Abstract
Background
Pericytes surround endothelial cells at the perivascular interface. Signaling between endothelial cells and pericytes is crucial for capillary homeostasis, as pericytes stabilize vessels and regulate many microvascular functions. Recently it has been shown that pericytes are able to detach from the vascular wall and contribute to fibrosis by becoming scar-forming myofibroblasts in many organs including the kidney. At the same time, the loss of pericytes within the perivascular compartment results in vulnerable capillaries which are prone to instability, pathological angiogenesis, and, ultimately, rarefaction.
Aims
This review will give an overview of pericyte-endothelial cell interactions, summarize the signaling pathways that have been identified to be involved in pericyte detachment from the vascular wall, and present pathological endothelial responses in the context of disease of the kidney.
Keywords: Pericytes, Endothelial cells, Fibrosis, Pericyte-endothelial interaction, Microcirculation
Introduction
Fibrogenesis is one of the key processes in all organs and it is closely associated with loss of organ function during chronic human disease. Matrix deposition in the interstitial space, including the pathological accumulation of collagen I, III, and V, fibrillin, and other matrix proteins that are normal constituents of basement membranes, is one of its characteristic features. The cell type responsible for fibrillar matrix deposition is predominantly myofibroblast [1–3]. Fibrosis occurs not only in chronic kidney disease, where it is linked to the decline of kidney function and hypertension [4], but also during remodeling processes after an injury of any kind, e.g. in liver cirrhosis, heart failure, idiopathic lung fibrosis, and pancreatic disease [5–8], as well as following a stroke or a central nervous system trauma [9]. In addition, one of the initial steps in atherosclerosis is the formation of a fibrous cap and matrix deposition of collagen and elastin into the media of the vascular wall [10]. Recent studies using conditional fate mapping in mice have identified perivascular mesenchymal cells or pericytes as a major source of scarforming myofibroblasts in multiple organs including the kidney [6, 9, 11–16]. Although others have reported contributions to myofibroblasts by leukocytes, epithelial cells, and endothelial cells [17], a consensus is emerging that these latter cell types contribute to fibrogenesis predominantly via indirect mechanisms, at least in the animal models studied, and that most myofibroblasts arise from mesenchymal cells embedded in the organ connective tissue (known as resident fibroblasts) or attached to capillary walls (known as pericytes) [9–15]. The study of pericytes is complicated by the lack of specific markers, the lack of a consensus on the definition, tissue-specific variations in morphology, and the requirement for electron microscopy to validate microvascular wall integration. In some studies pericytes are referred to as perivascular cells, yet in others they are named mesenchymal stromal cells [18]. While in the brain markers such as RGS5 and NG2 are useful for marking most pericytes, in other tissues such as the skin, kidney, or lung these markers only label a subpopulation of pericytes [17–19]. At this time, it remains unclear whether there are discrete subpopulations of mesenchymal cells attached to capillaries serving as pools of myofibroblast precursors or one population exhibits plasticity. These controversies have recently been reviewed in some detail and will not be discussed further [16].
While pericytes stabilize the microvasculature in health [20, 21], their detachment from the vascular wall and differentiation into myofibroblasts under pathological conditions not only leads to interstitial fibrosis but also leaves the vascular wall unprotected and vulnerable [18, 22, 23]. Therefore, it is not surprising that the onset of interstitial fibrosis is often accompanied by simultaneous disease of the microvasculature [22]. In this review, we will focus on processes at the microvascular interface and their consequences for the capillary. We will provide updates on the current understanding of pericyte function and interaction with the endothelium in health and disease in the kidney and then summarize the identified mechanisms involved in the separation of both cell types leading to pathological angiogenesis and ultimately to vascular rarefaction, which in turn promotes ischemia, inflammation, and further fibrogenic responses. Although this review will focus on the pathological mechanisms detected during kidney disease, similar microvascular changes occurring in other organs such as the lung and heart implicate perivascular cell detachment in microvascular disease and simultaneous fibrogenesis in multiple organs [9, 19, 24, 25].
Pericyte Nomenclature
The discovery of pericytes dates back to Rouget [26] in 1873, when he described the existence of perivascular cells surrounding the endothelium of small blood vessels in the rat. Fifty years later, Zimmerman [27] reported a perivascular cell, first called a ‘Rouget cell’, essential for the ultrastructural composition of capillaries. Due to the close apposition of this cell type to neighboring endothelial cells, he called them ‘peri-cytes’ (‘peri’ is Greek for ‘around’ and ‘cyte’ is New Latin for ‘cyta’ from the ancient Greek ‘kutos’ meaning ‘vessel’ but is used as a suffix for ‘cells’) and coined the nomenclature that is still used today. This nomenclature made the localization of these cells quite clear, defining a pericyte as a cell of the periendothelial compartment (fig. 1). Today it is known that this periendothelial compartment is not exclusively occupied by pericytes but rather other cell types, i.e. vascular smooth muscle cells, fibroblasts, and resident macrophages, may reside within this space [28]. In the kidney, pericytes were first described by Courtoy and Boyles [29] in 1983.
Fig. 1.
a, b Immunofluorescent micrograph of an uninjured kidney section in a collagen 1a1gfp transgenic mouse. In the interstitium of the kidney, peritubulary capillaries (CD31-cy3 staining) are in close apposition to coll1a1gfp-expressing cells. In most places, both cell types are separated by basal lamina (blue laminin staining) marked by arrows (a), while in some places no separation can be found (arrows in b). Note that long coll1a1gfp-positive processes can be found throughout the pericapillary compartment (scale bars = 10 µm). c Schematic of b representing pericyte (green)-endothelial cell (red) interaction; blue represents the CBM. Peg-and-socket junctions can be found in places where no separation via the CBM occurs.
