Abstract
A successful transition from childhood to adulthood requires adolescent maturation of social information processing. The neurobiological underpinnings of this maturational process remain elusive. This research employed the male Syrian hamster as a tractable animal model for investigating the neural circuitry involved in this critical transition. In this species, adult and juvenile males display different behavioral and neural responses to vaginal secretions, which contain pheromones essential for expression of sexual behavior in adulthood. These studies tested the hypothesis that vaginal secretions acquire positive valence over adolescent development via remodeling of neural circuits underlying sexual reward. Sexually naïve adult, but not juvenile, hamsters showed a conditioned place preference for vaginal secretions. Differences in behavioral response to vaginal secretions between juveniles and adults correlated with a difference in the vaginal secretion-induced neural activation pattern in mesocorticolimbic reward circuitry. Fos immunoreactivity increased in response to vaginal secretions in the medial amygdala and ventral tegmental dopaminergic cells of both juvenile and adult males. However, only in adults was there a Fos response to vaginal secretions in non-dopaminergic cells in interfascicular ventral tegmental area, nucleus accumbens core, and infralimbic medial prefrontal cortex. These results demonstrate that a socially relevant chemosensory stimulus acquires the status of an unconditioned reward during adolescence, and that this adolescent gain in social reward is correlated with experience-independent engagement of specific cell groups in reward circuitry.
Keywords: Syrian hamster, puberty, conditioned place preference, pheromone, dopamine
Introduction
A universal feature of mammalian adolescence is the restructuring of social spheres as interactions with peers become more salient than those with family (Nelson et al., 2005). This reallocation of interest involves maturation of social information processing, i.e., the perception of and responses to social stimuli. Social stimuli evoke different neural responses in human adolescents as compared with adults, particularly in the prefrontal cortex (Blakemore, 2008), but the neuronal mechanisms underlying this developmental change remain unclear. Given the importance of appropriately interpreting social stimuli in successful adult social interactions, the overall goal of this study is to determine which brain regions and neurotransmitter systems are associated with adolescent changes in the perception of social stimuli.
The male Syrian hamster is an ideal animal model for investigating the neural substrates of adolescent maturation of social information processing. Information regarding female reproductive status is conveyed via pheromone-containing vaginal secretions (VS). Appropriate neural processing of VS is required for the performance of male sexual behavior and is sufficient to induce a conditioned place preference (CPP) in sexually naïve adult male hamsters, indicating that VS are inherently rewarding to adults (Murphy & Schneider, 1970; Petrulis, 2009; Bell et al., 2010). In contrast, juvenile hamsters are not attracted to VS (Johnston & Coplin, 1979), nor do they mate with a receptive female when primed with exogenous testosterone (Meek et al., 1997; Schulz et al., 2009). Studies using the immediate early gene Fos as a proxy for neural activation reveal that juvenile hamsters do detect VS, as it elicits an increase in Fos expression in brain regions typically associated with processing of chemosensory social stimuli, e.g. the medial amygdala (Romeo et al., 1998). Thus, the behavioral responses to VS change across adolescent development, and this naturally occurring maturation of social information processing is critical for successful reproduction.
The neural underpinnings of these age-related changes in responses to VS are unknown. The ability of adults to form a CPP for VS suggests involvement of reward-related neural systems in the processing of this social stimulus. In particular, the rodent mesocorticolimbic dopaminergic and hypothalamic orexin systems are implicated in sexual, food and psychotropic drug reward (Meisel et al., 1996; Becker et al., 2001; Harris et al., 2005; Muschamp et al., 2007; Ikemoto, 2010; Lajtha & Sershen, 2010; Di Sebastiano et al., 2011), and these systems often operate in concert (Fadel & Deutch, 2002; Korotkova et al., 2003; Narita et al., 2006). Both dopaminergic and orexinergic circuitries undergo functional and structural changes during adolescence (Kuhn et al., 2010; Sawai et al., 2010); however, developmental changes in response to social stimuli, including VS, have not been examined within these circuitries. The present study seeks to determine if juveniles differ from adults in their proclivity to 1) show CPP for VS, and 2) express Fos in response to VS in mesocorticolimbic and hypothalamic reward circuits.
Materials and Methods
Animals
Syrian hamsters (Mesocricetus auratus) were obtained from Harlan Laboratories (Madison, WI) and were housed in temperature- and humidity-controlled vivaria with a shifted light:dark cycle (14:10, dark phase began at 1:00pm) and ad libitum access to food (Teklad Rodent diet 8640, Harlan, Madison, WI) and water. Juveniles in Exp 1 arrived at postnatal day 13 (P13) and were housed with their littermates and biological mother until weaning at P18. Adults in Exp 1 arrived at ages ranging from P56-62, juveniles in Exp 2 at P20, and adults in Exp 2 at P54. Weanlings and sexually naïve adult males were singly housed in clear polycarbonate cages (30.5 × 10.2 × 20.3 cm) as is typical for this solitary species. Sixty adult female hamsters, approximately 12 months old, were housed under similar conditions in separate vivaria and used as the source of VS. Female hamsters were ovariectomized several weeks before hormone administration and collection of VS. They were injected subcutaneously with 10 μg estradiol benzoate and 500 μg progesterone in sesame oil, 52 and 4 hours respectively, prior to collection of VS by gentle vaginal palpation. All experiments were conducted under <4 lux red light 1-5 hours into the dark phase. A total of 110 hamsters were treated in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals, and protocols were approved by the Michigan State University Institutional Animal Care and Use Committee.
Experiment 1: Conditioned Place Preference for potential rewards
Experimental Design
Place preference conditioning occurred as described previously (Bell et al., 2010) in an apparatus with one middle compartment and two outer compartments distinct in their visual, tactile and olfactory cues (Med Associates, St. Albans, VT). To acclimate subjects to handling and novel chambers, male hamsters were placed in glass aquaria in the behavioral testing room for 10 minutes every day, for three days prior to the start of the CPP regimen. A 17-minute pretest (2 minutes in the middle compartment followed by 15 minutes access to all compartments) was used to determine each hamster's initial compartment preference and to create groups with similar initial preferences, when possible. The outer compartment in which the hamster spent more time was defined as the initially preferred compartment. Hamsters that did not enter each compartment at least 5 times were excluded from further training. Following the pretest, the hamsters received a series of 30-minute conditioning sessions in the side compartments, one session per day on consecutive days, alternating no-stimulus or stimulus-paired sessions. During the no-stimulus conditioning sessions, hamsters in both the experimental and the control groups were placed in their initially preferred compartments, where they remained alone. During stimulus-paired conditioning sessions, hamsters in the experimental group were placed in the initially nonpreferred compartments with the stimulus. The hamsters in the control groups were also placed in their initially nonpreferred compartments but were not given the stimulus. This group served to quantify any change in preference or difference score across tests that were not due to conditioning. Twenty-four hours after the last conditioning session, hamsters were tested for their place preference following the same procedure used for the pretest. Pretest, conditioning sessions, and test all occurred at the same time of day (+/- an hour) for each hamster. VS and cocaine were used as stimuli.
