Abstract
The adult mammalian cerebellar cortex is generally assumed to have a uniform cytoarchitecture. Differences in cerebellar function are thought to arise, in the main, through distinct patterns of input and output connectivity, rather than as a result of variations in cortical microcircuitry. However, evidence from anatomical, physiological and genetic studies is increasingly challenging this orthodoxy and there are now various lines of evidence that the cerebellar cortex is non uniform. Here we develop the hypothesis that regional differences in cerebellar cortical microcircuit properties lead to important differences in information processing.
Introduction
The cerebellum is essential for the performance of smooth and accurate goal-directed movements, making postural adjustments in order to maintain balance, and learning new motor skills. It is also involved in a host of other activities, ranging from control of the autonomic system through associative learning to cognition1. This remarkable diversity of function is made possible by the massive extent of the outer shell – the cortex – of the mammalian cerebellum, which contains more neurons than the rest of the CNS put together2.
On a macro scale, the cerebellum consists of two hemispheres united in the midline by a region known as the vermis (Fig. 1a). Two deep fissures divide the cerebellar cortex into three lobes; the anterior and posterior lobes, which are divided by the primary fissure, and the flocculonodular lobe, which is separated from the posterior lobe by the posterolateral fissure. These regions are further subdivided into lobules by shallow fissures, and depending on the species certain lobules can be folded into sub-lobules or folia (Fig. 1b).
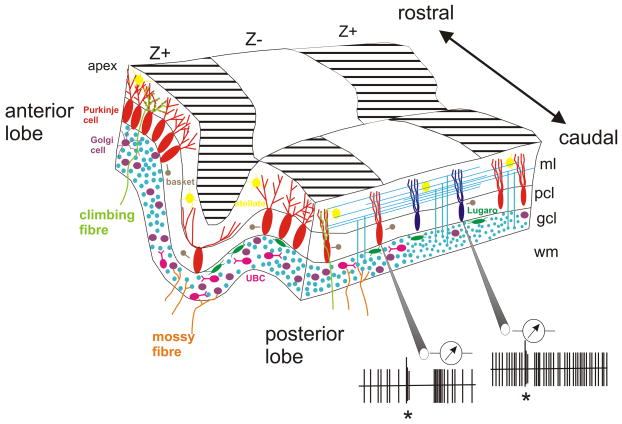
Figure 1. Classical view of cerebellar cytoarchitecture.
a| Dorso-posterior view of the rat cerebellum showing its major anatomical subdivisions. Note the bilateral organization with the anatomical midline (dashed line) dividing the cerebellar vermis into two halves and a hemisphere on each side. Dotted line shows the midline plane of the section in panel b. b| Drawing of midsagittal cross-section through the rat cerebellum, showing its lobular organization, with each lobe demarcated by Roman numerals183. Dotted line demarcates the cerebellar cortex from the white matter. c| The basic cerebellar cytoarchitecture is comprised of Purkinje cells, which are the output cells of the cerebellar cortex, granule cells, Golgi cells, Lugaro cells, unipolar brush cells, stellate cells and basket cell interneurons. The two main types of afferents that project to the cerebellum are the climbing fibres, which synapse directly with Purkinje cells, and the mossy fibres, which synapse with granule cells. The axons of the granule cells ascend up to the molecular layer where they bifurcate in a T-type manner to form parallel fibres, which extend for several millimetres along the folia of cerebellar lobules. COP, copula pyramidis; FL, flocculus; gcl, granular layer; LS, lobulus simplex; ml, molecular layer; pcl, Purkinje cell layer; PF, paraflocculus; PML, paramedian lobule; UBC, unipolar brush cell. Adapted, with permission, from REF184.
Throughout its extent the cerebellar cortex is divided into three distinct layers – the molecular layer, the Purkinje cell layer and the granular layer. Together these contain seven main neuronal cell types: Purkinje cells, granule cells, Golgi cells, Lugaro cells, unipolar brush cells (UBCs), basket cells and stellate cells3–6. Of these, Purkinje cells are considered to be the most functionally important because they provide the sole output of the cortex (Fig. 1c). They are arranged in a monolayer and possess an extensive fan-like dendritic tree that projects into the molecular layer where they receive input from two major types of excitatory neuronal fibres: climbing fibres and parallel fibres. Most Purkinje cell axons make inhibitory synaptic contact with neurons in the cerebellar nuclei located deep within the cerebellar white matter. In turn, neurons within the cerebellar nuclei form most of the output from the cerebellum, providing connections to a wide range of other CNS structures in order to control movement and influence many other functions.
Because of their central role in cerebellar function, Purkinje cells have been the most extensively studied type of cerebellar neuron and are the main focus of this review. Purkinje cells are highly unusual in the CNS because they generate two distinct types of action potential. Simple spikes fire spontaneously or as a result of activation of the mossy fibre–granule cell–parallel fibre pathway, and typically occur at high rates (30–100 Hz7, 8). In sharp contrast, complex spikes, which consist of an initial action potential that is usually followed by a series of smaller spikelets, occur at very low rates (typically ~1.0 Hz7). Complex spikes are generated as a result of activity in the inferior olive climbing fibre system9, which also imposes a precise topographical order on cerebellar circuits. Purkinje cells located in rostrocaudally oriented zones within the cortex each receive their climbing fibres from a specific part of the contralateral inferior olivary complex, and provide output to a distinct territory within the cerebellar and vestibular nuclei, thereby forming a series of olivo–cortico–nuclear modules10–12. Individual cortical zones can be further subdivided into smaller regions termed microzones which are thought to be the fundamental functional units of the cerebellar cortex (for references see10). The remaining cell types in the cortex are interneurons. Golgi cells, Lugaro cells, granule cells and UBCs are located below the Purkinje cells, within the granular layer, whereas stellate and basket cells are located superficial to the Purkinje cells in the molecular layer.
Conventional wisdom maintains that the various cell types and their connectivity are essentially the same throughout the cerebellar cortex, leading to the widely held assumption that the same neural computation (which has been termed a ‘universal cerebellar transform’, for example13) is performed throughout its extent, and that regional differences in function are to a large extent due to differences in afferent and efferent connectivity. In particular, the various subdivisions of the inferior olivary complex are dominated by afferent connections arising from different regions of the CNS and, in turn, each of these olivary subdivisions provides climbing fibres that terminate in different parts of the cerebellar cortex14, 15. However, the evidence that we will describe in this review indicates that this is not the full story, and that the concept of a universal cerebellar transform probably does not hold true. With a focus on neuronal organization within the adult mammalian cerebellar cortex we consider recent evidence and revisit older studies that suggest that variations in cerebellar cortical anatomy and physiology also make a significant contribution to regional differences in function.
Non-uniform cerebellar architecture
A number of regional differences in cerebellar cytoarchitecture have long been known to exist. The most notable of these is the finding that Purkinje cells display marked regional variations in packing density. For example, in the rat, fewer Purkinje cells are located in the base of each cerebellar folium than at the apex3, 16, 17, and packing density is greater in the anterior lobe than the posterior lobe16. Regional differences in the size of Purkinje cells have also been reported: Purkinje cell diameter and volume of their organelles is larger in phylogenetically older regions of the cerebellum, such as the vermis18–20. Such differences could profoundly alter the biophysical properties and energy consumption of individual Purkinje cells located in different cortical regions. In addition, systematic differences in the calibre of Purkinje cell axons between white matter compartments21 and in the morphology of the dendritic arbour of Purkinje cells located in the base compared to the apex of a folium have also been reported3, 22 (Fig. 2). Similarly, granule cells display regional differences in packing density, with greater numbers in the apex than in the base of a folium23, and larger granule cells are found in the vermis than in the hemispheres18. In relation to the final point it should be noted, however, that Lange18 did not take into account regional differences in distribution of UBCs (see below), although it seems unlikely that this finding were due to a misclassification, given the substantial size difference between granule cells and UBCs (larger granule cells are typically half the size of UBCs24).
Figure 2. Anatomical location determines the morphology of cerebellar Purkinje cells.

Line drawings and photomicrographs illustrating representative morphologies of Purkinje cells present in the apex (upper panel) and base (lower panel) of cerebellar folia in adult mice. To the right are shown examples of Purkinje cells labelled using an L7/Pcp2CreER tamoxifen inducible genetic approach141, which selectively induces green fluorescent protein expression in a small subset of Purkinje cells (green). The tissue was counterstained with calbindin, which marks all Purkinje cells (red). In contrast to Purkinje cells located in the apex (upper panel), those located in the base region of a folium usually have dendritic arbours with two main primary dendrites (lower panel). Scale bar in photomicrograph = 50μm. Line drawings reproduced, with permission from REF22. Photomicrographs produced using the methods described in REF141.
Systematic differences in regional distribution of cell packing density and morphologies is clearly at odds with the assumption that the cerebellar cortex is uniform in structure. However, it has been suggested that the differences in the packing density of Purkinje cells and the morphology of their dendritic fields allows each cell to receive similar numbers of parallel fibre inputs irrespective of its folial location, in order to maintain a constancy of input between the molecular and Purkinje cell layers3, 17. Alternatively, the variation could be related to the difference in size of the synaptic contacts made by climbing fibres and parallel fibres at different levels of the molecular layer25, or differences in parallel fibre length. Indeed parallel fibres in the deep parts of the molecular layer have been found to be thicker and only a third to half the length of those found superficially 26. Mossy fibres originating from the spinal cord versus the basal pontine nucleus have been shown to synapse with superficial and deep granule cells respectively3, 27. Whether differences in Purkinje cell morphology are also related to differences in mossy fibre terminations and in the way granule cells process their mossy fibre inputs is unknown. However, the lower density of Purkinje cells and greater overlap of their dendritic fields in the base of fissures may compensate for the need to integrate functionally diverse mossy fibre–parallel fibre signals that is required by adaptive filter models of cerebellar function (see Supplementary Information28), although empirical evidence for this remains to be determined.
Regional differences in inhibitory interneurons have also been reported. Probably the most striking example of this is the packing density of UBCs. They are most numerous in the nodulus (vermal lobules Xa and Xb), ventral uvula (vermal lobule IXd), flocculus, and ventromedial paraflocculus29. Additionally, three molecularly distinct UBC subtypes that express either calretinin, metabotropic glutamate receptor 1 α (mGluR1α) or phospholipase Cβ4 (PLCβ4) have been found, each with their own topographical distribution29–33, see REF 34 for a recent review). As UBCs are located primarily in areas related to vestibular and oculomotor function, this suggests that the operation of the cerebellar cortex is not solely related to regional differences in the pattern of inputs and outputs, and that certain areas may contain distinct cell types to allow different computational processes to aid specific functions. UBCs, for example, are thought to play an important role in head and body stability by providing signals related to velocity estimation34, 35.