Pericyte Structure and Interaction
Constituting the smallest unit within the vascular system, pericytes interact with endothelial cells (fig. 1). They can be found on capillaries but also on small precapillary arterioles and postcapillary venules. Together with endothelial cells, pericytes form the microvasculature, a system responsible for hydrostatic balance, the delivery of nutrition, and metabolic exchange. As pericytes originate from the mesenchyme of each organ, the functions of pericytes are specialized/adapted to their specific needs depending on their surroundings, making it unlikely that a single type of pericyte exists. More likely, pericytes show organ-specific functions and properties. Hepatic stellate cells in the liver are considered a specialized form of pericytes [30]. In the brain, it has been described that pericytes are essential for maintenance of the blood-brain barrier, inducing the polarization of astrocyte end feet [31]. In the glomerulus of the kidney, mesangial cells and podocytes are considered specialized forms of pericytes [32].
A ubiquitous pericyte feature, regardless of the residing organ, is that they interact closely with endothelial cells. In the kidney, pericytes and endothelial cells share a capillary basement membrane (CBM). Components of the CBM are produced by pericytes as well as by endothelial cells [33]. A lack of CBM coverage between pericytes and endothelial cells can be found in specialized locations, where pericytes are able to directly interact with endothelial cells [34]. Those specialized areas where direct cell-cell interaction takes place sometimes appear as ‘peg-and-socket’ junctions. They form when endothelial cell cytoplasm invaginations are filled with a pericyte process and vice versa in a ‘key-and-lock’-like manner and direct signaling via tight, gap, and adherence junctions occurs [29, 34] (fig. 1). In addition, long processes of pericytes make it possible for a pericyte not only to interact with one endothelial cell exclusively but also to span and signal with several endothelial cells, leading to a uniform endothelial cell reaction upon stimulation [35]. Observations from the kidney suggest that pericytes also signal to other cell types such as epithelial cells and coordinate endothelial and epithelial cell actions [36, 37]. By comparison, observations of the brain, where pericytes in combination with endothelial cells and astrocytes form the blood-brain barrier, have shown bicellular signaling of pericytes, leading to astrocyte end foot polarization [31], and recently an important role of pericytes in leukocyte migration was identified [38].
Pericyte Function
Coordination of tissue repair after injury is of utmost importance for the prevention of impairments in organ function and integrity. A hallmark of functional remodeling is sufficient angiogenesis. The initial step in this process is endothelial tube formation [39]. Shortly, vascular guidance tunnels appear within the extracellular matrix and guide the sprouting of an endothelial tube formed by tip and stalk cells [40]. This vascular sprout at the beginning is highly proliferative but unstable and prone to regression. Stabilization occurs when mural cells such as pericytes or vascular smooth muscle cells are recruited to the abluminal surface [41]. It is not surprising that for decades the endothelium has been the main target of vascular research, as endothelial cells build the inner layer of each blood vessel regardless of the size or the organ. Research focusing on the perivascular compartment has been relatively neglected by comparison. Part of the reason is that the extent of pericytes throughout the microvasculature of essentially all organs has only recently been appreciated through the identification of better markers and higher-resolution imaging of the microvasculature and because there are no truly specific pericyte markers [42] (table 1).
Table 1.
Identified pericyte markers
| Marker | Other cell types with marker expression |
References |
|---|---|---|
| PDGFR-β | Myofibroblasts, neurons and progenitors, mesenchymal cells, mesenchymal stem cells | Hirschi and Amore [99] Diaz-Flores et al. [100] Lin et al. [15] |
| PDGFR-α | Mesenchymal cells, neural stem cells/B cells | Takakura et al. [101] Jackson et al. [102] |
| NG2 | vSMC, adipocytes, neuronal progenitors, glial cells, developing bone, muscle skin | Armulik et al. [31] Bergers and Song [103] Ruiter et al. [104] Huang et al. [105] |
| Desmin | Skeletal muscle cells, cardiac smooth muscle cells, mesangial cells | Nehls et al. [106] Diaz-Flores et al. [100] |
| α-SMA | vSMC, myofibroblasts | Armulik et al. [31] Strutz and Zeisberg [107] |
| RGS5 | vSMC | Bondjers et al. [108] |
| Endosialin | Myofibroblasts, fibroblasts, vSMC | MacFadyen et al. [109] Smith et al. [110] |
| CD73 | Mesenchymal stem cells | Diaz-Flores et al. [100] |
| CD13 | vSMC, epithelial cells in the kidney, tumor endothelial cells | Dermietzel and Krause [111] Kunz et al. [112] Stefanovic et al. [113] |
| CD146 | Mesenchymal stem cells | Crisan et al. [51] |
| CD105 | Mesenchymal stem cells, endothelial cells, hematopoietic stem cells | Crisan et al. [51] Nassiri et al. [114] Pierelli et al. [115] |
| CD44 | Mesenchymal stem cells, lymphocytes, hematopoietic stem cells | Sackstein et al. [116] Jalkanen and Jalkanen [117] Dimitroff et al. [118] |
| ANGPT1 | Hematopoietic progenitor cells, glioblastoma tumor cells, mast cells | Sato et al. [119] Stratmann et al. [120] Nakayama et al. [121] |
| VEGF-A | Tumor cells, macrophages | Berse et al. [122] |
Among the most important homeostatic functions of pericytes are stabilization of the vascular wall and maintenance of vascular quiescence and vascular integrity [34, 41, 43–47]. This stabilization occurs via bidirectional signaling between pericytes and endothelial cells and by deposition of basement membrane. The signaling systems involved in pericyte/endothelial cell homeostasis have been investigated and reviewed in detail [28, 34]. Additional functions of pericytes include contraction of the vascular diameter and bridging of endothelial gaps [48–50] as well as leukocyte attraction and guidance [38]. It has been postulated that pericytes not only perform vascular stabilization functions but may also act as mesenchymal stem cells with the potential to differentiate in vitro into adipogenic, chondrogenic, or osteogenic cells [51, 52]. In bone marrow, it has been shown that mesenchymal stem cells reside in a vascular niche [53]. Crisan et al. [51] proposed that mesenchymal stem cells exist in other organs, and several groups have shown MSC potential within organ-specific pericyte cultures, placing pericytes as a central target in regeneration [12, 52] and promoting a ‘research renaissance’ for pericytes.