Experiment 1a: CPP for VS
To test for a CPP for VS, 22 sexually naïve adult and 18 juvenile hamsters were assigned to control and experimental groups, n = 9-11. In order to reduce the number of cohorts required and prevent exposing control animals to the smell of the stimuli, control animals were housed in a separate but similar room in which the dark phase began at 8:00am, and testing at 9:00am. Ten total conditioning sessions occurred, including 5 no-stimulus and 5 stimulus-paired sessions. Including the pretest and test, the experiment took place over 12 consecutive days, from P20 to 31 for juvenile animals and P63-69 to 74-80 for adult animals. An hour before use, VS were collected from 30 females and mixed together to total approximately 500μl. VS are composed of both non-volatile and volatile components, and both have been shown to have behaviorally relevant properties (Petrulis, 2009). Thus, to ensure exposure to both nonvolatile and volatile components of VS immediately prior to and for the duration of the training session, VS were delivered in two ways. Approximately 15 μl of VS were applied to water-moistened cotton gauze packed into a 2-ml Eppendorf tube, one tube for each male. Immediately before testing, the tube was placed out of reach from the male at the top of the back wall in the initially nonpreferred compartment in VS-paired conditioning sessions for the VS group. Empty Eppendorf tubes were used for the control group in all conditioning sessions and for the VS group in the no-stimulus conditioning sessions. To ensure exposure to nonvolatile components of VS, the remaining ∼200 μl of VS were mixed with 1 ml of mineral oil, and approximately 10 μl of this mixture was applied with a metal spatula directly onto the nose of hamsters in the VS group immediately before being placed in the VS-paired compartment. Only the VS group was present and all were restrained to their VS-paired compartment when VS was present in the behavior testing room, thus eliminating any concerns about odor diffusion and non-specific conditioning. Clean oil was applied to the nose of hamsters in the control group for all conditioning sessions and in the VS group for no-stimulus conditioning sessions. One hour after completion of the CPP test, hamsters were euthanized with an overdose of sodium pentobarbital (150 mg/kg, ip) and a terminal blood sample was collected via cardiac puncture for radioimmunoassay of circulating plasma testosterone.
Experiment 1b: CPP for cocaine
To test for a CPP for cocaine, 16 juvenile hamsters were assigned to control and experimental groups, n = 8. Six total conditioning sessions occurred, (3 no-stimulus and 3 stimulus-paired sessions on alternating days), and the experiment lasted 8 days from P23-30. Hamsters in the experimental group were injected intraperitoneally with cocaine (20mg/kg, Lannett Company, Inc. Philadelphia, PA) immediately before being placed in the initially nonpreferred compartment for stimulus-paired sessions, whereas they received a 0.9% saline vehicle injection before being placed in the initially preferred compartment for no-stimulus sessions. The control group received saline injections before being placed in either compartment in conditioning sessions.
Statistical analysis
To confirm that all stimulus and no-stimulus paired groups had similar initial preference and difference scores, a one way ANOVA was used. To assess whether the stimuli (VS or cocaine) induced a CPP, data from the pretests and final tests were used to calculate a preference score, defined as [time in the stimulus-paired compartment/(time in stimulus-paired compartment+time in no-stimulus compartment)], and a difference score, defined as [time in the no-stimulus compartment-time in the stimulus-paired compartment] (Martínez & Paredes, 2001; Meerts & Clark, 2007; Tenk et al., 2009; Bell et al., 2010; Parada et al., 2010). Changes in preference and difference scores were determined by subtracting pretest measures from test measures for each hamster. In the no-stimulus control animals, average change measures for preference score and difference score were determined to provide a standard for unconditioned change. Control change measures were then subtracted from each stimulus-paired experimental animal's scores to correct for any unconditioned change. Corrected changes in preference and difference scores were then used in one sample t-tests within each group, comparing the value to 0 to evaluate significant changes. These statistical procedures are similar to earlier studies that used paired t-tests to determine changes in preference and difference scores within a group (Meisel & Joppa, 1994; Martínez & Paredes, 2001; Kohlert & Olexa, 2005; Meerts & Clark, 2007; Tenk et al., 2009; Bell et al., 2010; Parada et al., 2010). In addition, correcting for unconditioned changes observed in control animals reduces the chances of false positives, as any initial preferences for an outer compartment can sometimes be reduced after repeated equivalent exposures to those chambers (Bell et al., 2010)(Bell et al., 2010)(Bell et al., 2010)(Bell et al., 2010)(Bell et al., 2010). Significant changes in both preference and difference scores were required to determine that a conditioned place preference had been established; for simplicity, only preference scores are presented in figure format here (Meisel & Joppa, 1994; Bell et al., 2010). Here and with all other reported analyses, p < 0.05 was considered significant, and all statistical analyses were done with SPSS software (PASW Statistics 20; SPSS: An IBM Company, Chicago, IL).
Experiment 2: Neural Responses to VS
Experimental Design
16 juvenile (P28) and 16 adult (P64) hamsters were weighed and randomly assigned to either the VS or control group, n = 8. VS were collected less than two hours before use and delivered via cotton applicator swabs to minimize effects of handling the animals on Fos expression. On the morning of swab exposures, hamsters were moved from their colony room to a separate behavior testing room. Four – seven hours later, VS-containing or clean blank swabs were dropped into VS or control hamsters' home cages, respectively, and behavior was monitored while the hamsters interacted with the swab for one hour. Hamsters were often observed to pick up the swab, chew on it, and place it in their cheek pouches for several minutes at a time. While behavior was not quantified, adults were observed to perform more vigorous investigation of the VS swab. Thus, the Fos response would represent the combined responses to olfactory stimuli as well as behavioral interactions with the swab. To prevent control hamsters from smelling volatile components of VS, they were given access to blank swabs and sacrificed for tissue collection prior to swab exposure for the VS-exposed hamsters. Thus, blank and VS-containing swabs were delivered 1-2 and 3-4 hours after lights off, respectively. One hour after introduction of swab into the cage, hamsters were euthanized with an overdose of sodium pentobarbital (150 mg/kg, ip) and a terminal blood sample was collected via cardiac puncture for radioimmunoassay of circulating plasma testosterone. Hamsters were perfused transcardially with heparinized buffered saline rinse followed by 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde for 24 hours and stored in 20% sucrose/phosphate buffered saline solution until sectioning.
Histological procedures
Brains were sectioned with a cryostat into 4 series of 40 μm thick sections and stored in cryoprotectant at -20°C until histological processing. The first series of sections was mounted onto glass slides, dehydrated with a series of ethanols, and stained with cresyl violet before coverslipping for identification of regions of interest. A second and third series of sections were used to double-label cFos with tyrosine hydroxylase (TH) and orexin-A immunoreactivity, respectively, with free-floating immunohistochemistry. cFos is an immediate early gene used to indicate transcriptional activation (Sheng & Greenberg, 1990; Hughes & Dragunow, 1995), and TH is the rate-limiting enzyme for catecholamine production. Dopamine-β-hydroxylase, the enzyme that converts dopamine to norepinephrine, is absent in the ventral tegmental area in hamsters (Vincent, 1988), thus TH immunoreactivity in the ventral tegmental area was used here to identify dopaminergic cells. Orexin-A is one of two active orexinergic peptides (de Lecea et al., 1998; Sakurai et al., 1998), and, in particular, has been implicated in sexual reward (Muschamp et al., 2007; Di Sebastiano et al., 2011).