Lugaro and Golgi cells have also been shown to have pronounced regional differences in distribution. In the human cerebellum Lugaro cells have a higher packing density in the posterior vermis and corresponding parts of the hemispheres than in other regions of the cerebellar cortex (with the exception of lobule VIa-c, and lobule X36). On the other hand, the packing densities of Golgi cells in a range of mammalian species, including human, are lower in the hemispheres (except for the flocculus) than in the corresponding vermal lobules, with the greatest density present in the flocculus and lobule IX18, 37 (see also38, 39). Golgi cell soma size is also smaller in the hemispheres than in the vermis40. However, because the greatest packing density of Golgi cells was found in these studies to be in the vermis, where UBCs are known to be present at high numbers, it has been argued that these regional differences may be attributable to the misclassification of UBCs34, 37. Clearly, further studies using markers of different cell types are required to resolve this issue. For example, mGluR2 is almost exclusively present in Golgi cells that have both a GABAergic and glycine, whereas the presence of neurogranin selectively labels Golgi cells that are only GABAergic40, 41. As discussed below, systematic differences in neurochemical phenotype could provide the basis for distinct regional circuits within the cerebellar cortex that are independent of afferent and efferent connections.
Patterned molecular marker expression
Prominent regional variations in the expression of various proteins and genes (molecular markers) also occur within the adult cerebellar cortex. In general, these molecular markers reveal that the cortex can be divided into an array of rostrocaudally oriented bands or stripes. The first such marker to be discovered, over 40 years ago, was the enzyme 5′-nucleotidase42. Since then numerous others have been identified, including heat shock protein (HSP) 2543, cocaine- and amphetamine-regulated transcript (CART44), mGluRs45, excitatory amino acid transporter 4 (EAAT4)46, phospholipase C47, 48, 1 inositol 1,4,5-trisphosphate receptor (IP3R1)49, protein kinase C50, 51, neuroplastin52, GABA receptors 53, 54, acetylcholinesterase55, 56, neuronal calcium sensor-157, microtubule-associated protein 1A58, neurogranin59, and various transgenes (e.g. REF 60), demonstrating that the mammalian cerebellar cortex contains a rich molecular topography.
To date, the most comprehensively studied of these molecules is zebrin II61 (subsequently identified as an antigen on the respiratory enzyme aldolase C62). Zebrin II is expressed by subsets of Purkinje cells, and in many areas of the cortex groups of Purkinje cells expressing zebrin II (zebrin II+) alternate with those that do not (zebrin II−), forming a striking array of rostrocaudally oriented zebrin II +/− bands (Fig. 3). Recent data from a knock-in mouse model suggests that the levels of zebrin II gene expression may vary across the bands in which it is expressed63. However, the general banding pattern is highly consistent and conserved across mammals and birds10, 64.
Figure 3. Patterned molecular marker expression in the cerebellar cortex.
Schematic illustrating the results of wholemount immunohistochemical staining of the mouse cerebellum, showing alternating bands of zebrin II expression (brown). The co-localization of zebrin II+ Purkinje cells (brown) and zebrin II− Purkinje cells with various other molecular makers is shown. NCS-1, EAAT4, excitatory amino acid transporter 4; GABABr2, GABA-B receptor subtype 2; MAP1a, microtubule-associated protein 1A; mGluR1B, metabotropic glutamate receptor R1b; NCS-1, neuronal calcium sensor-1; PLCB3, phospholipase Cβ3; PLCB4, phospholipase Cβ4. Schematic is based on data from REFS45, 47, 48, 52–54, 58, 59, 61.
Various other molecular markers are co-expressed with zebrin II. For example, the glutamate transporter EAAT4 is highly concentrated in the spines and thin dendrites of Purkinje cells located in zebrin II+ bands46. Likewise, components of intracellular signalling pathways, such as phospholipase Cβ3, also show restricted expression to zebrin II+ bands47. Conversely, some markers are expressed only in zebrin II− bands, including metabotropic glutamate receptor mGluR1b 45 and phospholipase Cβ447 (Fig. 3).
The diversity of markers within the cerebellar cortex is not only limited to Purkinje cells; markers have been shown to be heterogeneously expressed in subsets of UBCs, Golgi and granule cells (for a recent review see REF65). However, little is known about their localization in these cells in relation to zebrin II65 (but see REF66). Various markers and neuromodulators have also been found in subsets of cerebellar afferents (e.g. REF67–75). For example, climbing fibres in zebrin II+ bands have been shown to exhibit enhanced expression of the glutamate transporter vGluT272, whereas vGluT1 and vGluT2 are differentially expressed in bands of mossy fibres69. A body of evidence has also accumulated to indicate that the terminals of climbing fibres and mossy fibres arising from different sources align with cerebellar stripes of various molecular markers of Purkinje cells, most notably zebrin II (e.g. REF15, 76–81). More specifically, a correspondence exists between individual Purkinje cell zones (defined by their climbing fibre input, see above) and zebrin II expression. This includes the important finding that cortical zones in which Purkinje cells are zebrin II+, tend to receive climbing fibre input from regions of the inferior olive dominated by descending inputs, while cortical zones in which Purkinje cells are zebrin II−, receive climbing fibre input from regions of the inferior olive that receive predominantly peripheral inputs15, 82, 83.
It is also important to note that the patterns of expression of molecular markers of Purkinje cells are not solely confined to a striped arrangement. For example, the D3 type dopamine receptor and associated dopamine signalling proteins such as dopamine transporter (DAT) and monoamine transporter 2 (VMAT2) are all strongly restricted in the anterior-posterior cerebellar axis and expressed in Purkinje cells located mainly in lobules IX and X84. This finding, combined with corresponding differences in UBC packing density within the same cerebellar lobules, raises the possibility that information processing within cerebellar cortical circuits may differ not only at the level of zebrin II bands but also between cerebellar lobules.
Patterned physiology
Regional cerebellar cortical differences in the expression of molecular markers suggest that the synaptic physiology and intrinsic properties of neurons might also vary by region. Given the remarkable relationship between zebrin II expression and cerebellar afferent and efferent connectivity and patterns of expression of many other molecular markers, this issue has been addressed mainly by using zebrin II expression as a reference10, 14, 15, 77, 79, 85–88.
Among the best characterized of the proteins that co-localize with zebrin II is EAAT446, 89–91. EAAT4 is expressed exclusively by Purkinje cells and acts to limit the duration of action of glutamate at climbing fibre and parallel fibre synapses, and also to limit its diffusion to extrasynaptic receptors and to receptors in nearby synapses92–96. Since EAAT4 limits the time and spread of glutamate, in vitro studies have shown the ability of complex spikes to activate metabotropic glutamate receptors, and thereby induce synaptic plasticity, notably long term depression (LTD) of parallel fibre synaptic efficacy, is reduced in zebrin II+ bands97.
In vitro studies have also revealed regional variations in Purkinje cell intrinsic properties across cerebellar lobules. For example, the depolarization-induced slow current (DISC), which acts to increase Purkinje cell excitability, is present at high levels in Purkinje cells in the vermis of the posterior lobe, but only at low levels in the anterior lobe84, 98: this correlates with the pattern of expression of D3, DAT and VMAT284. Furthermore, comparisons of Purkinje cells in vermal lobules III–V with those in lobule X have found a number of differences in passive (input resistance and capacitance) and active (e.g. presence of A-type K+ current) membrane properties, leading to lobule X Purkinje cells being less excitable and displaying a greater variety of firing patterns in response to depolarizing current pulses (Fig. 4a, b)99.
Figure 4. Patterned electrophysiological activity in the cerebellar cortex.
The intrinsic properties and firing patterns of Purkinje cells vary in different regions of the cerebellar cortex. a| In brain slice preparation in juvenile rats (between postnatal days 21 and 23) firing patterns were determined in response to depolarizing currents injected from hyperpolarized holding potentials. Representative traces illustrate tonic firing, complex bursting, initial bursting, and gap firing patterns of activity. b| The pie charts show the relative proportion of each of the different firing patterns in lobules III–V and lobule X of the cerebellar vermis. Tonic firing and complex bursting patterns were found both in lobules III–V and X, whereas gap firing and initial bursting firing patterns were encountered only in lobule X99. c| Extracellular recordings of Purkinje cells in the presence of ketamine/xylazine that were localized to zebrin II− (Z−) and zebrin II+ (Z+) bands respectively. Asterisks indicate complex spikes. Dot indicates complex spike expanded above. Zebrin II− Purkinje cells have higher simple spike firing rates (spikes without asterisks). The graph shows the mean simple spike firing rates in Z− and Z+ bands. Error bars indicate one SD of the distribution. d| Graph shows the normalized complex spike-simple spike pause – the pause in simple spike activity following the occurrence of a complex spike – for Purkinje cells in Z− and Z+ bands. Purkinje cells in Z− bands have a relatively stronger active suppression of simple spikes than Purkinje cells in Z+ bands. The mean simple spike pause duration of each Purkinje cell was normalized by dividing by its mean simple spike interspike interval. The distribution of ratio is shown for Z− and Z+ Purkinje cells. Dashed line indicates the expected ratio if the latency from a complex spike to the first succeeding simple spike is solely a function of simple spike firing rate. The solid black lines indicate the overall median across each group, and the grey boxes indicate the interquartile range from 25% to 75%. e| Purkinje cell simple spike firing rates in relation to complex spikes recorded in awake adult mouse. Four different types of simple spike response types among the Purkinje cells were identified: normal, facilitation, suppression and oscillation. The percentage of different response types in Z and Z+ PCs are shown in the pie charts. The facilitation type occurs predominantly in Z PCs, whereas the suppression and oscillati on type are restricted to the Z+ PCs. a, b reproduced and adapted with permission from REF99; c, d reproduced, with permission from REF111 e; adapted with permission from REF110.
Whether these differences reflect lobular organization, or whether they are more fundamentally linked to zebrin compartmentalization is unclear, because the zebrin status of the recorded cells was not determined in these studies. Nevertheless, and taken together with the EAAT4 results, these findings provide clear evidence that the intrinsic and synaptic physiology of Purkinje cells varies considerably between cerebellar cortical regions. These systematic variations could in turn lead to regional differences in the spiking behaviour and computational characteristics of Purkinje cells. Indeed, marked variations in Purkinje cell spiking parameters are known to occur. For example, spontaneous simple spike rates for individual Purkinje cells range from 1 Hz to >150 Hz under in vivo and in vitro conditions7, 100–102; and the slopes of f-I (frequency-current) curves103 and the regularity of simple spike activity varies widely between Purkinje cells100, 104, 105. Purkinje cells also vary in their ability to transition between up and down states (e.g. REF7, 106–108). For instance, in the awake cat, about half of all recorded Purkinje cells show a firing pattern with periods of tonic activity separated by long pauses in simple spike activity, while the remainder display continuous simple spike activity109. But whether these and other parameters of Purkinje cell spike activity vary systematically (as opposed to randomly) between cerebellar cortical regions remains to be established.