Pericytes Regulate Vessel Growth
Pericytes are involved in vasculogenic (new vessel formation from angioblasts – during organ development), angiogenic (new vessel formation from existing vessels – in adults during growth), and pathological angiogenic processes such as vessel remodeling/formation after injury [21]. The angiogenic processes can be divided into 3 major steps: (a) initiation, (b) vascular sprouting and migration, and (c) maturation/termination. Initiation takes place when endothelial cells and pericytes are exposed to proangiogenic stimuli, leading to diminished contact of the pericyte and its processes with the CBM. Simultaneously, vascular sprouting and pericyte migration are started [54]. A common feature of sprouting is the diminished coverage of the vascular sprout by pericytes, a process that enables endothelial cell proliferation [21]. Only when angiogenesis is terminated do pericytes relocate to endothelial cells. Such coverage is associated with maturation and termination of the growth of the newly formed vessel [21, 55]. This maturation is accompanied by the deposition of basement membrane between endothelial cells and pericytes [33]. In other settings, such as corpus luteum growth or tumor microvessels, migratory pericytes pave the way for the developing vascular sprout by forming tunnels through the matrix [56, 57].
To conclude, the migration of pericytes from the vascular wall is permissive of endothelial cell sprouting and new vessel formation and can therefore be considered proangiogenic in many regenerative processes.
Pericyte Detachment, Vascular Rarefaction, and Interstitial Fibrosis Are Interrelated Processes
Fibrosis occurs as a reaction to chronic injuries [58]. Many hypotheses have been proposed to explain the appearance of myofibroblasts in the interstitial compartment [58]. One of the major models which has recently evolved is that pericytes give rise to myofibroblasts in the kidney and other organs (fig. 2). Lin et al. [15] used a classical fibrosis model (UUO: unilateral urethral obstruction) to provoke fibrosis in the kidney in a mouse with the Coll1a1-GFP transgene. In that mouse, each cell producing collagen I(α)1 protein (a major component of the fibrillar extracellular matrix in fibrosing processes) was labeled by the GFP protein. They identified cells in a normal kidney expressing GFP, and colabeling for CD73 as well as PDGFR-β, which under physiological conditions were in close apposition to peritubular capillaries and fulfilled the criteria of pericytes. Just 9 h after the initiation of kidney injury via unilateral urethral obstruction, these perivascular cells did not stay in a steady state within the vascular wall but became activated. This activation led to detachment of the pericytes from the perivascular compartment, as well as spreading and migration into the interstitial space. There, activated pericytes proliferated and produced mature pathological extracellular matrix components [15] typical of myofibroblasts (fig. 2). The results indicated that this process potentially results in a loss of pericyte function through the detachment of pericytes and functional transition to scar-forming cells. In addition, it is possible that not all pericytes that detach from the vascular wall will differentiate into myofibroblasts but they will undergo apoptosis, a common phenomenon seen in diabetic retinopathy [59, 60]. In experiments from our laboratory, pericytes purified from kidneys migrated to capillary tubes in 3-D cultures, integrated partially into the endothelial wall, stimulated the deposition of an organized CBM, and regulated the vascular diameter [22, 23]. When the stability of the capillaries was assessed by stressing them with serine protease treatment, pericytes promoted vascular stability [22, 23, 41]. These capacities were lost when pericyte-derived myofibroblasts from diseased kidneys were applied to the same assays. In preliminary studies, myofibroblasts may in fact promote vascular leakage and the death of capillaries. Therefore, for many reasons, pericyte detachment is deleterious to capillaries.
Fig. 2.
Schematic of pericyte detachment upon a disease stimulus followed by microvascular rarefaction and the occurrence of myofibroblasts and extracellular matrix within the interstitium, representing fibrosis.
Another possible outcome at the pericyte-endothelial interface is the transdifferentiation of injured endothelial cells into myofibroblasts. Although this remains a controversial topic, a consensus appears to be evolving. Initial reports in which the lineage of endothelial cells was traced using Cre/Lox systems relying on promoters to the angiopoietin receptor TIE1 or TIE2 suggested that as many as 100% of myofibroblasts were derived from the endothelium [61, 62]. However, closer examination of these studies has shown that very few ‘endothelium-restricted’ genes are truly restricted to the endothelium. TIE-1 and TIE-2 are both transiently expressed by myeloid lineages and stromal/smooth muscle lineages including pericytes and resident fibroblasts of the kidney [62]. More recently, this process of endothelial transdifferentiation was reassessed by the same authors using the VE-cadherin-Cre transgene. Those studies suggested that a more modest 15% of myofibroblasts were derived from the endothelium [17]. However, our laboratories have never identified an endothelial cell in transition in vivo or hallmark proteins such as CD31 or VE-cadherin in myofibroblasts. Since the authors did not use conditional Cre systems to map the fate of a cohort of endothelial cells, the contribution of endothelial cells to myofibroblasts remains unclear.
The loss of capillaries (known as microvascular rarefaction) is closely associated with chronic kidney disease and interstitial scarring and is likely to be an underappreciated central cause of chronic kidney disease progression as a result of the consequent tissue ischemia and loss of nephron function [63–65]. Following the discovery that pericytes not only migrate away from the vascular wall but are also a major source of myofibroblasts, further studies have been undertaken to identify the molecular mechanisms that regulate the loss of protective pericytes from the capillary wall. Such experiments have focused on the hypothesis that impaired pericyte-endothelial cross talk might lead to vascular instability. The next section will review the identified signaling pathways involved in microvascular homeostasis at the onset of kidney fibrosis.
