Abstract
Objective
Hematopoietic stem cell transplantation (HCT) is a stressful and rigorous medical procedure involving significant emotional and immune challenges. The endocannabinoid (eCB) signaling system is involved in regulation of both the immune system and emotional reactivity, yet little is known about its function during HCT. We investigated the role of the eCB signaling system in a group of HCT recipients.
Methods
A total of 19 HCT recipients were enrolled and provided psychosocial data and blood samples at three peri-transplant time points: prior to transplant, hospital discharge, and approximately 100 days post-transplant. Psychosocial factors, inflammatory molecules, and the eCBs were determined and assessed for changes over this period and association with each other.
Results
HCT recipients demonstrated significant changes over the peri-transplant period in inflammatory molecules and psychosocial functioning, but not in circulating concentrations of the eCBs. Associations among these variables were most likely to be present pre-transplant and least likely to be present immediately post-transplant, with depressive symptoms and inflammation most significantly associated. The eCB 2-arachidonoylglycerol (2-AG) was significantly, positively associated with both interleukin (IL)-6 and C-reactive protein (CRP) and negatively associated with depressive symptoms.
Conclusions
The eCB signaling system may have alternative sources and regulatory mechanisms in addition to the immune system. Given the significant associations with inflammatory molecules and depressive symptoms in the peri-transplant period, it is important to better understand this system and its potential implications in the setting of complex and stressful medical procedures such as HCT.
Keywords: Depressive symptoms, Psychosocial factors, Endocannabinoids, 2-AG, Pro-inflammatory molecules
1. Introduction
Hematopoietic stem cell transplantation (HCT) is an intensive medical procedure used to treat a variety of hematologic malignancies and disorders whereby stem cells are infused into the recipient to replace a damaged immune system. This infusion follows complete or near-complete ablation of the recipient's immune system with chemotherapy and/or radiation. HCT involves significant emotional and immune challenges, with the highest level of psychosocial stress usually occurring during the early post-transplant phase (first 30 days) (McQuellon et al., 1998). This period of time is also marked by significant immune suppression, high risk of infection, and other immunologic complications. The endocannabinoid (eCB) system is uniquely positioned to play a significant role in the peri-HCT period as it is a modulator of emotional reactivity and immune and inflammatory responses (Hillard et al., 2012). There are no reports of studies of the eCB system in the transplant setting.
Multiple psychosocial factors can affect outcomes after HCT (Hoodin et al., 2006); in particular, pre-transplant depression and anxiety are associated with worse post-transplant survival (Andrykowski et al., 1994, Loberiza et al., 2002). Previous studies demonstrate that pre-transplant depression and anxiety are also associated with altered immune function during the post-transplant period (Gregurek et al., 1996, Knight et al., 2014, McGregor et al., 2012, Pereira et al., 2010, Pulgar et al., 2012), with pro-inflammatory factors increasing in the immediate post-transplant period before gradually returning to baseline (Wang et al., 2008, Wang et al., 2014). Further, pro-inflammatory pathways can mediate the relationship between psychosocial stress and poor outcomes (Knight et al., 2013, Wang et al., 2008, Wang et al., 2014). These data suggest that the effects of pre-transplant psychosocial pathology negatively influence outcomes as a result of promoting a pro-inflammatory milieu.
The two most well-studied eCBs are 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamine or anandamide (AEA); both of these lipids are present in the circulation (Hillard et al., 2012). The immune system - specifically macrophages and platelets - is a significant source of eCB synthesis and release in response to endotoxins and cytokines (Varga et al., 1998). In support of these data, previous studies demonstrate that circulating concentrations of 2-AG are increased during inflammation (Bluher et al., 2006, Cote et al., 2007, Di Marzo et al., 2009, Weis et al., 2010). Other known sources include adipose tissue (Matias et al., 2008, Spoto et al., 2006) and reproductive organs (El-Talatini et al., 2010). Since the eCBs are lipophilic, it is likely that their concentrations in the circulation are also affected by overflow from tissues with high eCB contents, including the brain (Caille et al., 2007).
Previous studies of circulating eCB concentrations have demonstrated significant correlations with both depression and inflammation. Concentrations of 2-AG are significantly lower in individuals diagnosed with major depression (Hill et al., 2009, Hill et al., 2008). Other studies demonstrate that circulating concentrations of 2-AG are increased during pro-inflammatory states (Bluher et al., 2006, Cote et al., 2007, Di Marzo et al., 2009) and are positively correlated with concentrations of interleukin 6 (IL-6) (Weis et al., 2010). These data led us to hypothesize that the profound suppression of the immune system that occurs during the process of HCT results in loss of eCBs in the circulation. We further hypothesized that depression in this population would be associated with lower circulating 2-AG concentrations as has been seen in other populations.
