The impact of position was unremarkable with respect to oscillatory shear.
Discussion
Alterations in shear stress, aging, and atherosclerosis are linked such that reductions in mean shear stress increase the propensity for atherogenesis with age. However, the mechanisms involved in age-associated alterations in shear rate and the relative importance of antegrade compared to retrograde shear rate in the atherogenic process are unclear. Thus, this study sought to determine if aging alters the contribution of NO to leg shear rate patterns, as assessed in the common femoral artery, in the often assumed supine and seated positions. To our knowledge, this is the first mechanistic evaluation of age-related differences in shear rate patterns in the atherosclerotic-prone vasculature of the leg. The primary novel findings of this study are that age-related alterations in shear rate in the leg are characterized by reduced mean shear rate, driven primarily by diminished antegrade shear. The inhibition of NOS elicited reductions in antegrade shear rate such that age-associated differences were eliminated. In contrast, retrograde shear rate was not different between groups, and the magnitude of the increase in retrograde shear due to NOS inhibition was similar between the young and the old revealing an important, albeit age-independent, contribution of NO to retrograde shear rate. Finally, changing body position from supine to seated reduced mean shear rate in young, but not in the old subjects. Collectively, these data reveal that posture is an important modulator of shear rate in the young and that the age-related reduction in NO bioavailability contributes to the attenuation of mean shear rate by lowering the antegrade shear rate in the atherosclerotic-prone vasculature of the leg.
The contribution of NO to shear rate: limb-specific differences
Mean shear rate in the legs is 2 to 3 fold lower than in the arms as a result of a greater vessel diameter, reduction in antegrade shear rate, and an elevation in retrograde shear rate23, 24, 29, 30. This diminished mean shear rate in the vasculature of the legs has been proposed to reflect the heightened propensity for atherosclerosis in the legs compared to the arms30. Despite these limb-specific differences and the greater contribution of antegrade shear rate to overall mean shear rate, the age-related increase in atherosclerosis in the leg has been largely attributed to the deleterious and pro-atherogenic impact of augmented retrograde shear rate24, 25, 37. In the current study, mean shear rate was reduced with age (Figure 2A) due to a reduction in antegrade shear rate (Figure 2C) and not an elevation in retrograde shear rate as previously reported25. This discrepancy between the current study and Young et al.25 may be explained by several factors including the substantially smaller femoral artery diameter of the older subjects in the previous study (0.86 ± 0.03cm) likely due to their inclusion of women and the substantial age differences between the older groups in these two studies (15 yrs). In the current study, the reduction in antegrade shear appears to be largely explained by reduced NO bioavailability, as NOS inhibition abolished the age-related differences in antegrade shear rate. Despite this age-dependent role of NO in the regulation of antegrade shear rate, the reduction in mean shear due to NOS inhibition was similar between groups, also highlighting an important NO-independent component of mean shear rate. The underlying reason for differing regulatory pathways for mean and antegrade shear rate is not entirely clear, but identifies an important role of NO in the regulation of shear rate patterns in both young and old.
In-vivo animal data support the role of reduced mean shear rate, due to diminished antegrade shear rate, in the atherogenic process as larger and more vulnerable atherosclerotic lesions were evident in regions exposed to reduced mean shear rate compared to regions of elevated oscillatory shear rate38. Thus, important limb differences in shear rate must be considered when examining mechanisms contributing to the development and progression of atherosclerosis and question the generalizability of previous findings23, 24 performed in the vasculature of the arm. Indeed, a potentially critical role of reduced antegrade shear rate in the leg must be considered when examining the heightened propensity for atherosclerosis in the lower limbs with age. Additionally, vessel diameter, a key variable in the calculation of shear rate, must also be considered when comparing across limbs or when vessel diameter is dissimilar between groups.
The lack of an age-related difference in the regulation of retrograde shear rate by NO is surprising, as previously the inhibition of NOS in the arm abolished shear rate differences between young and old subjects24. In the current study, NOS inhibition in the young subjects altered all shear rate patterns such that the age-related differences were eliminated (Figure 2). However, NOS inhibition also evoked similar changes in mean, retrograde, and oscillatory shear in the old, indicating that the contribution of NO to these shear rates is independent of age in the leg. The NOS-induced changes in shear rate are not explained by elevated MAP during NOS-inhibition as the relatively small increases in MAP were similar between groups and within postures (Table 2). Thus, it appears that NO accounts for the age-related differences in antegrade shear and is additionally an important regulator of retrograde shear rate and overall shear rate patterns in the leg of both the young and old.