PDGF-B and VEGF-A Signaling
Cross talk between endothelial cells and pericytes via PDGF-B and VEGF-A signaling has been shown to promote angiogenesis under physiological conditions [66, 67]. In this regard endothelial cell-derived PDGF-B can attract various mural cells including pericytes through PDGFR-β signaling. This is important during angiogenesis, when stalk cells express more PDGF-B than tip cells, making sure that after sprouting processes pericytes are attracted back to the vascular sprout to terminate this process and initiate maturation [54]. Failure of pericyte attraction can lead to instability and regression [21]. Furthermore, mice lacking PDGFB showed microvascular defects and were not viable [21, 68], underlining the importance of this signaling cascade during development and physiological angiogenesis. In contrast, VEGF produced by pericytes induces endothelial cell proliferation, migration, and sprouting through VEGFR-2 signaling [44]. In 2008, Greenberg et al. [44] were able to link both signaling pathways. The authors found that, under PDGFB-mediated angiogenesis, VEGF led to a lack of pericytes at the vascular sprout and induced vessel destabilization through VEGFR-2 signaling. Activation of VEGFR-2 suppressed PDGFR-β signaling through the induction of a VEGFR-2-PDGFR-β complex [44]. Inhibition of VEGFR-2 could restore angiogenesis [44], and treatment with VEGF inhibitors in the tumor vasculature showed pericytes that were closely associated with the surviving tumor vessel [69, 70]. While VEGF/VEGFR-2 signaling can be regarded as proangiogenic in endothelial cells, it negatively influences pericyte coverage and pericyte-mediated vessel stabilization [44].
Under pathophysiological conditions in the kidney, pericytes migrate from the vascular wall and therefore might influence homeostasis in PDGF and VEGF signaling between both cell types (fig. 3). By blocking VEGFR-2 on endothelial cells and PDGFR-β on pericytes during the early phase of fibrosis (UUO), vascular rarefaction and interstitial fibrosis was attenuated. These observations highlight a link between pericyte detachment from the vascular wall and the appearance of myofibroblasts [71]. Furthermore, Lin et al. [71] provided evidence that the differentiation of pericytes into myofibroblasts was accompanied by a switch from VEGF164, which is proangiogenic to its isoforms VEGF120 and VEGF188 that lack the usual angiogenic properties.
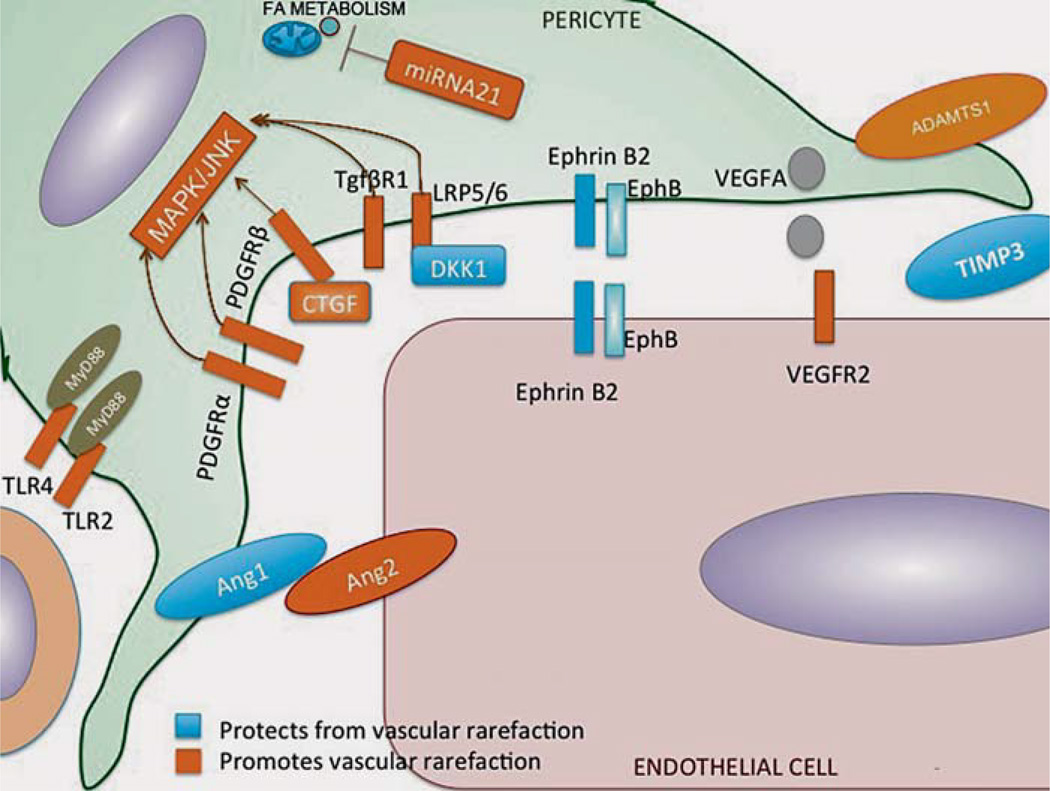
Fig. 3.
Schematic illustration of the pathways identified to be involved in pericyte detachment and vascular rarefaction at the pericyte-endothelial interface. ADAMTS-1/TIMP3 signaling [22], PDGF-B VEGF-A signaling [71], WNT signaling [88], ephrinB2 signaling [23], and ANGPT1/ANGPT2 signaling [93].
Metalloproteinase Activity
An important feature of angiogenesis is the ability of endothelial cells to dissolve basement membrane and local tissue structures to form tunnels (invasion) for new vessel formation. Regulation of matrix metalloproteinase (MMP) activity has been shown in several distinct assays to be important for this process [33, 40, 72–74]. At the interface of endothelial cells and pericytes, MMP are involved in the disruption of tight junctions, promoting pericyte detachment and proangiogenic endothelial cell proliferation [75]. At the same time, growth factors and cytokines with complementary effects are activated [76]. The current understanding of this process involves integrin-mediated recruitment of MMP to the endothelium-pericyte interface [75]. There, degradation of the extracellular matrix results in the activation of growth factors like TGF-β and VEGF165 [76, 77] and initiates proangiogenic as well as antiangiogenic signaling cascades [76].
