Summary
CD52 and CD20 antigens are important therapeutic targets for the monoclonal antibodies (mAbs) alemtuzumab and rituximab respectively. Circulating CD52 (cCD52) and CD20 (cCD20) have prognostic utility in lymphoid malignancies. The efficacy of mAb therapy in patients with chronic lymphocytic leukaemia (CLL) may be adversely affected by cCD52 or cCD20. In this report, blood and bone marrow (BM) cCD52 and cCD20 were measured at response assessment in previously treated (N = 235) patients with CLL who received fludarabine, cyclophosphamide, and rituximab (FCR). Univariate and multivariate statistical models evaluated correlations of pre- and response variables with progression-free (PFS) and overall survival (OS). Response variables included 1996 National Cancer Institute-Working Group (NCI-WG) response, polymerase chain reaction (PCR) for immunoglobulin heavy chain (IGHV) in BM, and cCD52 and cCD20 levels (blood and BM) at response assessment. Using multivariate analysis, response blood and BM cCD52, blood cCD20, and NCI-WG response were significant independent predictors of PFS. At the time of response assessment, BM cCD52 correlated with OS in univariate analysis. cCD52 and cCD20, therefore appear useful in predicting survival and may be important for monitoring patients following salvage FCR (fludarabine, cyclophosphamide, rituximab) therapy. These data further indicate that plasma may be a good target to evaluate for minimal residual disease using cCD52/cCD20 levels.
Keywords: CD20, CD52, CLL, fludarabine, cyclophosphamide and rituximab, salvage
The clinical course for patients with chronic lymphocytic leukaemia (CLL) is varied, with some patients having smouldering, asymptomatic disease and others having rapid progression. Identifying prognostic markers and factors that may be useful in guiding management and follow-up are important for patients with CLL. Prognostic factors that are being studied as potentially important predictors of clinical endpoints, such as time to treatment, response to treatment, remission duration, and overall survival (OS), include IGHV mutation status; expression of ZAP-70 and CD38 by leukaemia cells; plasma levels of β2 microglobulin (β2M) and circulating CD23; and presence of chromosome abnormalities, such as 17p deletion and 11q deletion by fluorescence in situ hybridization (FISH) analysis. These factors were initially studied in selected patient populations in retrospective analyses. True appreciation of the utility and importance of each factor will come with prospective analyses in unselected patients. Some of these factors are static and do not change through the clinical course, and others may change, such as acquisition of new chromosomal abnormalities (clonal evolution). Less is known about the importance of these prognostic factors in previously treated patients.
Chemoimmunotherapy, specifically the combination of anti-CD20 (rituximab) and/or anti-CD52 (alemtuzumab) monoclonal antibodies with chemotherapeutic agents, has shown impressive efficacy in the treatment of CLL in frontline and salvage settings (Keating et al, 2005; Wierda et al, 2005; Ravandi & O'Brien, 2006; Tam et al, 2008). CD20 is an integral membrane protein expressed on the surface of B lymphocytes and may play a role in proliferation, differentiation, and regulation of B lymphocyte activities (Golay et al, 1985; Tedder & Engel, 1994). Although it has four transmembrane-spanning regions, and therefore is highly membrane embedded, (Kehrl et al, 1994; Riley & Sliwkowski, 2000) high levels of circulating CD20 (cCD20) have been detected in plasma samples from patients with CLL, and have been correlated with advanced stage CLL and shorter survival (Manshouri et al, 2003). This cCD20 probably represents membrane fragments in plasma from apoptotic cells that result from cell turnover, rather than specifically shed protein (Manshouri et al, 2003). In vitro studies have shown that cCD20 can interfere with rituximab binding to CLL cells; therefore, high cCD20 levels may act as an in vivo sump, altering rituximab pharmacokinetics and minimizing the clinical response to rituximab (Manshouri et al, 2003).
The amount of circulating CD52 (cCD52) has prognostic value in patients with CLL (Albitar et al, 2004). CD52 is a glycosylphosphatidylinositol (GPI)-anchored cell protein expressed on the surface of haematopoietic cells, including T and B lymphocytes, monocytes, macrophages, and eosinophils (Gilleece & Dexter, 1993; Elsner et al, 1996; Rowan et al, 1998; Taylor et al, 2000). CD52 is also expressed on neoplastic cells of lymphoid and myeloid origin (Salisbury et al, 1994; Belov et al, 2001). cCD52 was detected at higher levels in patients with CLL, compared to healthy individuals. Furthermore, low levels of cCD52 were correlated with less aggressive disease, higher complete response (CR) rates following treatment and longer survival in patients with CLL (Albitar et al, 2004). In contrast to CD20, and in view of its GPI linkage, CD52 is thought to be actively shed from the surface of lymphocytes. cCD52 may also be detected as membrane fragments produced by apoptosis and cell turnover. cCD52 may interfere with alemtuzumab binding to CLL cells, and lower levels of cCD52 have been correlated with increased response to alemtuzumab (Albitar et al, 2004).
