Abstract
The interpretation of social cues must change during adolescence in order to promote appropriate social interactions in adulthood. For example, adult, but not juvenile, male Syrian hamsters find female pheromones contained in vaginal sections (VS) rewarding, and only adult hamsters engage in sexual behavior with a receptive female. We previously demonstrated that the rewarding value of VS is both testosterone- and dopamine-dependent. Additionally, VS induces Fos expression throughout the mesocorticolimbic circuit in adult but not juvenile hamsters. In this study, we determined whether or not treatment of juvenile male hamsters with testosterone is sufficient to promote adult-like neural responses to VS. Juvenile and adult male hamsters were gonadectomized and given empty or testosterone-filled subcutaneous capsules for one week. Hamsters were then exposed to either clean or VS-containing mineral oil on their nares, and brains were collected one hour later for immunohistochemistry to visualize Fos and tyrosine hydroxylase immunoreactive cells. Testosterone-treatment failed to promote adult-typical patterns of Fos expression in juvenile hamsters; indeed, in some brain regions, juveniles exposed to VS expressed less Fos compared to age-matched controls while, as expected, adults exposed to VS expressed greater Fos compared to age-matched controls. Age-related changes in tyrosine-hydroxylase expression were also observed. These data indicate that testosterone cannot activate the adult-typical pattern of Fos expression in response to female social cues in prepubertal males, and that additional neural maturation during adolescence is required for adult-typical mesocorticolimbic responses to female pheromones.
Keywords: testosterone, puberty, dopamine, pheromone, Fos
INTRODUCTION
The perception of and responses to social cues mature during puberty and adolescence. In particular, interactions with peers become more salient and gain sexual connotations (Spear, 2000). Thus, social interactions evolve during the juvenile-to-adult transition, but little is known about the underlying neurobiological mechanisms of this vital aspect of adolescence. To address this question, we have studied developmental changes in endocrine, neural, and behavioral responses of sexually naïve male Syrian hamsters to female hamster vaginal secretions (VS) (Bell et al., 2010; 2013). VS, which signal female receptivity, must be detected and neurally integrated with internal hormonal information by male hamsters for a successful sexual interaction to occur (Wood and Newman, 1995; Wood, 1997). Importantly, adult, but not juvenile, hamsters are attracted to, and form a conditioned place preference (CPP) for, VS (Johnston and Coplin, 1979; Bell et al., 2010; 2013), demonstrating a developmental transition in the positive valence of female pheromones contained in VS. Sexual reward is often associated with dopaminergic action within the mesocorticolimbic reward circuit, a hormone-sensitive circuit that is remodeled during puberty and adolescence (Meisel et al., 1996; Becker et al., 2001; Doremus-Fitzwater et al., 2010; Wahlstrom et al., 2010; Aubele and Kritzer, 2012). Therefore, the present study investigated the effects of adolescent development and gonadal hormone manipulation on neural responses to VS within the mesocorticolimbic circuit.
Immediate early gene expression, specifically Fos immunoreactivity, has been used as a proxy for neural activity to reveal neural correlates of male hamster behavioral responses to VS. Initial studies focused on hormone-sensitive brain regions previously implicated in chemosensory responses to social cues, including the bed nucleus of the stria terminalis, magnocellular region of the medial preoptic nucleus, and the posterior medial amygdala (MeP) (Fiber et al., 1993; Kollack-Walker and Newman, 1997). Both gonad-intact adult and juvenile hamsters express more Fos in all of these regions after exposure to VS compared with unexposed age-matched controls (Romeo et al., 1998). However, more recently, we found that juvenile and adult responses to VS diverge in the mesocorticolimbic circuit (Bell et al., 2013). In that study, VS again elicited a Fos response in the MeP of both juvenile and adult hamsters, and also in ventral tegmental area (VTA) dopamine-producing neurons. However, in non-dopamine cells in the VTA, nucleus accumbens (Acb) core, and the infralimbic region of the medial prefrontal cortex (mPFC), VS elicited a Fos response only in adults. These data suggest that during adolescent development, VS acquires positive valence through alterations in reward circuit processing of sensory information relayed from the medial amygdala.
The increase in gonadal hormone secretion that normally occurs during puberty modulates behavioral responses to VS. Juvenile hamsters treated with testosterone become attracted to and form a conditioned place preference for VS (Johnston and Coplin, 1979; Meek et al., 1997; Bell and Sisk, 2013). Moreover, the testosterone-dependent CPP for VS in juvenile males is blocked by a dopamine receptor antagonist (Bell and Sisk, 2013), further supporting the idea that testosterone-dopamine interactions underlie the development and expression of sexually motivated behaviors (Hull et al., 1995; Putnam et al., 2001; Kritzer et al., 2007). On the other hand, testosterone treatment of juvenile hamsters is insufficient to induce them to perform adult-like levels of sexual behaviors with a receptive female (Meek et al. 1997), suggesting that additional adolescent neural maturation is required for expression of sexual behavior. Therefore, the goal of the present study was to determine if treating juvenile hamsters with testosterone results in an adult-like pattern of mesocorticolimbic neural activation in response to VS.
METHODS
Animals
Sexually naïve male Syrian hamsters (Mesocricetus auratus) were obtained from Harlan Laboratories (Madison, WI) and singly housed in clear polycarbonate cages (30.5 × 10.2 × 20.3 cm) in temperature- and humidity-controlled vivaria with a shifted light:dark cycle (14:10, dark phase began at 2:00pm). They were provided with cotton nestlet enrichment and ad libitum access to food (Teklad Rodent diet 8640, Harlan, Madison, WI) and water. Juveniles arrived on postnatal day 18 (P18) or P19 and adults on P49-56. Subjects were habituated to handling procedures on two days prior to testing. Ten-month old adult female hamsters were housed under similar conditions in separate vivaria and used as the source of VS. All experiments were conducted under <4 lux red light 1–5 hours into the dark phase. Hamsters were treated in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals, and protocols were approved by the Michigan State University Institutional Animal Care and Use Committee.