In this pilot study, we were able to test these specific hypotheses: 1) concentrations of the eCBs are altered over time in HCT recipients; and 2) circulating concentrations of the eCBs are positively correlated with pro-inflammatory molecules and negatively correlated with depression in the peri-transplant period. We further explored the association between the eCBs and anxiety as well as changes in inflammatory markers, depression, and anxiety over time.
2. Methods
2.1. Patients
A total of 19 men and women who underwent HCT at the University of Rochester Medical Center (URMC) between May 2010 and May 2011 for any reason participated in this study. To be eligible for the study, participants had to be at least 18 years of age, English speaking, and able to complete self-report inventories. Exclusion criteria included conditions preventing meaningful participation in the research interview, for example, major, uncorrected sensory impairments, severe communication limitations such as aphasia, active psychosis, or acute substance intoxication at the time of the initial interview as assessed by the study PI (JMK). Participants were recruited through the study PI after being identified by their transplant physician at their pre-transplant visit in the URMC outpatient HCT clinic. Written informed consent was obtained prior to study participation.
2.2. Procedures
First, participants completed a packet of demographic information in addition to the clinical surveys. At the same time, a 30 ml blood sample was drawn by venipuncture of the antecubital vein or from central venous access, if available, by a trained nurse or phlebotomist (time point 1, T1). T1 ranged from 1 to 18 days prior to transplant (median = 7.5), and always preceded pre-conditioning regimens. Surveys were administered and blood collected at two additional time points: at the time of hospital discharge following initial transplant admission (T2) and as close to day 100 following transplant as possible (T3). T2 collection time ranged from day 9 to day 17 post-transplant (median = 12.5) and T3 ranged from day 91 to day 175 post-transplant (median = 104). Blood specimens were obtained between 0900 h and 1400 h and were centrifuged for 10 min at 1000 × g. The plasma was then aspirated, divided into aliquots, and stored at −80 °C. Plasma analyses were performed at the Medical College of Wisconsin (MCW). Both the URMC and MCW Institutional Review Boards approved these procedures.
2.3. Plasma endocannabinoid extraction and measurement
Concentrations of AEA and 2-AG were determined simultaneously in the same plasma sample. Plasma samples (0.5 ml each) were thawed and made up to 15% ethanol, to which the internal standards [2H8]-AEA (16.9 pmol) and [2H8]-2-AG (46.5 pmol) (Cayman Chemicals, Ann Arbor, MI) were added. Samples were vortexed and centrifuged at 1000 × g for 4 min. The supernatant was loaded onto Bond Elut C18 solid-phase extraction columns (1 ml; Varian Inc, Lake Forest, CA) which had been conditioned with 1 ml redistilled ethanol and 3 ml of double distilled water (ddH2O). The remaining pellet was washed with 100 μl of 15% ethanol and centrifuged again for 3 min. The resulting supernatant was also loaded onto the C18 column. Columns were washed with 5 ml ddH2O and eluted with 1 ml of ethyl acetate. The ethyl acetate layer in the resulting eluate was removed and dried under N2. Lipids in the residual ddH2O phase were extracted by mixing with an additional 1 ml of ethyl acetate, which was added to the original ethyl acetate solution. Once dried, samples were resuspended in 20 μl of methanol and stored at −80 °C. AEA and 2-AG were quantified using liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry (LC-APCI-MS); selective ion monitoring was used to quantify the biogenic lipids as described previously (Hill et al., 2013).
2.4. Quantitative determination of cytokines in human plasma
Cytokines were quantified in human plasma using Fluorokine MAP multiplex kits (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer's protocol. The Human High Sensitivity Cytokine Base Kit was used with corresponding Fluorokine MAP analyte-specific bead sets to detect interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-12p70, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF) protein concentrations in plasma samples diluted 2-fold. C-reactive protein (CRP) was detected using the Human Cardiac Base Kit B with Fluorokine MAP analyte-specific bead sets for CRP in plasma samples diluted 1000-fold. Detection and quantitative analysis of each analyte was performed on the Luminex 200 system with integrated software (v2.3) capable of generating a five parameter logistic curve-fit. Median Fluorescent Intensity (MFI) was collected by assigning the bead region for each analyte being measured at the following instrument settings: Sample Timeout of 100 s, Minimum beads = 50, Sample size of 50ul, and Doublet Discriminator gates set at 7500 and 15,500.
2.5. Psychosocial factors
The Demographic Questionnaire is a 12-item self-report questionnaire that contains items concerning demographics, socioeconomic status, and living circumstances.
Center for Epidemiologic Studies Depression Scale – Revised (CES-D-R) (Gallo and Rabins, 1999): A 20-item self-report questionnaire mapping items that form the diagnostic criteria for major depression; scores range from zero to 60, while a threshold score of 16 may be a sign of significant depression.
State-Trait Anxiety Inventory (STAI) (Spielberger, 1989): a 40-item self-report inventory that is widely used and thoroughly validated to measure anxiety in adults; we assessed the more general and long-standing “trait anxiety” (STAI-T) using 20 questions.