Besides being a source of matrix metalloproteinases [78], pericytes contribute to the production of tissue inhibitor of matrix metalloproteinase 3 (TIMP3) which stabilizes vascular tubes in combination with endothelial cells [41].
To investigate pericyte detachment at the onset of kidney fibrosis, the capacity of primary kidney pericytes and myofibroblasts to stabilize endothelial cell tubes in a 3-D tube formation assay was tested [22, 79]. While primary kidney pericytes completely prevented the collapse of the vascular network and were shown to have migrated to and attached to the capillary wall, primary kidney myofibroblasts did not prevent gel regression, indicating that myofibroblasts lose their capillary-stabilizing functions [22]. Microarray analysis of isolated cells showed that pericytes expressed TIMP3. The TIMP3 gene expression was downregulated in diseased cells, while the expression of a disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS-1), a metalloproteinase involved in cell migration and cleavage of CBM, was rapidly and highly upregulated [22]. While the addition of recombinant TIMP3 into the regression assay prevented gel regression, mimicking the stabilizing effect of pericytes, recombinant ADAMTS-1 accelerated regression. Furthermore, the addition of pericytes in the presence of recombinant ADAMTS-1 no longer prevented regression. The molecular mechanisms involved in pericyte stabilization of the capillary network were downregulation of VEGFR-2 signaling on endothelial cells and blockade of MMP activity in endothelial cells, strongly suggesting that the pericyte-stabilizing functions in kidney injury depend on the local regulation of MMP and VEGFR-2 activity. While ADAMTS-1 marks activated pericytes/myofibroblasts unable to stabilize vascular tubes, other members of the ADAMTS family are involved in myofibroblast transition in kidney disease [80]. ADAMTS-2 and ADAMTS-12 are highly upregulated in myofibroblasts. Although Grgic et al. [80] did not link both proteins to vascular rarefaction, ADAMTS-2 and ADAMTS-12 are involved in cleavage of extracellular collagen I and both are inhibited by TIMP3 [81]. It will be interesting to investigate their role in vascular rarefaction during kidney fibrosis in more detail.
EphrinB2 Signaling at the Microvascular Interface
A further mechanism identified in kidney pericyte-endothelial interaction and pericyte activation in combination with vascular rarefaction is signaling via ephrinB2 [23]. EphrinB2 reverse signaling is known to regulate VEGFR2 activity, and pericyte-restricted ephrinB2 deficiency leads to vascular instability with hemorrhaging during development [82], highlighting the importance of this receptor for normal microvascular pericyte coverage [83]. Furthermore, ephrinB2 is involved in the regulation of migration, spreading and adhesion of mural cells during vascular wall assembly [84]. EphrinB2 signaling can be mediated via tyrosine phosphorylation and/or PDZ domain-dependent signaling.
In kidney research, primary kidney pericytes from PDZ−/− mice showed enhanced proliferation and migration due to an overactivated pericyte status [23]. In contrast, primary microvascular endothelial cells proliferated less and were less migratory due to impaired VEGFR-2 function, pointing to ephrinB2 on microvascular endothelial cells as a regulator of VEGFR-2 activity [23]. In addition, ephrinB2 PDZ domain-deficient pericytes showed an impaired capillary-stabilizing capacity and contributed less to capillary basement protein synthesis [23]. EphB4-ephrinB2 bidirectional signaling had an impact on endothelial cell integrity through endothelial-endothelial interactions but not through pericyte-endothelial cell cross talk. Mice deficient in ephrinB2 phosphotyrosine signaling also showed a mildly impaired capillary stabilization capacity, emphasizing the predominance of PDZ-dependent signaling in pericyte-endothelial cell homeostasis. Collectively, these studies indicate that ephrinB2 is an important regulator of microvascular quiescence during kidney fibrosis (fig. 3) [23].
WNT Signaling
In kidney injury, WNT/β-catenin signaling pathways have been shown to be involved in tissue regeneration [85] as well as disease progression [14]. Studies not focusing on myofibroblasts and pericytes respectively showed that the WNT pathway is overactivated in kidney diseases [14], and transcriptional analysis indicated that myofibroblasts showed the highest levels of WNT signaling activity [86]. Dickkopf-related protein 1 (DKK1) is a ligand for the WNT coreceptors low-density lipoprotein receptor-related proteins 5 and 6 (LRP5, 6), believed to block WNT pathway activation by displacing WNT ligands from the LRP5/6 binding sites [87]. Systemic delivery of DKK1 to the kidney blocked the proliferation of preexisting myofibroblasts, stopped the progression of fibrosis, and attenuated capillary rarefaction in vivo [88]. In addition, it inhibited pericyte activation and detachment and the transition to myofibroblasts in vitro. When pericytes were tested in vitro for their response to profibrogenic cytokines, PDGF-B TGF-β or connective tissue growth factor, DKK1 markedly blunted the cell responses to these cytokines, though not to another factor, i.e. Cyr61 [88]. By dissecting the cell surface protein interactions and intracellular signaling, Ren et al. [88] suggested that the WNT/LRP6 signaling complex interacts with other receptor pathways, enabling effective signaling of cellular activation by stress kinase cascades rather than by canonical pathways. These findings suggest that DKK1 is potently protective against excessive myofibroblast activation via inhibition of noncanonical signaling pathways in pericytes (fig. 3). DiRocco et al. [89] also showed a role of WNT signaling in the onset of kidney fibrosis. They generated a mouse model in which they could activate WNT/β-catenin signaling in interstitial pericytes and fibroblasts, and kidneys showed spontaneous myofibroblast differentiation in the absence of injury. Others have shown that WNT/β-catenin signaling stimulates the chondrogenic differentiation and inhibits the adipogenic differentiation of pericytes [90]. These latter studies do not link WNT signaling to vascular regression but do show a direct effect on pericyte differentiation, making changes in endothelial pericyte interaction likely and worthy of investigation. Other studies in humans have shown an involvement of WNT signaling in epithelial-to-mesenchymal transition [91]. Therefore, WNT receptor blockade may have beneficial effects on the epithelium directly. It is worth noting that WNT inhibition may be associated with bone disease, and recent studies have suggested that antibodies against DKK1 may prevent some of the skeletal changes associated with chronic kidney disease progression [92].