Immunohistochemistry occurred at room temperature unless otherwise noted. Rinses with Trizma buffered saline (0.05M, pH = 7.6) occurred initially and between steps, and all antibodies were diluted in 2% of the appropriate serum and 0.3% Triton-X Trizma buffered saline (see Table 1). To visualize Fos and TH, residual aldehydes were removed with 0.1% sodium borohydride after the first series of Trizma buffered saline rinses, and endogenous peroxidase activity was quenched with 1% hydrogen peroxide. Tissue was blocked and made permeable with 20% goat serum and 0.3% Triton-X Trizma buffered saline, followed by incubation in the Fos primary antibody for 48 hours at 4°C. Tissue was then incubated consecutively in the Fos secondary antibody and avidin-biotin complex for one hour each. Lastly, sections were reacted for ∼2 minutes with 10 mg 3,3′-Diaminobenzideine tetrahydrochloride in 50 ml Trizma buffered saline and 45 μl of 30% hydrogen peroxide to produce a dark brown reaction product in the nucleus of Fos-immunoreactive (ir) cells. After rinsing, tissue was again blocked and made permeable and then incubated overnight in TH primary antibody. TH secondary antibody and avidin-biotin complex were then each applied consecutively for 1 hour. Finally, sections were reacted for ∼2 minutes with 1 drop of Vector SG enzyme substrate in 7 ml Trizma buffered saline and 50 μl 30% hydrogen peroxide to produce a cytoplasmic blue reaction product in TH-ir cells. To visualize Fos and orexin, a similar immunohistochemistry protocol was used, but with the appropriate reagents (see Table 1). Primary and secondary antibody deletion control studies were run on separate sections. Non-specific background staining was low or absent in these sections. Tissue sections were mounted onto glass slides and dehydrated with a series of ethanols before coverslipping.
Table 1. Immunohistochemical reagents.
|
| ||
| Fos/TH Immunohistochemistry | ||
|
| ||
| Fos | Serum | Normal goat serum. Pel-Freez Biologicals, Rogers, AR |
| Primary | c-Fos (4): rabbit, sc-52. 1:10,000, 0.02μg IgG/ml solution, Santa Cruz Biotech, Santa Cruz, CA | |
| Secondary | Biotinylated goat anti-rabbit IgG (H+L). 1:500, 3μg IgG/ml solution, Vector Laboratories, Burlingame, CA | |
| TH | Serum | Normal goat serum. Pel-Freez Biologicals, Rogers, AR |
| Primary | Mouse anti-TH monoclonal antibody. 1:2,000, Millipore-Chemicon, Billerica, MA | |
| Secondary | Biotinylated goat anti-mouse IgG (H+L). 1:500, 3μg IgG/ml solution, Vector Laboratories, Burlingame, CA | |
| Fos/orexin Immunohistochemistry | ||
|
| ||
| Fos | Serum | Normal donkey serum. Jackson ImmunoResearch Laboratories, West Grove, PA |
| Primary | c-Fos (4): rabbit, sc-52. 1:10,000, 0.02μg IgG/ml solution, Santa Cruz Biotech, Santa Cruz, CA | |
| Secondary | Biotinylated donkey anti-rabbit IgG (H+L). 1:500, 2.4μg IgG/ml solution, Jackson ImmunoResearch, West Grove, PA | |
| Orexin | Serum | Normal horse serum, S-2000. Vector Laboratories, Burlingame, CA |
| Primary | Orexin-A (C-19): goat, sc-8070, 1:5,000, 0.04μg IgG/ml solution, Santa Cruz Biotech, Santa Cruz, CA | |
| Secondary | Biotinylated horse anti-goat IgG (H+L), 1:500, 3μg IgG/ml solution, Vector Laboratories, Burlingame, CA | |
| Other reagents | ||
|
| ||
| Avid :Biotin Complex | Peroxidase- Vectastain ABC Kit PK-6100, Vector Laboratories, Burlingame, CA | |
| Enzyme Substrate | Peroxidase- Vector SG Substrate Kit SK-4700, Vector Laboratories, Burlingame, CA | |
| Enzyme Substrate | Peroxidase- 3,3′ Diaminobenzidine tetrahydrochloride, Sigma-Aldrich, St. Louis, MO | |
Microscopic analysis
Regions of interest included the nucleus accumbens (Acb), medial prefrontal cortex (mPFC), and ventral tegmental area (VTA) because they are primary components of the mesocorticolimbic dopamine circuitry (Fibiger & Phillips, 1988); the lateral hypothalamus (LH) because of its orexinergic cell population (Aston-Jones et al., 2009); the ventromedial hypothalamus (VMH) because of its role in gating reproductive and defensive behaviors (Choi et al., 2005); and the posterior medial amygdala (MeP) as a positive control region known to express Fos in response to VS in both juvenile and adult male hamsters (Romeo et al., 1998).
Regions were subdivided according to the hamster brain atlas (Morin & Wood, 2001), as indicated by previous research demonstrating distinct functional and anatomical characteristics of the subregions (Groenewegen et al., 1999; Bradley & Meisel, 2001; Heidbreder & Groenewegen, 2003; Balfour et al., 2006; Ikemoto, 2007). The mPFC included the anterior cingulate (Cg1), prelimbic (PrL), and infralimbic (IL) subregions; the Acb included the core (AcbC) and medial portion of the shell (AcbSh); the MeP included the dorsal (MePD) and ventral (MePV) subregions; the VMH included medial (VMHM) and lateral (VMHL) portions; and the VTA included interfasicular (IF), paranigral (PN), parabrachial pigmented (PBP), and Tail nuclei (Figure 1). The hypothalamic area containing orexinergic cells was subdivided into the dorsomedial hypothalamus and perifornical area (DM/PeF) and lateral hypothalamus (LH), relative to the lateral edge of the fornix because of functional specificity of these two populations of orexinergic neurons (Harris et al., 2005), Figure 1).
Figure 1.
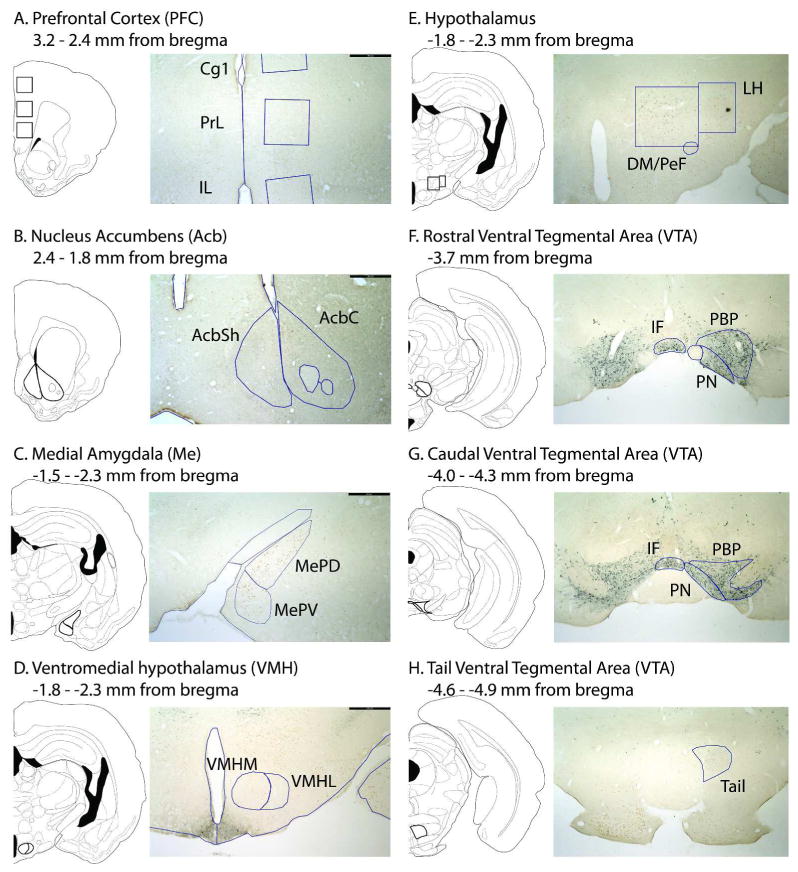
Representative subregion contours drawn over atlas diagrams (Morin and Wood, 2001) and low-magnification photomicrographs of immunohistochemically-treated tissue sections from VS-exposed adult animals; scale bar is 500μm.