There is a clear correlation between the response to treatment by 1996 National Cancer Institute-Working Group (NCI-WG) criteria (Cheson et al, 1996) and the progression-free survival (PFS) and OS for patients with CLL. One of the potential shortcomings of the 1996 NCI-WG response criteria is that residual disease is identified by microscopic examination of blood and bone marrow, which does not address residual disease in others sites, such as lymph node and spleen. New assays to evaluate response and predict for PFS and OS potentially could therefore be used in conjunction with 1996 NCI-WG response criteria to better predict these important clinical outcomes.
We evaluated the prognostic value of cCD20 and cCD52 in peripheral blood and BM at the time of response assessment from 235 previously treated patients (Wierda et al, 2005) who received the three-drug combination of fludarabine, cyclophosphamide and rituximab (FCR). Additionally, we evaluated the pre-treatment characteristics, which may be helpful in predicting PFS and OS following FCR therapy in patients with CLL.
Materials and methods
Patients and treatment protocol
Patients in this analysis included 177 patients from a previously published study investigating the efficacy of FCR in the salvage setting (Wierda et al, 2005) for whom we had cryopreserved samples, and 58 additional patients. All patients provided informed consent according to U.T. MD Anderson Cancer Center Institutional guidelines. Briefly, 235 previously treated patients with CLL were enrolled on a phase II clinical trial starting in November, 1999. Pre-treatment evaluation included history, physical examination, complete blood count (CBC) with differential, platelet (PLT) count, liver and kidney function tests, β2M, lactate dehydrogenase (LDH), and BM aspiration with immunophenotyping and BM biopsy. Refractoriness to fludarabine or alkylating agents was defined as failure to achieve at least partial remission (PR) with the last agent-based treatment or progression within 6 months of treatment.
Patients were to receive up to six courses of fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2, each daily for 3 d, and a single dose of rituximab 375 mg/m2, course 1 and 500 mg/m2 courses 2–6 (Keating et al, 2005; Wierda et al, 2005). Each course was 4 weeks as permitted by recovery of neutrophil and platelet counts.
Response to treatment and progression were evaluated by the 1996 NCI-WG response criteria (Cheson et al, 1996). CR required resolution of all palpable disease, normalization of blood counts and no evidence of disease in BM biopsy. Nodular partial remission (nPR) required the same criteria as for CR except that one or more lymphoid nodules or aggregates were present on bone marrow biopsy. PR criteria included at least 50% reduction in measurable disease and one or more of the following features: neutrophil count > 1·5 × 109/l or a 50% improvement over baseline, platelets ≥ 100 × 109/l or a 50% improvement over baseline, and haemoglobin ≥ 110 g/l or a 50% improvement over baseline without transfusions. Imaging studies (i.e. computed tomography scans) were not required to evaluate response. The response must have persisted for more than 2 months. Patients’ BM was also evaluated by 2-colour flow cytometry for presence of CD5/19+ cells as a measure of residual disease.
Blood and/or bone marrow (BM) samples for cCD20 and cCD52 evaluation were obtained pre-treatment in a limited number of patients, and at specific time points following treatment in greater proportion. Samples at the following time points from start of treatment were available for BM cCD20 measurement: pre-treatment (n = 50), 3 months (n = 29), 6 months (time of response assessment) (n = 66), 12 months (n = 71), 18 months (n = 49), 24 months (n = 48), and 36 months (n = 38); BM cCD52 measurement: pre-treatment (n = 50), 3 months (n = 29), 6 months (n = 67), 12 months (n = 71), 18 months (n = 49), 24 months (n = 48), and 36 months (n = 38); blood cCD20 measurement: pre-treatment (n = 27), 3 months (n = 22), 6 months (n = 61), 12 months (n = 68), 18 months (n = 46), 24 months (n = 54), and 36 months (n = 41); and blood cCD52 measurement: pre-treatment (n = 27), 3 months (n = 21), 6 months (n = 62), 12 months (n = 68), 18 months (n = 47), 24 months (n = 53), and 36 months (n = 42). To reduce the possibility that the clotting process may damage circulating cells and influence the levels of cCD20, plasma, rather than serum, was used for measuring cCD20 and cCD52 in blood. Anti-coagulated BM samples were centrifuged to obtain the non-cellular fluid phase used to measure BM cCD52 and cCD20. Despite the precautions taken during BM sample collection, dilution of BM samples by blood cannot be fully excluded.
