Abstract
Contrast-induced acute kidney injury (CIAKI) is a leading cause of iatrogenic renal failure. Multiple studies have shown that patients with diabetic nephropathy are at high risk of CIAKI. This Review presents an overview of the pathogenesis of CIAKI in patients with diabetic nephropathy and discusses the currently available and potential future strategies for CIAKI prevention.
Introduction
In 2008, contrast-induced acute kidney injury (CIAKI) was proposed as the consensus name for what was formerly termed ‘contrast-induced nephropathy’.1 CIAKI is the most common cause of iatrogenic, drug-induced, acute kidney injury (AKI) in hospitals.2–5 CIAKI primarily presents as a nonoliguric form of AKI with an increase in serum creatinine level within 48–72 h after administration of contrast medium. Serum creatinine levels typically return to baseline after 7–10 days, although some patients develop a chronic reduction in kidney function or a permanent need for renal replacement therapy. CIAKI is associated with a 1-year mortality rate of up to 30%.5–10 In a study of more than 8,000 patients,11 diabetes, female sex, and the amount of contrast agent administered were the strongest predictors of CIAKI-associated death. The mortality rate associated with CIAKI might even be >30% in patients with diabetes who receive intravenous contrast media.12 This Review presents an overview of the pathogenesis of CIAKI in patients with diabetic nephropathy and discusses both currently available therapies and potential future strategies for CIAKI prevention. Given the limited evidence that CIAKI is associated with the use of gadolinium-based contrast agents, this Review will focus on iodinated contrast media.
Types of contrast media
Gadolinium
Gadolinium, the contrast medium commonly used in MRI, was once considered to be non-nephrotoxic.13 However, reports of nephrotoxic and dermatotoxic14 effects of gadolinium have led to gadolinium being administered predominantly to patients with preserved renal function.15 Gadolinium-associated CIAKI has been reported in patients with diabetes,16 but supporting evidence is limited and is not discussed further in this article.
Iodinated agents
CIAKI can occur after intra-arterial and intravenous administration of iodinated contrast media.17 The risk of CIAKI in patients with diabetic nephropathy depends on the characteristics of the contrast medium, including its osmolality, viscosity and volume.3,18
The effects of osmolality and viscosity
High-osmolality contrast media (>800 mmol/kg) are associated with a higher incidence of CIAKI than low-osmolality (600–800 mmol/kg) contrast media in patients with diabetic nephropathy.2,19 Iso-osmolal contrast media (approximately 290 mmol/kg) were originally developed to reduce the risk of CIAKI. However, iso-osmolal contrast media have a higher viscosity than low-osmolal contrast media,20,21 and increased viscosity has been shown to reduce renal tubular flow and decrease glomerular filtration rate (GFR).22 The desirability of low-osmolality contrast agents has, therefore, been questioned.20,21,23,24
Early studies showed a lower incidence of CIAKI in patients with diabetic nephropathy who had received an iso-osmolar contrast medium than in patients who had received a low-osmolarity contrast medium;25,26 however, larger studies have not confirmed this finding.27,28 The results of the double-blind, multi-center CARE study27 showed no difference in the rate of CIAKI, defined as a >50% increase in serum creatinine level with respect to baseline, between patients with diabetic nephropathy who had received the low-osmolal contrast medium iopamidol and patients who had received the iso-osmolal contrast medium iodixanol. By contrast, in the VALOR trial,28 researchers studied the incidence of CIAKI in patients with chronic kidney disease (CKD) without diabetes (n = 145) and in patients with both CKD and diabetes (n = 154) receiving either the iso-osmolar contrast medium iodixanol or the low-osmolar contrast medium ioversol. CIAKI was defined as an increase in serum creatinine level from baseline of ≥44 μmol/l. In patients with CKD but without diabetes, no difference was observed in the incidence of CIAKI between the two treatment groups. However, in patients with both CKD and diabetes (most of whom were likely to have diabetic nephropathy29,30), the incidence of CIAKI tended to be lower in the iodixanol group than in the ioversol group (21.9% versus 26.4%; P = 0.57). Furthermore, the mean peak percentage increase in serum creatinine levels from baseline after administration of contrast medium was significantly lower in patients with CKD and diabetes who received iodixanol than in those who received ioversol (12.9% versus 22.4%; P = 0.01). The VALOR and CARE trials differ in several important aspects, however, which might account for their disparate findings. Some of these aspects are discussed below. In summary, although strong evidence is lacking, iso-osmolar contrast media might be safer than low-osmolar and high-osmolar contrast media.
Renal function assessment
The results of the CARE and VALOR studies also illustrate that the definition of CIAKI can make an important difference to trial outcomes and, therefore, to the reported incidence of CIAKI. Efforts to implement a reasonable classification and definition of CIAKI have, however, been made only in the past couple of years.6
All studies published to date have defined the development of CIAKI as an increase in serum creatinine level from baseline either in absolute terms (such as 44–88 μmol/l) or as a percentage increase (25% or 50%).3,4,31–34 However, serum creatinine level is an insensitive marker of GFR in patients with normal kidney function—these levels can vary by 10–20% in such individuals, depending on hydration status. Hyperfiltration occurs in a small subset of patients with diabetes, dependent on the degree of hyperglycemia.35 Furthermore, changes in serum creatinine level may take a day or more to become apparent after a substantial decrease in GFR has occurred. Creatinine clearance seems to be a more sensitive marker of GFR than serum creatinine level, but measurement of creatinine clearance requires 24 h urine collection, which is impractical in many settings. Studies of the short-term renal clearance of iothalamate suggest that this marker might be more promising than creatinine clearance as a measure of GFR. Measurement of serum cystatin C concentration might be a more accurate measure of GFR than serum creatinine level, although further studies are needed.36
The RIFLE criteria have been developed to standardize and improve care of patients with AKI.37,38 By using both GFR and urine output criteria, the RIFLE criteria avoid the pitfalls of using serum creatinine levels alone to categorize the severity of renal impairment. Patients are grouped according to the RIFLE acronym: at risk of AKI (R), with renal injury (I), with renal failure (F), with sustained loss of renal function (L), and with end-stage renal disease (E). Several studies have shown that the RIFLE criteria correlate with outcomes in different populations,39 including hospitalized patients,40 patients in intensive care units,41–43 cardiac surgery patients,44 and patients on continuous venovenous hemodialysis.45 Whether different etiologies of AKI, including CIAKI, affect the clinical outcome of each degree of renal dysfunction in the RIFLE classification is not yet known.37 Staging the severity of AKI according to the RIFLE criteria might be reasonable in future studies of CIAKI.
Pathogenesis of CIAKI
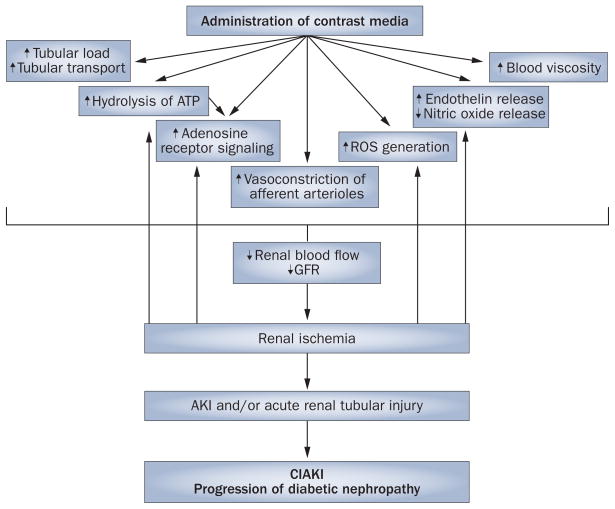
CIAKI is primarily an ischemic form of AKI caused by the vasoconstrictive properties of contrast media (Figures 1 and 2). In addition, contrast media can potentially have direct toxic effects on endothelial cells and renal tubules, reduce erythrocyte flexibility, and activate leukocyte adhesion molecules, which results in leukocytes binding to endothelial cells.2,3,46
Figure 1.
The pathways potentially underlying the pathogenesis of CIAKI in patients with diabetes. Abbreviations: AKI, acute kidney injury; CIAKI, contrast-induced acute kidney injury; GFR, glomerular filtration rate; ROS, reactive oxygen species.