This key issue has started to be addressed in vivo by comparing Purkinje cell activity in zebrin II+ and zebrin II− regions of the rodent cerebellar cortex. Substantial regional differences in the spiking patterns of Purkinje cells in both awake restrained mice110 and anaesthetised rats111 have been found. These include differences in simple spike firing rates: Purkinje cells located in zebrin II− bands display significantly higher firing frequencies than those located in zebrin II+ bands110, 111 (Fig 4c), and Purkinje cells in zebrin II+ bands display greater irregularity of simple spike firing111. However, using a different measure of spike train regularity, the study in awake mice110 did not find differences between zebrin II+ and zebrin II− regions across their entire dataset. A difference was only evident when the analysis was from a more restricted cerebellar region. This suggests that lobular as well as zebrin-related variations in simple spike regularity may exist.
Systematic differences in regularity of firing could have important implications for the transmission of information from the cerebellar cortex to the cerebellar nuclei, because zebrin II+ and zebrin II− Purkinje cells project to non-overlapping cerebellar nuclear regions85, 112. Purkinje cells are often assumed to use rate coding to transmit information. If this is the case, a greater irregularity of simple spike activity in zebrin II+ Purkinje cells would cause them to transmit information less efficiently, as rate code efficiency depends on the regularity of the spike train113. If the variability in the spike times is not correlated across Purkinje cells, such differences could be overcome by a large convergence in the Purkinje cell-cerebellar nuclear projection, which would allow averaging of signals. However, a recent estimate suggests that the convergence in this pathway is relatively low114. Instead, other coding mechanisms, such as spike rate modulation or temporal coding115, 116, may be used. Such strategies would depend on the intrinsic properties of Purkinje cells and their connectivity, which in turn could lead to differences in function.
An important additional consideration is that the simple spike activity of Purkinje cells occurs not only spontaneously, but also as a result of excitatory input from granule cells. The granule cell axon can be divided into ascending and parallel fibre portions, and each part has been hypothesized to represent the dominant excitatory drive to Purkinje cells (the radial and beam hypotheses, respectively3, 17, 117). Evidence for both hypotheses has been obtained118–120, and it has been hard to reconcile these conflicting results. However, a recent report suggests that differences in the synaptic efficacy of the parallel fibre-Purkinje cell synapse between zebrin II+ and zebrin II− regions may provide an explanation121. Stimulation of mossy fibres or sensory pathways evoked beam-like responses (the responding region was elongated along the longitudinal axis of the folium) in lobule crus I, which is primarily zebrin II+, but a more patch-like response distribution in crus II, where zebrin II expression forms a striped pattern.
Beyond this potential resolution of the radial and beam hypotheses, these results imply that the functioning of cerebellar cortical microzones (see Introduction)10, 122, may differ greatly according to cerebellar region. Specifically, in regions where zebrin II is expressed uniformly, parallel fibres that extend across the region would modulate all Purkinje cell simple spike activity in concert. In contrast, where zebrin exhibits a striped pattern, parallel fibres would link the simple spike activity of multiple zebrin II+ bands, while the activity in the intervening zebrin II− bands remains independent.
Of course Purkinje cells are not confined to firing only simple spikes, and one of the fundamental questions in cerebellar physiology is how simple spikes and complex spikes interact with one another over different timescales. Such interactions may allow the impact of the relatively infrequent complex spikes on the overall output of Purkinje cells to be amplified. On the shortest timescale (tens of milliseconds), complex spikes are typically followed by a pause in simple spikes123–125. Zebrin II+ Purkinje cells have longer absolute pauses, consistent with their slower firing rates110, 111; however, once normalized for differences in firing rate, zebrin II− Purkinje cells display a longer relative pause than zebrin II+ Purkinje cells, indicating that a stronger active suppression of simple spikes occurs in zebrin II− bands111 (Fig. 4d).
On a longer timescale (several hundreds of milliseconds), a modulation of simple spike activity often follows the initial complex spike induced pause. This modulation can be either a facilitation or depression of activity relative to baseline, and zebrin II+ and zebrin II− regions of the cortex also differ in the strength and dominant type of this post pause modulation; with Purkinje cells in zebrin II+ regions displaying greater variability in modulation characteristics110, 111 (Fig. 4e). Much longer term interactions (over seconds to minutes) can also occur between simple spikes and complex spikes, such as the inverse relationship between simple spike rates and sustained changes in complex spike rates104, 126–128; but whether regional differences exist in these interactions remains to be investigated.
What might be the underlying cellular mechanism that accounts for zebrin-related differences in Purkinje cell physiology? Zebrin itself could affect Purkinje cell activity: indeed, application of the products of the reaction catalysed by zebrin, glyceraldehyde-3-phosphate and dihydroxyacetone phosphate, increases the firing rates of both zebrin II+ and zebrin II− cells110. However, this would imply that zebrin II+ Purkinje cells should have higher spontaneous simple spike rates than zebrin II− cells, which is the opposite to the results obtained to date110, 111. Thus, any direct effect of zebrin on firing rates is likely to be minor. Instead, it appears that differences in expression or activity of transient receptor potential cation channel type C3 (TRPC3) may be involved110.
It is also important to note that Purkinje cells are not the only feature of the cerebellar cortex to show regional variations in physiology. Under in vitro conditions, climbing fibres in zebrin II+ bands release more glutamate than those in zebrin II− bands. As a result the complex spikes of Purkinje cells in zebrin II+ bands have a longer duration and are composed of more spikelets72, 110. With regard to cerebellar cortical interneurons, significant regional differences in their activity may also be present. For example, a striking difference in the ability of parallel fibre activity to drive the activity of molecular layer interneurons (basket and stellate cells) in zebrin II+ versus zebrin II− bands has been described129. Electrical stimulation of the cerebellar cortical surface triggers synchronized action potentials in the underlying parallel fibres, leading to a wave of successively excited Purkinje cells along the axis of the parallel fibres. This wave of excitation has been shown to be continuously flanked by regions of Purkinje cell inhibition due to the geometrical arrangement of the parallel fibres and the basket and stellate cell axons, which run perpendicular to each other130. Recent work has shown that the flanking inhibitory waves are not of constant amplitude, but rather wax and wane, with Purkinje cell inhibition being strongest in zebrin II+ bands and weak or absent in zebrin II− bands129, 131. This implies that the synaptic strength between parallel fibres and molecular layer interneurons varies between zebrin II bands and thereby shapes the spatial pattern of responsiveness of Purkinje cells to mossy fibre inputs. The functional significance of these intriguing findings remains to be determined; however, given the ability of the parallel fibre portion of the granule cell axon to drive both Purkinje cells and interneurons in zebrin II+ regions, this may be a mechanism to selectively enhance the influence of inputs in a spatially restricted manner.
Patterned neurodegeneration
Regional differences in the organizational plan of the cerebellar cortex have important implications for the pathogenesis of disease. Genetic and physical insults that damage the cerebellum typically result in Purkinje cell death, but the configuration of this cell death is often not random. A clear-cut anatomical organization of cell loss emerges 132–134 (Table 1) and the use of spontaneous mouse mutants has helped reveal how dying Purkinje cells become restricted into specific patterns.
Table 1.
Relationship between mutant type and patterned cerebellar neurodegeneration. Note than in some cases (e.g. Niemann-Pick type C and Borna disease model) the degeneration starts in one set of bands but eventually progresses to the neighbouring bands of opposite phenotype.
| Patterned neurodegeneration in the cerebellum | |||||
|---|---|---|---|---|---|
| Mutant | Type of mutant | Disease model | Degeneration bands | TH expression | Reference |
| leaner | spontaneous | cerebellar ataxia | zebrin II (−) | up regulated | 135, 136, 151 |
| nervous | spontaneous | postnatal Purkinje cell degeneration | zebrin II (+) | not known | 137 |
| Purkinje cell degeneration(pcd) | spontaneous | neurodegeneration | zebrin II (+) | not known | 137 |
| Niemann-Pick type C | spontaneous | childhood cholesterol storage disease | zebrin II (−) | up regulated | 143 |
| Global brain ischemia | induced (surgical) | brain ischemia | zebrin II (−) | not known | 134 |
| IP3R1 | conditional engineered mutant | dystonia | no degeneration | up regulated | 49, 182 |
| tottering | spontaneous | familial hemiplegic migraine and episodic ataxia type-2 (EA-2) | zebrin II (−) in lobule I, II zebrin II (+) in lobule IX |
up regulated | 151, 152, 154, 156 |
| Neonatal Borna Disease | engineered (viral injection) | neonatal borna disease | zebrin II (−) | not known | 144 |
| L7cre/vgatflox/flox | conditional engineered mutant | ataxia/dysequilibrium | no degeneration | up regulated | 157 |
| Slc9a6−/− | engineered | angelman syndrome | Zebrin II (−) | not known | 158 |
Of particular note is the leaner mutant, which carries an autosomal recessive mutation in the gene coding for the α(1A) pore forming subunit of the Ca(V)2.1 (P/Q-type) voltage-gated calcium channel. Purkinje cell death in this mutant is most pronounced and rapid in the anterior lobe135, and the cell loss is remarkably patterned, being mostly restricted to zebrin II− bands136. The pattern of degeneration in leaner is almost perfectly mirrored in nervous and Purkinje cell degeneration mice, in which cell loss is mainly restricted to zebrin II+ Purkinje cells137. Taken together these data suggest that the patterns of degeneration are genetically determined. Furthermore, their spatial organization fits with how the normal lineages of Purkinje cells arrange themselves during development, during which bands of Purkinje cells are specified by different genetic cues and are born on distinct embryonic days (Box 1)138–142.
Box 1. Embryonic origins of parasagittal bands.
The fundamental pattern of cerebellar bands is determined during development 174. Several developmental stages sequentially establish the final pattern. The first stage is highly dependent on when Purkinje cells are born; this occurs between ~ embryonic day 10–13 in mouse. Specific clusters of Purkinje cells are born on each day139, 175. At the next stage the clusters acquire distinct molecular properties that are under the control of at least two families of transcription factors: the atypical helix-loop-helix transcription factor early B-cell factor 2 (EBF2) and the homeodomain transcription factors Engrailed1 and 2 (EN1/2). EBF2 directs the fate of clusters towards a zebrin II+ versus zebrin II− lineage138, whereas EN1/2 control the anterior-posterior and medial-lateral positioning of marker expression in each cluster142, 176. However, the expression of Purkinje cell markers is temporally dynamic and, because their patterns change over time, they can be divided into early markers that demarcate embryonic clusters59, 60, 177, late markers that define adult bands47, 61, and constitutively expressed markers that bridge between the clusters and bands174, 178, 179. One result of this dynamic mode of patterning is that each cluster in the embryo contributes to multiple bands in the adult140, 141, 178–181. The final patterns are then refined by activity157.