ELISA for cCD20 and cCD52
Enzyme-linked immunosorbent assay (ELISA) was employed to detect plasma cCD20 and cCD52 levels as previously described (Manshouri et al, 2003; Albitar et al, 2004). Briefly, the sample was added to a 96-well polystyrene plate coated with anti-CD20 or anti-CD52, and incubated for 3 h with constant shaking at room temperature. Subsequently, the plates were washed with phosphate-buffered saline (PBS) containing 0·1% Tween 20 (PBS-T) and then incubated for 3 h with 20 μl of humanized anti-CD20 antibody (rituximab) or anti-CD52 (alemtuzumab) conjugated with horseradish peroxidase at a dilution of 1:400 in 2% bovine serum albumin (BSA) and 0·1% Tween 20. Following six more washes with PBS-T, 100 μl of colorimetric substrate was added to the wells and plates were then incubated with constant shaking for 15–30 min. Following the addition of 50 μl of 2 N HCl to quench the reaction, the plates were read at a wavelength of 450 nm.
Minimal Residual Disease (MRD) by PCR for IGHV
Assessment of IGHV for MRD was performed as previously described (Jilani et al, 2006). PCR was performed on DNA samples from blood and BM cells using 5′ fluorescently end-labelled consensus primers for the rearranged IGH CDRIII region, FR3A [5′-ACACGGC(C/T)(G/C) TGTATTACTGT-3′] and VLJH (5′-GTGACCAGGGTNCCTTGGCCCCAG-3′). PCR products were resolved on 3100 genetic analyzers (Applied Biosystems, Foster City, CA, USA), and peak areas and heights were determined by Genescan analysis software (Applied Biosystems). Fluorescently-tagged PCR amplification products were subsequently digested with the restriction enzyme HaeIII (New England Biolabs, Beverley, MA, USA).
Resulting fragments were resolved on a polyacrylamide gel, and then isolated using gel extraction kit from Qiagen (Valencia, CA, USA). Ligase chain reaction (LCR) using Taq DNA Ligase (New England Biolabs) was subsequently performed on patient's PCR amplified DNA, and products were resolved on the ABI 3100 under similar conditions described for the PCR assay. The ratio of the peak areas of VLJH to K-RAS (internal control) was reported as positive (ratio > 0·2), low positive (ratio < 0·2), or negative (ratio = 0). This ligase-based assay was reported to have similar sensitivity to allele-specific PCR (Jilani et al, 2006).
Statistical analysis
Patient characteristics were summarized using median (range) for numerical variables and frequency (percentage) for categorical variables. OS time was defined as the time interval from enrollment on protocol (or start of therapy) to death or last follow-up. Progression-free survival (PFS) was defined as the time interval from enrollment on protocol to progression (1996 NCI-WG criteria), death or last follow-up. Both cCD20 and cCD52 were treated as continuous variables in Cox regression analyses. OS or PFS probabilities were estimated by Kaplan and Meier analyses. The differences in OS or PFS among subgroups of patients were evaluated using the log-rank test. Cox proportional hazards regression model was used to assess the ability of patient characteristics to predict OS or PFS, with goodness-of-fit assessed by the Grambsch-Therneau test, Schoenfeld residual plots, and martingale residual plots. Predictive variables were transformed as appropriate based on these plots. CART (Categorization and Regression Tree) analysis was used to search for the optimal cutoff values for covariates. Tables I and II contain the full list of variables that were assessed in the univariate Cox models. Only variables with P-value ≤ 0·05 in the univariate fit were considered for the multivariate models. A multivariate Cox model was developed for OS and PFS by performing a backward elimination with P-value cutoff of 0·05, then allowing any variable previously deleted to enter the final model if its P-value was <0·05. Of note, we did not include pre- and post-treatment variables in the same multivariate analyses. Natural logarithmic (Ln)-transformation was used for some variables to minimize skewing of data points. All computations were carried out in S-Plus.
Table I.