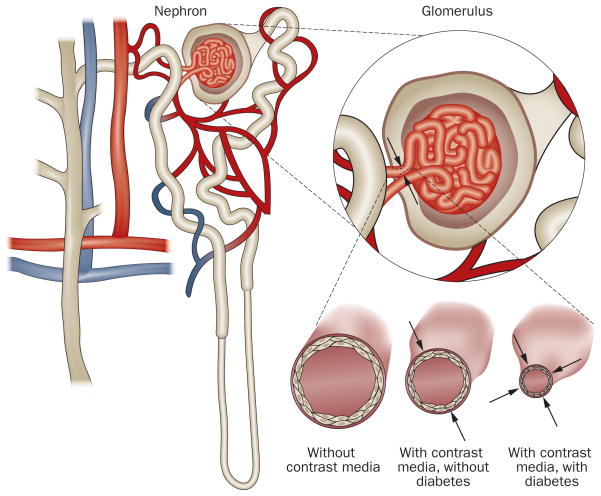
Figure 2.
Both contrast media and diabetes affect dilation of the renal vasculature. Permission obtained from International Scientific Literature Inc. © Pflueger, A. et al. Med. Sci. Monit. 15, RA125–RA136 (2009).
Animal studies have shown that after injection of contrast media into the renal vasculature, renal blood flow transiently increases followed by a substantial decrease to levels below the original baseline.47,48 This biphasic response of renal blood flow to contrast-medium injection is also characteristic of the response to adenosine in the renal vasculature,47–51 so adenosine has been postulated to have an important role in the pathogenesis of CIAKI.47–51 The overall net effect of infusion of either adenosine or contrast media into the renal artery is a prolonged reduction in renal blood flow.51–54
Iodine-based contrast media seem to cause renal vaso-constriction in the afferent arteriole via stimulation of 47,48,55–57 In addition to adenosine receptor A1 (Figure 1). stimulation of adenosine receptor A1, adenosine also causes renal vasodilation via stimulation of the adenosine receptor A2a in the efferent arteriolar and medullary capillaries. Contrast media seem to reduce renal blood flow directly through afferent arteriole vasoconstriction via activation of adenosine receptor A1.57
In animal studies, intravenous administration of contrast media increases renal excretion of adenosine47,48,58 and endothelin.46,59 Indirectly, contrast media are thought to induce the release of various renal vasoconstrictors within the kidney, including adenosine, endothelin, angiotensin II, and reactive oxygen species (ROS), which collectively cause renal hypoxia and increased adenosine generation through ATP hydrolysis.2,4,19,46–48 This hypothesis is supported by studies that demonstrate that CIAKI can be prevented by administration of adenosine-receptor antagonists.55,60–62
Furthermore, administration of contrast media results in increased renal tubular osmotic load, which can upregulate tubular transport mechanisms and ATP hydrolysis (Figure 1). The effects of contrast media on the renal tubules include diuresis, natriuresis, alterations in regulatory mechanisms, increases in intratubular pressure, tubular obstruction and necrosis.34 The role of direct toxic tubular effects in CIAKI remains controversial but they are probably a contributory factor.2,4,62–64
Risk factors for CIAKI
Since CIAKI is primarily caused by renal vasoconstriction, an individual with diminished renal vasodilatory capacity, such as a patient with diabetic nephropathy, is at increased risk of developing CIAKI. Numerous factors that increase the risk of CIAKI have been reported, including impaired renal function, diabetes, reduced intravascular volume, cardiovascular disease, use of diuretics, advanced age, female sex, multiple myeloma, hypertension, hyperuricemia and surgical interventions. Among these factors, pre-existing renal impairment seems to have the most prominent effect.65,66
CIAKI (when defined as an increase in baseline serum creatinine level of ≥25%) has an incidence <5% among individuals with normal renal function.3 However, the risk of CIAKI is increased in patients with CKD and in those with diabetic nephropathy.2,4,52 The Contrast-Induced Nephropathy (CIN) Consensus Working Panel concluded that the risk of CIAKI is significantly higher in patients with an estimated GFR (eGFR) of <60 ml/ min/1.73 m2 and is amplified when other risk factors are present.67
Diabetic nephropathy is an important risk factor for CIAKI.3,4,64,68,69 The incidence of CIAKI in patients with diabetic nephropathy is 9–40% in individuals with a serum creatinine level >124 μmol/l3,52,69 and 50–95% in those with a serum creatinine level >177 μmol/l.3,31,33,70
In addition, evidence suggests that even patients in a prediabetic state are at increased risk of CIAKI if they also have CKD. Toprak et al.65 showed that CIAKI occurred in 20% of patients with CKD and diabetes and in 11.4% of patients with CKD and prediabetes (defined as having a fasting glucose level of 5.55–6.94 mmol/l), versus 5.5% of patients with CKD but no evidence of diabetes or prediabetes. This observation suggests that mild glucose intolerance, which does not fulfill the current criteria for diabetes mellitus, increases the risk of CIAKI in the presence of renal impairment.
Whether patients with diabetes and normal renal function have an increased propensity to develop CIAKI remains controversial. The results of some studies suggest that these patients might have an increased risk of CIAKI,71 whereas other studies have failed to show this association.72 The heterogeneity of these findings might be due to the existence of different phenotypes of diabetic nephropathy, particularly in early stages of the disease, as is discussed below.
Pathogenesis of diabetic nephropathy
Patients with diabetes have an up to 40% lifetime risk of developing diabetic nephropathy.73 In one-third of patients, diabetic nephropathy is a progressive disease characterized by increasing proteinuria (mainly albuminuria) and a subsequent decline in GFR (Table 1). However, results from studies published in the past few years indicate that several phenotypes of diabetic nephropathy exist, and the majority of patients do not develop the classic phenotype of progressive diabetic nephropathy. For example, the renal function of some patients with diabetes is characterized by a decreased GFR and little or no albuminuria.74,75
Table 1.
Classification of diabetic nephropathy
| Stage of diabetic nephropathy | gFr (ml/min/1.73 m2) | Albuminuria (mg/g) |
|---|---|---|
| 1 | ≥90 | Absent or <30 |
| 2 | ≥60 | 30–300 |
| 3 | ≥60 | >300 |
| 4 | <60 | >3,000 |
| 5 | <15 | Absent or present |
Abbreviation: GFR, glomerular filtration rate.
Diabetic nephropathy is characterized by renal vascular dysfunction, which manifests as an increased sensitivity to renal vasoconstrictors and renal ischemia, and a decrease in nitric oxide-dependent vasodilation.51,76 Ishimura et al.77 demonstrated that patients with stage 1 diabetic nephropathy have increased renal resistive indices in their renal vasculature, which indicate a diminished renal vasodilatory blood flow reserve. Furthermore, Frauchiger et al.78 showed that patients with early diabetic nephropathy have a lower renal blood flow (vasodilatory) response to administration of glyceryl trinitrate than healthy controls. In addition, Epstein et al.79 elegantly demonstrated that renal blood oxygenation (as quantified by MRI techniques that are dependent on blood oxygenation levels) is lower in patients with stage 1 diabetic nephropathy than in healthy controls after a water load. This observation suggests that oxygen delivery is impaired in patients with early stages of diabetic nephropathy. This effect might be at least partly accounted for by vascular and/or endothelial dysfunction. Numerous other factors might also contribute to this impairment in renal blood oxygenation, including defective nitric oxide production, high concentrations of advanced glycosylation end products, increased generation of cytokines, and increased generation of ROS.80–82 Several animal studies have demonstrated that nitric-oxide-dependent renal vasodilation is critical in countervailing the vasoconstrictive effects of both adenosine51,76 and contrast media49 and that the responsiveness of this vasodilation mechanism is diminished in patients with diabetes (Figure 2).77,81–83
CIAKI causes renal ischemia, and repeated ischemic insults to the renal vasculature of patients with diabetes cause progression of diabetic nephropathy and deterioration of renal function.72 Prevention of CIAKI in patients with diabetes is, therefore, important for the long-term preservation of renal function. In addition, given the evidence from Toprak et al.’s study65 that the incidence of CIAKI is increased in patients in a prediabetic state as well as in those with diabetes, it seems likely that many patients with diabetes would also have renal vascular dysfunction, even in the absence of overt diabetic nephropathy. Such patients are frequently considered to have ‘normal renal function’ and thus the risk of CIAKI is not fully appreciated in this group. New tests must be devised to detect early renal dysfunction and identify patients at risk of CIAKI. In addition, therapeutic and preventative measures for CIAKI are potentially important for the prevention of diabetic nephropathy in patients with diabetes at risk of CIAKI. Consequently, until appropriate screening tests are available, a prudent approach would be to implement CIAKI prophylaxis in all patients with diabetes.