These striking patterns of degeneration extend beyond spontaneous mouse models: genetically engineered and inducible disease models also exhibit a similar neuropathology132. For instance, it has been reported that in a mouse model of the childhood cholesterol storage disease, Niemann-Pick type C, zebrin II− bands degenerate first. The progression of cell loss eventually spreads to zebrin II+ bands, but remarkably, the Purkinje cells in lobules IX and X exhibit a powerful resistance to death143. Zebrin II− Purkinje cells also die more frequently in a rodent model of global brain ischaemia134 and the same is true in neonatal rats that are used for an early-life infection model of Borna disease144. Why these particular Purkinje cells are susceptible to degeneration remains unknown, although their vulnerability may relate to some of the molecular markers described in the previous sections; notably, the expression of calcium-associated proteins such as mGluR, EAAT4 and IP3R197, 134, 145. However it is uncertain if these molecules play a direct role in marking specific Purkinje cells for death. What is becoming clear is that in neurodegeneration models such as the Purkinje cell degeneration mutant, the mechanism of death likely involves multiple pathways including autophagy146, mitochondrial dysfunction147, and DNA damage148. Whether these processes are restricted to particular zebrin bands and whether the proteins that mediate them are patterned into zebrin-like bands requires further study.
Conversely, why some Purkinje cells preferentially survive also remains unclear. However, the particular resistance to cell death in lobules IX and X may be related to the expression of the small heat shock protein HSP25143, 149, which can function as a chaperone. In neurons, chaperone function has been implicated in cell survival, particularly when the mechanism is triggered to protect against protein misfolding and apoptotic activity150.
The molecular defects that follow Purkinje cell damage and precede cell death are not fully understood. However, altering calcium homeostasis (and thereby the excitability of cerebellar neurons) leaves a molecular ‘imprint‘ on the affected cells151. Tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis, is abnormally expressed in the Purkinje cells of several types of ataxic mutant mice such as tottering152 mice and also in related genetic mutants in which calcium regulation is interrupted153. However, it appears that not all mutant Purkinje cells express TH, as only certain cells are damaged in a manner that induces TH expression. This expression reveals a series of striking parasagittal bands that overlap with zebrin II153–155. In the case of tottering mice, the up-regulation of TH far precedes the late-onset cell death, which is also regionally patterned, indicating that the presence of TH may predict the ultimate fate and demise of the cell in older mice156. However, Purkinje cells over expressing TH need not be destined for death. In a recent study, it was shown that genetically silencing Purkinje cell neurotransmission resulted in altered zebrin II band formation and a heavy induction of TH expression157. In contrast to the compromised Purkinje cells in tottering mice, Purkinje cells that were unable to communicate with the cerebellar nuclei did not die after TH was abnormally expressed in zebrin II+ Purkinje cells157. Thus, TH expression may be a good molecular indicator that can be used to link defective cell function to the status of the neuropathology in highly patterned circuits.
The relationship between regional degeneration of Purkinje cells and motor dysfunction is evidenced by mutants such as leaner mice. However, as indicated above, the cerebellum is probably also involved in non-motor functions1. It is therefore of interest that regional degeneration of Purkinje cells has been noted in a mouse model of Angelman syndrome, a disorder on the autism spectrum158. In humans, mutations in the solute carrier family 9 isoform 6 gene (SLC9A6), which is located on chromosome Xq26.3, are known to cause a number of neurological diseases involving complex and slowly progressive degeneration, including Angelman syndrome. Analyses of a knock-out mouse that lacks the encoded sodium-hydrogen exchanger 6 transporter are intriguing because, as in the classic motor degenerative models, Purkinje cells in the Slc9a6 mutant also exhibit loss of zebrin II− Purkinje cells158. A key unknown is how particular zebrin II bands are involved in motor versus non-motor function and related diseases. It is also important to determine how zebrin II expression in the human cerebellum159 relates to the patterns of cell loss and cell dysfunction found in autism spectrum disorders160, dystonia161, and multiple sclerosis162.
Concluding comments
This review has outlined evidence that regional differences exist in the adult mammalian cerebellar cortex that relate to cell type, morphology and expression of various molecular markers, most notably zebrin II expression by Purkinje cells (Fig. 5). Since Purkinje cells are the sole output of the cerebellar cortex they are the final determinant of any functional heterogeneity within the cortex. It is therefore important that systematic regional differences in physiology have been found between Purkinje cells, including distinct patterns of simple spike activity that depend on whether a cell is zebrin II+ or zebrin II−. In addition, mouse mutant models show that restricted patterns of Purkinje cell death occur in relation to both motor and potentially non-motor dysfunction. We therefore propose that variations in gene expression and related anatomical and physiological differences at a cellular level lead to regionally distinct cerebellar cortical microcircuits that have different information processing capabilities.
Figure 5. The non-uniform nature of cerebellar cortical cytoarchitecture and physiology.
Schematic showing a cross section of part of the cerebellar cortex, depicting key differences in anatomy and physiology. Larger Purkinje cells are present in the vermis (to the left), and there is a greater density of Purkinje cells in the anterior lobe and apex of the folia. The dendritic arbour of Purkinje cells also differs between the base and apex of folia. Granule cell density is greater in the apex of the folia and larger granule cells are found in the vermis compared to the hemispheres. Unipolar brush cells (UBCs) and Golgi cells are found mainly in the vermis of the posterior lobe also Lugaro cells have a higher packing density in the posterior lobe. Molecular markers such as excitatory amino acid transporter 4, GABA-B receptor subtype 2, phospholipase Cβ3 and neuronal calcium sensor-1 are co-expressed in rostro-caudally oriented bands with Purkinje cells positive for the molecular marker zebrin II (Z+); while metabotropic glutamate receptor R1b, microtubule-associated protein 1A, neurogranin, neuroplastin, and phospholipase Cβ4 are found in zebrin II− bands (Z−). Purkinje cell simple spike frequencies are higher in Purkinje cells located in Z− bands; while simple spike activity is less regular, the complex spike-induced pause in simple spikes is longer, and the post pause facilitation in simple spikes is greater in Z+ bands. Asterisk indicates complex spike; gcl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; white matter; Z+, zebrin II+ band; Z−, zebrin II− band. Schematic is based on data from REFS3, 18, 22, 36, 37, 46, 47, 61, 110, 111, 141
Information arriving via different cerebellar afferent systems that target zebrin II+ and zebrin II− regions to differing extents has the potential to be transformed by networks characterized by quite distinct operational states. Thus the cerebellar cortex may be more varied in the way it processes information than generally thought; that is, evidence is gathering that challenges the concept of a ‘universal cerebellar transform’13. Instead, at least two distinct forms of information transform are possible: one via zebrin II− Purkinje cells and the other via zebrin II+ Purkinje cells. It remains to be established whether they operate independently or co-operatively, given that pairs of zebrin II+ and zebrin II− bands have been found to form functional units in relation to patterns of optic flow163. But given the precise spatial organization that exists between climbing fibre and mossy fibre inputs and individual zebrin bands (the ‘one-map hypothesis’10, 77), it is possible that at least some inputs could be processed independently. In other words, since some projections target either positive or negative bands76, including olivo-cerebellar projections arising from ascending or descending sources15, 82, 83, such inputs have the potential to have different computations performed upon them. It is also possible that the same information is forwarded to both types of zebrin band but access to the information is regulated through selective gating of afferent transmission164, 165. The advent of new technologies such as optogenetics and imaging of particular neuronal phenotypes will help address these and related questions by allowing genetically targeted investigation of zebrin II+ versus zebrin II− territories in the cerebellar cortex.
In terms of possible function, physiological differences between zebrin II bands in Purkinje cell firing patterns are related to corresponding differences in expression of other molecular markers that, in turn, may reflect how different parts of the cerebellum process information. In particular, previous studies have established that paradigms that induce long term potentiation (LTP) at parallel fibre-Purkinje cell synapses are associated with zebrin II+ bands, while LTD of parallel fibre-Purkinje cell synapses occurs more readily in zebrin II− bands97, 131. Whether the corresponding variations in simple spike firing rates in zebrin II− and zebrin II+ bands contribute to these differences in cerebellar plasticity is unknown. However, in principle, Purkinje cells with lower simple spike firing frequencies potentially have a greater operational range to enable them to undergo LTP, whereas Purkinje cells with higher firing frequencies are unlikely to be able to increase their firing rates any further, and therefore may be predisposed to undergo LTD110.
Differences in simple spike firing rates between zebrin II− and zebrin II+ bands are also consistent with predictions from the adaptive-filter model of cerebellar function28, 166–169. This is because the adaptive-filter model requires both LTP and LTD, and empirical evidence has shown that Purkinje cell-parallel fibre synapses post-synaptically can undergo bidirectional plasticity170–172. During different phases of learning (acquisition, consolidation and extinction), zebrin II+ and zebrin II− Purkinje cells may play different roles, particularly since different rates of learning have been described for classical conditioning (e.g. REF173). Further experiments examining learning rates in zebrin II+ and zebrin II− Purkinje cells are necessary to test this idea.
Finally, one major question that needs to be addressed is how differences in microcircuit physiology within the cerebellar cortex influence neural processing at the level of the cerebellar nuclei. Given that zebrin II− and zebrin II+ Purkinje cells target distinct regions in the nuclei14, 88, 112 the possibility arises that regional differences in information processing also exist within the cerebellar nuclei. Zebrin II− bands, which have higher simple spike firing rates, are likely to inhibit their target regions of the cerebellar nuclei more strongly than zebrin II+ bands inhibit theirs, perhaps making them less responsive to other inputs. Such differences could cause the information processing gain of different cerebellar nuclear regions to be quite distinct. The higher gain zebrin II+ target nuclear regions may preferentially be used to make large, rapid changes in motor output, whereas the lower gain zebrin II− nuclear regions may be specialized for more subtle aspects of motor control. An analogy for such a dual system is the coarse and fine focus controls on a microscope; the differences in Purkinje cell phenotype may represent such an arrangement.
Supplementary Material
Acknowledgments
We thank the UK Medical Research Council (NLC, RA), Action Medical Research (NLC, RA), National Science Foundation (EJL), National Institutes of Health (RVS), and the Bachmann-Strauss Dystonia and Parkinson Foundation (RVS) for their financial support.