The impact of posture and the role of NO in determining shear rate in the leg
The average American spends nearly two-thirds of the day in an upright posture31, 32, exposing the vasculature of the legs to elevated blood pressure which may contribute to the increased propensity for atherosclerosis in the leg. Additionally, a recent report indicates that adults, on average, spend more than 8 hours per day during waking hours engaged in sedentary behavior, primarily prolonged sitting39. Therefore, the examination of alterations in shear rate patterns in the upright seated compared to supine position may provide clinically important information as prolonged sitting is an established risk factor for all-cause mortality40. The deleterious effects of sitting have been fundamentally linked to metabolic, and to a lesser extent, vascular dysfunction41; however, distinguishing between the effects of physical inactivity from prolonged sitting is inherently difficult as the two occur concomitantly. Thus, assessment of shear rates in two common postures, seated and supine, is important to fully elucidate the potential role of shear stress in the preferential development of atherosclerosis in the vasculature of the leg.
Based on the findings of this study and work by Newcomer et al.29 in young subjects, posture, independent of physical activity, appears to alter shear rate in healthy young, but not old subjects (Figure 2). Specifically, antegrade and mean shear rate were reduced in the young subjects in the seated compared to supine position. Additionally, the seated position reduced the contribution of NO to mean shear rate in the young such that the change in mean shear rate due to NOS inhibition was lower in the seated compared to the supine position. The current findings of a proatherogenic shear rate pattern in the seated compared to supine position may provide an explanation for the improvement in vascular function following bed rest42, while also supporting epidemiological evidence linking sitting to both elevated systemic blood pressure and atherosclerosis43. Thus, the seated position, per se, appears to evoke a proatherogenic shear pattern in the young similar to that which is consistently observed in the older subjects regardless of position. We can only speculate on the long-term adaptations to sitting that may occur as a consequence of the unfavorable hemodynamic environment (i.e. reduced mean shear rate and elevated blood pressure) over a lifetime. Importantly, although free from overt cardiovascular disease, the older subjects of the current study exhibited an elevated IMT, indicative of significant structural adaptations in the leg vasculature and supportive of a potential role of diminished mean shear rate in the early stages of atherosclerotic disease.
The contribution of sympathetic nervous activity (SNA) and α-adrenergic vasoconstriction in determining shear rate
This study reveals that NO clearly contributes to shear rate patterns in the leg of both young and old subjects (Figure 2), however, other mechanisms, including heightened SNA and α-adrenergic vasoconstriction may also play a role. Indeed, acute elevations in SNA, evoked by lower body negative pressure, elicited increases in retrograde and oscillatory shear in the arm of the young such that differences between young and old subjects were eliminated23, 44. Likewise, in the arm of young subjects, stimulation of α-adrenergic receptors increased retrograde and oscillatory shear while blockade of these receptors, by phentolamine, abolished retrograde and oscillatory shear23, 44. In contrast, α-adrenergic blockade reduced the accentuated retrograde and oscillatory shear in the old, but did not eliminate these shear pattern characteristics suggesting multiple factors are involved in the regulation of shear rate in the arm of the old23, 44. Altered SNA and α-adrenergic vasoconstriction may also contribute to the differences in leg shear rate observed in the current study, however, direct evidence supporting these mechanisms is lacking. Given the apparent limb-specific differences in the contribution of NO to shear and the heightened propensity of atherosclerosis in the leg with aging, future investigations of non-NO dependent mechanisms regulating shear rate patterns are warranted.
Shear stress, vascular function, and atherogenesis
Compelling in vitro and in vivo evidence underscores the detrimental impact of altered shear stress on vascular function and the development of atherosclerosis. However, the pattern and direction of shear stress (i.e. reduced antegrade, elevated retrograde) associated with a pro-atherogenic hemodynamic environment is equivocal, likely dependent upon the duration of exposure (acute vs. chronic) and experimental model employed (in vitro vs. in vivo). Studies utilizing endothelial cell culture and isolated arteries indicate that exposure to increased retrograde and oscillatory shear, as well as reduced mean shear, contribute to vascular dysfunction at the molecular, cellular, and functional levels6–14. In vivo evidence implicates augmented retrograde and oscillatory shear in impaired vascular function. Thijsen et al.18 demonstrated a dose dependent reduction in vascular function in response to graded elevations in retrograde shear rate. Conversely, acute increases in antegrade shear improved vascular function despite non-uniform alterations in retrograde shear15. Consequently, these authors15 suggested that the magnitude of antegrade shear is the primary contributor to alterations in flow mediated dilation and therefore vascular health in humans.