CIAKI prevention in diabetic patients
Although CIAKI is the most common form of iatrogenic renal failure, only about 40% of patients with an eGFR <60 ml/min/1.73 m2 receive any sort of CIAKI prophylaxis.1 Furthermore, even when prevention strategies are implemented, few patients receive a standardized regimen.17 The renal vasodilatory capacity represents a vital mechanism to counter the vasoconstrictive effects of contrast media. Potential therapeutic agents for the prevention of CIAKI in patients with diabetes are presented in Table 2 and discussed below. Further suggestions for reducing the risk of CIAKI are shown in Box 1.
Table 2.
Potential therapeutic agents for the prevention of CIAKI in patients with diabetes
| Agent | Administration | putative mechanism of action | evidence |
|---|---|---|---|
| Renal vasodilators (e.g. dopamine) | Oral or intravenous | Increased renal blood flow | Numerous studies failed to show benefit; some suggestions of harm94 |
| Sodium bicarbonate | Intravenous | Increased pH of tubular urine | Unclear; might cause harm through pro-oxidant properties96,100 |
| N-acetylcysteine | Oral or intravenous (treatment duration is controversial) | Antioxidant effects, reduction of reactive oxygen species | Mixed evidence, but seems to be beneficial101 |
| Adenosine-receptor antagonists (theophylline and antagonists of adenosine receptor A1) | Oral or intravenous | Antagonism of adenosine-mediated vasoconstriction | Theophylline: some evidence of benefit112,113 Selective adenosine receptor A1 antagonists: preliminary animal116 and human113 data suggest that these agents increase GFR |
| Statins (simvastatin and atorvastatin) | Oral | Pleiotropic, antioxidant, and anti-inflammatory effects | Conflicting results from trials125,126 |
Abbreviations: CIAKI, contrast-induced acute kidney injury; GFR, glomerular filtration rate.
Box 1. Suggestions for reducing the risk of CIAKI.
Imaging studies with contrast media should only be performed when the benefits outweigh the risks; alternative protocols that do not require iodinated contrast media should be considered if they can provide the necessary information
The use of iso-osmolar contrast media might reduce the incidence of CIAKI
Avoidance of concomitant administration of nephrotoxic agents, such as angiotensin-converting-enzyme inhibitors and NSAIDs, seems prudent, although no conclusive evidence exists to support such clinical decision making
Intravascular volume expansion should be provided, preferably via intravenous hydration, if this technique is not contraindicated
Our experience indicates that N-acetylcysteine should be administered at a dose of 1,200 mg orally twice daily for 7 days, starting 1 day before contrast-medium administration
Abbreviation: CIAKI, contrast-induced acute kidney injury.
Hydration
Hydration is the intervention best supported by evidence of a preventive effect on CIAKI and is the foundation of most approaches that prevent CIAKI. However, the mechanism by which hydration prevents CIAKI is unclear,84 although intravenous hydration seems to be more effective than unrestricted oral hydration.85 Standardized, prospective studies to determine the optimal hydration strategy and evaluate the cost-effectiveness of oral hydration are needed.84
In a randomized trial that included 1,620 patients, 217 of whom had diabetes, Mueller and colleagues86 showed that intravenously administered 0.9% saline solution was superior to 0.45% saline solution, although this difference was not apparent in the subgroup of patients with serum creatinine level >141 μmol/l regardless of diabetes status. Although data are limited, 0.9% saline solution is claimed to be superior to 0.45% saline solution for CIAKI prevention in general.84 Furthermore, two small studies suggest that sustained fluid administration within 12 h before and within 12 h after administration of contrast medium is superior to bolus administration at the time of contrast administration.87,88
Mannitol and diuretics
In the general population as well as in patients with diabetes, mannitol and furosemide diuresis have both been shown to increase the risk of CIAKI.27 Despite the lack of definitive evidence of a pathogenic link between CIAKI and administration of these two diuretics, these agents should be avoided in patients with diabetes.
Vasodilators
Renal vasodilators, including calcium-channel antagonists, are promising agents in the prevention of CIAKI;89 so far, however, their administration has failed to show conclusive evidence of a beneficial effect.90–93 Low-dose dopamine has a dilatory effect on renal vasculature, but confers no advantage over hydration in the prevention of CIAKI, and may be harmful in patients with peripheral vascular disease.94 These observations are not surprising since the renal vasodilatory response is impaired in patients with diabetes, and thus administration of renal vasodilators is likely to be of little or no benefit in the prevention of CIAKI.
Sodium bicarbonate
The intravenous administration of sodium bicarbonate solutions for CIAKI prevention was first studied by Merten and colleagues.94 In this small study, intravenous sodium bicarbonate administration was associated with a decreased incidence of CIAKI. Subsequent studies have failed to show any benefit of the intravenous administration of sodium bicarbonate over isotonic sodium chloride in CIAKI prevention.95,96 The rationale for a potential role of sodium bicarbonate in the prevention of CIAKI might involve the ability of bicarbonate to increase tubular fluid pH level and prevent the formation of free radicals, but whether these effects actually decrease risk of CIAKI is unclear and highly controversial. Typically, bicarbonate acts as a pro-oxidant, particularly in the presence of ROS; as the pathogenesis of CIAKI seems to involve both renal ischemia and ROS generation, the administration of a pro-oxidant seems counterintuitive and might be associated with an increased incidence of CIAKI.17
Furthermore, contrast-medium-induced apoptosis of renal cells is inhibited by the antioxidants N-acetylcysteine and ascorbic acid, but not by sodium bicarbonate.97 A small trial published in 2010 demonstrated that intravenous administration of sodium bicarbonate and 0.45% saline solution 48 h after elective coronary angiography was not superior to intravenous administration of 0.45% saline solution alone for the prevention of CIAKI; however, only about one-third of the participants had diabetes.98
Two meta-analyses have been published on the use of sodium bicarbonate in the prevention of CIAKI, and both reported substantial study heterogeneity and publication bias.99,100 In most studies, only a minority of patients had diabetes, and until larger controlled trials have been conducted to study the effects of sodium bicarbonate on the risk of CIAKI in patients with diabetes, the usefulness of this compound remains uncertain.
N-acetylcysteine
CIAKI and diabetic nephropathy are both associated with increased generation of ROS and thus some researchers have suggested that antioxidants, such as N-acetylcysteine, might potentially prevent or treat CIAKI. The first study of the use of N-acetylcysteine for CIAKI prevention was conducted by Tepel and colleagues.101 In this study, the investigators compared the efficacy of oral administration of 600 mg of N-acetylcysteine twice daily on the day before and on the day of contrast-medium administration alongside intravenous hydration with 0.45% saline with the efficacy of intravenous hydration with 0.45% saline alone. 48 h after administration of contrast medium, patients who received saline hydration alone experienced a small, insignificant increase in serum creatinine level from 212 μmol/l to 220 μmol/l (P = 0.18), whereas patients in the N-acetylcysteine group experienced a significant decrease in serum creatinine level from 221 μmol/l to 186 μmol/l (P = 0.01). Several subsequent studies demonstrated ambiguous results on the effectiveness of N-acetylcysteine in preventing CIAKI.102,103
Several factors might contribute to these disparate results, among them the dose administered and treatment duration. N-acetylcysteine is commonly only given for 2 days101 on the assumption that ROS production occurs for only a short time after being induced by contrast media. However, this concept may need to be revised since the effects of ROS induction may last much longer than previously assumed, particularly in patients with diabetes. El-Osta et al.104 demonstrated that short-term (1 h) exposure to high glucose concentrations induced long-lasting ROS-mediated activation of the transcription factor nuclear factor κB both in vitro and in vivo, and was associated with increases in the expression of C–C motif chemokine 2 and vascular cell adhesion molecule 1 that persisted for at least 6 days. Whether ongoing ROS generation that might be a target for antioxidant therapy continues after glucose levels normalize has yet to be proven, but this evidence illustrates that ROS-mediated processes could propagate long after the initial insult. On the other hand, achieving a sufficiently high serum concentration of N-acetylcysteine at the time of contrast-medium exposure might be more important than the duration of N-acetylcysteine administration. Unfortunately, levels of N-acetylcysteine are difficult to measure accurately, as this compound has a very short half-life in plasma owing to extensive first-pass metabolism. This characteristic might explain why this agent seems to have greater efficacy in the prevention of CIAKI when it is administered intravenously rather than orally.102,103,105–107 Two daily doses of oral N-acetylcysteine might, therefore, be insufficient to achieve consistent renoprotective effects.