Experimental design
The experiment used a 2 × 2 × 2 × 9 factorial design, with age (juvenile or adult), hormone status (testosterone or blank capsule treated), and stimulus exposure (VS or clean oil) as between subject variables, and brain region as a within subject variable. Because of the large number of animals, stimulus exposure and tissue collection were performed in cohorts on two consecutive days, with all groups represented evenly each day. The pubertal transition in hamsters occurs roughly between P28 and P56 (Miller et al 1977); therefore, we tested subjects’ responses to VS at P28 (juveniles) and P58-66 (adults) after one week of testosterone or empty capsule treatment. This duration of testosterone exposure is sufficient to promote a conditioned place preference to VS in juvenile animals (Bell and Sisk 2013) and robust sex behavior in adult animals that have been without testosterone for several weeks (Schulz et al 2009). Two or three days after arrival, on P21 (juveniles) and P51-58 (adults), hamsters were gonadectomized under isoflurane anesthetic. Bilateral longitudinal scrotal incisions were made and the testes were removed with a cut distal to ligature (adults) or cauterization (juveniles). At the same time, animals received subcutaneous implants of two blank or testosterone-containing silastic capsules (one 7 mm and one 15 mm of testosterone, Sigma-Aldrich, St. Louis, MO, sealed on each end with 4mm of silastic adhesive; inner diameter 1.98 mm; outer diameter 3.18 mm). These capsule lengths were chosen to provide concentrations of testosterone in the high end of physiological range, as has been used successfully in the past (Bell and Sisk 2013, Schulz et al 2009). Subjects received a subcutaneous injection of ketoprofen analgesic at the time of surgery and again 24 hours after. One week after surgery, half of each age/hormone group was randomly assigned to exposure to either clean or VS-containing mineral oil.
Stimulus exposure
Thirty female hamsters were ovariectomized on P70 and used as stimulus animals in other experiments for several months before hormone administration and collection of VS for use in the two exposure days in this study. They were injected subcutaneously with 10 μg estradiol benzoate and 500 μg progesterone in sesame oil 52 and 4 hours, respectively, to mimic hormonal estrus prior to collection of VS. VS were collected by gentle vaginal palpation and mixed together (total of ~0.5ml VS) and stored at −20°C for two weeks prior to thawing and mixing with 1.5 ml of mineral oil one hour prior to exposure. Clean mineral oil was used as the control for VS exposure. The morning of stimulus exposure, hamsters were weighed and moved from their colony room to a separate behavior testing room. Approximately 10 μl of clean or VS oil were applied with a metal spatula directly onto the nose of hamsters in a quick and minimally stressful handling procedure. While handling is known to affect Fos responses (Kollack-Walker et al 1997), this procedure was necessary to ensure equivalent exposure to nonvolatile components of VS across age groups and was applied equally across all groups. To prevent control hamsters from smelling volatile components of VS, they were given clean oil and sacrificed for tissue collection prior to doing the same for the VS-exposed hamsters. Thus, clean oil and VS-containing oil were delivered 1–2 and 3–4 hours after lights off, respectively.
Tissue collection and analysis
Hamsters were euthanized with an overdose of sodium pentobarbital (150 mg/kg, intraperitoneal) sixty minutes after stimulus exposure to allow for peak Fos expression (Sheng and Greenberg, 1990; Hughes and Dragunow, 1995). Flank gland lengths and seminal vesicle weights were recorded as bioassays of peripheral testosterone levels (Vandenbergh, 1973). A terminal blood sample was collected via cardiac puncture, held on ice in a heparinized tube for less than four hours, and centrifuged to isolate plasma, which was later used for radioimmunoassay of circulating plasma testosterone. Hamsters were perfused transcardially with heparinized and buffered saline rinse followed by 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde for 12 hours and stored in 20% sucrose/phosphate buffered saline solution until sectioning.
Histological procedures
Brains were sectioned with a cryostat into 4 series of 40 μm thick sections and stored in cryoprotectant at −20°C until histological processing. One series of sections was used to double-label Fos and tyrosine hydroxylase (TH) immunoreactivity by free-floating immunohistochemistry. TH is the rate-limiting enzyme for catecholamine production; dopamine-β-hydroxylase, the enzyme that converts dopamine to norepinephrine, is absent in the VTA in hamsters (Vincent, 1988), thus TH immunoreactivity in the VTA was used here to identify dopaminergic cells.
Immunohistochemistry was performed as in (Bell et al., 2013). Briefly, all work occurred at room temperature unless otherwise noted, rinses in Trizma buffered saline (TBS, 0.05M, pH = 7.6) occurred initially and between steps, and all antibodies were diluted in 2% normal goat serum (Pel-Freez Biologicals, Rogers, AR) and 0.3% Triton-X TBS. To visualize Fos and TH, residual aldehydes were removed and endogenous peroxidase activity was quenched before tissue was blocked and made permeable with 20% goat serum and 0.3% Triton-X TBS. Tissue was then incubated in the cFos primary antibody (c-Fos (4): rabbit, sc-52. 1:10,000, 0.02μg IgG/ml solution, Santa Cruz Biotech, Santa Cruz, CA) for 48 hours at 4°C, cFos secondary antibody (biotinylated goat anti-rabbit IgG (H+L), 1:500, 3μg IgG/ml solution, Vector Laboratories, Burlingame, CA) for one hour, and avidin-biotin complex (Peroxidase-Vectastain ABC Kit PK-6100, Vector) for one hour, consecutively. Then, tissue was reacted with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO) to produce a dark brown reaction product in the nucleus of Fos-immunoreactive (ir) cells. After rinsing, tissue was again blocked and made permeable and then incubated overnight in TH primary antibody (mouse anti-TH monoclonal antibody, 1:2,000, Millipore-Chemicon, Billerica, MA). TH secondary antibody (biotinylated goat anti-mouse IgG (H+L), 1:500, 3μg IgG/ml solution, Vector) and avidin-biotin complex (same as above) were then each applied consecutively for 1 hour, and sections were reacted with SG enzyme substrate (Kit SK-4700, Vector) to produce a cytoplasmic blue reaction product in TH-ir cells. Primary and secondary antibody deletion control studies were run on separate sections; non-specific background staining was low or absent in these sections. Tissue sections were mounted onto glass slides and dehydrated with a series of ethanols before coverslipping.