Glossary of terms
- Long term depression
A long lasting decrease in the response of neurons to stimulation of their afferents following a brief patterned stimulus (for example, a 1-Hz stimulus)
- Rate coding
A form of neural representation where the frequency or rate of action potentials over a given time period carries the relevant information. Any information possibly encoded in the temporal structure of the spike train is ignored
- Spike rate modulation
Changes in frequency or rate of action potentials which are thought to encode information
- Temporal coding
A form of neuronal representation where the timing of action potentials carries the relevant information. The time of action potentials can be referenced with respect to other action potentials of the same cell or those of other cells
- Chaperone
A protein that mediates the folding or assembly of another polypeptide, but does not form part of the completed structure or participate in its biological function
- Optogenetics
A series of recently developed tools that make use of light-activated proteins. Most frequently, light-sensitive ion channels and membrane pumps are used to control the firing rate of neurons, but increasingly other types of proteins are placed under similar light control
- One-map hypothesis
The topography of the cerebellar cortex has been defined by different patterns of climbing fibre input, mossy fibre input, and Purkinje cell phenotype. Based on embryological development, the one-map hypothesis proposes that the basic units of each map align in the adult animal to form one unitary map
References
- 1.D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits. 2012;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. Springer-Verlag; Berlin: 1967. [Google Scholar]
- 4.Hatten ME, Roussel MF. Development and cancer of the cerebellum. Trends Neurosci. 2011;34:134–42. doi: 10.1016/j.tins.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. Springer; Berlin: 1974. [Google Scholar]
- 6.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–22. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DM, Rawson JA. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979;289:425–48. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thach WT., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967;30:675–96. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- 9.Eccles JC, Llinás R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966;182:268–96. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–81. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- 11.Buisseret-Delmas C, Angaut P. The cerebellar olivo-corticonuclear connections in the rat. Prog Neurobiol. 1993;40:63–87. doi: 10.1016/0301-0082(93)90048-w. [DOI] [PubMed] [Google Scholar]
- 12.Voogd J, Bigaré F. In: The Inferior Olivary Nucleus, Anatomy and Physiology. Courville J, de Montigny C, Lamarre Y, editors. Raven Press; New York: 1980. pp. 207–234. [Google Scholar]
- 13.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–60. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 14.Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar nuclei: analysis by three-dimensional mapping of the olivonuclear projection and aldolase C labeling. J Neurosci. 2007;27:9696–710. doi: 10.1523/JNEUROSCI.1579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voogd J, Pardoe J, Ruigrok TJ, Apps R. The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: congruence of climbing fiber cortical zones and the pattern of zebrin banding within the rat cerebellum. J Neurosci. 2003;23:4645–56. doi: 10.1523/JNEUROSCI.23-11-04645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong DM, Schild RF. A quantitative study of the Purkinje cells in the cerebellum of the albino rat. J Comp Neurol. 1970;139:449–56. doi: 10.1002/cne.901390405. Histological study showing that there is considerable local variations in Purkinje cell packing density. [DOI] [PubMed] [Google Scholar]
- 17.Braitenberg V, Atwood RP. Morphological observations on the cerebellar cortex. J Comp Neurol. 1958;109:1–33. doi: 10.1002/cne.901090102. [DOI] [PubMed] [Google Scholar]
- 18.Lange W. In: The Cerebellum: New Vistas. Palay SL, Chan-Palay V, editors. Springer-Verlag; Berlin: 1982. pp. 93–105. An anatomical study summarising the differences in Purkinje cell, granule cell and Golgi cell density and cell size between the vermis and hemispheres of the cerebellum. [Google Scholar]
- 19.Muller U, Heinsen H. Regional differences in the ultrastructure of purkinje cells of the rat. Cell Tissue Res. 1984;235:91–8. doi: 10.1007/BF00213728. Regional differences in morphology of Purkinje cells in the vermis versus the hemisphere. [DOI] [PubMed] [Google Scholar]
- 20.Parma A. The size of cell corpuscles of Purkinje cells in the paleocerebellum and neocerebellum of some mammals as compared to man. Acta Anat Suppl (Basel) 1969;56:337–46. [PubMed] [Google Scholar]
- 21.Voogd J. Cerebellar zones: a personal history. Cerebellum. 2011;10:334–50. doi: 10.1007/s12311-010-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedelescu H, Abdelhack M. Comparative morphology of dendritic arbors in populations of Purkinje cells in mouse sulcus and apex. Neural Plast. 2013;2013:948587. doi: 10.1155/2013/948587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friede R. Quantitative displacement of the layers within the convolutions of the cerebellar cortex and its biologic importance. Acta Anat (Basel) 1955;25:65–72. [PubMed] [Google Scholar]
- 24.Jaarsma D, Wenthold RJ, Mugnaini E. Glutamate receptor subunits at mossy fiber-unipolar brush cell synapses: light and electron microscopic immunocytochemical study in cerebellar cortex of rat and cat. J Comp Neurol. 1995;357:145–60. doi: 10.1002/cne.903570113. [DOI] [PubMed] [Google Scholar]
- 25.Van der Want JJ, Vrensen GF, Voogd J. Differences in synaptic size in the superficial and deep layers of the molecular layer of the cerebellar cortex of the cat. An electronmicroscopic and autoradiographic study. Anat Embryol (Berl) 1985;172:303–9. doi: 10.1007/BF00318978. [DOI] [PubMed] [Google Scholar]
- 26.Pichitpornchai C, Rawson JA, Rees S. Morphology of parallel fibres in the cerebellar cortex of the rat: an experimental light and electron microscopic study with biocytin. J Comp Neurol. 1994;342:206–20. doi: 10.1002/cne.903420205. [DOI] [PubMed] [Google Scholar]
- 27.Altman J. In: The Cerebellum New Vistas. Palay SL, Palay V, editors. Springer-Verlag; Berlin: 1982. pp. 8–49. [Google Scholar]
- 28.Dean P, Porrill J, Ekerot CF, Jörntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11:30–43. doi: 10.1038/nrn2756. [DOI] [PubMed] [Google Scholar]
- 29.Diño MR, Willard FH, Mugnaini E. Distribution of unipolar brush cells and other calretinin immunoreactive components in the mammalian cerebellar cortex. J Neurocytol. 1999;28:99–123. doi: 10.1023/a:1007072105919. [DOI] [PubMed] [Google Scholar]
- 30.Braak E, Braak H. The new monodendritic neuronal type within the adult human cerebellar granule cell layer shows calretinin-immunoreactivity. Neurosci Lett. 1993;154:199–202. doi: 10.1016/0304-3940(93)90206-z. [DOI] [PubMed] [Google Scholar]
- 31.Jaarsma D, Diño MR, Ohishi H, Shigemoto R, Mugnaini E. Metabotropic glutamate receptors are associated with non-synaptic appendages of unipolar brush cells in rat cerebellar cortex and cochlear nuclear complex. J Neurocytol. 1998;27:303–27. doi: 10.1023/a:1006982023657. [DOI] [PubMed] [Google Scholar]
- 32.Nunzi MG, Shigemoto R, Mugnaini E. Differential expression of calretinin and metabotropic glutamate receptor mGluR1alpha defines subsets of unipolar brush cells in mouse cerebellum. J Comp Neurol. 2002;451:189–99. doi: 10.1002/cne.10344. [DOI] [PubMed] [Google Scholar]
- 33.Sekerková G, Watanabe M, Martina M, Mugnaini E. Differential distribution of phospholipase C beta isoforms and diaglycerol kinase-beta in rodents cerebella corroborates the division of unipolar brush cells into two major subtypes. Brain Struct Funct. 2014;219:719–49. doi: 10.1007/s00429-013-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mugnaini E, Sekerková G, Martina M. The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev. 2011;66:220–45. doi: 10.1016/j.brainresrev.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekerková G, Ilijic E, Mugnaini E, Baker JF. Otolith organ or semicircular canal stimulation induces c-fos expression in unipolar brush cells and granule cells of cat and squirrel monkey. Exp Brain Res. 2005;164:286–300. doi: 10.1007/s00221-005-2252-7. [DOI] [PubMed] [Google Scholar]
- 36.Braak H. On the intermediate cells of lugaro within the cerebellar cortex of man. A pigmentarchitectonic study. Cell Tissue Res. 1974;149:399–411. doi: 10.1007/BF00226773. An assessment of the distribution of Lugaro cells in the cerebellar cortex. Lugaro cells were found to have a higher packing density in the posterior cerebellum. [DOI] [PubMed] [Google Scholar]
- 37.Lange W. Regional differences in the distribution of golgi cells in the cerebellar cortex of man and some other mammals. Cell Tissue Res. 1974;153:219–26. doi: 10.1007/BF00226610. [DOI] [PubMed] [Google Scholar]
- 38.Brodal A, Drablos PA. Two Types of Mossy Fiber Terminals in the Cerebellum and Their Regional Distribution. J Comp Neurol. 1963;121:173–87. doi: 10.1002/cne.901210203. [DOI] [PubMed] [Google Scholar]
- 39.Fox SS, Liebeskind JC, O’Brien JH, Dingle RD. Mechanisms for limbic modification of cerebellar and cortical afferent information. Prog Brain Res. 1967;27:254–80. doi: 10.1016/S0079-6123(08)63104-0. [DOI] [PubMed] [Google Scholar]
- 40.Geurts FJ, Timmermans J, Shigemoto R, De Schutter E. Morphological and neurochemical differentiation of large granular layer interneurons in the adult rat cerebellum. Neuroscience. 2001;104:499–512. doi: 10.1016/s0306-4522(01)00058-6. [DOI] [PubMed] [Google Scholar]
- 41.Simat M, Parpan F, Fritschy JM. Heterogeneity of glycinergic and gabaergic interneurons in the granule cell layer of mouse cerebellum. J Comp Neurol. 2007;500:71–83. doi: 10.1002/cne.21142. [DOI] [PubMed] [Google Scholar]
- 42.Scott TG. A unique pattern of localization within the cerebellum. Nature. 1963;200:793. doi: 10.1038/200793a0. The distribution of 5′-nucleotidase enzyme activity in the cerbellar cortex. One of the very first studies to demonstrate a striped gene expression in the cerebellum. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Constitutive expression of the 25-kDa heat shock protein Hsp25 reveals novel parasagittal bands of purkinje cells in the adult mouse cerebellar cortex. J Comp Neurol. 2000;416:383–97. doi: 10.1002/(sici)1096-9861(20000117)416:3<383::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Reeber SL, Sillitoe RV. Patterned expression of a cocaine- and amphetamine-regulated transcript peptide reveals complex circuit topography in the rodent cerebellar cortex. J Comp Neurol. 2011;519:1781–96. doi: 10.1002/cne.22601. [DOI] [PubMed] [Google Scholar]