The doses of N-acetylcysteine that have been investigated might also be too low to achieve a meaningful reduction in ROS. N-acetylcysteine has been successfully used in the treatment of acetaminophen-induced toxic effects on the liver, which are mediated by ROS. Daily doses of N-acetylcysteine for this indication are typically 40-fold higher than those currently recommended for CIAKI prevention (1,200 mg daily or 7 × 10–3 mol). Daily physiological production of the superoxide anion is estimated to be 1.75 kg or 5.5 × 104 mol.108 As ROS production is increased to above this amount in patients with diabetes and CIAKI, the presumption that only 7 × 10–3 mol/l of N-acetylcysteine would cause a meaningful reduction in a daily ROS generation of ≥5.5 × 104 mol/l of super-oxide anion seems to be ill-founded. Marenzi et al.109 demonstrated that 1,200 mg of N-acetylcysteine given twice daily is more effective in CIAKI prevention than 600 mg of N-acetylcysteine given twice daily.109 Given also the favorable adverse-effect profile110 and low cost of N-acetylcysteine, higher doses of this antioxidant and longer treatment periods than those currently implemented would, therefore, seem appropriate to improve CIAKI prevention. Current practice in our clinic is to administer 1,200 mg of N-acetylcysteine orally twice daily on the day before contrast-medium administration, and on days 1–7 after. This regimen is associated with good tolerability and outcomes (Calvin, A. D. et al., unpublished data).
Adenosine-receptor antagonists
Since adenosine-induced vasoconstriction seems to be markedly enhanced in patients with diabetes, and as CIAKI is thought to cause renal vasoconstriction by an adenosine-mediated mechanism (see above), adenosine-receptor antagonists have been investigated as a potential option for CIAKI prevention. When given before contrast media, oral or intravenously administered theophylline, a nonselective adenosine-receptor antagonist, reduces the incidence of CIAKI.55,56,61 The use of theophylline to prevent CIAKI might be limited by inter-individual variability in drug metabolism, drug–drug interactions, and risk of tachyarrhythmias,111 although adverse cardiac effects associated with theophylline administration seem to be rare at the doses given in these studies (single doses of 5 mg/kg intravenously,55 2.88 mg/kg orally,56 and 165 mg intravenously61). The conclusions of a meta-analysis stated that these and other studies provide some evidence of a beneficial effect of theophylline administration in the prevention of CIAKI.112 However, factors such as differences in theophylline dose, volume and type of contrast medium administered, hydration status, comorbidities such as diabetes, and the lack of a consistent method to measure plasma levels of theophylline might contribute to the variability of results obtained in different studies.
Selective antagonists of adenosine receptor A1 inhibited adenosine-induced renal vasoconstriction and restored renal blood flow after renal ischemia in non-diabetic and diabetic animals.51,76 Furthermore, antagonists of adenosine receptor A1 have protective effects on the renal tubular system by increasing sodium excretion in humans with heart failure113 and in diabetic animal models.114 Both of these effects could help to prevent CIAKI. The diuretic and natriuretic properties of selective antagonists of adenosine receptor A1 are of particular interest, since natriuresis occurs without increased potassium excretion. By contrast, loop diuretics increase potassium excretion, which could aggravate cardiac arrhythmias. Administration of selective antagonists of adenosine receptor A1 might have additional beneficial properties, such as improving glucose tolerance (as shown in animal models of diabetes)115 and inhibiting platelet aggregation.3 Newly developed selective antagonists of adenosine receptor A1 do not seem to be associated with adverse cardiac effects55,56,61 and cardioprotective effects have even been demonstrated in animal models of ischemia–reperfusion injury.116–119 Although not yet clinically available, several selective antagonists of adenosine receptor A1 (DPCPX, FK453 and FR113452) have been studied in both animals and in humans.115–118 The intravenous administration of FK453 increased GFR and fractional excretion of sodium in animals117 and in humans120 without disturbing potassium homeostasis. Other selective antagonists of adenosine receptor A1 (BW-1433 and CVT-124) are under investigation.121–123
In summary, CIAKI seems to result, at least in part, from renal vasoconstriction mediated by stimulation of adenosine receptor A1. Nonselective adenosine-receptor antagonists, such as theophylline, reduce the risk of CIAKI in patients with diabetes. Administration of novel, investigational, selective antagonists of adenosine receptor A1 might prevent CIAKI in patients with diabetes and could have both higher efficacy in preventing CIAKI and fewer adverse effects than theophylline.
Statins
Statin administration might reduce the progression of CKD and diabetic nephropathy,124 perhaps through pleiotropic, antioxidant and anti-inflammatory effects. Statins might also have beneficial effects in the prevention of CIAKI. In an animal model of ischemia–reperfusion injury, administration of pravastatin limited renal damage via inhibition of the mevalonate–isoprenoid pathway, independently of the lipid-lowering action of statins.125
In a retrospective study that included more than 29,000 patients who underwent percutaneous coronary angiography, Khanal et al.126 found that patients who were on statin therapy before the procedure had a lower incidence of CIAKI than patients who were not taking a statin at that time. These results are in line with those of a prospective, observational study that included 434 participants, in which patients who were taking statins before undergoing coronary angiography had a lower rate of CIAKI (defined as an increase in serum creatinine level of ≥44 mmol/l or >25% from baseline). This association was also evident among the 161 patients with diabetes included in the study.127 In the PROMISS study,128 however, researchers administered simvastatin or placebo to patients before they underwent coronary angiography and observed no reduction in CIAKI (defined as an increase in serum creatinine of ≥25% or an absolute increase of ≥44 mmol/l within 48 h after contrast-medium administration) in the statin-treated group compared with the placebo-treated group. Only 59 of the 247 participants in the PROMISS study had diabetes, and in this subgroup a trend towards a beneficial effect of simvastatin was observed (the incidence of CIAKI was 6.3% in the treatment group versus 11.1% in the placebo group, P = 0.65). However, a 2010 trial of atorvastatin in 304 patients with CKD (estimated creatinine clearance <60 ml/min) failed to demonstrate benefits of treatment with atorvastatin versus placebo. In the subgroup of 64 patients with diabetes, the incidence of CIAKI did not differ between the treatment and placebo groups.129
Hemodialysis and hemofiltration
Hemodialysis is effective in removing contrast media from the circulation130–133 and some reports indicate that hemodialysis can prevent CIAKI.134 However, several studies have failed to show that prophylactic hemodialysis soon after contrast-medium exposure is effective in preventing CIAKI in patients who are not already on renal replacement therapy.135–137 Hemodialysis has failed to prevent CIAKI even when dialysis was performed within 1 h of contrast-medium administration138 or concurrently with coronary angiography,139 and results from one study suggested that prophylactic hemodialysis might actually cause harm when performed after administration of contrast medium to patients with renal insufficiency.140 However, the CIN Consensus Working Panel agreed that in patients with severe renal impairment (eGFR <20 ml/min) who require contrast-medium administration, hemodialysis should be undertaken if CIAKI develops.67
The role of hemofiltration in CIAKI prevention has been assessed in a single study.141 Patients undergoing coronary angiography with a baseline serum creatinine level >177 μmol/l and eGFR <50 ml/min, 30% of whom had diabetes, were randomly assigned to receive usual care (intravenous high-volume hydration with 1 ml kg–1/h isotonic saline solution for most patients or 0.5 ml kg–1/h isotonic saline solution for patients with a left ventricular ejection fraction <40%), starting 6–8 h before the procedure and for 24 h afterwards, or preemptive venovenous hemofiltration before contrast-medium administration and for 18–24 h afterwards. Patients who received continuous hemofiltration were less likely to experience CIAKI, defined as a rise in serum creatinine level of >25%, than patients receiving standard care. In-hospital and 1-year mortality rates were significantly lower in patients who underwent hemofiltration than in those who received standard care. However, these results have been called into question as hemofiltration is generally ineffective in the removal of contrast media,130 and CIAKI is thought to occur immediately upon exposure to contrast media,130 whereas in this study hemofiltration was interrupted during contrast-medium administration. The benefits of hemofiltration observed in this study have been suggested to be due to the concomitant administration of heparin,142 which has anti-inflammatory effects143 and might reduce ROS generation.144 Furthermore, patients randomly assigned to hemofiltration also underwent controlled, high-volume hydration and monitoring in the intensive care unit, both of which could reduce the incidence of CIAKI. The CIN Consensus Working Panel concluded that hemofiltration merits further study.67
Conclusions
CIAKI incidence and CIAKI-associated mortality are high in patients with diabetes and diabetic nephropathy, and good screening tools for early diabetic nephropathy are lacking. We recommend, therefore, that CIAKI prophylaxis should be considered in all patients with diabetes who require either intra-arterial or intravenous administration of contrast media, regardless of renal function. To date, the only intervention clearly proven to prevent CIAKI is hydration. Unfortunately, the optimal hydration strategy has not yet been established, although intravenous hydration seems to be superior to oral hydration. Although additional studies are needed, administration of high-dose N-acetylcysteine also seems to be effective in preventing CIAKI. Statins might reduce the risk of CIAKI, and continuing statin treatment in patients who are already taking these agents seems reasonable. Currently, the administration of sodium bicarbonate for CIAKI prevention cannot be recommended in patients with diabetes or other conditions in which there is an increased generation of ROS. Selective antagonists of adenosine receptor A1 have shown promise in preventing CIAKI, but are not yet commercially available.