Angiopoietin 1, Angiopoietin 2, and Cognate Receptor Tie2 Signaling
Recent work by Ziegler et al. [93] showed that microvascular pericyte coverage can be influenced by angiopoietin 2 (ANGPT2). The angiopoietin/Tie2 system has two principal ligands, i.e angiopoietin 1 (ANGPT1), which is vasculoprotective and is produced by pericytes [94], and ANGPT2, the antagonist of ANGPT1 produced in endothelial cells [95]. Both ligands compete for their receptor Tie2 [96, 97]. In sepsis, rhANGPT1 treatment improves a number of sepsis-induced organ dysfunctions [98] including capillary leakage. Taking this into account, Ziegler et al. [93] designed a mouse in which they could induce ANGPT2 overexpression in endothelial cells (until 12 weeks of age) and switch off the overexpression via doxycycline. Indeed, those mice showed vascular rarefaction and pericyte loss in the heart. The switch-off of overexpressed ANGPT2 to normal ANGPT2 states prevented pericyte loss and vascular rarefaction at 24 weeks of age. In addition, pericyte loss at the microvascular locus was associated with increased capillary leakage, hypotensive hypercirculation, and pathological cardiac hypertrophy. Cardiomyocyte-specific ANGPT2 overexpression altered the cardiac microvascular architecture but did not cause any changes in peripheral pericyte-endothelial cell interactions. The addition of ANGPT1 or PDGFB at week 12 increased the endothelial coverage with pericytes at 24 weeks of age and normalized the permeability in ear capillaries. Furthermore, in 2 sepsis models (lipopolysaccharide injection and cecal puncture and ligation) the addition of an ANGPT2-blocking antibody decreased the pericyte loss and increased the microvascular integrity. These experiments demonstrate that the balance of ANGPT1 and ANGPT2 is essential for pericyte-endothelial cell integrity and links capillary leakage directly to the absence of pericytes at the microvascular locus (fig. 3).
Conclusion
Although the research focusing on injury-related alterations in pericyte-endothelial interactions is still in its infancy, it is clear that the appearance of myofibroblasts and fibrosis is intimately associated with capillary instability which may result in microvascular rarefaction. Pericytes with mesenchymal stem cell properties and the capacity to differentiate into myofibroblasts play a central role both in fibrogenesis and in the maintenance of vascular quiescence. While pericytes stabilize the microvasculature in health, their differentiation into myofibroblasts leads to a lack of pericytes at the microvascular interface, resulting in vulnerability and endothelial destabilization. The pathways involved in pericyte-endothelial cell disassembly include the VEGF and PDGF signaling pathways, WNT signaling, ephrinB2 signaling, and the ANGPT1/ANGPT2 Tie2 signaling cascade but also include pathways involved in metalloproteinase activity (TIMP3 and ADAMTS-1). It will be the aim of further research to target these pathways and find new treatment options to prevent pericyte detachment and maintain a functioning perivascular environment.
Acknowledgements
We thank Axel Haverich for ongoing support and Ulrike Boer for helpful discussions.
Disclosure Statement
C.S. is funded by HiLF of Hannover Medical School. J.S.D. holds patents for the use of WNT and microRNA regulators in the treatment of fibrotic diseases, is cofounder of Muregen LLC, and serves as an advisor for Promedior Inc., Regulus Therapeutics, Abbvie, Takeda, Bristol-Myers Squibb, Biogen Idec, and Boehringer Ingelheim.
Footnotes
This review is based on a lecture presented by C.S. at a symposium on vascular remodeling organized by the DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, in Munich, Germany, in February 2013.
References
- 1.Roberts IS, Burrows C, Shanks JH, Venning M, McWilliam LJ. Interstitial myofibroblasts: predictors of progression in membranous nephropathy. J Clin Pathol. 1997;50:123–127. doi: 10.1136/jcp.50.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. 1. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 3.Badid C, Desmouliere A, Babici D, Hadj-Aissa A, McGregor B, Lefrancois N, Touraine JL, Laville M. Interstitial expression of alpha-SMA: an early marker of chronic renal allograft dysfunction. Nephrol Dial Transplant. 2002;17:1993–1998. doi: 10.1093/ndt/17.11.1993. [DOI] [PubMed] [Google Scholar]
- 4.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 5.Siao CJ, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen-Gould L, Parrish DC, Habecker BA, Nykjaer A, Ellenson LH, Tessarollo L, Hempstead BL. ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. J Exp Med. 2012;209:2291–2305. doi: 10.1084/jem.20111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachem MG, Zhou Z, Zhou S, Siech M. Role of stellate cells in pancreatic fibrogenesis associated with acute and chronic pancreatitis. J Gastroenterol Hepatol. 2006;21(suppl 3):S92–S96. doi: 10.1111/j.1440-1746.2006.04592.x. [DOI] [PubMed] [Google Scholar]
- 8.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr161. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 9.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 11.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan A, Lemos DR, Rossi FM. Fibro/adipogenic progenitors: a double-edged sword in skeletal muscle regeneration. Cell Cycle. 2010;9:2045–2046. doi: 10.4161/cc.9.11.11854. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2011;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffield J. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 19.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 22.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24:559–572. doi: 10.1681/ASN.2012080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ieronimakis N, Hays AL, Janebodin K, Mahoney WM, Jr, Duffield JS, Majesky MW, Reyes M. Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFβ1 signaling in the mdx mouse model of Duchenne muscular dystrophy. J Mol Cell Cardiol. 2013;63:122–134. doi: 10.1016/j.yjmcc.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–R1123. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouget C. Mémoire sur le développement, la structure et les propriétés physiologiques des capillaries sanguins et lymphatiques. Arch Physiol Norm Pathol. 1873;5:603–663. [Google Scholar]