Patient characteristics (N = 235).
| Pre-treatment characteristics | Number (%) |
|---|---|
| Gender | |
| Male | 169 (72) |
| Rai Stage | |
| 0–II | 129 (55) |
| III–IV | 107 (45) |
| Number of enlarged nodal sites | |
| 0–1 | 43 (19) |
| 2–3 | 188 (81) |
| ECOG performance status | |
| 0 | 36 (15) |
| 1 | 185 (79) |
| 2–3 | 11 (5) |
| Number of prior treatments | |
| 1 | 98 (42) |
| 2 | 67 (29) |
| ≥3 | 70 (30) |
| Refractory to alkylating agent | |
| No | 107 (46) |
| Yes | 59 (25) |
| No Rx | 66 (28) |
| Refractory to Fludarabine | |
| No | 141 (60) |
| Yes | 39 (17) |
| No Rx | 51 (22) |
| Median (range) | |
|---|---|
| Age (years) | 59 (31–82) |
| Liver (cm) | 0 (0–17) |
| Spleen (cm) | 0 (0–25) |
| Haemoglobin (g/l) | 124 (68–165) |
| PLT (×109/l) | 125 (6·0–391) |
| ALC (×109/l) | 36 (0·2–414) |
| Alkaline phosphatase (i/u per litre) | 79 (41–195) |
| Albumin (g/l) | 40 (20–49) |
| Creatinine (μmol/l) | 97·24 (44·2–291·72) |
| β2M (mg/l) | 4·4 (1·9–19·6) |
| LDH (i/u per litre) | 575 (36–2859) |
| BM cellularity (%) | 60 (5–100) |
ECOG, Eastern Cooperative Oncology Group; ALC, absolute lymphocyte count; BM, bone marrow; β2M, Beta-2 Microglobulin; LDH, lactate dehydrogenase; PLT, platelet.
Table II.
Response to treatment.
| Number (%) | |
|---|---|
| NCI-WG response to treatment | |
| CR | 70 (30) |
| nPR | 35 (15) |
| PR | 71 (30) |
| BM two-colour flow cytometry | |
| <1% CD5/19+ | 49 (46) |
| 1–5% CD5/19+ | 30 (28) |
| >5% CD5/19+ | 28 (26) |
| BM PCR for IGHV | |
| Negative/low positive | 64 (55) |
| Positive | 53 (45) |
NCI-WG, 1996 National Cancer Institute Working Group; CR, Complete remission; nPR, nodular partial remission; OR, Overall response; BM, Bone marrow; PCR, polymerase chain reaction.
Results
Patient characteristics
Pre-treatment patient characteristics are presented in Table I. Routine blood studies, included blood counts and chemistries, physical examination findings and BM biopsy were evaluated at study entry. Seventy percent of patients were male, Rai stages were well represented, and most patients had a performance status (PS) of 0 or 1. Seventy percent of patients had received ≤ 2 prior treatments, and 25% and 17% were considered refractory to alkylating agents and fludarabine respectively. Significant differences were noted between BM (median = 100 nmol/l) and peripheral blood cCD20 (median = 257 nmol/l) (P = 0·02, Wilcoxn rank-sum test) as well as peripheral blood cCD20 and cCD52 (Spearman correlation coefficient = 0·54; P-value < 0·001).
Response to treatment
The majority (63%) of patients completed ≥ 4 courses of FCR. Responses to treatment are shown in Table II. CR and PR rates were both 30%; nodular PR was seen in 15% of patients. Measures of residual disease, in addition to 1996 NCI-WG response criteria, were used to evaluate patients. Two-colour flow cytometry evaluation of the BM for CD5/CD19 at response assessment or 2 months following the last cycle of FCR for those that did not receive 6 cycles, and PCR for IGHV yielded similar frequencies of patients with MRD (25% vs. 23% respectively). Distribution of cCD20 and cCD52 levels is shown in Table III. The majority of patients had cCD20 and cCD52 values available at the time of response assessment; however, too few patients had pre-treatment values to perform meaningful analyses. We performed univariate and multivariate analyses using cCD20 and cCD52 levels at the time of response assessment. The 1st and 3rd quartile values are shown to demonstrate the distribution of cCD20 and cCD52 measurements used in these analyses (Table III). For a given patient, there was a significant difference between the BM and blood cCD20 at the time of response assessment (P = 0·02, Wilcoxon rank-sum test), but no significant differences were noted for BM and blood cCD52 at the time of response assessment (P = 0·72, Wilcoxon rank-sum test). Additionally, to determine the correlation between number of FCR cycles and blood cCD20 and cCD52 levels, patients were grouped into 3 categories based on the number of FCR cycles received: (i) ≤3 cycles; (ii) 4 or 5 cycles; and (iii) ≥6 cycles. There was a significant association between blood cCD20 at the time of response assessment and the number of FCR cycles (P-value = 0·04; Kruskal–Wallis test), but no significant association between blood CD52 at 6 months and the number of FCR cycles (P-value = 0·95; Kruskal–Wallis test).
Table III.