Key points.
Contrast-induced acute kidney injury (CIAKI) is caused by the intra-arterial and intravenous administration of contrast media and is associated with a high risk of mortality
Diabetes, even in the absence of renal impairment, might increase the risk of CIAKI, and CIAKI might favor progression of diabetic nephropathy
CIAKI prophylaxis should be considered in all patients with diabetes who require intra-arterial or intravenous administration of contrast medium
Intravenous hydration is the cornerstone of CIAKI prophylaxis, whereas the administration of sodium bicarbonate is of unclear benefit and might be harmful in patients with diabetes owing to its pro-oxidant properties
N-acetylcysteine administration might have protective effects and has low toxicity and should be considered for CIAKI prevention in patients with diabetes
Adenosine A1 receptor antagonists seem to be promising agents for CIAKI prophylaxis, but additional studies in humans are needed
Review criteria.
Articles were identified through a search of the PubMed database using the search terms “contrast-induced nephropathy”, “radio-contrast nephropathy”, “contrast nephropathy”, and “contrast medium-induced nephropathy”, using the ‘related articles’ function, and through review of consensus documents and published guidelines. No restrictions were imposed with regard to language or year of publication.
Acknowledgments
A. D. Calvin’s research is supported by the Mayo Clinic Clinician–Investigator Training Program. The authors gratefully acknowledge D. Mackenburg and E. Pflueger, who contributed to this Review with editorial support, including help with references and figures. Both D. Mackenburg and E. Pflueger are affiliated with the Mayo Clinic College of Medicine, Rochester, MN, USA.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
A. D. Calvin and A. Pflueger researched data for the article, made substantial contributions to the discussion of content, wrote the article and reviewed/ edited the manuscript before submission. S. Misra made substantial contribution to the discussion of content and wrote the article.
References
- 1.Weisbord SD, et al. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med. 2008;168:1325–1332. doi: 10.1001/archinte.168.12.1325. [DOI] [PubMed] [Google Scholar]
- 2.Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188:171–178. doi: 10.1148/radiology.188.1.8511292. [DOI] [PubMed] [Google Scholar]
- 3.Pflueger A, et al. Role of adenosine in contrast media-induced acute renal failure in diabetes mellitus. Mayo Clin Proc. 2000;75:1275–1283. doi: 10.4065/75.12.1275. [DOI] [PubMed] [Google Scholar]
- 4.Rudnick MR, Berns JS, Cohen RM, Goldfarb S. Contrast media-associated nephrotoxicity. Curr Opin Nephrol Hypertens. 1996;5:127–133. doi: 10.1097/00041552-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Rudnick M, Feldman H. Contrast-induced nephropathy: what are the true clinical consequences? Clin J Am Soc Nephrol. 2008;3:263–272. doi: 10.2215/CJN.03690907. [DOI] [PubMed] [Google Scholar]
- 6.Harjai KJ, et al. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol. 2008;101:812–819. doi: 10.1016/j.amjcard.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 7.Gruberg L, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542–1548. doi: 10.1016/s0735-1097(00)00917-7. [DOI] [PubMed] [Google Scholar]
- 8.Gruberg L, et al. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2001;52:409–416. doi: 10.1002/ccd.1093. [DOI] [PubMed] [Google Scholar]
- 9.Kowalczyk J, et al. Risk stratification according to the type of impaired renal function in patients with acute myocardial infarction treated with percutaneous coronary intervention. Kardiol Pol. 2007;65:635–643. [PubMed] [Google Scholar]
- 10.Rihal CS, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 11.Iakovou I, et al. Impact of gender on the incidence and outcome of contrast-induced nephropathy after percutaneous coronary intervention. J Invasive Cardiol. 2003;15:18–22. [PubMed] [Google Scholar]
- 12.From AM, Bartholmai BJ, Williams AW, Cha SS, McDonald FS. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc. 2008;83:1095–1100. doi: 10.4065/83.10.1095. [DOI] [PubMed] [Google Scholar]
- 13.Kane GC, et al. Comparison between gadolinium and iodine contrast for percutaneous intervention in atherosclerotic renal artery stenosis: clinical outcomes. Nephrol Dial Transplant. 2008;23:1233–1240. doi: 10.1093/ndt/gfm725. [DOI] [PubMed] [Google Scholar]
- 14.Idée JM, et al. Possible involvement of gadolinium chelates in the pathophysiology of nephrogenic systemic fibrosis: a critical review. Toxicology. 2008;248:77–88. doi: 10.1016/j.tox.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Broome DR. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. Eur J Radiol. 2008;66:230–234. doi: 10.1016/j.ejrad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Reed PS, Dixon SR, Boura JA, O’Neill WW, Kahn JK. Comparison of the usefulness of gadodiamide and iodine mixture versus iodinated contrast alone for prevention of contrast-induced nephropathy in patients with chronic kidney disease undergoing coronary angiography. Am J Cardiol. 2007;100:1090–1093. doi: 10.1016/j.amjcard.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 17.From AM, et al. Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at mayo clinic. Clin J Am Soc Nephrol. 2008;3:10–18. doi: 10.2215/CJN.03100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ghonaim M, Pannu N. Prevention and treatment of contrast-induced nephropathy. Tech Vasc Interv Radiol. 2006;9:42–49. doi: 10.1053/j.tvir.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Rudnick MR, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995;47:254–261. doi: 10.1038/ki.1995.32. [DOI] [PubMed] [Google Scholar]
- 20.Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68:14–22. doi: 10.1111/j.1523-1755.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 21.Liss P, Persson PB, Hansell P, Lagerqvist B. Renal failure in 57 925 patients undergoing coronary procedures using iso-osmolar or low-osmolar contrast media. Kidney Int. 2006;70:1811–1817. doi: 10.1038/sj.ki.5001887. [DOI] [PubMed] [Google Scholar]
- 22.Ueda J, et al. Iodine concentrations in the rat kidney measured by X-ray microanalysis. Comparison of concentrations and viscosities in the proximal tubules and renal pelvis after intravenous injections of contrast media. Acta Radiol. 1998;39:90–95. doi: 10.1080/02841859809172157. [DOI] [PubMed] [Google Scholar]
- 23.Solomon R. The role of osmolality in the incidence of contrast-induced nephropathy: a systematic review of angiographic contrast media in high risk patients. Kidney Int. 2005;68:2256–2263. doi: 10.1111/j.1523-1755.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 24.Voeltz MD, Nelson MA, McDaniel MC, Manoukian SV. The important properties of contrast media: focus on viscosity. J Invasive Cardiol. 2007;19:1A–9A. [PubMed] [Google Scholar]
- 25.Aspelin P, et al. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491–499. doi: 10.1056/NEJMoa021833. [DOI] [PubMed] [Google Scholar]
- 26.Chalmers N, Jackson RW. Comparison of iodixanol and iohexol in renal impairment. Br J Radiol. 1999;72:701–703. doi: 10.1259/bjr.72.859.10624328. [DOI] [PubMed] [Google Scholar]
- 27.Solomon RJ, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115:3189–3196. doi: 10.1161/CIRCULATIONAHA.106.671644. [DOI] [PubMed] [Google Scholar]