Microscopic analysis
Acb, mPFC and VTA were subdivided according to the hamster brain atlas (Morin and Woods, 2001). The mPFC included the anterior cingulate (Cg1), prelimbic (PrL), and infralimbic (IL) subregions; the Acb included the core (AcbC) and medial portion of the shell (AcbSh); and the VTA included interfasicular (IF), paranigral (PN), and parabrachial pigmented (PBP) nuclei (Figure 1). The VTA was further subdivided along its rostro-caudal extent by the presence of the interpeduncular nucleus in caudal sections because of previous reports of rostral/caudal functional differences in rats, mice, and hamsters (Olson et al., 2005; Ikemoto, 2007). Upon completion of microscopic inspection and analysis, virtually all main effects and interactions were observed in the caudal portion of each VTA subregion, and therefore only data from caudal sections are presented here.
Figure 1.
Representative subregion contours placed over atlas diagrams (Morin and Wood, 2001).
Anatomically matched tissue sections throughout the extent of the Acb (3 sections), mPFC (3 sections), caudal VTA (cVTA; 2 sections), and posterodorsal medial amygdala (MePd, 1 section, as a control region) were selected at 4x objective and indicated in Figure 1. In all regions, 0.064mm2 rectangular contours were placed bilaterally and consistently according to neuroanatomical landmarks visible in immunohistochemically processed tissue. Because of the large size of Acb and MePd regions, two boxes were placed in each of those subregions and counts from them were added together within a hemisection.
Images were taken within the contour with an UPlanSApo 40x (0.9NA) objective; representative images of the AcbC across groups are shown in Figure 2. In the MePd, Acb, and mPFC, only single-labeled Fos-ir cells were observed, and ImageJ (NIH Bethesda, MD) was used to quantify Fos-ir cells. Color images were converted to grayscale, and the image threshold was adjusted to be three standard deviations above and below the minimum and maximum gray values for each image, which consistently detected Fos-ir cells. Particle size was defined as above 200 and below 2000 pixels2, excluding ones on the edge of the image. All particles above threshold were visually confirmed by an experimenter familiar with Fos-ir staining but blind to group assignment. In the VTA, both Fos- and TH-ir cells were present, which precluded ImageJ analysis. Therefore, cells were counted manually using Neurolucida (version 9; Microbrightfield, Williston, VT). Cells were considered Fos-ir if they had a distinct nucleus with visible puncta stained dark red-brown and TH-ir if the cytoplasm was stained gray-blue, as shown in Figure 3. All analyses were performed on an Olympus BX51 microscope under brightfield illumination, and images captured with an Optronics MicroFire camera.
Figure 2.
Representative images of Fos-ir cells at 40x magnification in accumbens core in testosterone-treated juveniles (left column) and adults (right column) exposed to clean oil (top row) or VS oil (bottom row). A Fos-ir cell is indicated by an arrowhead. Scale bar is 50um.
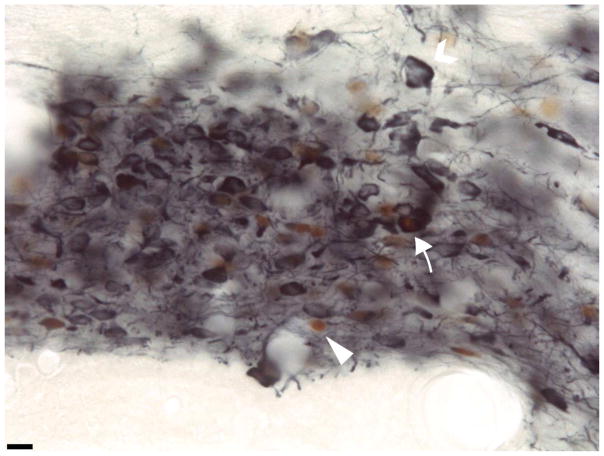
Figure 3.
Representative image of Fos and TH-ir cells from a VS-exposed and testosterone-treated adult at 40x magnification in interfascicular VTA. A Fos-ir cell shows dark red-brown staining in nuclei, and is indicated by an arrowhead. A TH-ir cell shows blue cytoplasm and is indicated by an unfilled arrow. A Fos/TH-ir cell is indicated by a filled arrow. Scale bar is 50um.
The number of single-labeled Fos-ir cells (excluding double-labeled cells in the VTA) within each subregion contour was divided by the area of that contour to create a measure of cell density within each hemisection. In the VTA, single-labeled TH-ir cells and cells double-labeled for both TH and Fos, here called TH/Fos-ir, were also counted and converted to density measures as with the Fos-ir cells. In all subregions, density measures were averaged across hemispheres and sections to create one measurement per subregion per hamster.
Plasma testosterone measures
Plasma testosterone was measured in duplicate 50 μl plasma samples in a single radioimmunoassay using the Coat-A-Count Total Testosterone Kit (Diagnostic Products, Los Angeles, CA). The minimum detectable concentration was 0.1ng/ml and the intra-assay coefficient of variation was 4.7%.
Statistical analysis
To provide an integrated assessment of independent variable effects on Fos expression across all brain regions studied, multilevel modeling (MLM) was used as in (Bell et al., 2013). The model treated hamster as the upper-level sampling unit and brain region as the lower-level sampling unit, with age, hormone status, and stimulus exposure, and brain region as independent variables and Fos-ir cell density as the dependent variable. The error structure was modeled to impose the traditional homoscedasticity assumption used in ANOVA. MLM provides a more powerful analysis than a traditional repeated measures analysis of variance (ANOVA) that uses a within-subject factor, because it integrates non-independence between samples from the same subject in the model. Interactions were followed up by separate MLMs; interactions that involved age were followed up by determining other effects within juveniles and adults separately. Interactions involving brain regions were followed-up by separate analyses for effects of age, hormonal status, or stimulus within a region. However in these analyses, there is just one sample per subject, in which case ANOVA provides the exact same modeling and statistical outcome as MLM. ANOVA was therefore used for analyses and follow up tests of interactions within region. Three way ANOVAs were also used to examine effects of age, hormone, and stimulus on TH and Fos/TH-ir in the VTA. P < 0.05 was considered significant, and all statistical analyses were done with SPSS software (PASW Statistics 19; SPSS: An IBM Company, Chicago, IL).