Based on these previous in vivo and in vitro experiments it appears that the detrimental impact of altered shear stress at both the cellular and functional level involves a balance between reduced antegrade and elevated retrograde shear stress6–8, 14, 15, 18. The relative importance of alterations in antegrade and retrograde shear rate and the translation of such changes to the heightened propensity for atherosclerosis in the leg of older individuals is not well understood. With aging, retrograde shear rate appears to be increased by ~ 2 to 3 sec−1 in the leg, while the reduction in antegrade shear rate is several fold higher, ~ 6 to 20 sec−1. Clearly the magnitude of change in antegrade shear rate is far greater, however the impact of large changes in antegrade verse small changes in retrograde shear rate in the atherogenic process is not clear. Based on the current findings the role of reduced antegrade shear is expected to contribute to the atherogenic process as there were no significant age-related differences in retrograde shear rate. Additionally, under normal physiological conditions (i.e. without the acute modulation of shear stress), it could be argued that the reduction in antegrade shear rate leading to the attenuation of mean shear rate in conduit arteries may be largely responsible for the increased propensity for atherosclerosis observed in the lower limbs and with age26–28. Indeed, in the current study, differences between young and old subjects with respect to antegrade and mean shear rate were 2 – 3 times greater than the differences in retrograde shear suggesting that the age-related decrease in mean shear appears to be primarily driven by reduced antegrade shear rate (Figure 2).
Perspectives
This study reveals that the attenuated mean shear rate with age in the atherosclerotic-prone vasculature of the leg is driven primarily by reduced antegrade shear rate. Reduced NO bioavailability, as evidenced by NOS inhibition, appears to account for this age-related reduction in antegrade shear rate. Interestingly, while not accounting for age-related differences in retrograde shear, NO does also appear to play an important role in modulating mean and retrograde shear rate across the lifespan. Posture differentially alters shear rate in an age-dependent manner such that in the seated position the young exhibit reductions in shear rate that reflects the shear rate pattern observed in the old. Thus, the seated position may be detrimental for vascular health and promote the development of atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is New?
Attenuated mean shear stress observed with aging is largely due to reductions in antegrade shear stress in the atherosclerotic vasculature of the legs.
Nitric oxide (NO) accounts for the age-associated reduction in antegrade shear stress.
Sitting evokes a proatherogenic shear pattern in healthy young adults.
What is Relevant?
Aging, reduced NO bioavailability, and endothelial dysfunction are linked to altered shear stress and development of cardiovascular disease including hypertension and atherosclerosis.
Summary
This study reveals that age-related reductions in mean shear rate, assessed in the atherosclerotic-prone vasculature of the leg, are largely explained by reductions in antegrade shear as a result of reduced NO bioavailability in the elderly.
Acknowledgments
The authors would like to thank all the participants for their time, effort, and commitment and D. Walter Wray, Ph.D. for input regarding manuscript preparation.
Sources of Funding
J. D. Trinity and S. J. Ives were supported by the Advanced Fellowship in Geriatrics awarded by the Veterans Affairs Medical Center. This work was funded by National Institutes of Health PO1 HL-091830 (to R. S. Richardson), VA Merit Award E6910R (to R. S. Richardson).
Footnotes
Disclosure Statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Blackshear WM, Phillips DJ, Strandness DE. Pulsed Doppler assessment of normal human femoral artery velocity patterns. J Surg Res. 1979;27:73–83. doi: 10.1016/0022-4804(79)90113-6. [DOI] [PubMed] [Google Scholar]
- 2.McDonald DA. The relation of pulsatile pressure to flow in arteries. J Physiol. 1955;127:533–552. doi: 10.1113/jphysiol.1955.sp005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornhill JF, Roach MR. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976;23:489–501. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 4.Stone PH, Coskun AU, Yeghiazarians Y, Kinlay S, Popma JJ, Kuntz RE, Feldman CL. Prediction of sites of coronary atherosclerosis progression: In vivo profiling of endothelial shear stress, lumen, and outer vessel wall characteristics to predict vascular behavior. Curr Opin Cardiol. 2003;18:458–470. doi: 10.1097/00001573-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Chiu J-J, Chien S. Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiological Reviews. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. PNAS. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and Steady Laminar Shear Stress Differentially Affect Human Endothelial Redox State : Role of a Superoxide-Producing NADH Oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 9.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol. 2006;290:H2320–2328. doi: 10.1152/ajpheart.00486.2005. [DOI] [PubMed] [Google Scholar]
- 10.Godbole AS, Lu X, Guo X, Kassab GS. NADPH oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol. 2009;296:H152–158. doi: 10.1152/ajpheart.01251.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 12.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile Versus Oscillatory Shear Stress Regulates NADPH Oxidase Subunit Expression: Implication for Native LDL Oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory Shear Stress Stimulates Endothelial Production of O2- from p47phox-dependent NAD(P)H Oxidases, Leading to Monocyte Adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of Oscillatory and Unidirectional Flow Environments on the Expression of Endothelin and Nitric Oxide Synthase in Cultured Endothelial Cells. Arterioscler Thromb Vasc Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]