The VTA was further subdivided along its rostrocaudal extent because of previous reports of functional specificity in rats and mice (Olson et al., 2005; Ikemoto, 2007) and a relative lack of region-specific analysis in the hamster. Rostral sections were defined as having TH cells adjacent to the fasciculus retroflexus prior to the onset of the interpeduncular nucleus; caudal sections were defined as having interpeduncular nucleus present prior to the medial lemniscus merging with the cerebral peduncle; tail sections were defined as having a rounded interpeduncular nucleus prior to the oral part of the pontine nuclei (Figure 1). Upon completion of microscopic inspection and analysis, similar effects of age and swab exposure were found in the rostral and caudal portions of each VTA subregion; therefore, data from rostral and caudal IF, PN, and PBP sections were combined within subregion for statistical analysis and presentation here.
Anatomically matched tissue sections throughout the extent of each region of interest (2-5 sections per subregion, depending on size) were selected at 4x objective. In the Acb, Me, and VMH, subregion contours were manually traced bilaterally according to the atlas and cytoarchitecture in Nissl-stained sections and then overlaid onto corresponding immunohistochemically-treated tissue sections for cell counting. In the mPFC, 600 × 600 μm boxes were placed in the mPFC relative to the medial brain edge and corpus callosum. In the hypothalamus, boxes were drawn to surround all orexin-ir cells medial or lateral to the lateral edge of the fornix in immunohistochemically-treated tissue sections. In the VTA, contours were drawn unilaterally in immunohistochemically treated tissue sections.
Cells counts were made within a contour by a single experimenter blind to hamster treatment with an UPlanSApo 40x (0.9NA) objective on an Olympus BX51 microscope under brightfield illumination using Neurolucida (version 7; Microbrightfield, Williston, VT). All quantification was performed on double-labeled immunohistochemically treated tissue; cells were considered Fos-ir if they had a distinct nucleus with visible puncta stained dark red-brown and TH- or orexin-ir if the cytoplasm was stained gray-blue. In all regions, single-labeled Fos-ir cells were counted; the number of Fos-ir cells within each subregion contour was divided by the area of that contour to create a measure of cell density within a section. These density data control for any change in subregion area with age, and generally detect similar effects of treatment as do cell count data. In the VTA, single-labeled TH-ir cells and cells double-labeled for both TH and Fos, here called TH/Fos-ir, were counted and used in the same density calculations as the Fos-ir cells. In the LH, single-labeled orexin-ir cells and cells double-labeled for both orexin and Fos, here called orexin/Fos-ir, were counted within an area defined by the presence of the orexin-ir cells. Therefore number, not density, of orexin-ir and orexin/Fos-ir cells per section is reported here. Double-labeled cells in the VTA and LH were not included in measures of Fos-ir cells in order to provide non-dopaminergic or -orexinergic cell phenotype-specific insights. Measurements from each tissue section were averaged across sections to create one measurement per subregion per hamster.
Statistical Analysis
With data from so many subregions within each hamster, one goal of our statistical approach was to simplify the data and present it at a circuit level by identifying clusters of regions that showed similar patterns of Fos expression across animals. To do so, we used a combination of factor analysis and descriptive correlational analyses to complement previous functional and anatomical findings. Factor analysis, with principal axis factoring and a promax rotation, identified two clusters of subregions. Cluster 1 included Cg1, PrL, IL, AcbC, AcbSh, MePD, MePV, IF, PN, PBP, and Tail, and we refer to regions in this cluster as mesocorticolimbic. Cluster 2 included DM/PeF, LH, VMHM, and VMHL, and we refer to regions in this cluster as hypothalamic.
We then computed the correlations among the regions within each cluster as well as between the two clusters. The average within-cluster correlation was 0.34 in the MCL cluster and 0.42 in the hypothalamic cluster, based on 55 and 6 correlations, respectively. These indicate that Fos expression in subregions within the same cluster were consistently correlated with one another. We also examined correlations between regions falling into the two different clusters, and here the average of the 44 between-cluster correlations was 0.05, supporting the idea that Fos responses in these two clusters are relatively independent.
Fos-ir cell density was next analyzed with multilevel modeling treating animal as the upper-level sampling unit and brain region as the lower-level sampling unit. In this analysis, the cluster the region belonged to (mesocorticolimbic vs. hypothalamic) was treated as a within-subject variable, and age (juvenile vs. adult) and swab (blank vs. VS) were treated as between-subject independent variables. Multilevel modeling provides a more powerful analysis than a traditional repeated measures analysis of variance (ANOVA) because it allows for analysis even if data from all subregions were unavailable for each hamster (as was the case in two juvenile and one adult hamsters due to poor quality tissue sections). The error structure was modeled to impose the traditional homoscedasticity assumption used in ANOVA. Our hypotheses predicted that swab (blank vs. VS) will differentially affect Fos expression in adults and juvenile animals in some subregions. Therefore, we also tested a set of planned comparisons within each subregion, comparing swab vs. blank groups within juvenile and adult animals separately.
A different statistical approach was preferred when analyzing densities of TH-ir and TH/Fos-ir cells and numbers of orexin-ir and orexin/Fos-ir cells because they were only quantified in one region per animal. Therefore, two way ANOVAs were used to analyze the effects of age (juvenile vs. adult) and swab (blank vs. VS) on these variables within each subregion.
Plasma testosterone measures
Duplicate 50 μl samples of plasma testosterone were analyzed within a single assay using the Coat-A-Count Total T Kit (Diagnostic Products, Los Angeles, CA). The minimum detectable concentration was 0.1ng/ml. The intra-assay coefficient of variation was 6.4% and 6.7% for Experiments 1 and 2, respectively. Two way ANOVA (age × swab) was used to analyze plasma testosterone concentrations between groups.
Results
Experiment 1: CPP for potential rewards
Expt 1a. CPP for VS
Adult hamsters showed a CPP for VS (Figure 2). In VS-conditioned adults, one sample t-tests showed that the corrected changes in preference (t(10) = 3.71, p < 0.01) and difference (t(10) = -3.11, p < 0.05) scores were significantly different from 0. On the other hand, juvenile hamsters did not show a CPP for VS (Figure 2). In juveniles, one sample t-tests showed that neither the corrected change in preference (t(8) = 1.23, NS) or difference (t(8) = -2.22, NS) scores were significantly different from 0. Adult and juvenile control and stimulus-paired groups did not differ in their initial preference score (F(3,39) = 0.53, NS) or difference score (F(3,39) = 0.72, NS).
Figure 2.
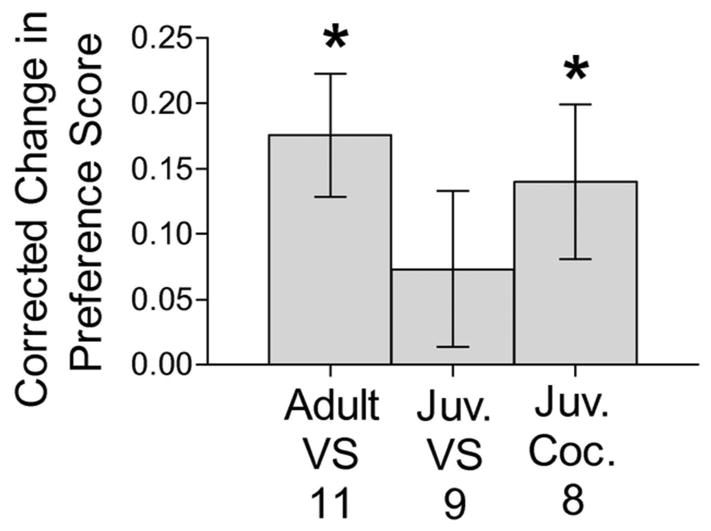
Corrected change in preference scores when tested for a CPP for VS and cocaine. Adult males showed a CPP for VS, whereas juvenile males did not; however, juvenile males did show a CPP for cocaine. * indicates a significant difference between corrected change in preference score to 0, within a group, p < 0.05 with one-sample t-test.