RESULTS
Fos-ir analysis
Multilevel modeling revealed multiple main effects and two-way interactions (Table 1). Notably, neither main effects of hormone status nor any interactions with hormone status were observed. Of the three two-way interactions observed, only the age x stimulus interaction was independent of brain region and could be followed-up without analyzing brain regions separately. Therefore, the age x stimulus interaction was probed to assess how the Fos response to VS was affected by age, taking all brain regions of interest into account. This analysis revealed an effect of stimulus in adults (F(1,30) = 18.61, p < 0.01) but not juveniles (F(1,28) = 1.55, NS), such that adults, but not juveniles, expressed more Fos-ir cells in response to VS than to clean oil (Figure 4). A three-way interaction of age x stimulus x region was also observed and followed-up with separate two-way ANOVAs (age x stimulus) for each region. For every region other than MePD, all main effects were qualified by an age x stimulus interaction (Table 1); the appropriate interaction follow-up is described for each region below.
Table 1.
Complete list of age, hormone, stimulus and region effects with interactions on Fos-ir. Salient interactions are followed up and described in text.
| Region
|
Effect
|
F value
|
df
|
p value
|
|---|---|---|---|---|
| all 9 | age | 63.66 | 1, 54 | < 0.01 |
| hormone | 0.55 | 1, 54 | ns | |
| stimulus | 4.32 | 1, 54 | ns | |
| region | 360.89 | 7, 378 | < 0.01 | |
| age x hormone | 0.30 | 1, 54 | ns | |
| age x stimulus | 14.83 | 1, 54 | < 0.01 | |
| age x region | 49.66 | 7, 378 | < 0.01 | |
| hormone x stimulus | 1.87 | 1, 54 | ns | |
| hormone x region | 0.48 | 7, 378 | ns | |
| stimulus x region | 6.14 | 7, 378 | < 0.01 | |
| age x hormone x stimulus | 0.03 | 1, 54 | ns | |
| age x hormone x region | 0.34 | 7, 378 | ns | |
| age x stimulus x region | 6.41 | 7, 378 | < 0.01 | |
| hormone x stimulus x region | 1.74 | 7, 378 | ns | |
| age x hormone x stimulus x region | 0.11 | 7, 378 | ns | |
|
| ||||
| MePD | age | 5.80 | 1,49 | 0.02 |
| stimulus | 166.19 | 1,49 | <0.01 | |
| age x stimulus | 0.40 | 1,49 | ns | |
|
| ||||
| AcbC | age | 1.24 | 1, 54 | ns |
| stimulus | 0.01 | 1, 54 | ns | |
| age x stimulus | 11.56 | 1, 54 | <0.01 | |
|
| ||||
| AcbSh | age | 0.37 | 1, 54 | ns |
| stimulus | 0.15 | 1, 54 | ns | |
| age x stimulus | 4.54 | 1, 54 | 0.04 | |
|
| ||||
| Cg1 | age | 1.81 | 1, 54 | ns |
| stimulus | 4.28 | 1, 54 | 0.04 | |
| age x stimulus | 6.78 | 1, 54 | 0.01 | |
|
| ||||
| PrL | age | 0.17 | 1, 54 | ns |
| stimulus | 0.76 | 1, 54 | ns | |
| age x stimulus | 16.62 | 1, 54 | <0.01 | |
|
| ||||
| IL | age | 0.82 | 1, 54 | ns |
| stimulus | 0.85 | 1, 54 | ns | |
| age x stimulus | 9.13 | 1, 54 | <0.01 | |
|
| ||||
| IF | age | 56.70 | 1, 54 | <0.01 |
| stimulus | 6.62 | 1, 54 | 0.01 | |
| age x stimulus | 7.99 | 1, 54 | <0.01 | |
|
| ||||
| PN | age | 26.60 | 1, 54 | <0.01 |
| stimulus | 0.04 | 1, 54 | ns | |
| age x stimulus | 4.62 | 1, 54 | 0.04 | |
|
| ||||
| PBP | age | 1.62 | 1, 54 | ns |
| stimulus | 0.01 | 1, 54 | ns | |
| age x stimulus | 1.11 | 1, 54 | ns | |
Figure 4.

Fos-ir cell density using data from all brain regions analyzed as sampling units as in MLM, mean +/− SE, * indicates p < 0.01. An age x stimulus interaction was found, such that only in adults was there a significant increase in the number of Fos-ir cells in response to VS.
In the IL and IF (Figure 5, left column), the age x stimulus follow-ups revealed an effect of stimulus in adults (F(1,28) = 5.88, p = 0.02 and F(1,30) = 15.51, p < 0.01, respectively), such that adults expressed more Fos-ir cells when in response to VS compared to clean oil. The effect of stimulus in juveniles was not significant in IF, and fell just short of statistical significance in IL (F(1,28) = 0.03, NS and F(1,28) = 3.963, p = 0.056, respectively).
Figure 5.
Fos-ir cell density in all brain regions analyzed, in blank- or testosterone (T)- treated juveniles and adults exposed to clean or VS-oil, mean +/− SE. In the MePD, main effects of age and stimulus were detected. In all other regions but PBP, interactions between age and stimulus were detected. To probe these interactions, effects of stimulus on hamsters within age (* p < 0.05), or effects of age within stimulus (+ p < 0.05) were assessed. Interactions occurred either when the effect of stimulus was opposite for the two age groups (AcbC, PrL), when an effect of stimulus was present only in one age (IL, IF, Cg), or when an effect of age was present in only one stimulus group (AcbSh). In the PN, an age x stimulus interaction was detected, but no significant effects of stimulus were found upon follow-up within each age. An interaction was likely detected because the effects of VS were in opposite directions in juveniles and adults.