Distribution of bone marrow (BM) and blood cCD20 and cCD52 at response assessment.
| N | Median (nmol/l) (Range) | 25th Percentile | 75th Percentile | |
|---|---|---|---|---|
| BM cCD20 | 66 | 100 (0–7142) | 44 | 227 |
| Blood cCD20 | 61 | 256 (0–4990) | 56 | 492 |
| BM cCD52 | 67 | 51 (0–7075) | 10 | 129 |
| Blood cCD52 | 62 | 79 (0–3213) | 9 | 190 |
BM, bone marrow.
Progression-free and overall survival
At the time of these analyses, the median follow up time was 75 months [95% confidence interval (CI): 72–81]. One hundred and seventeen patients (66%) of 177 responders (CR, nPR, or PR) have relapsed and the median time to progression was 29 months (95% CI = 27–38 months). Of the 233 patients analysed, 146 patients (63%) have died and the median survival time was 47 months (95% CI = 41–58 months).
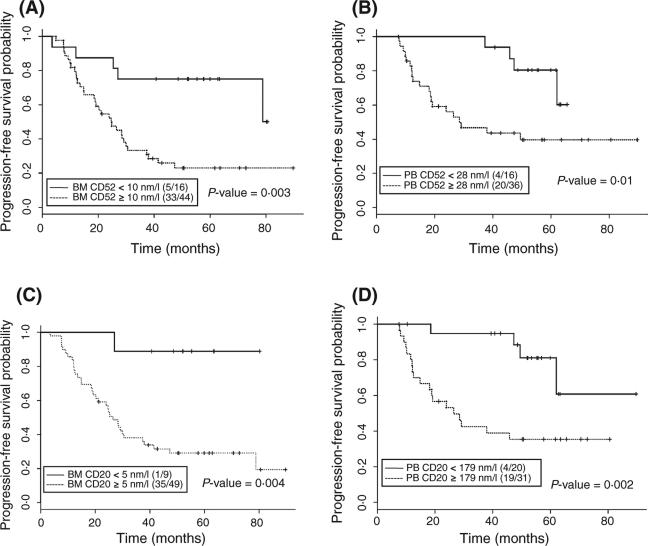
We determined cutoff values for BM CD52 and CD20, as well as blood CD52 and CD20, and correlated these values with PFS (Fig 1). Cutoff values for BM CD52, BM CD20, blood CD52 and blood CD20 at response assessment were 10, 5, 28 and 179 nmol/l respectively. The cutoff values for cCD20 or cCD52 were chosen using CART (classification and regression tree) analysis, which gives a more robust analysis compared to using median values or quartiles, the latter limited by the small number of patients for whom we had measured values at the indicated quartiles. PFS was significantly longer in patients with lower CD20 and CD52 levels in BM and blood at end of treatment (Fig 1). Although BM CD20 was significant in univariate analysis, it did not achieve statistical significance in the multivariate model. The use of rituximab in the treatment of all patients in this study may have affected the measurement of CD20 and therefore the outcomes of the analysis.
Fig 1.
Kaplan–Meier estimates for PFS. PFS by (A) BM cCD52, (B) Blood cCD52, (C) BM cCD20, and (D) Blood CD20 at Response Assessment. Cutoff value was found using CART analysis. Ratios indicate the number of events per total number of patients analysed.
Survival was correlated with 1996 NCI–WG response status (Cheson et al, 1996); those who achieved a CR had the longest PFS, while patients with PR had the shorter PFS (data not shown). Similarly, a significantly longer OS was noted in patients who achieved CR/nPR, compared with those who achieved PR. Patients who did not respond to treatment [non-responder/early death (NR/ED)] had the shortest OS (data not shown).
Predictors of PFS and OS
Univariate analyses showed the following pretreatment characteristics to be correlated with shorter PFS: advanced age (relative risk (RR) = 1·020; P = 0·02), elevated β2M (ln β2M) (RR = 1·808; P = 0·008) and elevated LDH (RR = 1·733; P = 0·05); smaller spleen size was correlated with longer PFS (RR = 0·948; P = 0·04). Table IVA shows factors evaluated at end of treatment that correlated with longer PFS, namely lower cCD20 and cCD52 in blood and bone marrow and lower level of residual disease by two-colour flow cytometry for CD5/CD19+ cells and PCR for IGHV in bone marrow.
Table IV.