2.6. Statistics
The cytokine and endocannabinoid measurements were found to have a wide dynamic range and highly skewed distribution. Since these findings complicated and/or invalidated planned analyses, the biomarker measurements were transformed using a base-10 logarithmic transformation. Values below the lower limit of the detection of the biomarkers were replaced by the lower limit of detection; this is a conservative approach that is unlikely to lead to overestimation of differences. Biomarkers with over 25% of values below the limit of detection (IL-1β, IFN-γ, IL-12p70) were excluded from the analysis. The psychological questionnaire scores were not transformed.
Descriptive statistics (mean ± SD, median and range) were calculated separately for each of the three time points. The effect of time was tested separately for each variable by using a generalized estimating equations (GEE) model with an exchangeable working correlation structure to account for the repeated measurements within each subject. An ANOVA-like hypothesis of no change over time versus any patterns of change was tested. Within each time-point, Pearson's correlation analysis was also used to examine the cross-sectional relationship between the eCBs and the psychosocial factors and inflammatory molecules.
3. Results
Demographic data for study participants are presented in Table 1. Nineteen subjects were enrolled, with 17 providing baseline demographic data. Subjects had a mean age of 55 (SD 12.5), were entirely Caucasian (100%), and the majority were married (76%). Less than half of the participants were employed full time (47%). Their diagnoses included acute myelogenous leukemia (5), lymphoma (4), and multiple myeloma (10). Fourteen patients received autologous, and 5 received allogeneic (4 sibling, 1 matched unrelated donor) transplants; all received peripheral blood stem cells and all but two patients received inpatient transplants.
Table 1.
Demographic characteristics of sample population.
| N = 17 | |
|---|---|
| Mean Age | 55 (SD 12.5) |
| Gender | |
| Male | 9 (53%) |
| Female | 8 (47%) |
| Race/Ethnicity | |
| Caucasian | 17 (100%) |
| Marital Status | |
| Married | 13 (76%) |
| Divorced | 3 (18%) |
| Single | 1 (6%) |
| Living Arrangements | |
| Spouse/significant other | 9 (53%) |
| Spouse/significant other and children | 4 (23.5%) |
| Other | 4 (23.5%) |
| Education (mean years) | 14.47 (2.0 SD) |
| Employment status | |
| Full time | 8 (47%) |
| Retired | 3 (18%) |
| Disability | 4 (23%) |
| Other | 2 (12%) |
| Income | |
| $10,000–$49,999 | 4 (24%) |
| $50,000–$99,999 | 7 (41%) |
| ≥$100,000 | 3 (17.5%) |
| Don't know/No response | 3 (17.5%) |
3.1. Changes over time of biomarkers and psychosocial factors
The means and standard deviations of the biomarkers and psychosocial measurements at each time point T1-T3 (before transplant, at the time of hospital discharge, and 100 days following transplant) are reported in Table 2, which also illustrates the direction of change over time for each variable. Among the biomarkers evaluated, TNF-α, IL-6, IL-10, CRP, and VEGF significantly varied among the three time points, with T2 being a significant inflection point for most inflammatory molecules. The pattern of change for these significant variables, except VEGF and TNF-α, was to increase from T1 to T2 and decrease again at T3; VEGF was significantly decreased at T2 compared to T1 or T3 and TNF-α did not decline at T3. Circulating eCB concentrations were not significantly different among these three time points.
Table 2.
Summary statistics for biomarkers and psychosocial factors across time points. A GEE model was used separately for each variable to test for the effect of time.