In the AcbC and PrL (Figure 5, middle column), the age by stimulus follow-ups revealed an effect of stimulus in both adults (F(1,28) = 5.57, p = 0.03 and F(1,28) = 11.62, p = 0.02, respectively), and juveniles (F(1,30) = 6.75, p = 0.01 and F(1,30) = 5.01, p = 0.03, respectively). However, the effects of VS were opposite at the two ages: juveniles expressed fewer Fos-ir cells in response to VS compared to clean oil, but adults expressed more Fos-ir cells in response to VS compared to clean oil. In the PN (Figure 5, middle column), no significant effects of stimulus were detected in either age group, however the results follow the same general trend as in AcbC and PrL, with juveniles expressing fewer, and adults more, Fos-ir cells in response to VS.
In the AcbSh (Figure 5, right column), the age x stimulus follow-up revealed no effect of stimulus in either age group. Therefore, the effect of age (juvenile or adult) was determined in clean oil- and VS- exposed hamsters separately. In clean oil-exposed hamsters, an effect of age was observed, F(1,28) = 4.55, p = 0.04, such that juveniles expressed more Fos-ir cells than did adults. In VS-exposed hamsters, no effect of age was detected.
In the Cg1 (Figure 5, right column), the age x stimulus follow-up revealed an effect of stimulus in juveniles, F(1,28) = 9.90, p < 0.01, such that they expressed fewer Fos-ir cells in response to VS compared to clean oil. No effect of stimulus was detected in adult hamsters.
In the MePD (Figure 5, left column) and PBP (Figure 5, right column), no age x stimulus interactions was observed. A main effect of stimulus was observed in the MePD, such that hamsters expressed more Fos-ir cells in response to VS than to clean oil, regardless of age. A main effect of age was also observed in the MePD, such that juveniles expressed more Fos-ir cells than adult hamsters.
TH-ir and Fos/TH-ir analysis
TH-ir
Main effects of age were observed on single-labeled TH-ir cells in the IF and PN, such that juveniles expressed more TH-ir cells than did adults, F(1,54) = 28.08, p < 0.01 and F(1,54) = 8.23, p < 0.01 respectively (Figure 6, top row). A main effect of hormone was also observed on TH-ir cells in the IF, such that blank capsule treated hamsters expressed more TH-ir cells than did T-treated hamsters, F(1,54) = 4.27, p = 0.04. No effects of stimulus or other interactions were observed in any of the three VTA subregions.
Figure 6.
TH- and Fos/TH-ir cell density in the VTA, in blank- or testosterone- treated juveniles and adults exposed to clean or VS-oil. Mean +/− SE, p < 0.05. In the IF and PN, main effects of age were detected (+) on TH-ir cells such that juveniles expressed more than did adults. In the IF, a main effect of hormone was also detected on TH-ir cells, such that blank-treated hamsters expressed more than did testosterone-treated. In all three regions, main effects of age were detected (+) on Fos/TH-ir cells, such that adults expressed more than did juveniles. Finally, in the PN, an age x stimulus interaction was detected on Fos/TH-ir cells, such that an effect of age was observed in VS exposed hamsters but not clean oil exposed hamsters.
Fos/TH-ir
Main effects of age were observed on double-labeled Fos/TH-ir cells in the IF, PN, and PBP such that more Fos/TH-ir cells were present in adults than juveniles, F(1,54) = 45.96, p < 0.01; F(1,54) = 6.31, p = 0.02; and F(1,54) = 10.68, p < 0.01 respectively (Figure 6, bottom row). An age x stimulus interaction was also observed on Fos/TH-ir cells in the PN, F(1,54) = 7.72, p < 0.01. Follow-up revealed an effect of age in VS-exposed hamsters, F(1,30) = 16.28, p < 0.01, but not in clean oil-exposed hamsters; when exposed to VS, adults expressed more Fos/TH-ir cells than did juveniles.
Peripheral tissue and testosterone measures
Physiological measures are shown in Table 2, and confirm efficacy of testosterone capsules in raising circulating testosterone and increasing flank glands in both ages. Gonadectomy resulted in a decrease in seminal vesicle weight only in adults. Groups of the same age did not differ in body weight.
Table 2.
Physiological measures of hamsters at time of sacrifice. Mean and standard deviations (SD) of body weight, flank gland length, seminal vesicle weight, and plasma testosterone (T). Adults are heavier, have longer flank glands, heavier seminal vesicles, and less circulating testosterone. Testosterone treated hamsters have longer flank glands, heaver seminal vesicles, and more circulating testosterone.
| Age | Capsule | Stimulus | N | Body Weight (g)
|
Flank gland length (mm)
|
Seminal Vesicle Weight (g)
|
Plasma T (ng/ml)
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
|
|
|
|
|
|
|
|
|
|
|
||
| Juv. | Empty | Blank | 7 | 51.03 | 4.04 | 3.39 | 0.82 | < 0.01 | - | <0.10 | - |
| Juv. | Empty | VS | 8 | 50.60 | 4.63 | 3.30 | 2.03 | < 0.01 | - | <0.10 | - |
| Juv. | T | Blank | 7 | 50.27 | 3.03 | 4.99 | 1.27 | 0.03 | 0.05 | 7.76 | 0.87 |
| Juv. | T | VS | 8 | 50.56 | 5.41 | 4.64 | 0.71 | 0.01 | 0.04 | 7.99 | 1.19 |
| Adult | Empty | Blank | 8 | 99.28 | 13.87 | 6.44 | 2.21 | 0.03 | 0.05 | <0.10 | - |
| Adult | Empty | VS | 8 | 96.95 | 10.57 | 7.04 | 1.20 | 0.08 | 0.09 | <0.10 | - |
| Adult | T | Blank | 8 | 93.05 | 8.63 | 8.41 | 1.85 | 0.25 | 0.27 | 6.19 | 1.34 |
| Adult | T | VS | 8 | 89.34 | 18.41 | 7.28 | 0.86 | 0.21 | 0.20 | 6.19 | 1.19 |
DISCUSSION
This study demonstrates that mesocorticolimbic responses to VS are immature in juvenile hamsters, even when circulating testosterone is experimentally elevated to adult-like levels. Specifically, we observed 1) greater VS-induced Fos expression in adult, but not juvenile, hamsters in the infralimbic mPFC and interfascicular VTA, and 2) opposite Fos responses to VS in adult (greater expression) and juvenile (reduced expression) hamsters in Acb core, prelimbic mPFC, and paranigral VTA (Figure 5). In addition, density of Fos/TH-ir cells in the paranigral VTA differed between VS-exposed adult and VS-exposed juvenile hamsters, demonstrating that dopaminergic cell responses to VS also mature with age (Figure 6).