- 15.Tinken TM, Thijssen DHJ, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of Shear Rate Modulation on Vascular Function in Humans. Hypertension. 2009;54:278–285. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol. 2009;297:H1103–1108. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins NT, Padilla J, Boyle LJ, Credeur DP, Laughlin MH, Fadel PJ. Disturbed Blood Flow Acutely Induces Activation and Apoptosis of the Human Vascular Endothelium. Hypertension. 2013;61:615–621. doi: 10.1161/HYPERTENSIONAHA.111.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thijssen DHJ, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde Flow and Shear Rate Acutely Impair Endothelial Function in Humans. Hypertension. 2009;53:986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 19.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular Aerobic Exercise Prevents and Restores Age-Related Declines in Endothelium-Dependent Vasodilation in Healthy Men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 20.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-Related Reduction of NO Availability and Oxidative Stress in Humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 21.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and Endothelial Function in Normotensive Subjects and Patients With Essential Hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 22.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casey DP, Padilla J, Joyner MJ. {alpha}-Adrenergic Vasoconstriction Contributes to the Age-Related Increase in Conduit Artery Retrograde and Oscillatory Shear * Novelty and Significance. Hypertension. 2012;60:1016–1022. doi: 10.1161/HYPERTENSIONAHA.112.200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of Aging on Conduit Artery Retrograde and Oscillatory Shear at Rest and During Exercise: Role of Nitric Oxide. Hypertension. 2011;57:484–489. doi: 10.1161/HYPERTENSIONAHA.110.165365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211:390–392. doi: 10.1016/j.atherosclerosis.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic Lesions Are More Frequent in Femoral Arteries than in Carotid Arteries Independent of Increasing Number of Risk Factors. Angiology. 1999;50:649–654. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 27.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984;114:79–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis: A Report From the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 29.Newcomer SC, Sauder CL, Kuipers NT, Laughlin MH, Ray CA. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1833–1839. doi: 10.1152/ajpheart.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging. 2004;19:188–193. doi: 10.1002/jmri.10441. [DOI] [PubMed] [Google Scholar]
- 31.Harris AM, Lanningham-Foster LM, McCrady SK, Levine JA. Nonexercise movement in elderly compared with young people. Am J Physiol Endocrinol Metab. 2007;292:E1207–1212. doi: 10.1152/ajpendo.00509.2006. [DOI] [PubMed] [Google Scholar]
- 32.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual Variation in Posture Allocation: Possible Role in Human Obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 33.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP, Ganz P. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 34.Cooke JP. Is atherosclerosis an arginine deficiency disease? J Investig Med. 1998;46:377–380. [PubMed] [Google Scholar]
- 35.Drexler H. Nitric oxide and coronary endothelial dysfunction in humans. Cardiovasc Res. 1999;43:572–579. doi: 10.1016/s0008-6363(99)00152-2. [DOI] [PubMed] [Google Scholar]
- 36.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Credeur DP, Dobrosielski DA, Arce-Esquivel AA, Welsch MA. Brachial artery retrograde flow increases with age: relationship to physical function. Eur J Appl Physiol. 2009;107:219–225. doi: 10.1007/s00421-009-1117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJAP, Krams R, de Crom R. Atherosclerotic Lesion Size and Vulnerability Are Determined by Patterns of Fluid Shear Stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 39.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Objectively Measured Light-Intensity Physical Activity Is Independently Associated With 2-h Plasma Glucose. Diabetes Care. 2007;30:1384–1389. doi: 10.2337/dc07-0114. [DOI] [PubMed] [Google Scholar]
- 40.van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. SItting time and all-cause mortality risk in 222Â 497 australian adults. Archives of Internal Medicine. 2012;172:494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton MT, Hamilton DG, Zderic TW. Role of Low Energy Expenditure and Sitting in Obesity, Metabolic Syndrome, Type 2 Diabetes, and Cardiovascular Disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 42.Bleeker MWP, De Groot PCE, Rongen GA, Rittweger J, Felsenberg D, Smits P, Hopman MTE. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol. 2005;99:1293–1300. doi: 10.1152/japplphysiol.00118.2005. [DOI] [PubMed] [Google Scholar]
- 43.Pletcher MJ, Bibbins-Domingo K, Lewis CE, Wei GS, Sidney S, Carr JJ, Vittinghoff E, McCulloch CE, Hulley SB. Prehypertension during Young Adulthood and Coronary Calcium Later in Life. Annals of Internal Medicine. 2008;149:91–99. doi: 10.7326/0003-4819-149-2-200807150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol. 2010;298:H1128–1135. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