| Biomarkers | Time 1a N = 18 Mean (SD) Median (min–max) |
Time 2a N = 14 Mean (SD) Median (min–max) |
Time 3a N = 14 Mean (SD) Median (min–max) |
p-valueb |
|---|---|---|---|---|
| TNF-α (pg/mL) | 9.0 (2.3) 8.8 (5.2–13.6) |
13.9 (6.3) 10.0 (7.6–23.0) |
13.9 (13.0) 9.1 (4.6–57.0) |
<0.01 |
| IL-6 (pg/mL) | 3.7 (3.9) 2.1 (1.0–14.8) |
9.5 (7.3) 8.5 (1.9–28.9) |
4.0 (4.5) 2.8 (1.0–18.2) |
<0.0001 |
| IL-8 (pg/mL) | 24.6 (35.1) 11.9 (5.4–134.4) |
14.7 (14.5) 9.1 (3.8–51.4) |
19.3 (16.9) 13.3 (2.6–62.6) |
0.15 |
| IL-10 (pg/mL) | 1.5 (1.7) 1.0 (0.5–7.4) |
11.3 (13.9) 2.4 (0.5–33.8) |
1.3 (0.9) 1.2 (0.5–4.0) |
<0.0001 |
| CRP (μg/mL) | 11.5 (11.7) 8.1 (0.1–38.1) |
66.7 (38.7) 62.7 (6.5–105.0) |
9.1 (9.9) 4.3 (0.2–33.1) |
<0.001 |
| VEGF (pg/mL) | 64.3 (31.5) 61.9 (15.7–141.4) |
51.4 (31.3) 43.3 (12.5–106.6) |
89.8 (61.5) 70.4 (23.9–272.0) |
<0.0001 |
| 2-AG (pmol/mL) | 152.5 (208.1) 98.4 (17.8–848.2) |
146.6 (169.0) 60.9 (32.1–549.6) |
116.1 (156.1) 59.1 (17.6–604.4) |
0.69 |
| AEA (pmol/mL) |
1.7 (1.0) 1.3 (0.8–5.0) |
1.4 (0.5) 1.5 (0.5–2.3) |
1.2 (0.6) 0.9 (0.6–3.1) |
0.18 |
| Psychosocial factors Mean (SD) |
N = 15 |
N = 10 |
N = 9 |
|
| CES-D | 9.3 (9.4) 6 (1–27) |
17.8 (13.6) 15 (3–42) |
5.6 (5.1) 5 (0–15) |
<0.01 |
| STAI-T | 39.0 (12.1) 35 (25–61) |
33.8 (11.4) 32.5 (22–60) |
31.9 (8.7) 29 (20–47) |
0.01 |
Time 1 = Before transplant (Day −1 – Day −8); Time 2 = Time of hospital discharge (Day +9 – Day +17); Time 3 = 100 days post-transplant (Day +91 – Day +175).
GEE-based test for equal mean at all time-points.
Depressive symptoms (CES-D) and anxiety (STAI-T) changed significantly over time (Table 2). Anxiety was highest preceding transplant (T1) while depressive symptoms (CES-D) peaked at hospital discharge (T2). Four participants (26.7%) reported significant depressive symptoms (CES-D ≥ 16; (Cohen et al., 2006)) at T1; 50% reported such symptoms at T2 and 0% at T3. There was greatest study attrition at T3; this appeared to be a more prevalent pattern among those endorsing significant depressive symptoms at T2. Clinically meaningful cut-points for the STAI-T have not been adequately demarcated in the literature.
3.2. The eCBs and their correlation with psychosocial factors and inflammatory molecules
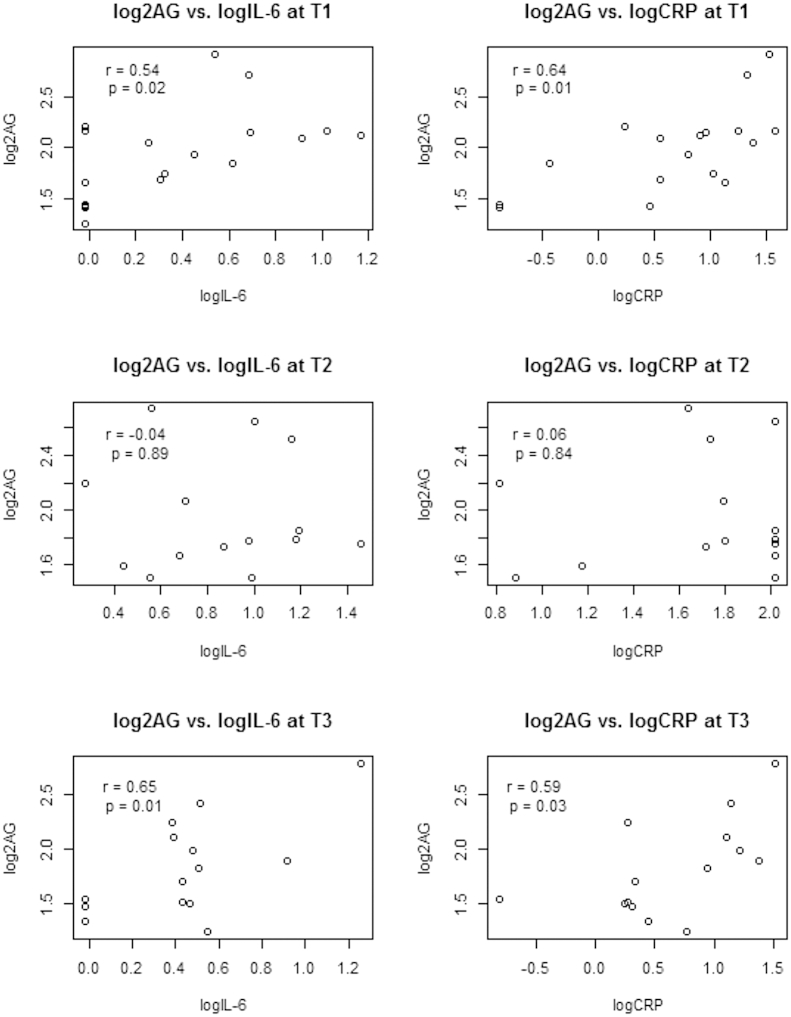
Correlations between the eCBs and the pro-inflammatory mediators were examined individually at the three time points. Pearson correlation analyses demonstrated a significant positive association between 2-AG concentrations and IL-6 and CRP at T1 (IL-6, r = 0.54, p = 0.02; CRP, r = 0.64, p < 0.01) and T3 (IL-6, r = 0.64, p = 0.01; CRP, r = 0.59, p = 0.03), but not at T2 (Fig. 1). The correlations between psychosocial factors and the eCBs demonstrate a significant association between greater depressive symptoms and lower 2-AG levels at T1 (r = −0.64, p = 0.01; data not shown). There were no significant associations between 2-AG and depressive symptoms at T2 or T3. There was no significant relationship between anxiety and the eCBs at any of the time points.