The mesocorticolimbic circuit includes complex and reciprocal dopaminergic and non-dopaminergic projections between the VTA, the Acb, mPFC, and MeP (Swanson, 1982; Oades and Halliday, 1987; Thompson and Swanson, 2010). In rodents, the MeP is responsible for initial evaluation of a chemosensory social stimulus and integration with hormonal information (Wood and Newman, 1995), and it then relays this information to the VTA, mPFC, and Acb (Phillipson, 1979; Kevetter and Winans, 1981; Coolen and Wood, 1998; Geisler and Zahm, 2005). Results from the present experiment support previous reports that VS elicits comparable responses in juvenile and adult male hamsters in MeP, and that over the course of adolescence and independent of social experience, VS exposure comes to engage non-dopamingeric cells in the IF and PN nuclei in the VTA, and downstream targets in the IL and PrL of the mPFC, and core of the Acb. Here we extend these findings to show that adult-typical neural responses to VS cannot be activated in juvenile male hamsters by testosterone, and therefore propose that other adolescent developmental processes, besides gonadal maturation, are required for adult-typical mesocorticolimbic responses to VS.
A one-week period of testosterone treatment, similar to that used here, is sufficient to cause juvenile males to be attracted to and show a CPP for VS (Johnston and Coplin, 1979; Bell and Sisk, 2013), but not to induce adult-typical sexual behavior (Meek et al., 1997; Schulz and Sisk, 2006). That is, the developmental changes in VS-induced Fos expression parallel the developmental changes in display of sexual behavior (i.e., neither can be activated by testosterone prior to puberty), and not the development of VS reward. Therefore, elevated testosterone is necessary and sufficient to render VS a rewarding social stimulus, but VS reward, although perhaps necessary, is not sufficient to motivate performance of sexual behavior. Given the involvement of the mesocorticolimbic circuit in linking motivation with motor output (Sesack and Grace, 2010), we propose that the differential mesocorticolimbic Fos responses to VS in adult and juvenile male hamsters reflect differences in their drive to perform motor components sexual behavior. Nineteen days of testosterone treatment is not sufficient to induce sexual behavior in P28 animals (Schulz et al 2009), suggesting that even a longer exposure to testosterone in juvenile animals in the current experiment would still be insufficient to induce adult-like Fos responses to VS.
Neural mechanisms of adolescent shift in VS response
Adolescent development of mesocorticolimbic neural circuitry could explain changes in the pattern of Fos responses to VS across adolescence. Although adult hamsters generally showed elevated corticolimbic Fos in response to VS compared to clean oil, juvenile hamsters unexpectedly showed reduced Fos-ir in AcbC, Prl and PN in response to VS exposure compared to clean oil (Figure 4). A reduction in Fos in response to a sensory stimulus is uncommon (or at least not often reported), but could be the result of reorganization of corticolimbic synapses during adolescent development (cite review). However, given the known dopaminergic involvement in responses to VS (Schulz et al 2004, Bell and Sisk 2013) and enhanced transcriptional activity in putative dopaminergic cells in adults compared to juveniles in the current study, we can hypothesize about a dopaminergic mechanism behind the developmental shift in Fos responses to VS.
A shift in the balance of D1/D2 receptor activation in the striatum and cortex shifts during adolescence may explain a reduction in Fos expression in response to VS in juvenile hamsters. While receptor binding studies have yielded somewhat inconsistent results, most find an increase in D2 receptor expression that either peaks at mid-adolescence or continues into adulthood and that the D2:D1 binding ratios are higher in juveniles than adolescents (Andersen et al., 1997; 2000; Tarazi and Baldessarini, 2000). Additionally, juveniles have less dopamine and glutamate innervation of the accumbens and cortex than adults (Giorgi et al., 1987; Kalsbeek et al., 1988; Cunningham et al., 2002; Brenhouse et al., 2008; Mathews et al., 2009). Both these factors would favor enhanced D2 receptor activation in juveniles compared to adults. This D2 biased activation may result in differential Fos responses in juveniles compared to adults: D2 specific agonists have been observed to reduce Fos expression (Wirtshafter and Asin, 1994; Keefe and Gerfen, 1995) and D2 antagonists often induce an increase in Fos expression (Bertran-Gonzalez et al., 2008). Therefore, the reduction in Fos expression in response to VS may result from a higher ratio of D2 receptor activation in juveniles and a more balanced D1/D2 receptor activation in adult hamsters.
Age and hormonal status affect constitutive Fos and TH expression
The number of Fos/TH-ir cells was greater in adults than juveniles, independent of VS exposure, which may be indicative of heightened vigilance or sensitivity to a range of stimuli in adulthood (mineral oil, in this study). The same effect of age on constitutive Fos expression in dopaminergic cells was observed in gonad intact hamsters (Bell et al., 2013); therefore, the present study supports the idea that adolescent maturation and not circulating testosterone is required for greater activity in TH-ir VTA cells. This age-dependent dopaminergic cell activity correlates with a gain in downstream activation in response to VS and the performance of sexual behavior.