- 28.Rudnick MR, Davidson C, Laskey W, Stafford JL, Sherwin PF. Nephrotoxicity of iodixanol versus ioversol in patients with chronic kidney disease: the Visipaque Angiography/Interventions with Laboratory Outcomes in Renal Insufficiency (VALOR) Trial. Am Heart J. 2008;156:776–782. doi: 10.1016/j.ahj.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Chawarnkul O, Vareesangthip K, Ongajyooth L, Cheunsuchon B, Parichatikanond P. Non-diabetic glomerular disease in type II DM: 10 years experience. J Med Assoc Thai. 2009;92 (Suppl 2):S57–S60. [PubMed] [Google Scholar]
- 30.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27:322–328. doi: 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 31.Harkonen S, Kjellstrand CM. Exacerbation of diabetic renal failure following intravenous pyelography. Am J Med. 1977;63:939–946. doi: 10.1016/0002-9343(77)90549-6. [DOI] [PubMed] [Google Scholar]
- 32.Harnish PP, Fountaine H, Ebrahimi R. Iodixanol. Experience in 1,259 patients in the United States. Invest Radiol. 1994;29 (Suppl 2):S236–S237. doi: 10.1097/00004424-199406001-00080. [DOI] [PubMed] [Google Scholar]
- 33.Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615–620. doi: 10.1016/0002-9343(90)90180-l. [DOI] [PubMed] [Google Scholar]
- 34.Morcos SK. Contrast media-induced nephrotoxicity--questions and answers. Br J Radiol. 1998;71:357–365. doi: 10.1259/bjr.71.844.9659127. [DOI] [PubMed] [Google Scholar]
- 35.Hostetter TH, Troy JL, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981;19:410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- 36.Chudleigh RA, et al. Use of cystatin C-based estimations of glomerular filtration rate in patients with type 2 diabetes. Diabetologia. 2009;52:1274–1278. doi: 10.1007/s00125-009-1379-7. [DOI] [PubMed] [Google Scholar]
- 37.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta RL, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2007;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 40.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 41.Ahlström A, et al. Comparison of 2 acute renal failure severity scores to general scoring systems in the critically ill. Am J Kidney Dis. 2006;48:262–268. doi: 10.1053/j.ajkd.2006.04.086. [DOI] [PubMed] [Google Scholar]
- 42.Hoste EA, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abosaif NY, Tolba YA, Heap M, Russell J, Nahas AM. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis. 2005;46:1038–1048. doi: 10.1053/j.ajkd.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettilä V. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542–546. doi: 10.1016/j.athoracsur.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 45.Bell M, et al. Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant. 2005;20:354–360. doi: 10.1093/ndt/gfh581. [DOI] [PubMed] [Google Scholar]
- 46.Heyman SN, et al. Radiocontrast agents induce endothelin release in vivo and in vitro. J Am Soc Nephrol. 1992;3:58–65. doi: 10.1681/ASN.V3158. [DOI] [PubMed] [Google Scholar]
- 47.Arend LJ, Bakris GL, Burnett JC, Jr, Megerian C, Spielman WS. Role for intrarenal adenosine in the renal hemodynamic response to contrast media. J Lab Clin Med. 1987;110:406–411. [PubMed] [Google Scholar]
- 48.Deray G, et al. A role for adenosine and calcium and ischemia in radiocontrast-induced intrarenal vasoconstriction. Am J Nephrol. 1990;10:316–322. doi: 10.1159/000168126. [DOI] [PubMed] [Google Scholar]
- 49.Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94:1069–1075. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan Z, Li S. Role of increased cytosolic calcium in rabbit proximal tubule cell injury induced by diatrizoate and protection by chlorpromazine. Abstract 279 presented at the 12th International Congress of Nephrology. [Google Scholar]
- 51.Pflueger AC, Schenk F, Osswald H. Increased sensitivity of the renal vasculature to adenosine in streptozotocin-induced diabetes mellitus rats. Am J Physiol. 1995;269:F529–F535. doi: 10.1152/ajprenal.1995.269.4.F529. [DOI] [PubMed] [Google Scholar]
- 52.Barrett BJ, et al. Contrast nephropathy in patients with impaired renal function: high versus low osmolar media. Kidney Int. 1992;41:1274–1279. doi: 10.1038/ki.1992.189. [DOI] [PubMed] [Google Scholar]
- 53.McCoy DE, et al. The renal adenosine system: structure, function, and regulation. Semin Nephrol. 1993;13:31–40. [PubMed] [Google Scholar]
- 54.Osswald H. Regulatory Function of Adenosine. In: Berne RM, Rall TW, Rubio R, editors. Adenosine and Renal Function. Martinus Nijhof Publishers; The Hague: 1983. pp. 399–415. [Google Scholar]
- 55.Erley CM, et al. Adenosine antagonist theophylline prevents the reduction of glomerular filtration rate after contrast media application. Kidney Int. 1994;45:1425–1431. doi: 10.1038/ki.1994.186. [DOI] [PubMed] [Google Scholar]
- 56.Katholi RE, et al. Nephrotoxicity from contrast media: attenuation with theophylline. Radiology. 1995;195:17–22. doi: 10.1148/radiology.195.1.7892462. [DOI] [PubMed] [Google Scholar]
- 57.Lee HT, et al. A1 adenosine receptor knockout mice are protected against acute radiocontrast nephropathy in vivo. Am J Physiol Renal Physiol. 2006;290:F1367–F1375. doi: 10.1152/ajprenal.00347.2005. [DOI] [PubMed] [Google Scholar]
- 58.Richardson DE, Regino CA, Yao H, Johnson JV. Methionine oxidation by peroxymonocarbonate, a reactive oxygen species formed from CO2/bicarbonate and hydrogen peroxide. Free Radic Biol Med. 2003;35:1538–1550. doi: 10.1016/j.freeradbiomed.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Heyman SN, et al. Effects of ioversol versus iothalamate on endothelin release and radiocontrast nephropathy. Invest Radiol. 1993;28:313–318. doi: 10.1097/00004424-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Erley CM, et al. Prevention of radiocontrast-media-induced nephropathy in patients with pre-existing renal insufficiency by hydration in combination with the adenosine antagonist theophylline. Nephrol Dial Transplant. 1999;14:1146–1149. doi: 10.1093/ndt/14.5.1146. [DOI] [PubMed] [Google Scholar]
- 61.Kolonko A, Wiecek A, Kokot F. The nonselective adenosine antagonist theophylline does prevent renal dysfunction induced by radiographic contrast agents. J Nephrol. 1998;11:151–156. [PubMed] [Google Scholar]
- 62.Brady HR, Brenner BM, Lieberthal W. In: Brenner & Rector’s The Kidney. 5. Brenner BM, editor. Vol. 2. WB Saunders Co; Philadelphia: 1996. pp. 1200–1252. [Google Scholar]
- 63.Cronin RE, Henrich WL. In: Brenner & Rector’s The Kidney. 5. Brenner BM, editor. Vol. 2. WB Saunders Co; Philadelphia: 1996. pp. 1680–1711. [Google Scholar]
- 64.Moreau JF, Helenon O, Kinkel K, Melki P. In: Oxford Textbook of Clinical Nephrology. 2. Davison AM, et al., editors. Vol. 1. Oxford Medical Publications; 1998. pp. 93–132. [Google Scholar]
- 65.Toprak O, et al. Impact of diabetic and pre-diabetic state on development of contrast-induced nephropathy in patients with chronic kidney disease. Nephrol Dial Transplant. 2007;22:819–826. doi: 10.1093/ndt/gfl636. [DOI] [PubMed] [Google Scholar]
- 66.Toprak O. Risk markers for contrast-induced nephropathy. Am J Med Sci. 2007;334:283–290. doi: 10.1097/MAJ.0b013e318068ddf9. [DOI] [PubMed] [Google Scholar]
- 67.McCullough PA, et al. Risk prediction of contrast-induced nephropathy. Am J Cardiol. 2006;98:27K–36K. doi: 10.1016/j.amjcard.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 68.Heyman SN, Rosen S, Brezis M. Radiocontrast nephropathy: a paradigm for the synergism between toxic and hypoxic insults in the kidney. Exp Nephrol. 1994;2:153–157. [PubMed] [Google Scholar]