- 45.Mateos JM, et al. Parasagittal compartmentalization of the metabotropic glutamate receptor mGluR1b in the cerebellar cortex. Eur J Anat. 2001;5:15–21. Study showing that the metabotropic glutamate receptor, mGluR1b, is restricted to longitudinal bands that are immunonegative for zebrin. [Google Scholar]
- 46.Dehnes Y, et al. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18:3606–19. doi: 10.1523/JNEUROSCI.18-10-03606.1998. First demonstration that the Purkinje cell specific glutamate transporter EAAT4 is expressed in a zebrin II band pattern. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarna JR, Marzban H, Watanabe M, Hawkes R. Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. J Comp Neurol. 2006;496:303–13. doi: 10.1002/cne.20912. First demonstration that key components in the transduction of type 1 metabotropic glutamate receptor-mediated responses, phospholipase Cβ3 and phospholipase Cβ4 are confined to zebrin II+ and zebrin II− Purkinje cells respectively. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka O, Kondo H. Localization of mRNAs for three novel members (beta 3, beta 4 and gamma 2) of phospholipase C family in mature rat brain. Neurosci Lett. 1994;182:17–20. doi: 10.1016/0304-3940(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 49.Furutama D, et al. Expression of the IP3R1 promoter-driven nls-lacZ transgene in Purkinje cell parasagittal arrays of developing mouse cerebellum. J Neurosci Res. 2010;88:2810–2825. doi: 10.1002/jnr.22451. [DOI] [PubMed] [Google Scholar]
- 50.Barmack NH, Qian Z, Yoshimura J. Regional and cellular distribution of protein kinase C in rat cerebellar Purkinje cells. J Comp Neurol. 2000;427:235–54. doi: 10.1002/1096-9861(20001113)427:2<235::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Hillman DE. Compartmentation of the cerebellar cortex by protein kinase C delta. Neuroscience. 1993;56:177–88. doi: 10.1016/0306-4522(93)90572-w. [DOI] [PubMed] [Google Scholar]
- 52.Marzban H, et al. Expression of the immunoglobulin superfamily neuroplastin adhesion molecules in adult and developing mouse cerebellum and their localisation to parasagittal stripes. J Comp Neurol. 2003;462:286–301. doi: 10.1002/cne.10719. [DOI] [PubMed] [Google Scholar]
- 53.Chung SH, Kim CT, Hawkes R. Compartmentation of GABA B receptor2 expression in the mouse cerebellar cortex. Cerebellum. 2008;7:295–303. doi: 10.1007/s12311-008-0030-3. [DOI] [PubMed] [Google Scholar]
- 54.Fritschy JM, et al. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–8. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 55.Gorenstein C, Bundman MC, Bruce JL, Rotter A. Neuronal localization of pseudocholinesterase in the rat cerebellum: sagittal bands of Purkinje cells in the nodulus and uvula. Brain Res. 1987;418:68–75. doi: 10.1016/0006-8993(87)90963-2. [DOI] [PubMed] [Google Scholar]
- 56.Marani E, Voogd J. An acetylcholinesterase band-pattern in the molecular layer of the cat cerebellum. J Anat. 1977;124:335–45. [PMC free article] [PubMed] [Google Scholar]
- 57.Jinno S, Jeromin A, Roder J, Kosaka T. Compartmentation of the mouse cerebellar cortex by neuronal calcium sensor-1. J Comp Neurol. 2003;458:412–24. doi: 10.1002/cne.10585. [DOI] [PubMed] [Google Scholar]
- 58.Touri F, Hawkes R, Riederer BM. Differential distribution of MAP1a and aldolase c in adult mouse cerebellum. Eur J Neurosci. 1996;8:61–8. doi: 10.1111/j.1460-9568.1996.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 59.Larouche M, Che PM, Hawkes R. Neurogranin expression identifies a novel array of Purkinje cell parasagittal stripes during mouse cerebellar development. J Comp Neurol. 2006;494:215–27. doi: 10.1002/cne.20791. [DOI] [PubMed] [Google Scholar]
- 60.Oberdick J, et al. Control of segment-like patterns of gene expression in the mouse cerebellum. Neuron. 1993;10:1007–18. doi: 10.1016/0896-6273(93)90050-2. [DOI] [PubMed] [Google Scholar]
- 61.Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291:538–52. doi: 10.1002/cne.902910405. The first identification of the canonical stripe antigen zebrin II. [DOI] [PubMed] [Google Scholar]
- 62.Ahn AH, Dziennis S, Hawkes R, Herrup K. The cloning of zebrin II reveals its identity with aldolase C. Development. 1994;120:2081–90. doi: 10.1242/dev.120.8.2081. [DOI] [PubMed] [Google Scholar]
- 63.Fujita H, et al. Detailed expression pattern of aldolase C (Aldoc) in the cerebellum, retina and other areas of the CNS studied in Aldoc-Venus knock-in mice. PLoS One. 2014;9:e86679. doi: 10.1371/journal.pone.0086679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sillitoe RV, et al. Conservation of the architecture of the anterior lobe vermis of the cerebellum across mammalian species. Prog Brain Res. 2005;148:283–97. doi: 10.1016/S0079-6123(04)48022-4. [DOI] [PubMed] [Google Scholar]
- 65.Consalez GG, Hawkes R. The compartmental restriction of cerebellar interneurons. Front Neural Circuits. 2012;6:123. doi: 10.3389/fncir.2012.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sillitoe RV, Chung SH, Fritschy JM, Hoy M, Hawkes R. Golgi cell dendrites are restricted by Purkinje cell stripe boundaries in the adult mouse cerebellar cortex. J Neurosci. 2008;28:2820–6. doi: 10.1523/JNEUROSCI.4145-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barmack NH, Baughman RW, Eckenstein FP. Cholinergic innervation of the cerebellum of rat, rabbit, cat, and monkey as revealed by choline acetyltransferase activity and immunohistochemistry. J Comp Neurol. 1992;317:233–49. doi: 10.1002/cne.903170303. [DOI] [PubMed] [Google Scholar]
- 68.Bishop GA, Ho RH. The distribution and origin of serotonin immunoreactivity in the rat cerebellum. Brain Res. 1985;331:195–207. doi: 10.1016/0006-8993(85)91545-8. [DOI] [PubMed] [Google Scholar]
- 69.Gebre SA, Reeber SL, Sillitoe RV. Parasagittal compartmentation of cerebellar mossy fibers as revealed by the patterned expression of vesicular glutamate transporters VGLUT1 and VGLUT2. Brain Struct Funct. 2012;217:165–80. doi: 10.1007/s00429-011-0339-4. Combined anatomical and immunohistochemical study demonstrating that sensory projections to the cerebellum via the spinocerebellar and dorsal column nuclei mossy fibres differentially express VGLUT1 and VGLUT2, and additionally, terminate in molecularly distinct bands that respect common compartmental boundaries in the adult mouse cerebellar cortex. [DOI] [PubMed] [Google Scholar]
- 70.Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51:719–28. doi: 10.1016/0306-4522(92)90310-x. [DOI] [PubMed] [Google Scholar]
- 71.Jaarsma D, et al. Cholinergic innervation and receptors in the cerebellum. Prog Brain Res. 1997;114:67–96. doi: 10.1016/s0079-6123(08)63359-2. [DOI] [PubMed] [Google Scholar]
- 72.Paukert M, Huang YH, Tanaka K, Rothstein JD, Bergles DE. Zones of enhanced glutamate release from climbing fibers in the mammalian cerebellum. J Neurosci. 2010;30:7290–9. doi: 10.1523/JNEUROSCI.5118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sawada K, Fukui Y, Hawkes R. Spatial distribution of corticotropin-releasing factor immunopositive climbing fibers in the mouse cerebellum: analysis by whole mount immunohistochemistry. Brain Res. 2008;1222:106–17. doi: 10.1016/j.brainres.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 74.Cummings SL, Young WS, 3rd, Bishop GA, De Souza EB, King JS. Distribution of corticotropin-releasing factor in the cerebellum and precerebellar nuclei of the opossum: a study utilizing immunohistochemistry in situ hybridization histochemistry and receptor autoradiography. J Comp Neurol. 1989;280:501–21. doi: 10.1002/cne.902800402. [DOI] [PubMed] [Google Scholar]
- 75.King JS, Ho RH, Bishop GA. Anatomical evidence for enkephalin immunoreactive climbing fibres in the cerebellar cortex of the opossum. J Neurocytol. 1986;15:545–59. doi: 10.1007/BF01611856. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong CL, et al. A novel somatostatin-immunoreactive mossy fiber pathway associated with HSP25-immunoreactive purkinje cell stripes in the mouse cerebellum. J Comp Neurol. 2009;517:524–38. doi: 10.1002/cne.22167. [DOI] [PubMed] [Google Scholar]
- 77.Cerminara NL, Aoki H, Loft M, Sugihara I, Apps R. Structural basis of cerebellar microcircuits in the rat. J Neurosci. 2013;33:16427–42. doi: 10.1523/JNEUROSCI.0861-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chedotal A, Bloch-Gallego E, Sotelo C. The embryonic cerebellum contains topographic cues that guide developing inferior olivary axons. Development. 1997;124:861–70. doi: 10.1242/dev.124.4.861. [DOI] [PubMed] [Google Scholar]
- 79.Ji Z, Hawkes R. Developing mossy fiber terminal fields in the rat cerebellar cortex may segregate because of Purkinje cell compartmentation and not competition. J Comp Neurol. 1995;359:197–212. doi: 10.1002/cne.903590202. [DOI] [PubMed] [Google Scholar]
- 80.Pijpers A, Apps R, Pardoe J, Voogd J, Ruigrok TJ. Precise spatial relationships between mossy fibers and climbing fibers in rat cerebellar cortical zones. J Neurosci. 2006;26:12067–80. doi: 10.1523/JNEUROSCI.2905-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sotelo C, Chedotal A. Development of the olivocerebellar system: migration and formation of cerebellar maps. Prog Brain Res. 2005;148:1–20. doi: 10.1016/S0079-6123(04)48001-7. [DOI] [PubMed] [Google Scholar]
- 82.Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci. 2004;24:8771–85. doi: 10.1523/JNEUROSCI.1961-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voogd J, Ruigrok TJ. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol. 2004;33:5–21. doi: 10.1023/B:NEUR.0000029645.72074.2b. [DOI] [PubMed] [Google Scholar]
- 84.Kim YS, Shin JH, Hall FS, Linden DJ. Dopamine signaling is required for depolarization-induced slow current in cerebellar Purkinje cells. J Neurosci. 2009;29:8530–8. doi: 10.1523/JNEUROSCI.0468-09.2009. Study showing D3 dopamine receptors and dopamine plasma membrane transporters are more strongly expressed in the posterior cerebellum, particularly in lobules IX and X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chung SH, Marzban H, Hawkes R. Compartmentation of the cerebellar nuclei of the mouse. Neuroscience. 2009;161:123–38. doi: 10.1016/j.neuroscience.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 86.Pakan JM, Graham DJ, Wylie DR. Organization of visual mossy fiber projections and zebrin expression in the pigeon vestibulocerebellum. J Comp Neurol. 2010;518:175–98. doi: 10.1002/cne.22192. [DOI] [PubMed] [Google Scholar]
- 87.Quy PN, Fujita H, Sakamoto Y, Na J, Sugihara I. Projection patterns of single mossy fiber axons originating from the dorsal column nuclei mapped on the aldolase C compartments in the rat cerebellar cortex. J Comp Neurol. 2011;519:874–99. doi: 10.1002/cne.22555. [DOI] [PubMed] [Google Scholar]
- 88.Sugihara I. Compartmentalization of the deep cerebellar nuclei based on afferent projections and aldolase C expression. Cerebellum. 2011;10:449–63. doi: 10.1007/s12311-010-0226-1. [DOI] [PubMed] [Google Scholar]