Univariate cox proportional hazards models of post-treatment predictors for progression-free (A) (N = 179) or Overall (B) (N = 232) survival.
| Variable | RR | P-value |
|---|---|---|
| A. PFS | ||
| Ln BM cCD20 at response (nmol/l) | 1·189 | 0·03 |
| Ln BM cCD52 at response (nmol/l) | 1·290 | 0·002 |
| Ln Blood cCD20 at response (nmol/l) | 1·257 | 0·03 |
| Ln Blood cCD52 at response (nmol/l) | 1·304 | 0·02 |
| BM CD5/19+ >5% (vs. ≤5%) | 2·863 | 0·001 |
| BM PCR for IGHV (Positive versus negative/low positive) | 2·614 | <0·001 |
| B. OS | ||
| Ln BM cCD52 at Response | 1·194 | 0·04 |
| BM CD19 > 5% (vs. ≤ 5%) | 1·910 | 0·02 |
| BM PCR for IGHV (Positive versus negative/low positive) | 2·266 | 0·002 |
BM, bone marrow; RR, relative risk; PFS, progression-free survival; OS, overall survival; IGHV, immunoglobulin heavy chain variable gene; Ln, natural log.
Univariate analyses for pretreatment characteristics that correlated with shorter OS were advanced age (RR = 1·035; P ≤ 0·001); advanced Rai stage (high-risk) (RR = 1·8; P < 0·001); low haemoglobin (RR = 0·86; P ≤ 0·001); low albumin (RR = 0·62; P = 0·006); elevated alk phos (RR = 1·006; P = 0·03); elevated creatinine (ln Cr) (RR = 2·201; P = 0·03); elevated LDH (RR = 1·87; P = 0·002); elevated β2M (ln β2M) (RR = 2·67; P ≤ 0·001); more than one prior treatment (RR = 1·47; P = 0·03); and refractoriness to alkylating agents (RR = 1·67; P = 0·007). Table IVB shows factors evaluated at end of treatment that correlated with longer PFS, namely lower cCD52 in bone marrow and lower level of residual disease by two-colour flow cytometry for CD5/CD19+ cells and PCR for IGHV in bone marrow.
Multivariate analysis of pre- and post-treatment variables evaluated separately demonstrated that elevated β2M and splenomegaly were independently associated with shorter PFS (Table V). Shorter PFS was independently associated with an inferior response to treatment (NCI-WG criteria), higher blood cCD20 and cCD52 at end of treatment and higher BM cCD52 at end of treatment (Table V). Elevated β2M (ln β2M) (RR = 2·46; P < 0·001), lower albumin level (RR = 0·66; P = 0·05) and refractoriness to alkylating agents (RR = 1·68; P = 0·009) were significant independent pre-treatment predictors for shorter OS in multivariate analysis. The strongest post-treatment predictor for a shorter OS was achievement of PR/NR/ED (versus CR/nPR-1996 NCI-WG response status) (RR = 4·70; P < 0·0001). Neither cCD52 nor cCD20 added to this multivariate model. The lack of association of cCD52 and cCD20 with OS may be attributed to that fact that many patients received subsequent salvage therapy, which can impact survival.
Table V.
Fitted multivariate cox proportional hazards model for progression-free survival using Pre- (A) or Post (B–D)-treatment characteristics.
| RR | P-value | |
|---|---|---|
| A. | ||
| Pre-treatment characteristic (N = 164) | ||
| Spleen size (cm) | 0·93 | 0·006 |
| Ln β2M (mg/l) | 2·24 | <0·001 |
| B. | ||
| Post-treatment characteristic (N = 51) | ||
| NCI-WG response: PR/NR/ED (versus CR/nPR) | 4·81 | <0·001 |
| Ln Blood cCD20 at response | 1·24 | 0·04 |
| C. | ||
| Post-treatment characteristic (N = 52) | ||
| NCI-WG response: PR/NR/ED (versus CR/nPR) | 4·61 | <0·001 |
| Ln Blood cCD52 at response | 1·28 | 0·04 |
| D. | ||
| Post-treatment characteristic (N = 61) | ||
| NCI-WG response: PR/NR/ED (versus CR/nPR) | 2·85 | 0·005 |
| Ln BM cCD52 at response | 1·17 | 0·05 |
β2M, beta-2 microglobulin; PR, partial remission; CR, complete remission; nPR, nodular partial remission; BM, bone marrow; RR, relative risk; Ln, natural log.
Discussion
cCD20 and cCD52 appear to be important biological markers in CLL. We have previously analysed patient characteristics in this cohort of patients that predict for survival (Wierda et al, 2006); however, the data presented in this study demonstrate for the first time that cCD20 and cCD52 levels at the time of response assessment are predictors for post-treatment survival (PFS or OS) in CLL patients treated with FCR. Most notably, BM and blood cCD52 as well as blood cCD20 at response assessment were independent predictors for PFS in patients treated with FCR. Although our data seem to validate previously reported pre-treatment clinical and laboratory parameters as being significant prognostic factors for patients with CLL, this is the first report to describe that cCD20 and cCD52 at response assessment are important predictors of disease outcomes in CLL.