Age also affected the number of single-labeled VTA TH-ir cells, such that there were more in juvenile than adult hamsters. These results agree with previous findings in gonad-intact animals (Bell et al., 2013). This differential labeling could indicate either reduced TH expression within existing cells or reduced TH-expressing cell number in adult hamsters. One mechanism of differential TH expression could be increased dopamine release in adulthood, which then feeds back to reduce TH-expression (Stork et al., 1994), or adolescent changes in ion channel activity known to regulate TH-expression (Aumann et al., 2011).
An effect of hormone was observed on TH-ir cells in IF, in that testosterone-exposed hamsters expressed fewer TH-ir cells than blank-treated hamsters, independent of age. Some evidence suggests testosterone may suppress TH expression, as 30 days of gonadectomy causes an increase in the number of TH-ir cells in adult male rats (Johnson et al., 2010). The castration-induced increase in TH-ir cells was not seen after 14 days of gonadectomy (McArthur et al., 2007), a time-span that more closely matches 7 days of treatment in this study. However, the temporal pace of responses to hormone removal is often slower than that of responses to hormone replacement (Putnam et al., 2003).
Gonadectomy does not affect Fos responses to VS in adults
VS-induced Fos-ir in MePd, NA, mPFC, or VTA in adult hamsters was not reduced by one week of gonadal hormone absence. The lack of an effect in the MePd is not surprising in light of a previous study in which VS-induced Fos-ir in MeP was still observed 12 weeks after gonadectomy (Fiber and Swann, 1996). However, the Fos response to VS in a medial preoptic nucleus was reduced in gonadectomized vs. testosterone treated males, demonstrating that responses to VS are hormone sensitive in at least some brain regions (Fiber and Swann, 1996). Testosterone also modulates dopamine-dependent PFC function in male rats, but in a temporally specific pattern: TH innervation and extracellular dopamine is decreased 4 days after gonadectomy, but is increased 28 days after gonadectomy (Aubele and Kritzer, 2011). Short-term (10–14 days) castration does not affect tissue concentrations of dopamine and DOPAC in the ventral striatum (Engel et al., 1979; Mitchell and Stewart, 1989), however long-term (28–56 days) castration generally reduces dopamine and DOPAC concentrations in NA tissue (Alderson and Baum, 1981; Mitchell and Stewart, 1989) but see (Baum et al., 1986). Taken together, it is possible that 1) mesocorticolimbic responses to VS are not modulated by circulating testosterone, as is the case in the MePd, or 2) mesocorticolimbic responses to VS may not occur when testicular hormones are absent for a longer period of time than examined here.
Method of delivery affects neural responses to VS
In a previous study, increased Fos expression in response to VS was observed in AcbC, IL mPFC, non-dopamingeric cells in the IF VTA, PN VTA and PBP VTA in adult hamsters (Bell et al., 2013). A similar pattern was observed in the current study, with a few exceptions: an adult response in the PrL was a new observation, and adult Fos responses in the PN and PBP were not present. In addition, Fos/TH-ir responses to VS in the VTA were observed in a previous but not the present study. These different results may be due to different methods of VS delivery. In the current study, in order to more completely control for equivalent exposure of volatile and non-volatile components of VS across the groups, VS or oil was applied directly to hamsters’ nares, instead of presented on a cotton swab dropped into their home cage as in the previous study. More robust VTA responses may be observed after active VS swab investigation, compared to involuntary exposure, as in oil application in the current study. In fact, a similar phenomenon is seen when in response to electrical stimulation of the medial forebrain bundle in male rats. Animals voluntarily seeking self-stimulation showed greater Fos-ir in the VTA than yoked animals; no differences were seen between active and yoked rats in the mPFC and Acb (Hunt and McGregor, 1997). Voluntary exposure may also promote burst firing of dopaminergic VTA cells. Thus, voluntary approach to VS swabs may activate psychological or motor responses not seen in the current study. Therefore, these differential results present an interesting line of questions for future work.
The present study demonstrates that testosterone does not activate adult-typical Fos responses to a rewarding social cue in juvenile male hamsters, indicating that adolescent developmental programming underlies activation of mesocorticolimbic brain regions by female chemosensory cues in adults. These Fos responses may be a neural correlate for motivation or potential to perform sexual behavior, which is also not activated by testosterone prior to adolescent development. Normal expression of sexual behaviors is dependent on exposure to testosterone during puberty, as it organizes the brain for adult-typical expression of behavior. Therefore, mesocorticolimbic structures may be a target for these organizational effects of testosterone, and ongoing studies will investigate responses of the Acb to sexual experience in hamsters deprived of pubertal testosterone.
Acknowledgments
This work was supported by National Institutes of Health grants R01-MH068764 (C.L.S.), T32-MH070343 (M.R.B and S.H.M), T32-NS44928 (M.R.B.), and Michigan State University Provost Fellowship (S.H.M). Many thanks to Dana Gradl for capturing the Acb and PFC images; Jane Venier for performing the RIA; Rayson Figueira, Dr. Heather Molenda-Figueira, and Joshua Olsewisz for their help with tissue collection; Dr. Deborah Kashy for her statistical expertise, and Maggie Mohr and Kayla De Lorme, for their helpful comments on drafts of this manuscript.
Footnotes
Authors do not have any conflicts of interest to declare.