- 89.Nagao S, Kwak S, Kanazawa I. EAAT4, a glutamate transporter with properties of a chloride channel, is predominantly localized in Purkinje cell dendrites, and forms parasagittal compartments in rat cerebellum. Neuroscience. 1997;78:929–33. doi: 10.1016/s0306-4522(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 90.Chaudhry FA, et al. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–20. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 91.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–53. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–43. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 93.Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–16. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi M, Kovalchuk Y, Attwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J Neurosci. 1995;15:5693–702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi M, Sarantis M, Attwell D. Postsynaptic glutamate uptake in rat cerebellar Purkinje cells. J Physiol. 1996;497:523–30. doi: 10.1113/jphysiol.1996.sp021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takayasu Y, Iino M, Ozawa S. Roles of glutamate transporters in shaping excitatory synaptic currents in cerebellar Purkinje cells. Eur J Neurosci. 2004;19:1285–95. doi: 10.1111/j.1460-9568.2004.03224.x. [DOI] [PubMed] [Google Scholar]
- 97.Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–34. doi: 10.1038/nn1539. First demonstration of differential synaptic plasticity in zebrin II+ versus zebrin II− areas of the cerebellum. [DOI] [PubMed] [Google Scholar]
- 98.Shin JH, Kim YS, Linden DJ. Dendritic glutamate release produces autocrine activation of mGluR1 in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 2008;105:746–50. doi: 10.1073/pnas.0709407105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim CH, et al. Lobule-specific membrane excitability of cerebellar Purkinje cells. J Physiol. 2012;590:273–88. doi: 10.1113/jphysiol.2011.221846. An in vitro electrophysiological study showing that Purkinje cells can display four distinct firing patterns, and that the differences in electrophysiological firing patterns are lobule-specific. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Häusser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–78. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 101.McKay BE, Turner RW. Kv3 K+ channels enable burst output in rat cerebellar Purkinje cells. Eur J Neurosci. 2004;20:729–39. doi: 10.1111/j.1460-9568.2004.03539.x. [DOI] [PubMed] [Google Scholar]
- 102.Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31:785–97. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- 103.Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–95. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cerminara NL, Rawson JA. Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. J Neurosci. 2004;24:4510–7. doi: 10.1523/JNEUROSCI.4530-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22:10603–12. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loewenstein Y, et al. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci. 2005;8:202–11. doi: 10.1038/nn1393. [DOI] [PubMed] [Google Scholar]
- 107.Schonewille M, et al. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci. 2006;9:459–61. doi: 10.1038/nn0406-459. author reply 461. [DOI] [PubMed] [Google Scholar]
- 108.Cao Y, Maran SK, Dhamala M, Jaeger D, Heck DH. Behavior-related pauses in simple-spike activity of mouse Purkinje cells are linked to spike rate modulation. J Neurosci. 2012;32:8678–85. doi: 10.1523/JNEUROSCI.4969-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yartsev MM, Givon-Mayo R, Maller M, Donchin O. Pausing purkinje cells in the cerebellum of the awake cat. Front Syst Neurosci. 2009;3:2. doi: 10.3389/neuro.06.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou H, et al. Cerebellar modules operate at different frequencies. eLife. 2014;3:e02536. doi: 10.7554/eLife.02536. One of the first comparisons of the firing characteristics of Purkinje cells between zebrin II bands in awake mice showing that spiking activity of Purkinje cells differs and correlates with the expression of zebrin II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao J, et al. Systematic regional variations in Purkinje cell spiking patterns. PLoS One. 2014;9:e105633. doi: 10.1371/journal.pone.0105633. One of the first comparisons of the firing characteristics of Purkinje cells between zebrin II bands in vivo. This electrophysiological study showed that Purkinje cells recorded in zebrin II+ and zebrin II− bands, and the A2 and C1 zones (which are largely Z+ and Z− respectively) displayed significant differences in simple spike activity, irregularity of simple spike firing, post-complex spike pauses in simple spikes and post-pause modulation of simple spikes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sugihara I, et al. Projection of reconstructed single Purkinje cell axons in relation to the cortical and nuclear aldolase C compartments of the rat cerebellum. J Comp Neurol. 2009;512:282–304. doi: 10.1002/cne.21889. [DOI] [PubMed] [Google Scholar]
- 113.Stein RB. The information capacity of nerve cells using a frequency code. Biophys J. 1967;7:797–826. doi: 10.1016/S0006-3495(67)86623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2012;481:502–5. doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heck DH, De Zeeuw CI, Jaeger D, Khodakhah K, Person AL. The neuronal code(s) of the cerebellum. J Neurosci. 2013;33:17603–9. doi: 10.1523/JNEUROSCI.2759-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steuber V, Jaeger D. Modeling the generation of output by the cerebellar nuclei. Neural Netw. 2013;47:112–9. doi: 10.1016/j.neunet.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Llinás RR. In: The Cerebellum: New Vistas. Palay SL, Chan-Palay V, editors. Springer-Verlag; Berlin: 1982. pp. 189–192. [Google Scholar]
- 118.Bower JM, Woolston DC. Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol. 1983;49:745–66. doi: 10.1152/jn.1983.49.3.745. [DOI] [PubMed] [Google Scholar]
- 119.Cohen D, Yarom Y. Patches of synchronized activity in the cerebellar cortex evoked by mossy-fiber stimulation: questioning the role of parallel fibers. Proc Natl Acad Sci U S A. 1998;95:15032–6. doi: 10.1073/pnas.95.25.15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garwicz M, Andersson G. Spread of synaptic activity along parallel fibres in cat cerebellar anterior lobe. Exp Brain Res. 1992;88:615–22. doi: 10.1007/BF00228190. [DOI] [PubMed] [Google Scholar]
- 121.Cramer SW, Gao W, Chen G, Ebner TJ. Reevaluation of the beam and radial hypotheses of parallel fiber action in the cerebellar cortex. J Neurosci. 2013;33:11412–24. doi: 10.1523/JNEUROSCI.0711-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Oscarsson O. Functional units of the cerebellum- sagittal zones and microzones. Trends in Neuroscience. 1979;2:144–145. [Google Scholar]
- 123.Granit R, Phillips CG. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956;133:520–47. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bloedel JR, Roberts WJ. Action of climbing fibers in cerebellar cortex of the cat. J Neurophysiol. 1971;34:17–31. doi: 10.1152/jn.1971.34.1.17. [DOI] [PubMed] [Google Scholar]
- 125.Latham A, Paul DH. Spontaneous activity of cerebellar Purkinje cells and their responses to impulses in climbing fibres. J Physiol. 1971;213:135–56. doi: 10.1113/jphysiol.1971.sp009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Colin F, Manil J, Desclin JC. The olivocerebellar system. I. Delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibers. Brain Res. 1980;187:3–27. doi: 10.1016/0006-8993(80)90491-6. [DOI] [PubMed] [Google Scholar]
- 127.Montarolo PG, Palestini M, Strata P. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol. 1982;332:187–202. doi: 10.1113/jphysiol.1982.sp014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rawson JA, Tilokskulchai K. Suppression of simple spike discharges of cerebellar Purkinje cells by impulses in climbing fibre afferents. Neurosci Lett. 1981;25:125–30. doi: 10.1016/0304-3940(81)90319-0. [DOI] [PubMed] [Google Scholar]
- 129.Gao W, Chen G, Reinert KC, Ebner TJ. Cerebellar cortical molecular layer inhibition is organized in parasagittal zones. J Neurosci. 2006;26:8377–87. doi: 10.1523/JNEUROSCI.2434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eccles JC, Llinás R, Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1:17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- 131.Wang X, Chen G, Gao W, Ebner TJ. Parasagittally aligned, mGluR1-dependent patches are evoked at long latencies by parallel fiber stimulation in the mouse cerebellar cortex in vivo. J Neurophysiol. 2011;105:1732–46. doi: 10.1152/jn.00717.2010. Combined electrophysiological and fluorescent imaging study that demonstrates that zebrin II+ and zebrin II− bands differ markedly in their responses to parallel fibre stimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sarna JR, Hawkes R. Patterned Purkinje cell death in the cerebellum. Prog Neurobiol. 2003;70:473–507. doi: 10.1016/s0301-0082(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 133.Sillitoe RV, Hawkes R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. J Histochem Cytochem. 2002;50:235–44. doi: 10.1177/002215540205000211. [DOI] [PubMed] [Google Scholar]
- 134.Welsh JP, et al. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–59. This paper provided the first extensive discussion about the molecular and functional differences/susceptibilities of zones in different mouse models and in a rat model of stroke that exhibits patterned cell death. [PubMed] [Google Scholar]
- 135.Herrup K, Wilczynski SL. Cerebellar cell degeneration in the leaner mutant mouse. Neuroscience. 1982;7:2185–96. doi: 10.1016/0306-4522(82)90129-4. One of the earliest studies to show in a mouse mutant zebrin II− Purkinje cells are preferentially sensitive and die first. [DOI] [PubMed] [Google Scholar]
- 136.Heckroth JA, Abbott LC. Purkinje cell loss from alternating sagittal zones in the cerebellum of leaner mutant mice. Brain Res. 1994;658:93–104. doi: 10.1016/s0006-8993(09)90014-2. [DOI] [PubMed] [Google Scholar]
- 137.Wassef M, Sotelo C, Cholley B, Brehier A, Thomasset M. Cerebellar mutations affecting the postnatal survival of Purkinje cells in the mouse disclose a longitudinal pattern of differentially sensitive cells. Dev Biol. 1987;124:379–89. doi: 10.1016/0012-1606(87)90490-8. [DOI] [PubMed] [Google Scholar]
- 138.Croci C, et al. Cerebellar neurons and glial cells are transducible by lentiviral vectors without decrease of cerebellar functions. Dev Neurosci. 2006;28:216–21. doi: 10.1159/000091919. [DOI] [PubMed] [Google Scholar]
- 139.Hashimoto M, Mikoshiba K. Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. J Neurosci. 2003;23:11342–51. doi: 10.1523/JNEUROSCI.23-36-11342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Namba K, Sugihara I, Hashimoto M. Close correlation between the birth date of Purkinje cells and the longitudinal compartmentalization of the mouse adult cerebellum. J Comp Neurol. 2011;519:2594–614. doi: 10.1002/cne.22640. [DOI] [PubMed] [Google Scholar]