Fig. 1.
Associations between the endocannabinoid 2-arachidonoylglycerol (2-AG) and the inflammatory molecules interleukin-6 (IL-6) and C-reactive protein (CRP) pre-transplant, immediately following transplant, and around Day +100 post-transplant (T1–T3).
4. Discussion
In the present HCT sample, 2-AG and IL-6 concentrations are significantly, positively correlated in the peri-HCT setting. IL-6 and 2-AG concentrations are significantly correlated at T1, prior to transplantation, and T3, approximately 100 days post transplant. This correlation is consistent with that seen in individuals undergoing cardiopulmonary bypass, another markedly pro-inflammatory medical condition (Weis et al., 2010). 2-AG is also significantly positively associated with the pro-inflammatory marker CRP in samples taken at T1 and T3, a relationship not previously reported in the literature. The strength of the correlations between 2-AG and both IL-6 and CRP are remarkably similar at T1 and T3. However, there was no correlation between 2-AG and either IL-6 or CRP at T2, when the concentrations of the cytokines were the highest of the time points sampled. We hypothesize that the loss of correlation at this time point reflects a change in the source of either the cytokines or 2-AG, resulting in an uncoupling of this relationship. Consistent with prior literature (Hill et al., 2009), 2-AG demonstrates an inverse relationship with depressive symptoms in the present HCT sample but only at T1.
As demonstrated in prior studies with HCT patients (Wang et al., 2008, Wang et al., 2014), pro-inflammatory molecules are elevated at T2 in the present sample despite a decrease in bone marrow-derived hematopoiesis and general immune cell production. Indeed, cytokines are produced by many different types of cells; Kupffer cells, the resident tissue macrophages of the liver, are able to synthesize a variety of cytokines (Ramadori and Armbrust, 2001) and perivascular fat locally produces pro-inflammatory cytokines (Iantorno et al., 2014). Pre-transplant chemotherapy regimens may evoke this large cytokine response. Interestingly, however, the circulating concentration of 2-AG was unchanged at T2 compared to T1 and T3. This is in contrast to our hypothesis, which predicted that 2-AG concentrations would be reduced when the bone marrow-derived cell numbers are suppressed. This suggests that the mechanisms regulating circulating concentrations of 2-AG are complex and include non-inflammatory processes. In addition, the present findings suggest that the immune system may not be the primary source of circulating eCBs in the context of HCT, as their levels remain consistent despite significant immune suppression.
As previously identified in non-transplant populations (Hill et al., 2008, Hillard et al., 2012), circulating 2-AG concentrations are negatively correlated with depressive symptoms in this study of HCT recipients. The relationship between 2-AG and depression is present only at T1. Preclinical data support the hypothesis that 2-AG reduces depressive symptoms as a result of activation of brain CB1 receptors (CB1R) (Hillard and Liu, 2014). Since 2-AG concentrations did not fall during T2, it is possible that CB1R in the brain have lost responsivity to 2-AG during this period. This hypothesis is supported by the down regulation of CB1R in animals exposed to chronic unpredictable stress (Hill et al., 2005), as would be similar to the clinical scenario of a stem cell transplant. Alternatively, the data could suggest that a relatively intact immune system is necessary for this relationship between depressive symptoms and 2-AG to occur, as evidenced by the absence of this relationship at assessment time points following transplantation when the host's immune system is compromised. In sum, additional work is required to ascertain whether peripheral or central changes - or both - contribute to this change in relationship between 2-AG and depression just after transplant.
At T1 and T3, our data demonstrate a significant and positive association between the concentrations of IL-6 and 2-AG. Several recent studies provide evidence to support the hypothesis that 2-AG potentiates IL-6 production and suggest that 2-AG could be up-stream of IL-6. For example, 2-AG containing sera increases LPS-induced IL-6 expression in a macrophage cell line (Marazzi et al., 2011). Inhibition of 2-AG catabolism fully blocked diclofenac-induced increases in gastric concentrations of IL-6, an effect that required the CB1 receptor (Kinsey et al., 2011). Activation of CB1R in human adipocyte cultures produces a delayed increase in IL-6 protein release (Gonzalez-Muniesa et al., 2010). Finally, CB2 receptor deficiency results in reduced IL-6 production and mortality in polymicrobial sepsis (Csóka et al., 2009).