Expt. 1b. CPP for cocaine
Juvenile hamsters showed a CPP for cocaine (Figure 2). One sample t-tests showed that the corrected changes in preference (t(7) = 2.38, p < 0.05) and difference (t(7) = -2.55, p < 0.05) scores were significantly different from 0. Groups did not differ in their initial preference score (F(1,17) = 0.90, NS) or difference score (F(1.17) = 0.131, NS).
Experiment 2: Neural Responses to VS
Fos-ir
Multilevel modeling revealed a main effect of cluster (F(1,429) = 13.86, p < 0.01), but no main effect of age or swab on Fos-ir cell density (Figure 3). This main effect of cluster was qualified by an interaction between cluster and swab (F(1,429) = 10.53, p < 0.01), such that the effect of swab varied depending on the cluster (Figure 3). Follow-up multilevel modeling, analyzing Cluster 1 and 2 separately, indicated an increase in Fos-ir cell density in response to VS in the mesocorticolimbic cluster (F(1,30) = 20.366, p < 0.01), but no effect of swab in the hypothalamic cluster (F(1,28) = 2.41, NS).
Figure 3.
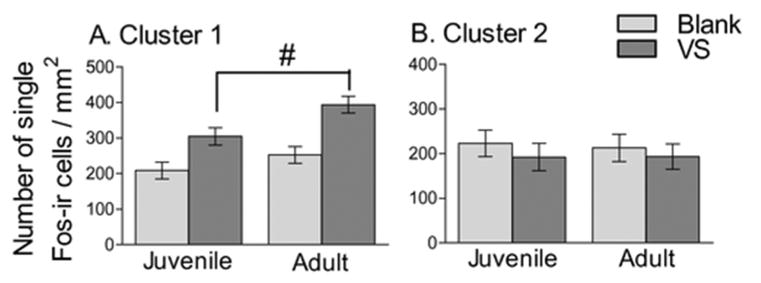
Fos-ir cell density in mesocorticolimbic (A) and hypothalamic (B) clusters. There was a greater density of Fos-ir cell in VS exposed hamsters compared to blank hamsters in the mesocorticolimbic but not hypothalamic clusters. # indicates a main effect of swab, p < 0.01 with multilevel modeling analysis.
Because the a priori hypotheses predicted that adult and juvenile hamsters would show different responses to VS, planned contrasts were performed to analyze differences in Fos-ir cell density between blank and VS-exposed animals within an age for each region of interest, n = 7-8 for all groups. Within the mesocorticolimbic cluster, in both juvenile and adult hamsters, VS elicited an increase in Fos-ir cell density in the MePD (t(26) =5.33, p < 0.01 and t(26) = 6.61, p < 0.01, respectively; Figure 4 and 5C), MePV (t(26) =5.49, p < 0.01 and t(26) = 5.06, p < 0.01, respectively; Figure 5C), and PN (t(28) =2.16, p < 0.05 and t(28) = 2.490, p < 0.05, respectively; Figure 5D). In contrast, the Fos response to VS in other mesocorticolimbic cluster subregions between adult and juvenile responses diverged. Adult, but not juvenile, hamsters showed greater Fos-ir cell density when exposed to VS compared to blank swabs in the IL (t(26) = 2.26, p = 0.03 and t(26) = 1.35, NS, respectively, Figure 5A), AcbC (t(26) = 2.33, p = 0.03 and t(26) = 0.78, NS, respectively, Figure 4 and 5B), IF (t(28) = 4.61, p < 0.01 and t(28) = 1.746, NS, respectively, Figure 4 and 5D), and PBP (t(28) = 3.56, p < 0.01 and t(28) = 1.53, NS, respectively, Figure 5D). VS did not elicit a Fos response in either juvenile or adult hamsters in the remaining mesocorticolimbic cluster subregions, which included Cg1, PrL, AcbSh, and VTA Tail (Figure 5). VS did not evoke a Fos response in any hypothalamic cluster subregions in either age group, as indicated by similar Fos-ir cell densities in the blank- and VS-exposed groups in both ages (data not shown).
Figure 4.
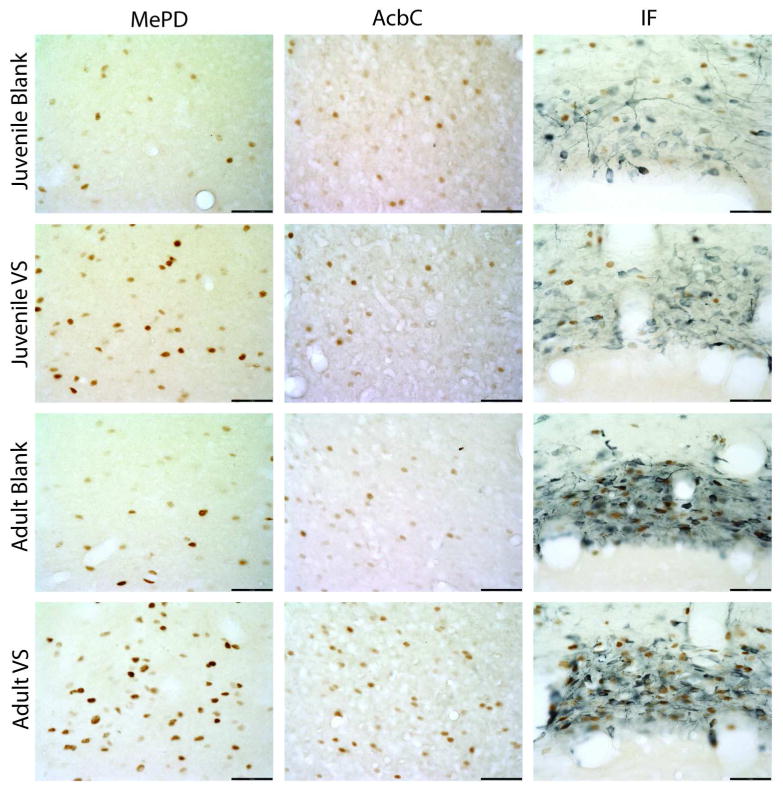
High magnification photomicrographs of representative Fos- (brown nuclei) and TH- (blue cytoplasm) immunoreactive tissue sections from juvenile and adult animals exposed either blank or VS swabs in the AcbC, MePD, and IF. Scale bar is 50μm.
Figure 5.