- 27.Zimmerman K. Der feinere Bau der Blutcapillaren. Z Anat Entwicklungsgesch. 1923;68:29–36. [Google Scholar]
- 28.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Courtoy PJ, Boyles J. Fibronectin in the microvasculature: localization in the pericyte-endothelial interstitium. J Ultrastruct Res. 1983;83:258–273. doi: 10.1016/s0022-5320(83)90133-8. [DOI] [PubMed] [Google Scholar]
- 30.Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- 31.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 35.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaissling B, Hegyi I, Loffing J, Le Hir M. Morphology of interstitial cells in the healthy kidney. Anat Embryol (Berl) 1996;193:303–318. doi: 10.1007/BF00186688. [DOI] [PubMed] [Google Scholar]
- 37.Rhodin JA. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968;25:452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- 38.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 39.Davis GE, Saunders WB. Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc. 2006;11:44–56. doi: 10.1038/sj.jidsymp.5650008. [DOI] [PubMed] [Google Scholar]
- 40.Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009;114:237–247. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SW, Chand S, Savage CO. Biology of the renal pericyte. Nephrol Dial Transplant. 2012;27:2149–2155. doi: 10.1093/ndt/gfs134. [DOI] [PubMed] [Google Scholar]
- 43.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayden MR, Karuparthi PR, Habibi J, Lastra G, Patel K, Wasekar C, Manrique CM, Ozerdem U, Stas S, Sowers JR. Ultrastructure of islet microcirculation, pericytes and the islet exocrine interface in the hip rat model of diabetes. Exp Biol Med (Maywood) 2008;233:1109–1123. doi: 10.3181/0709-RM-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caruso RA, Fedele F, Finocchiaro G, Pizzi G, Nunnari M, Gitto G, Fabiano V, Parisi A, Venuti A. Ultrastructural descriptions of pericyte/endothelium peg-socket interdigitations in the microvasculature of human gastric carcinomas. Anticancer Res. 2009;29:449–453. [PubMed] [Google Scholar]
- 47.Suzuki K, Masawa N, Sakata N, Takatama M. Pathologic evidence of microvascular rarefaction in the brain of renal hypertensive rats. J Stroke Cerebrovasc Dis. 2003;12:8–16. doi: 10.1053/jscd.2003.1. [DOI] [PubMed] [Google Scholar]
- 48.Tilton RG, Kilo C, Williamson JR, Murch DW. Differences in pericyte contractile function in rat cardiac and skeletal muscle microvasculatures. Microvasc Res. 1979;18:336–352. doi: 10.1016/0026-2862(79)90042-6. [DOI] [PubMed] [Google Scholar]
- 49.Kelley C, D’Amore P, Hechtman HB, Shepro D. Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. J Cell Biol. 1987;104:483–490. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sims DE, Miller FN, Donald A, Perricone MA. Ultrastructure of pericytes in early stages of histamine-induced inflammation. J Morphol. 1990;206:333–342. doi: 10.1002/jmor.1052060310. [DOI] [PubMed] [Google Scholar]
- 51.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Cai X, Lin Y, Friedrich CC, Neville C, Pomerantseva I, Sundback CA, Zhang Z, Vacanti JP, Hauschka PV, Grottkau BE. Bone marrow derived pluripotent cells are pericytes which contribute to vascularization. Stem Cell Rev. 2009;5:437–445. doi: 10.1007/s12015-009-9097-6. [DOI] [PubMed] [Google Scholar]
- 53.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 54.Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- 55.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amselgruber WM, Schafer M, Sinowatz F. Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study. Anat Histol Embryol. 1999;28:157–166. doi: 10.1046/j.1439-0264.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 57.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin ES, Huang Q, Gurel Z, Palenski TL, Zaitoun I, Sorenson CM, Sheibani N. STAT1-mediated Bim expression promotes the apoptosis of retinal pericytes under high glucose conditions. Cell Death Dis. 2014;5:e986. doi: 10.1038/cddis.2013.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang L, Noseda M, Higginson M, Ly M, Patenaude A, Fuller M, Kyle AH, Minchinton AI, Puri MC, Dumont DJ, Karsan A. Differentiation of vascular smooth muscle cells from local precursors during embryonic and adult arteriogenesis requires notch signaling. Proc Natl Acad Sci USA. 2012;109:6993–6998. doi: 10.1073/pnas.1118512109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- 64.Mallamaci F, Benedetto FA, Tripepi G, Cutrupi S, Pizzini P, Stancanelli B, Seminara G, Bonanno G, Rapisarda F, Fatuzzo P, Malatino LS, Zoccali C. Vascular endothelial growth factor, left ventricular dysfunction and mortality in hemodialysis patients. J Hypertens. 2008;26:1875–1882. doi: 10.1097/HJH.0b013e328307c3d2. [DOI] [PubMed] [Google Scholar]
- 65.Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N. Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol. 2002;13:1795–1805. doi: 10.1097/01.asn.0000018408.51388.57. [DOI] [PubMed] [Google Scholar]
- 66.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 67.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 68.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 71.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS. Targeting endothelium-pericyte crosstalk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 73.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 75.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takata F, Dohgu S, Matsumoto J, Takahashi H, Machida T, Wakigawa T, Harada E, Miyaji H, Koga M, Nishioku T, Yamauchi A, Kataoka Y. Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-alpha, releasing matrix metalloproteinase-9 and migrating in vitro. J Neuroinflammation. 2011;8:106. doi: 10.1186/1742-2094-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 2008;443:83–101. doi: 10.1016/S0076-6879(08)02005-3. [DOI] [PubMed] [Google Scholar]
- 80.Grgic I, Krautzberger AM, Hofmeister A, Lalli M, Dirocco DP, Fleig SV, Liu J, Duffield JS, McMahon AP, Aronow B, Humphreys BD. Translational profiles of medullary myofibroblasts during kidney fibrosis. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013101143. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang WM, Ge G, Lim NH, Nagase H, Greenspan DS. TIMP-3 inhibits the procollagen N-proteinase ADAMTS-2. Biochem J. 2006;398:515–519. doi: 10.1042/BJ20060630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by SRC kinases and PTP-BL phosphatase. Mol Cell. 2002;9:725–737. doi: 10.1016/s1097-2765(02)00488-4. [DOI] [PubMed] [Google Scholar]