2-AG and its primary receptors, CB1 and CB2, exert complex effects on the immune system. Both anti-inflammatory and pro-inflammatory effects have been reported, depending upon the cell type and the state of underlying inflammation. For example, loss of CB2 receptors on T cells results in higher T cell entry into the brain and greater cytokine concentrations in a mouse model of multiple sclerosis (Maresz et al., 2007). Inhibition of 2-AG catabolism reduces LPS-induced increases in brain cytokine production; an effect that is attenuated by CB1 receptor blockade (Kerr et al., 2013). On the other hand, CB2 receptor deficiency leads to reduced neuroinflammation in a mouse model of Alzheimer's Disease (Schmöle et al., 2015). As described above, data that 2-AG can increase release of IL-6 are consistent with the findings of this study. However, the results of this study are purely correlative and do not shed light on potential causative relationships between 2-AG and IL-6 or CRP.
It is noteworthy that during the time of greatest psychosocial distress (immediately following transplant) and immune suppression, the relationships among the eCB system and emotion response and immune function becomes uncoupled. Given the preclinical data of stress buffering effects of the eCBs (Hillard and Liu, 2014), it is possible that the dysregulated neuroimmune-2-AG relationship renders HCT recipients more vulnerable to psychosomatically mediated disease processes during the immediate peri-HCT time period. The potential for the eCB system to buffer against psychosocially mediated inflammation is important given the contribution of inflammation to adverse medical outcomes among HCT recipients, including early mortality, graft-versus-host disease, graft rejection, infection, and disease relapse (Cavet et al., 1999, Keen et al., 2004, Knight et al., 2013, Nagler et al., 1995, Schots et al., 1998).
This dysregulation may also contribute to the previously observed increased rates of psychopathology among HCT recipients in the immediate post-transplant time period due to a lack of eCB buffering of emotional reactivity. Since the eCB effect on depression was lost without a reduction in 2-AG at T2, this suggests that the CB1R in the brain may have lost responsivity during this period. The present findings provide a basis for further evaluation in rodent models to clarify the specific neuroimmune and behavioral implications of our preliminary findings. It is worthwhile to continue investigation of the interplay of these pathways as the eCB system may hold promise as a possible therapeutic target in this and other medically vulnerable populations.
The major limitations of the present study are the small and heterogeneous sample, thus these data are exploratory. Due to the sample size limitation, we were unable to control for possibly confounding medical and demographic variables. Despite these limitations, the present results are consistent with previous research involving patterns of change in psychosocial and inflammatory molecules, suggesting that the present data on circulating eCBs – a biomarker not previously investigated in the HCT setting – may indeed be generalizable to other HCT populations. Future studies with HCT patients investigating the eCB system should aim to recruit larger samples of more homogeneous diseases to control for possible patient and disease confounders such as preparative regimens, transplant type, disease and disease status, and other contributors to inflammatory status including body mass index (BMI), medications, and smoking. Despite its inherent limitations, the current data provide information suggestive for future study regarding the role of the eCB signaling system in the peri-HCT period.
5. Conclusions
2-AG is significantly, positively associated with both IL-6 and CRP in HCT recipients. However, this relationship is lost immediately post-transplant at the time of greatest immunosuppression. The fact that these correlations are not consistent suggests that multiple mechanisms regulate circulating 2-AG concentrations. 2-AG is negatively correlated with depressive symptoms in HCT recipients, as has been demonstrated in other populations. Further work is needed to understand the etiology and regulatory pathways involved in eCB signaling in the immediate post-transplant period to elucidate potential roles for risk prognostication, stress buffering, and therapeutic targets.
Acknowledgments
This works was funded in part by NIH grants 5R24 AG031089-02, T32 073452, R21 AT000895 as well as The Advancing a Healthier Wisconsin Endowment and the Department of Psychiatry at the Medical College of Wisconsin.