- 69.Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994;45:259–265. doi: 10.1038/ki.1994.32. [DOI] [PubMed] [Google Scholar]
- 70.Shafi T, Chou SY, Porush JG, Shapiro WB. Infusion intravenous pyelography and renal function. Effects in patients with chronic renal insufficiency. Arch Intern Med. 1978;138:1218–1221. [PubMed] [Google Scholar]
- 71.Lautin EM, et al. Radiocontrast-associated renal dysfunction: incidence and risk factors. AJR Am J Roentgenol. 1991;157:49–58. doi: 10.2214/ajr.157.1.2048539. [DOI] [PubMed] [Google Scholar]
- 72.Parfrey PS, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 73.Parving HH, et al. In: Brenner & Rector’s The Kidney. 5. Brenner BM, editor. Vol. 2. WB Saunders Co; Philadelphia: 1996. pp. 1864–1892. [Google Scholar]
- 74.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME, Mathew TH. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study) Med J Aust. 2006;185:140–144. doi: 10.5694/j.1326-5377.2006.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 75.Rosolowsky ET, et al. Between hyperfiltration and impairment: demystifying early renal functional changes in diabetic nephropathy. Diabetes Res Clin Pract. 2008;82 (Suppl 1):S46–S53. doi: 10.1016/j.diabres.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 76.Pflueger AC, Osswald H, Knox FG. Adenosine-induced renal vasoconstriction in diabetes mellitus rats: role of nitric oxide. Am J Physiol. 1999;276:F340–F346. doi: 10.1152/ajprenal.1999.276.3.F340. [DOI] [PubMed] [Google Scholar]
- 77.Ishimura E, et al. Intrarenal hemodynamic abnormalities in diabetic nephropathy measured by duplex Doppler sonography. Kidney Int. 1997;51:1920–1927. doi: 10.1038/ki.1997.261. [DOI] [PubMed] [Google Scholar]
- 78.Frauchiger B, Nussbaumer P, Hugentobler M, Staub D. Duplex sonographic registration of age and diabetes-related loss of renal vasodilatory response to nitroglycerine. Nephrol Dial Transplant. 2000;15:827–832. doi: 10.1093/ndt/15.6.827. [DOI] [PubMed] [Google Scholar]
- 79.Epstein FH, Veves A, Prasad PV. Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care. 2002;25:575–578. doi: 10.2337/diacare.25.3.575. [DOI] [PubMed] [Google Scholar]
- 80.Kanwar YS, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 81.Dai FX, Diederich A, Skopec J, Diederich D. Diabetes-induced endothelial dysfunction in streptozotocin-treated rats: role of prostaglandin endoperoxides and free radicals. J Am Soc Nephrol. 1993;4:1327–1336. doi: 10.1681/ASN.V461327. [DOI] [PubMed] [Google Scholar]
- 82.Diederich D. In: Nitric Oxide and the Kidney: Physiology and Pathophysiology. Goligorsky MS, Gross SS, editors. Chapman & Hall; New York: 1997. pp. 349–367. [Google Scholar]
- 83.Pflueger AC, Larson TS, Hagl S, Knox FG. Role of nitric oxide in intrarenal hemodynamics in experimental diabetes mellitus in rats. Am J Physiol. 1999;277:R725–R733. doi: 10.1152/ajpregu.1999.277.3.R725. [DOI] [PubMed] [Google Scholar]
- 84.Weisbord SD, Palevsky PM. Prevention of contrast-induced nephropathy with volume expansion. Clin J Am Soc Nephrol. 2008;3:273–280. doi: 10.2215/CJN.02580607. [DOI] [PubMed] [Google Scholar]
- 85.Trivedi HS, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93:C29–C34. doi: 10.1159/000066641. [DOI] [PubMed] [Google Scholar]
- 86.Mueller C, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 87.Bader BD, et al. What is the best hydration regimen to prevent contrast media-induced nephrotoxicity? Clin Nephrol. 2004;62:1–7. doi: 10.5414/cnp62001. [DOI] [PubMed] [Google Scholar]
- 88.Krasuski RA, Beard BM, Geoghagan JD, Thompson CM, Guidera SA. Optimal timing of hydration to erase contrast-associated nephropathy: the OTHER CAN study. J Invasive Cardiol. 2003;15:699–702. [PubMed] [Google Scholar]
- 89.Landoni G, et al. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Am J Kidney Dis. 2007;49:56–68. doi: 10.1053/j.ajkd.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 90.Cacoub P, Deray G, Baumelou A, Jacobs C. No evidence for protective effects of nifedipine against radiocontrast-induced acute renal failure. Clin Nephrol. 1988;29:215–216. [PubMed] [Google Scholar]
- 91.Khoury Z, et al. The effect of prophylactic nifedipine on renal function in patients administered contrast media. Pharmacotherapy. 1995;15:59–65. [PubMed] [Google Scholar]
- 92.Madsen JK, et al. Effect of nitrendipine on renal function and on hormonal parameters after intravascular iopromide. Acta Radiol. 1998;39:375–380. doi: 10.1080/02841859809172448. [DOI] [PubMed] [Google Scholar]
- 93.Stone GW, et al. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA. 2003;290:2284–2291. doi: 10.1001/jama.290.17.2284. [DOI] [PubMed] [Google Scholar]
- 94.Merten GJ, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 95.Brar SS, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300:1038–1046. doi: 10.1001/jama.300.9.1038. [DOI] [PubMed] [Google Scholar]
- 96.Maioli M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52:599–604. doi: 10.1016/j.jacc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 97.Romano G, et al. Contrast agents and renal cell apoptosis. Eur Heart J. 2008;20:2569–2576. doi: 10.1093/eurheartj/ehn197. [DOI] [PubMed] [Google Scholar]
- 98.Vasheghani-Farahani A, et al. Sodium bicarbonate in preventing contrast nephropathy in patients at risk for volume overload: a randomized controlled trial. J Nephrol. 2010;23:216–223. [PubMed] [Google Scholar]
- 99.Zoungas S, et al. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Ann Intern Med. 2009;151:631–638. doi: 10.7326/0003-4819-151-9-200911030-00008. [DOI] [PubMed] [Google Scholar]
- 100.Kunadian V, Zaman A, Spyridopoulos I, Qiu W. Sodium bicarbonate for the prevention of contrast induced nephropathy: a meta-analysis of published clinical trials. Eur J Radiol. doi: 10.1016/j.ejrad.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 101.Tepel M, et al. Prevention of radiographic- contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 102.Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. 2008;3:281–287. doi: 10.2215/CJN.02590607. [DOI] [PubMed] [Google Scholar]
- 103.Sterling KA, Tehrani T, Rudnick MR. Clinical significance and preventive strategies for contrast-induced nephropathy. Curr Opin Nephrol Hypertens. 2008;17:616–623. doi: 10.1097/MNH.0b013e32830f45a3. [DOI] [PubMed] [Google Scholar]
- 104.El-Osta A, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moldéus P, Cotgreave IA. N-acetylcysteine. Methods Enzymol. 1994;234:482–492. doi: 10.1016/0076-6879(94)34119-2. [DOI] [PubMed] [Google Scholar]
- 106.Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34:77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- 107.Borgström L, Kågedal B, Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31:217–222. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- 108.Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am J Med. 1994;26:5S–13S. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 109.Marenzi G, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 110.Kanter MZ. Comparison of oral and i.v acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm. 2006;63:1821–1827. doi: 10.2146/ajhp060050. [DOI] [PubMed] [Google Scholar]