Prior reports demonstrated the prognostic significance of both cCD20 and cCD52 in patients with lymphoid malignancies. Albitar et al (2004) showed that cCD52 was detectable at baseline in plasma from patients with CLL and that higher levels significantly correlated with advanced Rai stage, higher β2M, immunoglobulin mutation status, and worse survival. Similarly, higher baseline cCD20 levels in plasma were shown to correlate positively with β2M, leukaemia cell expression of CD38, advanced stage disease (Rai and Binet), and significantly shorter survival (Manshouri et al, 2003). In patients with non-Hodgkin (NHL) and Hodgkin lymphoma, pre- and post-treatment levels of serum cCD52 had no predictive value for survival, while post- treatment serum cCD20 levels were highly correlated with survival in NHL patients (Giles et al, 2003). Our results further extend these findings, specifically highlighting the prognostic value of cCD20 and cCD52 at the time of response assessment following therapy with FCR in previously treated patients. Unlike the aforementioned report examining post-treatment cCD20 and cCD52 levels, where patients were treated with various regimens not including rituximab or alemtuzumab, (Giles et al, 2003) all patients included in our study were treated uniformly with FCR. Furthermore, we did not use previously reported cutoff values for cCD20 and cCD52 in our analyses but examined values at the time of response assessment and general trends over time. In fact, the median values measured in our studies for cCD52 and cCD20 were much lower than those previously reported (1875 nmol/l and 2336 nmol/l for cCD20 and cCD52 respectively); (Manshouri et al, 2003; Albitar et al, 2004) this is probably secondary to our reported values representing post-treatment cCD20 and cCD52. Previously well-documented prognostic factors including age, haemoglobin, β2M, and NCI-WG response status were confirmed in our analyses. Although BM samples may be more difficult to obtain in clinical practice, BM values are part of the 1996 NCI-WG response criteria, and therefore BM cCD20 and cCD52 were included in our study.
cCD20 and cCD52 levels were correlated with PFS in multivariate analysis (Table V). Continued follow up may demonstrate that cCD20 and cCD52 are important prognostic factors for OS, even though they were not significantly correlated with OS in this analysis. In addition to cCD20 and cCD52, our results also showed PCR for IGHV to be a significant prognostic factor in previously treated patients, with higher levels correlated with shorter survival. Detection of IGHV is a sensitive marker of residual disease, and may signify failure of FCR to fully eradicate the malignant clones. Nevertheless, when used simultaneously with cCD52 and cCD20 in the multivariate analysis models (Table V), IGHV lost its significance as a predictor of PFS (versus cCD20 or cCD52), thereby indicating that, in comparison with IGHV, cCD20 and cCD52 may be better prognostic factors for PFS.
In addition to PFS and OS, we also analysed the association of CD5/CD19, cCD20 and cCD52 levels with MRD. There was a significant association between CD5/CD19 level (categorized as low, intermediate and high) and PCR for MRD (P = 0·0002, Fisher's exact test) as well as a significant association between blood (P = 0·03, Kruskal–Wallis test) and BM (P = 0·006, Kruskal–Wallis test) cCD52 at response assessment and PCR for MRD (data not shown). There were no significant associations between PCR for MRD and blood (P = 0·65, Kruskal–Wallis test) and BM (P = 0·56, Kruskal–Wallis test) cCD20 levels at response assessment (data not shown). We are prospectively studying the role of CD20 and CD52 in the detection of MRD, in comparison with the more standard approaches, which include four-colour flow cytometry (CD5, CD19, CD43 and CD20) and allele-specific PCR.
In summary, cCD20 and cCD52 levels at the time of response assessment had prognostic value in previously treated patients receiving FCR and potentially other treatment regimens. Elevated cCD20 or cCD52 levels at the time of response assessment following treatment with FCR indicate higher likelihood for relapse or shorter survival; in such cases additional treatment may be warranted. Further ongoing studies focusing on the association of cCD20 and cCD52 with rituximab therapy, as well as role of pre-treatment cCD20 and cCD52 are needed to determine their prognostic value in patients receiving FCR.