- 141.Sillitoe RV, Gopal N, Joyner AL. Embryonic origins of ZebrinII parasagittal stripes and establishment of topographic Purkinje cell projections. Neuroscience. 2009;162:574–88. doi: 10.1016/j.neuroscience.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sillitoe RV, Stephen D, Lao Z, Joyner AL. Engrailed homeobox genes determine the organization of Purkinje cell sagittal stripe gene expression in the adult cerebellum. J Neurosci. 2008;28:12150–62. doi: 10.1523/JNEUROSCI.2059-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sarna JR, et al. Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J Comp Neurol. 2003;456:279–91. doi: 10.1002/cne.10522. An example where an inherited, autosomal recessive, lipid-storage disorder where Purkinje cell death is a prominent feature of the neuropathology. The progressive pattern of Purkinje cell death is complex but reflects the fundamental compartmentation of the cerebellum. [DOI] [PubMed] [Google Scholar]
- 144.Williams BL, Yaddanapudi K, Hornig M, Lipkin WI. Spatiotemporal analysis of purkinje cell degeneration relative to parasagittal expression domains in a model of neonatal viral infection. J Virol. 2007;81:2675–87. doi: 10.1128/JVI.02245-06. This study examined Purkinje cell death in a model of neonatal viral infection and found Purkinje cell loss occurred in with zebrin II and EAAT4 Purkinje cell phenotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schorge S, van de Leemput J, Singleton A, Houlden H, Hardy J. Human ataxias: a genetic dissection of inositol triphosphate receptor (ITPR1)-dependent signaling. Trends Neurosci. 2010;33:211–9. doi: 10.1016/j.tins.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berezniuk I, et al. CCP1/Nna1 functions in protein turnover in mouse brain: Implications for cell death in Purkinje cell degeneration mice. FASEB J. 2010;24:1813–23. doi: 10.1096/fj.09-147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chakrabarti L, et al. Mitochondrial dysfunction in NnaD mutant flies and Purkinje cell degeneration mice reveals a role for Nna proteins in neuronal bioenergetics. Neuron. 2010;66:835–47. doi: 10.1016/j.neuron.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baltanas FC, et al. Purkinje cell degeneration in pcd mice reveals large scale chromatin reorganization and gene silencing linked to defective DNA repair. J Biol Chem. 2011;286:28287–302. doi: 10.1074/jbc.M111.246041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Duffin CA, McFarland R, Sarna JR, Vogel MW, Armstrong CL. Heat shock protein 25 expression and preferential Purkinje cell survival in the lurcher mutant mouse cerebellum. J Comp Neurol. 2010;518:1892–907. doi: 10.1002/cne.22309. [DOI] [PubMed] [Google Scholar]
- 150.Armstrong CL, Duffin CA, McFarland R, Vogel MW. Mechanisms of compartmental purkinje cell death and survival in the lurcher mutant mouse. Cerebellum. 2011;10:504–14. doi: 10.1007/s12311-010-0231-4. [DOI] [PubMed] [Google Scholar]
- 151.Hess EJ, Wilson MC. Tottering and leaner mutations perturb transient developmental expression of tyrosine hydroxylase in embryologically distinct Purkinje cells. Neuron. 1991;6:123–32. doi: 10.1016/0896-6273(91)90127-l. [DOI] [PubMed] [Google Scholar]
- 152.Fureman BE, Campbell DB, Hess EJ. Regulation of tyrosine hydroxylase expression in tottering mouse Purkinje cells. Neurotox Res. 2003;5:521–8. doi: 10.1007/BF03033162. [DOI] [PubMed] [Google Scholar]
- 153.Jeong YG, Kim MK, Hawkes R. Ectopic expression of tyrosine hydroxylase in Zebrin II immunoreactive Purkinje cells in the cerebellum of the ataxic mutant mouse, pogo. Brain Res Dev Brain Res. 2001;129:201–9. doi: 10.1016/s0165-3806(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 154.Abbott LC, Isaacs KR, Heckroth JA. Co-localization of tyrosine hydroxylase and zebrin II immunoreactivities in Purkinje cells of the mutant mice, tottering and tottering/leaner. Neuroscience. 1996;71:461–75. doi: 10.1016/0306-4522(95)00444-0. [DOI] [PubMed] [Google Scholar]
- 155.Sawada K, Fukui Y. Zebrin II expressing Purkinje cell phenotype-related and -unrelated cerebellar abnormalities in Cav2.1 mutant, rolling mouse Nagoya. ScientificWorldJournal. 2010;10:2032–8. doi: 10.1100/tsw.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sawada K, et al. Striking pattern of Purkinje cell loss in cerebellum of an ataxic mutant mouse, tottering. Acta Neurobiol Exp (Wars) 2009;69:138–45. doi: 10.55782/ane-2009-1736. The first anatomical study that demonstrated that in the ataxic tottering mouse that the pattern of Purkinje cell loss was characterized by a selective loss of Purkinje cells in zebrin II− bands in the anterior vermis and in zebrin II+ bands in the posterior vermis. [DOI] [PubMed] [Google Scholar]
- 157.White JJ, et al. Cerebellar zonal patterning relies on Purkinje cell neurotransmission. J Neurosci. 2014;34:8231–45. doi: 10.1523/JNEUROSCI.0122-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stromme P, et al. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain. 2011;134:3369–83. doi: 10.1093/brain/awr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marzban H, Hawkes R. On the architecture of the posterior zone of the cerebellum. Cerebellum. 2011;10:422–34. doi: 10.1007/s12311-010-0208-3. [DOI] [PubMed] [Google Scholar]
- 160.Carper RA, Wideman GM, Courchesne E. In: Structural Neuroimaging. Moldin SO, Rubenstein JLR, editors. CRC Press Taylor and Francis; Boca Raton: 2006. pp. 347–377. [Google Scholar]
- 161.Prudente CN, et al. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kutzelnigg A, et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17:38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Graham DJ, Wylie DR. Zebrin-immunopositive and -immunonegative stripe pairs represent functional units in the pigeon vestibulocerebellum. J Neurosci. 2012;32:12769–79. doi: 10.1523/JNEUROSCI.0197-12.2012. An electrophysiological study showing that Purkinje cell complex spike activity of a given zebrin II stripe pair in the uvula of the pigeon responds to particular patterns of optic flow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Apps R, Atkins MJ, Garwicz M. Gating of cutaneous input to cerebellar climbing fibres during a reaching task in the cat. J Physiol. 1997;502:203–14. doi: 10.1111/j.1469-7793.1997.203bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Apps R, Lee S. Gating of transmission in climbing fibre paths to cerebellar cortical C1 and C3 zones in the rostral paramedian lobule during locomotion in the cat. J Physiol. 1999;516:875–83. doi: 10.1111/j.1469-7793.1999.0875u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anderson SR, et al. An internal model architecture for novelty detection: implications for cerebellar and collicular roles in sensory processing. PLoS One. 2012;7:e44560. doi: 10.1371/journal.pone.0044560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dean P, Anderson S, Porrill J, Jörntell H. An adaptive filter model of cerebellar zone C3 as a basis for safe limb control? J Physiol. 2013;591:5459–74. doi: 10.1113/jphysiol.2013.261545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dean P, Porrill J. Adaptive-filter models of the cerebellum: computational analysis. Cerebellum. 2008;7:567–71. doi: 10.1007/s12311-008-0067-3. [DOI] [PubMed] [Google Scholar]
- 169.Dean P, Porrill J. Evaluating the adaptive-filter model of the cerebellum. J Physiol. 2011;589:3459–70. doi: 10.1113/jphysiol.2010.201574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jörntell H, Ekerot CF. Receptive Field Remodeling Induced by Skin Stimulation in Cerebellar Neurons in vivo. Front Neural Circuits. 2011;5:3. doi: 10.3389/fncir.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jörntell H, Ekerot CF. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J Neurosci. 2003;23:9620–31. doi: 10.1523/JNEUROSCI.23-29-09620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jörntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806. doi: 10.1016/s0896-6273(02)00713-4. [DOI] [PubMed] [Google Scholar]
- 173.Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27:2493–502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.White JJ, Sillitoe RV. Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdiscip Rev Dev Biol. 2013;2:149–64. doi: 10.1002/wdev.65. [DOI] [PubMed] [Google Scholar]
- 175.Sudarov A, et al. Ascl1 genetics reveals insights into cerebellum local circuit assembly. J Neurosci. 2011;31:11055–69. doi: 10.1523/JNEUROSCI.0479-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Millen KJ, Hui CC, Joyner AL. A role for En-2 and other murine homologues of Drosophila segment polarity genes in regulating positional information in the developing cerebellum. Development. 1995;121:3935–45. doi: 10.1242/dev.121.12.3935. [DOI] [PubMed] [Google Scholar]
- 177.Wassef M, Zanetta JP, Brehier A, Sotelo C. Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev Biol. 1985;111:129–37. doi: 10.1016/0012-1606(85)90441-5. [DOI] [PubMed] [Google Scholar]
- 178.Fujita H, Morita N, Furuichi T, Sugihara I. Clustered fine compartmentalization of the mouse embryonic cerebellar cortex and its rearrangement into the postnatal striped configuration. J Neurosci. 2012;32:15688–703. doi: 10.1523/JNEUROSCI.1710-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marzban H, Chung S, Watanabe M, Hawkes R. Phospholipase Cbeta4 expression reveals the continuity of cerebellar topography through development. J Comp Neurol. 2007;502:857–71. doi: 10.1002/cne.21352. [DOI] [PubMed] [Google Scholar]
- 180.Larouche M, Hawkes R. From clusters to stripes: the developmental origins of adult cerebellar compartmentation. Cerebellum. 2006;5:77–88. doi: 10.1080/14734220600804668. [DOI] [PubMed] [Google Scholar]
- 181.Ozol K, Hayden JM, Oberdick J, Hawkes R. Transverse zones in the vermis of the mouse cerebellum. J Comp Neurol. 1999;412:95–111. [PubMed] [Google Scholar]
- 182.Hisatsune C, et al. IP3R1 deficiency in the cerebellum/brainstem causes basal ganglia-independent dystonia by triggering tonic Purkinje cell firings in mice. Front Neural Circuits. 2013;7:156. doi: 10.3389/fncir.2013.00156. In a conditional knockout mouse lacking IP3R1 specifically in the cerebellum and brainstem and resulting in dystonia, Purkinje cells display a banded expression of tyrosine hydroxylase expression that is restricted to the posterior cerebellum i.e. lobules VI–X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Larsell O. The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol. 1952;97:281–356. doi: 10.1002/cne.900970204. [DOI] [PubMed] [Google Scholar]
- 184.Reeber SL, Otis TS, Sillitoe RV. New roles for the cerebellum in health and disease. Front Syst Neurosci. 2013;7:83. doi: 10.3389/fnsys.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.