References
- Andrykowski M.A., Brady M.J., Henslee-Downey P.J. Psychosocial factors predictive of survival after allogeneic bone marrow transplantation for leukemia. Psychosom. Med. 1994;56:432–439. doi: 10.1097/00006842-199409000-00008. [DOI] [PubMed] [Google Scholar]
- Bluher M., Engeli S., Kloting N., Berndt J., Fasshauer M., Batkai S., Pacher P., Schon M.R., Jordan J., Stumvoll M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S., Alvarez-Jaimes L., Polis I., Stouffer D.G., Parsons L.H. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J. Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavet J., Middleton P.G., Segall M., Noreen H., Davies S.M., Dickinson A.M. Recipient tumor necrosis factor-alpha and interleukin-10 gene polymorphisms associate with early mortality and acute graft-versus-host disease severity in HLA-matched sibling bone marrow transplants. Blood. 1999;94:3941–3946. [PubMed] [Google Scholar]
- Cohen L.S., Soares C.N., Vitonis A.F., Otto M.W., Harlow B.L. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch. Gen. Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- Cote M., Matias I., Lemieux I., Petrosino S., Almeras N., Despres J., Di Marzo V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- Csóka B., Németh Z.H., Mukhopadhyay P., Spolarics Z., Rajesh M., Federici S., Deitch E.A., Bátkai S., Pacher P., Haskó G. CB2 cannabinoid receptors contribute to bacterial invasion and mortality in polymicrobial sepsis. PLoS One. 2009;4:e6409. doi: 10.1371/journal.pone.0006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V., Cote M., Matias I., Lemieux I., Arsenault B., Cartier A., Piscitelli F., Petrosino S., Almeras N., Després J. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52:213–217. doi: 10.1007/s00125-008-1178-6. [DOI] [PubMed] [Google Scholar]
- El-Talatini M.R., Taylor A.H., Konje J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. 2010;93:1989–1996. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Gallo J.J., Rabins P.V. Depression without sadness: alternative presentations of depression in late life. Am. Fam. Physician. 1999;60:820–828. [PubMed] [Google Scholar]
- Gonzalez-Muniesa P., Bing C., Trayhurn P. Upregulation of the expression of inflammatory and angiogenic markers in human adipocytes by a synthetic cannabinoid. JTE. 2010;907 doi: 10.1055/s-0030-1255119. [DOI] [PubMed] [Google Scholar]
- Gregurek R., Labar B., Mrsić M., Batinić D., Ladika I., Bogdanić V., Nemet D., Skerlev M., Jakić-Razumović J., Klain E. Anxiety as a possible predictor of acute GVHD. Bone Marrow Transplant. 1996;18:585. [PubMed] [Google Scholar]
- Hill M.N., Miller G.E., Carrier E.J., Gorzalka B.B., Hillard C.J. Circulating endocannabinoids and< i> N</i>-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Patel S., Carrier E.J., Rademacher D.J., Ormerod B.K., Hillard C.J., Gorzalka B.B. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Bierer L.M., Makotkine I., Golier J.A., Galea S., McEwen B.S., Hillard C.J., Yehuda R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the world trade center attacks. Psychoneuroendocrinology. 2013;38:2952–2961. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Miller G., Ho W., Gorzalka B., Hillard C. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J., Liu Q.S. Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr. Pharm. Des. 2014;20:3795–3811. doi: 10.2174/13816128113196660735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J., Weinlander K.M., Stuhr K.L. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–229. doi: 10.1016/j.neuroscience.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoodin F., Uberti J.P., Lynch T.J., Steele P., Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transpl. 2006;38:255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- Iantorno M., Campia U., Di Daniele N., Nistico S., Forleo G., Cardillo C., Tesauro M. Obesity, inflammation and endothelial dysfunction. J. Biol. Regul. Homeost. Agents. 2014;28:169–176. [PubMed] [Google Scholar]
- Keen L.J., DeFor T.E., Bidwell J.L., Davies S.M., Bradley B.A., Hows J.M. Interleukin-10 and tumor necrosis factor alpha region haplotypes predict transplant-related mortality after unrelated donor stem cell transplantation. Blood. 2004;103:3599–3602. doi: 10.1182/blood-2002-11-3568. [DOI] [PubMed] [Google Scholar]