- 83.Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A, Tosato G. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–1716. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 85.Poss KD. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet. 2010;11:710–722. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bao J, Zheng JJ, Wu D. The structural basis of DKK-mediated inhibition of Wnt/LRP signaling. Sci Signal. 2012;5:pe22. doi: 10.1126/scisignal.2003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ren S, Johnson BG, Kida Y, Ip C, Davidson KC, Lin SL, Kobayashi A, Lang RA, Hadjantonakis AK, Moon RT, Duffield JS. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci USA. 2013;110:1440–1445. doi: 10.1073/pnas.1211179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. 2013;24:1399–1412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101:581–589. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 91.Kim MK, Maeng YI, Sung WJ, Oh HK, Park JB, Yoon GS, Cho CH, Park KK. The differential expression of TGF-beta1, ILK and wnt signaling inducing epithelial to mesenchymal transition in human renal fibrogenesis: an immunohistochemical study. Int J Clin Exp Pathol. 2013;6:1747–1758. [PMC free article] [PubMed] [Google Scholar]
- 92.Fang Y, Ginsberg C, Seifert M, Agapova O, Sugatani T, Register TC, Freedman BI, Monier-Faugere MC, Malluche H, Hruska KA. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013080818. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, Rohwedder I, Hinkel R, Gross L, Lee S, Hu J, Soehnlein O, Franz WM, Sperandio M, Pohl U, Thomas M, Weber C, Augustin HG, Fassler R, Deutsch U, Kupatt C. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest. 2013 doi: 10.1172/JCI66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 95.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 96.Thurston G. Role of angiopoietins and tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61–68. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 97.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 98.David S, Park JK, Meurs M, Zijlstra JG, Koenecke C, Schrimpf C, Shushakova N, Gueler F, Haller H, Kumpers P. Acute administration of recombinant angiopoietin-1 ameliorates multiple-organ dysfunction syndrome and improves survival in murine sepsis. Cytokine. 2011;55:251–259. doi: 10.1016/j.cyto.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- 100.Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 101.Takakura N, Yoshida H, Ogura Y, Kataoka H, Nishikawa S. PDGFR alpha expression during mouse embryogenesis: immunolocalization analyzed by whole-mount immunohistostaining using the monoclonal antimouse PDGFR alpha antibody APA5. J Histochem Cytochem. 1997;45:883–893. doi: 10.1177/002215549704500613. [DOI] [PubMed] [Google Scholar]
- 102.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruiter DJ, Schlingemann RO, Westphal JR, Denijn M, Rietveld FJ, De Waal RM. Angiogenesis in wound healing and tumor metastasis. Behring Inst Mitt. 1993:258–272. [PubMed] [Google Scholar]
- 105.Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev Biol. 2010;344:1035–1046. doi: 10.1016/j.ydbio.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270:469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- 107.Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol. 2006;17:2992–2998. doi: 10.1681/ASN.2006050420. [DOI] [PubMed] [Google Scholar]
- 108.Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162:721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.MacFadyen JR, Haworth O, Roberston D, Hardie D, Webster MT, Morris HR, Panico M, Sutton-Smith M, Dell A, van der Geer P, Wienke D, Buckley CD, Isacke CM. Endosialin (TEM1, CD248) is a marker of stromal fibroblasts and is not selectively expressed on tumour endothelium. FEBS Lett. 2005;579:2569–2575. doi: 10.1016/j.febslet.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 110.Smith SW, Eardley KS, Croft AP, Nwosu J, Howie AJ, Cockwell P, Isacke CM, Buckley CD, Savage CO. CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney Int. 2011;80:199–207. doi: 10.1038/ki.2011.103. [DOI] [PubMed] [Google Scholar]
- 111.Dermietzel R, Krause D. Molecular anatomy of the blood-brain barrier as defined by immunocytochemistry. Int Rev Cytol. 1991;127:57–109. doi: 10.1016/s0074-7696(08)60692-0. [DOI] [PubMed] [Google Scholar]
- 112.Kunz J, Krause D, Kremer M, Dermietzel R. The 140-kDa protein of blood-brain barrier-associated pericytes is identical to amino-peptidase N. J Neurochem. 1994;62:2375–2386. doi: 10.1046/j.1471-4159.1994.62062375.x. [DOI] [PubMed] [Google Scholar]
- 113.Stefanovic V, Vlahovic P, Ardaillou N, Ronco P, Ardaillou R. Cell surface aminopeptidase A and N activities in human glomerular epithelial cells. Kidney Int. 1992;41:1571–1580. doi: 10.1038/ki.1992.227. [DOI] [PubMed] [Google Scholar]
- 114.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K, Lloyd RV. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–2290. [PubMed] [Google Scholar]
- 115.Pierelli L, Bonanno G, Rutella S, Marone M, Scambia G, Leone G. CD105 (endoglin) expression on hematopoietic stem/progenitor cells. Leuk Lymphoma. 2001;42:1195–1206. doi: 10.3109/10428190109097744. [DOI] [PubMed] [Google Scholar]
- 116.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 117.Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992;116:817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sato A, Iwama A, Takakura N, Nishio H, Yancopoulos GD, Suda T. Characterization of TEK receptor tyrosine kinase and its ligands, Angiopoietins, in human hematopoietic progenitor cells. Int Immunol. 1998;10:1217–1227. doi: 10.1093/intimm/10.8.1217. [DOI] [PubMed] [Google Scholar]
- 120.Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol. 1998;153:1459–1466. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakayama T, Yao L, Tosato G. Mast cell-derived angiopoietin-1 plays a critical role in the growth of plasma cell tumors. J Clin Invest. 2004;114:1317–1325. doi: 10.1172/JCI22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]