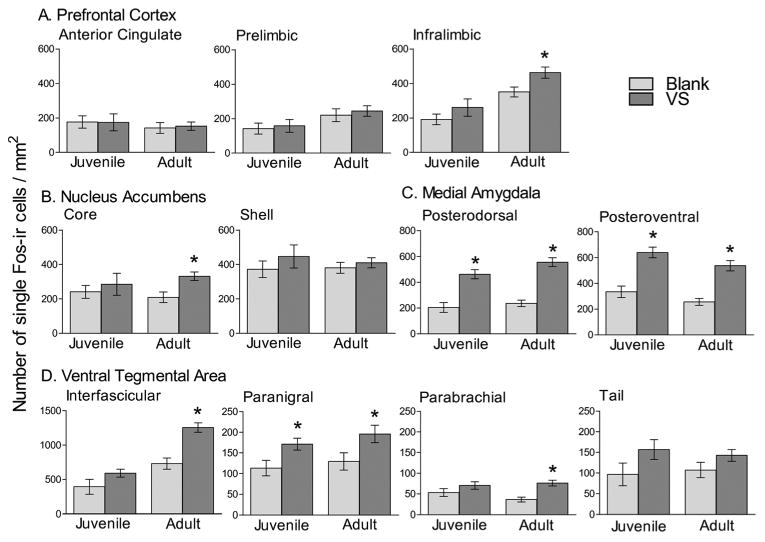
Single-labeled Fos-ir cell density in mesocorticolimbic cluster regions, PFC (A), Acb (B), MeP(C), and VTA (D). There was a greater density of Fos-ir cells in animals exposed to VS swabs compared to those exposed to blank swabs in the MeP and PN in both juveniles and adults, and in the AcbC, IL, and IF in only adults. * indicates difference between Blank and VS swab with planned contrasts within an age group, p < 0.05. Note differences in y-axis in D.
TH-ir and TH/Fos-ir cells
The densities of TH-ir and TH/Fos-ir cells were calculated for VTA and analyzed by a two-way ANOVA, n = 8 for all groups. In IF, a main effect of age was observed on the density of TH/Fos-ir cells (F(1,28) =88.246, p < 0.01, Figure 6a). Specifically, adults showed greater density of double-labeled cells independent of swab exposure. No effect of age was observed on density of TH-ir cells, and no significant effects of swab or age × swab interaction was observed on TH-related measures in IF.
Figure 6.
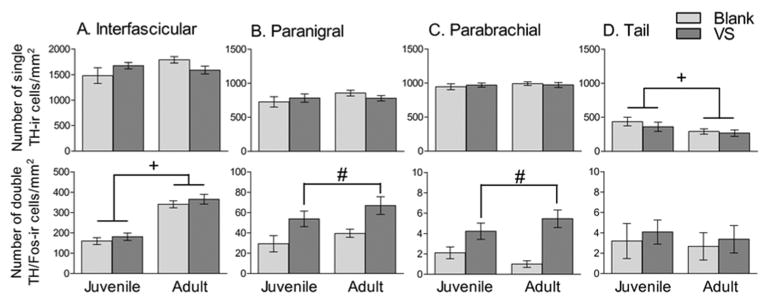
TH-ir measures in IF (A), PN (B), PBP (C) and Tail (D) VTA. First row shows number of single-labeled TH-ir cells and second row shows number of double-labeled TH/Fos-ir cells. In IF and Tail, adults and juveniles differ in immunoreactivity, independent of swab exposure; in PN and PBP, both adults and juveniles show greater immunoreactivity when exposed to a VS swab compared to a blank swab. + indicates main effect of age, # indicates main effect of swab, p < 0.05 with a two-way ANOVA. Note different y axis scales in throughout.
In PN and PBP, a main effect of swab was observed on the density of TH/Fos-ir cells (F(1,28) = 12.51, p < 0.01, Figure 6b and F(1,28) = 23.63, p < 0.01, Figure 6c, respectively), such that hamsters exposed to VS expressed a greater density of double-labeled cells compared to those exposed to a blank swab, regardless of age. No effect of swab was observed on density of TH-ir cells, and no effect of age or age × swab interaction was observed on any TH-related measure in PN or PBP.
In Tail, a main effect of age was observed on TH-ir cell density (F(1,28) = 4.524, p < 0.05), such that juvenile hamsters expressed a greater TH-ir cell density than adults, regardless of swab condition (Figure 6d). No effect of age was observed on density of TH/Fos-ir cells, and no effect of swab or age × swab interaction was observed on any TH-related measure in Tail.
Orexin-ir and orexin/Fos-ir cells
The number of orexin-ir cells and orexin/Fos-ir cells was determined in the LH and analyzed by two-way ANOVA, n = 7-8 for all groups (Table 2). A main effect of age was observed on number of orexin-ir cells in both LHM (F(1,25) = 35.80, p < 0.01) and LHL (F(1,25)=17.79, p < 0.01), such that juvenile hamsters expressed a greater number of orexin-ir cells than adults, independent of swab condition. There was also a main effect of age on the number (F(1,25) = 7.12, p = 0.01) of orexin/Fos-ir cells in LHM, such that adults expressed greater levels than juvenile hamsters. There was no effect of age on number of orexin/Fos-ir cells in the LHL, nor was there an effect of swab or age × swab interaction on any measure in LHM and LHL.
Table 2. Number of single- and double-labeled orexin-ir cells.
| Juvenile | Adult | |
|---|---|---|
|
|
|
|
| Single-labeled orexin-ir cells | ||
|
|
||
| DM/PeF | 77.21 +/- 17.30 | 43.80 +/- 13.00 * |
| LH | 52.92 +/- 10.13 | 36.50 +/- 11.09 * |
| Double-labeled orexin/fos-ir cells | ||
|
|
||
| DM/PeF | 37.12 +/- 13.54 | 50.72 +/- 13.26 * |
| LH | 14.63 +/- 6.40 | 12.81 +/-5.31 |
mean +/- standard deviation
indicates effect of age, p <0.05
Plasma testosterone concentration
Plasma testosterone measures revealed a main effect of age in both Expt 1a (F(1,35) = 30.164, p < 0.01) and Expt 2 (F(1,26) = 40.52, p < 0.01), such that adult hamsters had greater testosterone concentrations than juvenile hamsters (Table 3). In addition in Exp 2, a main effect of swab was observed (F(1,26) = 5.16, p = 0.03), in which hamsters exposed to VS had greater testosterone concentrations than those exposed to blank swabs. This main effect appears to be driven solely by an increase in testosterone in VS-exposed adults, although no statistically significant age × swab interaction was detected.
Table 3. Plasma testosterone concentrations.
| Exp | Group | Juvenile | Adult |
|---|---|---|---|
|
|
|
||
| 1a | No Stimulus: | 0.24 +/- 0.08 | 0.68 +/- 0.10 * |
| Stimulus Paired: | 0.09 +/- 0.04 | 0.84 +/- 0.16 | |
| 2 | Blank Swab: | 0.29 +/- 0.07 | 0.73 +/- 0.13 * |
| VS Swab: | 0.34 +/- 0.06 | 1.12 +/- 0.08 # |
ng/ml, mean +/- standard error,
indicates effect of age, p <0.05,
indicates ME of swab, p <0.05
Discussion
This report is the first demonstration that adolescent maturation of social information processing includes a transformation of a species-specific, socially-relevant sensory signal from a neutral stimulus to an unconditioned reward in the absence of social experience. This perceptual shift is accompanied by a gain in the ability of the social stimulus to activate midbrain, ventral striatal, and prefrontal components of the mesocorticolimbic reward pathway, indicating that these particular regions are recruited to mediate the adolescent gain in the perception of VS as rewarding (Figure 7).
Figure 7.