References
- Alderson LM, Baum MJ. Differential effects of gonadal steroids on dopamine metabolism in mesolimbic and nigro-striatal pathways of male rat brain. Brain Res. 1981;218:189–206. doi: 10.1016/0006-8993(81)91300-7. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Gonadectomy and hormone replacement affects in vivo basal extracellular dopamine levels in the prefrontal cortex but not motor cortex of adult male rats. Cereb Cortex. 2011;21:222–232. doi: 10.1093/cercor/bhq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level. Cereb Cortex. 2012;22:1799–1812. doi: 10.1093/cercor/bhr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumann TD, Egan K, Lim J, Boon WC, Bye CR, Chua HK, Baban N, Parish CL, Bobrovskaya L, Dickson P, Horne MK. Neuronal activity regulates expression of tyrosine hydroxylase in adult mouse substantia nigra pars compacta neurons. J Neurochem. 2011;116:646–658. doi: 10.1111/j.1471-4159.2010.07151.x. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Melamed E, Globus M. Dissociation of the effects of castration and testosterone replacement on sexual behavior and neural metabolism of dopamine in the male rat. Brain Res Bull. 1986;16:145–148. doi: 10.1016/0361-9230(86)90025-0. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, De Lorme KC, Figueira RJ, Kashy DA, Sisk CL. Adolescent gain in positive valence of a socially relevant stimulus: engagement of the mesocorticolimbic reward circuitry. Eur J Neurosci. 2013;37:457–468. doi: 10.1111/ejn.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Meerts SH, Sisk CL. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Horm Behav. 2010;58:410–414. doi: 10.1016/j.yhbeh.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Sisk CL. Dopamine mediates testosterone-induced social reward in male Syrian hamsters. Endocrinology. 2013;154:1225–1234. doi: 10.1210/en.2012-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault J-A. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Creese I, Sibley DR, Leff S. Classification of dopamine receptors. Adv Biochem Psychopharmacol. 1983;37:255–266. [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Ahlenius S, Almgren O, Carlsson A, Larsson K, Södersten P. Effects of gonadectomy and hormone replacement on brain monoamine synthesis in male rats. Pharmacol Biochem Behav. 1979;10:149–154. doi: 10.1016/0091-3057(79)90181-3. [DOI] [PubMed] [Google Scholar]
- Fiber JM, Adames P, Swann JM. Pheromones induce c-fos in limbic areas regulating male hamster mating behavior. Neuroreport. 1993;4:871–874. doi: 10.1097/00001756-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Fiber JM, Swann JM. Testosterone differentially influences sex-specific pheromone-stimulated Fos expression in limbic regions of Syrian hamsters. Horm Behav. 1996;30:455–473. doi: 10.1006/hbeh.1996.0050. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, Mele S, Calderini G, Toffano G, Biggio G. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Brain Res. 1987;432:283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GE, McGregor IS. Rewarding brain stimulation induces only sparse Fos-like immunoreactivity in dopaminergic neurons. Neuroscience. 1997;83:501–515. doi: 10.1016/s0306-4522(97)00409-0. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Day AE, Ho CC, Walker QD, Francis R, Kuhn CM. Androgen decreases dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22:238–247. doi: 10.1111/j.1365-2826.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE, Coplin B. Development of responses to vaginal secretion and other substances in golden hamsters. Behav Neural Biol. 1979;25:473–489. doi: 10.1016/s0163-1047(79)90242-5. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Gerfen CR. D1–D2 dopamine receptor synergy in striatum: effects of intrastriatal infusions of dopamine agonists and antagonists on immediate early gene expression. Neuroscience. 1995;66:903–913. doi: 10.1016/0306-4522(95)00024-d. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32:481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–55. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Luo Z, Volkow ND, Heintz N, Pan Y, Du C. Acute cocaine induces fast activation of D1 receptor and progressive deactivation of D2 receptor striatal neurons: in vivo optical microprobe [Ca2+]i imaging. J Neurosci. 2011;31:13180–13190. doi: 10.1523/JNEUROSCI.2369-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews IZ, Waters P, McCormick CM. Changes in hyporesponsiveness to acute amphetamine and age differences in tyrosine hydroxylase immunoreactivity in the brain over adolescence in male and female rats. Dev Psychobiol. 2009;51:417–428. doi: 10.1002/dev.20381. [DOI] [PubMed] [Google Scholar]
- McArthur S, Murray HE, Dhankot A, Dexter DT, Gillies GE. Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem. 2007;100:678–692. doi: 10.1111/j.1471-4159.2006.04226.x. [DOI] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm Behav. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA, Rowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur J Pharmacol. 1996;309:21–24. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- Miller LL, Whitsett JM, Vandenberg JG, Colby DR. Physical and Behavioral Aspects of Sexual Maturation in Male Golden Hamsters. J Comp Phy Psych. 1977;91:245–259. doi: 10.1037/h0077315. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Stewart J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Res. 1989;491:116–127. doi: 10.1016/0006-8993(89)90093-0. [DOI] [PubMed] [Google Scholar]
- Morin LP, Woods R. A Stereotaxic Atlas of The Golden Hamster Brain. London, UK: Academic Press; 2001. [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Du J, Sato S, Hull EM. Testosterone restoration of copulatory behavior correlates with medial preoptic dopamine release in castrated male rats. Horm Behav. 2001;39:216–224. doi: 10.1006/hbeh.2001.1648. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Sato S, Hull EM. Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Horm Behav. 2003;44:419–426. doi: 10.1016/j.yhbeh.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Parfitt DB, Richardson HN, Sisk CL. Pheromones elicit equivalent levels of Fos-immunoreactivity in prepubertal and adult male Syrian hamsters. Horm Behav. 1998;34:48–55. doi: 10.1006/hbeh.1998.1463. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Current directions in psychological science 2000 [Google Scholar]
- Stork O, Hashimoto T, Obata K. Haloperidol activates tyrosine hydroxylase gene-expression in the rat substantia nigra, pars reticulata. Brain Res. 1994;633:213–222. doi: 10.1016/0006-8993(94)91542-3. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci USA. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG. Effects of gonadal hormones on the flank gland of the golden hamster. Horm Res. 1973;4:28–33. doi: 10.1159/000178287. [DOI] [PubMed] [Google Scholar]
- Vincent SR. Distributions of tyrosine hydroxylase-, dopamine-beta-hydroxylase-, and phenylethanolamine-N-methyltransferase-immunoreactive neurons in the brain of the hamster (Mesocricetus auratus) J Comp Neurol. 1988;268:584–599. doi: 10.1002/cne.902680408. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Interactive effects of stimulation of D1 and D2 dopamine receptors on fos-like immunoreactivity in the normosensitive rat striatum. Brain Res Bull. 1994;35:85–91. doi: 10.1016/0361-9230(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32:40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. J Neurosci. 1995;15:7261–7269. doi: 10.1523/JNEUROSCI.15-11-07261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]