- Kerr D., Harhen B., Okine B., Egan L., Finn D., Roche M. The monoacylglycerol lipase inhibitor JZL184 attenuates LPS-induced increases in cytokine expression in the rat frontal cortex and plasma: differential mechanisms of action. Br. J. Pharmacol. 2013;169:808–819. doi: 10.1111/j.1476-5381.2012.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey S.G., Nomura D.K., O'Neal S.T., Long J.Z., Mahadevan A., Cravatt B.F., Grider J.R., Lichtman A.H. Inhibition of monoacylglycerol lipase attenuates nonsteroidal anti-inflammatory drug-induced gastric hemorrhages in mice. J. Pharmacol. Exp. Ther. 2011;338:795–802. doi: 10.1124/jpet.110.175778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J.M., Lyness J.M., Sahler O.J.Z., Liesveld J.L., Moynihan J.A. Psychosocial factors and hematopoietic stem cell transplantation: potential biobehavioral pathways. Psychoneuroendocrinology. 2013;38:2383–2393. doi: 10.1016/j.psyneuen.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J.M., Moynihan J.A., Lyness J.M., Xia Y., Tu X., Messing S., Hunter B.C., Huang L., Obi R.O., Liesveld J.L. Peri-transplant psychosocial factors and neutrophil recovery following hematopoietic stem cell transplantation. PLoS One. 2014;9:e99778. doi: 10.1371/journal.pone.0099778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loberiza F.R., Jr., Rizzo J.D., Bredeson C.N., Antin J.H., Horowitz M.M., Weeks J.C., Lee S.J. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J. Clin. Oncol. 2002;20:2118. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- Marazzi J., Kleyer J., Paredes J.M.V., Gertsch J. Endocannabinoid content in fetal bovine sera—unexpected effects on mononuclear cells and osteoclastogenesis. J. Immunol. Methods. 2011;373:219–228. doi: 10.1016/j.jim.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Maresz K., Pryce G., Ponomarev E.D., Marsicano G., Croxford J.L., Shriver L.P., Ledent C., Cheng X., Carrier E.J., Mann M.K. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Matias I., Petrosino S., Racioppi A., Capasso R., Izzo A.A., Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Mol. Cell. Endocrinol. 2008;286:S66–S78. doi: 10.1016/j.mce.2008.01.026. [DOI] [PubMed] [Google Scholar]
- McGregor B.A., Syrjala K.L., Dolan E.D., Langer S.L., Redman M. The effect of pre-transplant distress on immune reconstitution among adult autologous hematopoietic cell transplantation patients. Brain Behav. Immun. 2013;30:S142–S148. doi: 10.1016/j.bbi.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuellon R.P., Russell G.B., Rambo T.D., Craven B.L., Radford J., Perry J.J., Cruz J., Hurd D.D. Quality of life and psychological distress of bone marrow transplant recipients: the 'time trajectory' to recovery over the first year. Bone Marrow Transpl. 1998;21:477–486. doi: 10.1038/sj.bmt.1701115. [DOI] [PubMed] [Google Scholar]
- Nagler A., Or R., Nisman B., Kalickman I., Slavin S., Barak V. Elevated inflammatory cytokine levels in bone marrow graft rejection. Transplantation. 1995;60:943–948. [PubMed] [Google Scholar]
- Pereira D.B., Christian L.M., Patidar S., Bishop M.M., Dodd S.M., Athanason R., Wingard J.R., Reddy V.S. Spiritual absence and 1-year mortality after hematopoietic stem cell transplant. Biol. Blood Marrow Transplant. 2010;16:1171–1179. doi: 10.1016/j.bbmt.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Pulgar A., Garrido S., Alcala A., Reyes del Paso G.A. Psychosocial predictors of immune response following bone marrow transplantation. Behav. Med. 2012;38:12–18. doi: 10.1080/08964289.2011.647118. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Armbrust T. Cytokines in the liver. Eur. J. Gastroenterol. Hepatol. 2001;13:777–784. doi: 10.1097/00042737-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Schmöle A., Lundt R., Ternes S., Albayram Ö., Ulas T., Schultze J.L., Bano D., Nicotera P., Alferink J., Zimmer A. Cannabinoid receptor 2 deficiency results in reduced neuroinflammation in an Alzheimer's disease mouse model. Neurobiol. Aging. 2015 Feb;36(2):710–719. doi: 10.1016/j.neurobiolaging.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Schots R., Kaufman L., Van Riet I., Lacor P., Trullemans F., De Waele M., Van Camp B. Monitoring of C-reactive protein after allogeneic bone marrow transplantation identifies patients at risk of severe transplant-related complications and mortality. Bone Marrow Transplant. (Basingstoke) 1998;22:79–85. doi: 10.1038/sj.bmt.1701286. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. Consulting Psychologists Press; Palo Alto, CA: 1989. State-trait Anxiety Inventory: a Comprehensive Bibliography. [Google Scholar]
- Spoto B., Fezza F., Parlongo G., Battista N., Sgro E., Gasperi V., Zoccali C., Maccarrone M. Human adipose tissue binds and metabolizes the endocannabinoids anandamide and 2-arachidonoylglycerol. Biochimie. 2006;88:1889–1897. doi: 10.1016/j.biochi.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Varga K., Wagner J.A., Bridgen D.T., Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Wang X.S., Shi Q., Williams L.A., Cleeland C.S., Mobley G.M., Reuben J.M., Lee B.N., Giralt S.A. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113:2102–2109. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.S., Shi Q., Shah N.D., Heijnen C.J., Cohen E.N., Reuben J.M., Orlowski R.Z., Qazilbash M.H., Johnson V.E., Williams L.A., Mendoza T.R., Cleeland C.S. Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin. Cancer Res. 2014;20:1366–1374. doi: 10.1158/1078-0432.CCR-13-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis F., Beiras-Fernandez A., Hauer D., Hornuss C., Sodian R., Kreth S., Briegel J., Schelling G. Effect of anaesthesia and cardiopulmonary bypass on blood endocannabinoid concentrations during cardiac surgery. Br. J. Anaesth. 2010;105:139–144. doi: 10.1093/bja/aeq117. [DOI] [PubMed] [Google Scholar]