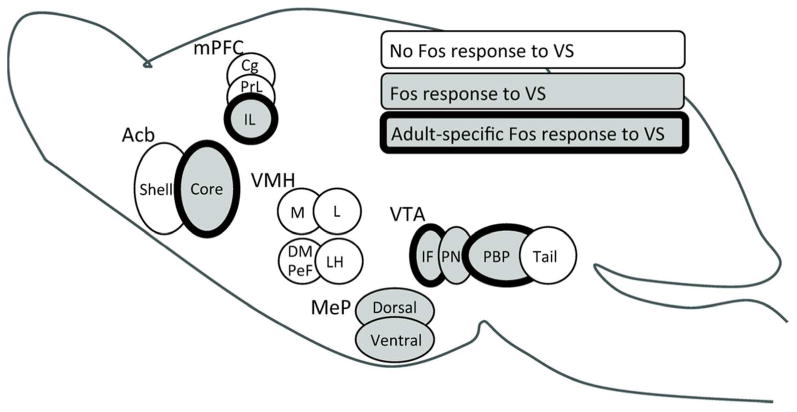
Schematic diagram of adolescent changes in VS-induced Fos responses (indicated by shading). VS activated the posterior medial amygdala and paranigral VTA neurons similarly in juvenile and adult male hamsters, but activated non-dopaminergic interfascicular and parabrachial VTA neurons, nucleus accumbens core, and infralimbic medial prefrontal cortex only in adults (bold outline). This pattern of selective neural activation in adulthood correlates with the ability of VS to serve as an unconditioned stimulus for reward in adult, but not juvenile males, indicating recruitment of these specific cell groups in the adolescent development of social reward.
Adolescent gain in the positive valence of VS
Juvenile male hamsters failed to show a CPP for VS. However, they did show a CPP to cocaine, demonstrating a pre-adolescent ability to show a place preference for a pharmacological reward. This is consistent with previous reports that demonstrate enhanced sensitivity to cocaine, nicotine and ethanol reward during adolescence (Doremus-Fitzwater et al., 2010). As expected, adult males did form a CPP for VS, leading to the conclusion that adult, but not prepubertal, male hamsters perceive VS as rewarding. These results provide strong evidence that in the absence of sexual experience, a species-specific social stimulus that is a relatively weak reward or neutral in valence to juveniles becomes a potent unconditioned reward as a consequence of adolescent maturation. This report also extends earlier studies on the development of hamsters' attraction to VS, where adults, but not juveniles, spend significantly more time investigating VS than control stimuli. Preferences for VS are present only after males reach 40 days of age, by which time circulating levels of testosterone are elevated as a result of puberty onset (Johnston & Coplin, 1979). Whether or not elevated testosterone levels influence the perception of VS as rewarding is an open and testable question. However, it appears that organizational effects of testosterone are not necessary for the rewarding interpretations of VS, as hamsters that are gonadectomized prior to the onset of puberty and given replacement testosterone in adulthood still show a CPP to VS (De Lorme et al., 2012). Therefore, either activational effects of testosterone are sufficient to promote VS reward or gonadal hormone-independent development during adolescence is required. In either case, the transformation of VS as a rewarding social stimulus during adolescence is likely critical for successful social interactions in adulthood.
Adolescent maturation of VS-induced neural activation patterns
Factor analysis of Fos expression in the fifteen brain areas analyzed in this study identified two functionally related clusters of cell groups. One cluster included the MeP and members of a complex network of limbic, tegmental, and cortical projections that coordinate reward, incentive motivation and adaptive behavior (reviewed in (Berridge & Robinson, 1998; Ikemoto & Panksepp, 1999; Wise, 2004). This cluster was characterized by neural responsiveness to VS. Within this cluster, the adolescent gain of rewarding properties of VS was correlated with different patterns of VS-induced neural activation between adults and juveniles. However, the second cluster, which included the hypothalamic subregions, was characterized by an absence of responsiveness to VS. Thus, developmental dynamics within the mesocorticolimbic cluster appear to underlie the developmental gain in positive valence of VS.
The mesocorticolimbic reward system includes extensive dopaminergic and non-dopaminergic projections from the VTA to the Acb, mPFC, and MeP, all of which are complexly and reciprocally connected via recurrent circuits (Swanson, 1982; Oades & Halliday, 1987; Thompson & Swanson, 2010). In rodents, the flow of social chemosensory information to this circuit begins with direct projections from the main and accessory olfactory bulbs to the MeP, which integrates sensory information with the internal hormonal milieu for initial evaluation of the social stimulus (Wood & Newman, 1995). This first pass evaluation can then be relayed either directly or via preoptic and hypothalamic cell groups to the VTA (Phillipson, 1979; Kevetter & Winans, 1981; Coolen & Wood, 1998; Geisler & Zahm, 2005). Placing our data within the framework of this circuitry, we propose that VS acquires positive valence through experience-independent alterations in mesocorticolimbic responses to the initial evaluation of a social stimulus by the amygdala. We base this hypothesis first on the observation that early stage evaluation of VS by the MeP appears to be in place in juveniles and similar to that of adults, because VS elicited similar Fos responses in the amygdala and one of the downstream areas, the VTA PN. Subsequently, over the course of adolescence and in the absence of social experience, VS stimulation comes to engage the IF and PBP nuclei in the VTA, IL of the mPFC, and core of the Acb. This observation suggests that the responses of IF, PBB, IL, and AcbC in evaluating transmissions from the amygdala are altered by developmentally programmed or testosterone-induced maturational changes, thus associating these cell groups with a positive valence of VS in adulthood. There are several possible neurobiological mechanisms that could produce the adult-typical behavioral and neural responses to VS, including developmental changes in corticolimbic dopaminergic receptors and mesocorticolimbic dopaminergic projections; these that are reviewed extensively elsewhere (Doremus-Fitzwater et al., 2010; Kuhnet al., 2010)
The apparent lack of involvement of the hypothalamic cluster cell groups in mediating adolescent change in social information processing is surprising, given their roles in expression of social behaviors and reward. The VMH is involved in sexual behavior (Harding & McGinnis, 2005) and shows increased Fos expression in response to estrous odors in adult male rats (Kippin et al., 2003). However, the rats in this study were sexually experienced, whereas hamsters in the current study were sexually naïve, suggesting that a VMH response to estrous odors may be conditioned as a result of previous experience. Hypothalamic orexin is involved in expression of sexual behavior and reward (Muschamp et al., 2007; Di Sebastiano et al., 2011), but the finding that orexinergic neurons were not responsive to VS suggests that the rewarding value of VS is somehow distinct from general sexual reward.
Adolescent maturation of mesolimbic dopamine and orexin
The number of single-labeled Tail VTA TH-ir and orexin-ir neurons was greater in juveniles than in adults. These results are somewhat difficult to interpret because a reduction in cytoplasmic immunoreactivity could be indicative of either reduced protein expression or reduced cytoplasmic levels of protein secondary to enhanced protein transport to the axon terminal. The current study also found that, compared with juveniles and independent of VS exposure, adults had greater numbers of Fos-expressing TH-ir and orexin-ir cells in Tail VTA and DM/PeF, respectively. These results may be indicative of heightened vigilance or sensitivity to non-specific stimuli in adulthood (e.g., a clean cotton swab in this study), as both dopamine and DM/PeF orexin have been implicated in general arousal as reviewed in (Harris & Aston-Jones, 2006; Ikemoto, 2007; Boutrel et al., 2010).
Conclusion
Previous studies have documented adolescent changes in the rewarding properties of drugs of abuse in animals, but less attention has been paid to natural or social rewards (Doremus-Fitzwater et al., 2010). The present study demonstrates an experience-independent gain in the unconditioned rewarding value of a social stimulus over the course of adolescent development, and provides a neuroanatomical basis for the hypothesis that maturational changes within the mesocorticolimbic system mediate this shift in behavioral responses to VS. Because maturation of social information processing is a decisive component of mammalian adolescence, and perturbations in this aspect of development are associated with maladaptive behaviors and certain mental illnesses associated with adolescence, further research into the mechanisms by which social stimuli gain rewarding properties during this critical period of development is warranted.