References
- Albitar M, Do KA, Johnson MM, Giles FJ, Jilani I, O'Brien S, Cortes J, Thomas D, Rassenti LZ, Kipps TJ, Kantarjian HM, Keating M. Free circulating soluble CD52 as a tumor marker in chronic lymphocytic leukemia and its implication in therapy with anti-CD52 antibodies. Cancer. 2004;101:999–1008. doi: 10.1002/cncr.20477. [DOI] [PubMed] [Google Scholar]
- Belov L, de la Vega O, dos Remedios CG, Mulligan SP, Christopherson RI. Immunophenotyping of leukemias using a cluster of differentiation antibody microarray. Cancer Research. 2001;61:4483–4489. [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, Rai KR. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- Elsner J, Hochstetter R, Spiekermann K, Kapp A. Surface and mRNA expression of the CD52 antigen by human eosinophils but not by neutrophils. Blood. 1996;88:4684–4693. [PubMed] [Google Scholar]
- Giles FJ, Vose JM, Do KA, Johnson MM, Manshouri T, Bociek G, Bierman PJ, O'Brien SM, Keating MJ, Kantarjian HM, Armitage JO, Albitar M. Circulating CD20 and CD52 in patients with non-Hodgkin's lymphoma or Hodgkin's disease. British Journal Haematology. 2003;123:850–857. doi: 10.1046/j.1365-2141.2003.04683.x. [DOI] [PubMed] [Google Scholar]
- Gilleece MH, Dexter TM. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood. 1993;82:807–812. [PubMed] [Google Scholar]
- Golay JT, Clark EA, Beverley PC. The CD20 (Bp35) antigen is involved in activation of B cells from the G0 to the G1 phase of the cell cycle. Journal of Immunology. 1985;135:3795–3801. [PubMed] [Google Scholar]
- Jilani I, Keating M, Day A, William W, Kantarjian H, O'Brien S, Giles FJ, Albitar M. Simplified sensitive method for the detection of B-cell clonality in lymphoid malignancies. Clinical and Laboratory Haematology. 2006;28:325–331. doi: 10.1111/j.1365-2257.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- Keating MJ, O'Brien S, Albitar M, Lerner S, Plunkett W, Giles F, Andreeff M, Cortes J, Faderl S, Thomas D, Koller C, Wierda W, Detry MA, Lynn A, Kantarjian H. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. Journal of Clinical Oncology. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Kehrl JH, Riva A, Wilson GL, Thevenin C. Molecular mechanisms regulating CD19, CD20 and CD22 gene expression. Immunology Today. 1994;15:432–436. doi: 10.1016/0167-5699(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Manshouri T, Do KA, Wang X, Giles FJ, O'Brien SM, Saffer H, Thomas D, Jilani I, Kantarjian HM, Keating MJ, Albitar M. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood. 2003;101:2507–2513. doi: 10.1182/blood-2002-06-1639. [DOI] [PubMed] [Google Scholar]
- Ravandi F, O'Brien S. Alemtuzumab in CLL and other lymphoid neoplasms. Cancer Investigation. 2006;24:718–725. doi: 10.1080/07357900600981414. [DOI] [PubMed] [Google Scholar]
- Riley JK, Sliwkowski MX. CD20: a gene in search of a function. Seminars in Oncology. 2000;27:17–24. [PubMed] [Google Scholar]
- Rowan W, Tite J, Topley P, Brett SJ. Cross-linking of the CAMPATH-1 antigen (CD52) mediates growth inhibition in human B- and T-lymphoma cell lines, and subsequent emergence of CD52-deficient cells. Immunology. 1998;95:427–436. doi: 10.1046/j.1365-2567.1998.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury JR, Rapson NT, Codd JD, Rogers MV, Nethersell AB. Immunohistochemical analysis of CDw52 antigen expression in non-Hodgkin's lymphomas. Journal of Clinical Pathology. 1994;47:313–317. doi: 10.1136/jcp.47.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ML, Noble PW, White B, Wise R, Liu MC, Bochner BS. Extensive surface phenotyping of alveolar macrophages in interstitial lung disease. Clin Immunol. 2000;94:33–41. doi: 10.1006/clim.1999.4803. [DOI] [PubMed] [Google Scholar]
- Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunology Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Wierda W, O'Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D, Do KA, Cortes J, Koller C, Beran M, Ferrajoli A, Giles F, Lerner S, Albitar M, Kantarjian H, Keating M. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. Journal of Clinical Oncology. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- Wierda W, O'Brien S, Faderl S, Ferrajoli A, Wang X, Do KA, Garcia-Manero G, Thomas D, Cortes J, Ravandi-Kashani F, Giles F, Lerner S, Kantarjian H, Keating M. A retrospective comparison of three sequential groups of patients with Recurrent/Refractory chronic lymphocytic leukemia treated with fludarabine-based regimens. Cancer. 2006;106:337–345. doi: 10.1002/cncr.21554. [DOI] [PubMed] [Google Scholar]