- 111.Schlienger RG, Wyser C, Ritz R, Haefeli WE. Clinico-pharmacological case (4) Epileptic seizure as an unwanted drug effect on theophylline poisoning. Praxis. 1996;85:1407–1412. [PubMed] [Google Scholar]
- 112.Bagshaw SM, Ghali WA. Theophylline for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Arch Intern Med. 2005;165:1087–1093. doi: 10.1001/archinte.165.10.1087. [DOI] [PubMed] [Google Scholar]
- 113.Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC. The effects of KW-3902, an adenosine A1-receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol. 2007;50:1551–1560. doi: 10.1016/j.jacc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 114.Pflueger AC, Berndt TJ, Knox FG. Effect of renal interstitial adenosine infusion on phosphate excretion in diabetes mellitus rats. Am J Physiol. 1998;274:R1228–R1235. doi: 10.1152/ajpregu.1998.274.5.R1228. [DOI] [PubMed] [Google Scholar]
- 115.Xu B, Berkich DA, Crist GH, LaNoue KF. A1 adenosine receptor antagonism improves glucose tolerance in Zucker rats. Am J Nephrol. 1998;274:E271–E279. doi: 10.1152/ajpendo.1998.274.2.E271. [DOI] [PubMed] [Google Scholar]
- 116.Gare M, et al. The renal effect of low-dose dopamine in high-risk patients undergoing coronary angiography. J Am Coll Cardiol. 1999;34:1682–1688. doi: 10.1016/s0735-1097(99)00422-2. [DOI] [PubMed] [Google Scholar]
- 117.Kuan CJ, Herzer WA, Jackson EK. Cardiovascular and renal effects of blocking A1 adenosine receptors. J Cardiovasc Pharmacol. 1993;21:822–828. doi: 10.1097/00005344-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 118.Maczewski M, Beresewicz A. The role of adenosine and ATP-sensitive potassium channels in the protection afforded by ischemic preconditioning against the post-ischemic endothelial dysfunction in guinea-pig hearts. J Mol Cell Cardiol. 1998;30:1735–1747. doi: 10.1006/jmcc.1998.0736. [DOI] [PubMed] [Google Scholar]
- 119.Neely CF, DiPierro FV, Kong M, Greelish JP, Gardner TJ. A1 adenosine receptor antagonists block ischemia-reperfusion injury of the heart. Circulation. 1996;94(Suppl):II376–II380. [PubMed] [Google Scholar]
- 120.Balakrishnan VS, Coles GA, Williams JD. Effects of intravenous adenosine on renal function in healthy human subjects. Am J Physiol. 1996;271:F374–F381. doi: 10.1152/ajprenal.1996.271.2.F374. [DOI] [PubMed] [Google Scholar]
- 121.Pfister JR, et al. Synthesis and biological evaluation of the enantiomers of the potent and selective A1-adenosine antagonist 1,3-dipropyl-8-[2-(5,6-epoxynorbonyl)]-xanthine. J Med Chem. 1997;40:1773–1778. doi: 10.1021/jm970013w. [DOI] [PubMed] [Google Scholar]
- 122.Chang LC, et al. 2,4,6-Trisubstituted pyrimidines as a new class of selective adenosine A1 receptor antagonists. J Med Chem. 2004;47:6529–6540. doi: 10.1021/jm049448r. [DOI] [PubMed] [Google Scholar]
- 123.Weyler S, et al. Improving potency, selectivity, and water solubility of adenosine A1 receptor antagonists: xanthines modified at position 3 and related pyrimido[1,2,3-cd]purinediones. ChemMedChem. 2006;1:891–902. doi: 10.1002/cmdc.200600066. [DOI] [PubMed] [Google Scholar]
- 124.Agarwal R. Effects of statins on renal function. Mayo Clin Proc. 2007;82:1381–1390. doi: 10.4065/82.11.1381. [DOI] [PubMed] [Google Scholar]
- 125.Sharyo S, et al. Pravastatin improves renal ischemia-reperfusion injury by inhibiting the mevalonate pathway. Kidney Int. 2008;74:577–584. doi: 10.1038/ki.2008.210. [DOI] [PubMed] [Google Scholar]
- 126.Khanal S, et al. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med. 2005;118:843–849. doi: 10.1016/j.amjmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 127.Patti G, et al. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2008;101:279–285. doi: 10.1016/j.amjcard.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 128.Jo SH, et al. Prevention of radiocontrast medium-induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial--a randomized controlled study. Am Heart J. 2008;155:499. doi: 10.1016/j.ahj.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 129.Toso A, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol. 2010;105:288–292. doi: 10.1016/j.amjcard.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 130.Schindler R, et al. Removal of contrast media by different extracorporeal treatments. Nephrol Dial Transplant. 2001;16:1471–1474. doi: 10.1093/ndt/16.7.1471. [DOI] [PubMed] [Google Scholar]
- 131.Donnelly PK, et al. Hemodialysis and iopamidol clearance after subclavian venography. Invest Radiol. 1993;28:629–632. doi: 10.1097/00004424-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 132.Furukawa T, Ueda J, Takahashi S, Sakaguchi K. Elimination of low-osmolality contrast media by hemodialysis. Acta Radiol. 1996;37:996–971. doi: 10.1177/02841851960373P2104. [DOI] [PubMed] [Google Scholar]
- 133.Moon SS, Bäck SE, Kurkus J, Nilsson-Ehle P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron. 1995;70:430–437. doi: 10.1159/000188641. [DOI] [PubMed] [Google Scholar]
- 134.Lee PT, et al. Renal protection for coronary angiography in advanced renal failure patients by prophylactic hemodialysis. A randomized controlled trial. J Am Coll Cardiol. 2007;50:1015–1020. doi: 10.1016/j.jacc.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 135.Lehnert T, et al. Effect of haemodialysis after contrast medium administration in patients with renal insufficiency. Nephrol Dial Transplant. 1998;13:358–362. doi: 10.1093/oxfordjournals.ndt.a027830. [DOI] [PubMed] [Google Scholar]
- 136.Sterner G, Frennby B, Kurkus J, Nyman U. Does post-angiographic hemodialysis reduce the risk of contrast-medium nephropathy? Scand J Urol Nephrol. 2000;34:323–326. doi: 10.1080/003655900750048350. [DOI] [PubMed] [Google Scholar]
- 137.Reinecke H, et al. A randomized controlled trial comparing hydration therapy to additional hemodialysis or N-acetylcysteine for the prevention of contrast medium-induced nephropathy: the Dialysis-versus-Diuresis (DVD) Trial. Clin Res Cardiol. 2007;96:130–139. doi: 10.1007/s00392-007-0473-4. [DOI] [PubMed] [Google Scholar]
- 138.Huber W, et al. Haemodialysis for the prevention of contrast-induced nephropathy: outcome of 31 patients with severely impaired renal function, comparison with patients at similar risk and review. Invest Radiol. 2002;37:471–481. doi: 10.1097/01.RLI.0000023572.58117.55. [DOI] [PubMed] [Google Scholar]
- 139.Frank H, et al. Simultaneous hemodialysis during coronary angiography fails to prevent radiocontrast-induced nephropathy in chronic renal failure. Clin Nephrol. 2003;60:176–182. doi: 10.5414/cnp60176. [DOI] [PubMed] [Google Scholar]
- 140.Vogt B, et al. Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am J Med. 2001;111:692–698. doi: 10.1016/s0002-9343(01)00983-4. [DOI] [PubMed] [Google Scholar]
- 141.Marenzi G, et al. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003;349:1333–1340. doi: 10.1056/NEJMoa023204. [DOI] [PubMed] [Google Scholar]
- 142.Jacobs F. Hemofiltration and the prevention of radiocontrast-agent-induced nephropathy. N Engl J Med. 2004;19:836–838. [PubMed] [Google Scholar]
- 143.Derhaschnig U, et al. Evaluation of antiinflammatory and antiadhesive effects of heparins in human endotoxemia. Crit Care Med. 2003;31:1108–1112. doi: 10.1097/01.CCM.0000059441.70680.DC. [DOI] [PubMed] [Google Scholar]
- 144.Sela S, et al. Oxidative stress during hemodialysis: effect of heparin. Kidney Int Suppl. 2001;78:S159–S163. doi: 10.1046/j.1523-1755.2001.59780159.x. [DOI] [PubMed] [Google Scholar]