Acknowledgments
This work was supported by National Institutes of Health grants R01-MH068764 (C.L.S.), T32-MH070343 (M.R.B), T32-NS44928 (M.R.B.). Many thanks to Jane Venier, Dr. Heather Molenda-Figueira, Dr. Sarah Meerts, Maggie Mohr, Bradley Lawrence, Dana Gradl, Allison Melkonian, Genivieve Trombly, Robyn Weston, Jennifer La, and Christine Azizhkan.
Abbreviations
- Acb
nucleus accumbens
- AcbC
nucleus accumbens core
- AcbSh
nucleus accumbens shell
- Cg1
anterior cingulate medial prefrontal cortex
- CPP
conditioned place preference
- DM/PeF
dorsomedial hypothalamus / perifornical area
- IL
infralimbic medial prefrontal cortex
- LH
lateral hypothalamus
- MeP
posterior medial amygdale
- MePD
posterdorsal medial amygdale
- MePV
posteroventral medial amygdala
- mPFC
medial prefrontal cortex
- PrL
prelimbic medial prefrontal cortex
- TH
tyrosine hydroxylase
- VMH
ventromedial hypothalamus
- VMHL
lateral ventromedial hypothalamus
- VMHM
medial ventromedial hypothalamus
- VS
vaginal secretions
- VTA
ventral tegmental area
- IF
interfascicular nucleus of the ventral tegmental area
- PN
paranigral nucleus of the ventral tegmental area
- PBP
parabrachial pigmented nucleus of the ventral
- Tail
tail nucleus of the ventral tegmental area
References
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Supplement 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour ME, Brown JL, Yu L, Coolen LM. Potential contributions of efferents from medial prefrontal cortex to neural activation following sexual behavior in the male rat. Neuroscience. 2006;137:1259–1276. doi: 10.1016/j.neuroscience.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ. The Role of Dopamine in the Nucleus Accumbens and Striatum during Sexual Behavior in the Female Rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Meerts SH, Sisk CL. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Horm Behav. 2010;58:410–414. doi: 10.1016/j.yhbeh.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–111. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KC, Meisel RL. Sexual Behavior Induction of c-Fos in the Nucleus Accumbens and Amphetamine-Stimulated Locomotor Activity Are Sensitized by Previous Sexual Experience in Female Syrian Hamsters. The Journal of Neuroscience. 2001;21:2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 Delineates a Pathway Mediating Innate Reproductive Behaviors from the Amygdala to the Hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: Simultaneous anterograde and retrograde tract tracing. The Journal of Comparative Neurology. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory-activity. Proceedings of the National Academy of Sciences. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorme KC, Bell MR, Sisk CL. Maturation of social reward in adult male Syrian hamsters does not depend on organizational effects of pubertal testosterone. Horm Behav. 2012;62:180–185. doi: 10.1016/j.yhbeh.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sebastiano AR, Wilson-Pérez HE, Lehman MN, Coolen LM. Lesions of orexin neurons block conditioned place preference for sexual behavior in male rats. Horm Behav. 2011;59:1–8. doi: 10.1016/j.yhbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Phillips AG. Mesocorticolimbic Dopamine Systems and Reward. Ann N Y Acad Sci. 1988;537:206–215. doi: 10.1111/j.1749-6632.1988.tb42107.x. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the ratanatomical substratum for integrative functions. The Journal of Comparative Neurology. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AVJ, Voorn P. Convergence and Segregation of Ventral Striatal Inputs and Outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Microlesions of the ventromedial nucleus of the hypothalamus: effects on sociosexual behaviors in male rats. Behav Neurosci. 2005;119:1227–1234. doi: 10.1037/0735-7044.119.5.1227. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Groenewegen H. The medial prefrontal cortex in the rat: evidence for a doros-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behaviors: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Coplin B. Development of Responses to Vaginal Secretion and Other Substances in Golden Hamsters. Behavioral and Neural Biology. 1979;25:473–489. doi: 10.1016/s0163-1047(79)90242-5. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. The Journal of Comparative Neurology. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Cain SW, Pfaus JG. Estrous odors and sexually conditioned neutral odors activate separate neural pathways in the male rat. Neuroscience. 2003;117:971–979. doi: 10.1016/s0306-4522(02)00972-7. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Olexa N. The role of vaginal stimulation for the acquisition of conditioned place preference in female Syrian hamsters. Physiol Behav. 2005;84:135–139. doi: 10.1016/j.physbeh.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of Ventral Tegmental Area Dopaminergic and Nondopaminergic Neurons by Orexins/Hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58:122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha A, Sershen H. Heterogeneity of Reward Mechanisms. Neurochem Res. 2010;35:851–867. doi: 10.1007/s11064-009-0096-4. [DOI] [PubMed] [Google Scholar]
- Martínez I, Paredes RG. Only Self-Paced Mating Is Rewarding in Rats of Both Sexes. Horm Behav. 2001;40:510–517. doi: 10.1006/hbeh.2001.1712. [DOI] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of Testosterone in Prepubertal and Postpubertal Male Hamsters: Dissociation of Effects on Reproductive Behavior and Brain Androgen Receptor Immunoreactivity. Horm Behav. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Meerts SH, Clark AS. Female rats exhibit a conditioned place preference for nonpaced mating. Horm Behav. 2007;51:89–94. doi: 10.1016/j.yhbeh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56:1115–1118. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA, Rowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur J Pharmacol. 1996;309:21–24. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Murphy MR, Schneider GE. Olfactory Bulb Removal Eliminates Mating Behavior in the Male Golden Hamster. Science. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A Role for Hypocretin (Orexin) in Male Sexual Behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct Involvement of Orexinergic Systems in the Activation of the Mesolimbic Dopamine Pathway and Related Behaviors Induced by Morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of Drug Reward by cAMP Response Element-Binding Protein: Evidence for Two Functionally Distinct Subregions of the Ventral Tegmental Area. The Journal of Neuroscience. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Chamas L, Censi S, Coria-Avila G, Pfaus JG. Clitoral stimulation induces conditioned place preference and Fos activation in the rat. Horm Behav. 2010;57:112–118. doi: 10.1016/j.yhbeh.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Petrulis A. Neural mechanisms of individual and sexual recognition in Syrian hamsters (Mesocricetus auratus) Behav Brain Res. 2009;200:260–267. doi: 10.1016/j.bbr.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: A horseradish peroxidase study in the rat. The Journal of Comparative Neurology. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Parfitt DB, Richardson HN, Sisk CL. Pheromones Elicit Equivalent Levels of Fos-Immunoreactivity in Prepubertal and Adult Male Syrian Hamsters. Horm Behav. 1998;34:48–55. doi: 10.1006/hbeh.1998.1463. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sawai N, Ueta Y, Nakazato M, Ozawa H. Developmental and aging change of orexin-A and -B immunoreactive neurons in the male rat hypothalamus. Neurosci Lett. 2010;468:51–55. doi: 10.1016/j.neulet.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone Programs Adult Social Behavior before and during, But Not after, Adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Greenberg MF. The Regulation and Function of c-fos and Other Immediate Early Genes in the Nervous System. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined flourescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: Effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–97. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R, Swanson L. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci U S A. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR. Distributions of Tyrosine Hydroxylase-, Dopamine beta Hydroxylase-, and Phenylethanolamine-N-Methyltransferase- Immunoreactive Neurons in the Brain of the Hamster (Mesocricetus auratus) The Journal of Comparative Neurology. 1988;268:584–599. doi: 10.1002/cne.902680408. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. J Neurosci. 1995;15:7261–7269. doi: 10.1523/JNEUROSCI.15-11-07261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


