Abstract
Aims
Enhanced cardiac late Na current (late INa) and increased sarcoplasmic reticulum (SR)-Ca2+-leak are both highly arrhythmogenic. This study seeks to identify signalling pathways interconnecting late INa and SR-Ca2+-leak in atrial cardiomyocytes (CMs).
Methods and results
In murine atrial CMs, SR-Ca2+-leak was increased by the late INa enhancer Anemonia sulcata toxin II (ATX-II). An inhibition of Ca2+/calmodulin-dependent protein kinase II (Autocamide-2-related inhibitory peptide), protein kinase A (H89), or late INa (Ranolazine or Tetrodotoxin) all prevented ATX-II-dependent SR-Ca2+-leak. The SR-Ca2+-leak induction by ATX-II was not detected when either the Na+/Ca2+ exchanger was inhibited (KBR) or in CaMKIIδc-knockout mice. FRET measurements revealed increased cAMP levels upon ATX-II stimulation, which could be prevented by inhibition of adenylyl cyclases (ACs) 5 and 6 (NKY 80) but not by inhibition of phosphodiesterases (IBMX), suggesting PKA activation via an AC-dependent increase of cAMP levels. Western blots showed late INa-dependent hyperphosphorylation of CaMKII as well as PKA target sites at ryanodine receptor type-2 (-S2814 and -S2808) and phospholamban (-Thr17, -S16). Enhancement of late INa did not alter Ca2+-transient amplitude or SR-Ca2+-load. However, upon late INa activation and simultaneous CaMKII inhibition, Ca2+-transient amplitude and SR-Ca2+-load were increased, whereas PKA inhibition reduced Ca2+-transient amplitude and load and additionally slowed Ca2+ elimination. In atrial CMs from patients with atrial fibrillation, inhibition of late INa, CaMKII, or PKA reduced the SR-Ca2+-leak.
Conclusion
Late INa exerts distinct effects on Ca2+ homeostasis in atrial myocardium through activation of CaMKII and PKA. Inhibition of late INa represents a potential approach to attenuate CaMKII activation and decreases SR-Ca2+-leak in atrial rhythm disorders. The interconnection with the cAMP/PKA system further increases the antiarrhythmic potential of late INa inhibition.
Keywords: Late Na current, Antiarrhythmic drugs, Atrial fibrillation, Protein kinases, SR-Ca2+-leak
1. Introduction
The late Na+ current (late INa) is a small Na+ inward current, which follows the peak Na+ current, persists throughout the action potential1 and plays a small electrophysiological role under physiological conditions. However, an enhanced late INa significantly contributes to total Na+ influx.2 Late INa was shown to be increased in ischemia3 and oxidative stress4 as well as in heart failure5 and atrial fibrillation (AF).6 Under these conditions, the total amount of Na+ entering the cell via late INa can even exceed that of peak INa.7 In addition, an increased late INa prolongs action potential duration (APD)8 and promotes early afterdepolarizations (EADs).9 EADs and triggered atrial activity constitute a pathomechanism for the initiation and perpetuation of AF.10 Another relevant mechanism in AF is an increased SR-Ca2+-leak,11 which occurs when altered ryanodine receptor type-2 (RyR2) spontaneously opens in diastole releasing small amounts of Ca2+ (Ca2+ sparks). The increased diastolic Ca2+ leak in AF is caused by CaMKII-dependent hyperphosphorylation of RyR2, which triggers delayed afterdepolarizations (DADs) via activation of the Na+/Ca2+ exchanger (NCX) leading to transient inward currents (Iti).12 Furthermore, it has been suggested that PKA-dependent phosphorylation of RyR2 decreases the amount of RyR2-bound calstabin-2 and also contributes to the instability of RyR2.13 Thus, both arrhythmic triggers (increased late INa and increased SR-Ca2+-leak) coexist in AF. This study investigates if both cellular phenomena are linked and elucidates the underlying pathways.
2. Methods
2.1. Human myocardial tissue
All procedures performed conform to the Declaration of Helsinki and were approved by the local ethics committee. Written informed consent was received from all participants prior to inclusion. Human atrial myocardium was obtained from resections of the left atrial appendage during open heart surgery. The samples were immediately placed in cooled cardioplegic solution (in mmol/L: NaCl 110, KCl 16, MgCl2 16, NaHCO3 16, CaCl2 1.2, and glucose 11) and kept cool until cell isolation started or instantly frozen in liquid nitrogen and stored at −80°C for western blot analysis.
2.2. Myocyte isolation
2.2.1. Mouse
The animal study was approved by the local or ethics review board and procedures were performed in accordance with the NIH guidelines (guide for the care and use of laboratory animals) and the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Mice were killed in isoflurane 133 anaesthesia (5%) by cervical dislocation. To ensure sufficient digestion of atrial myocardium, mice were administered 100 IU heparin by an intraperitoneal injection prior to isolation. A Langendorff apparatus was used to retrogradely perfuse the explanted hearts with an initially Ca2+-free solution containing (in mmol/L) NaCl 113, KCl 4.7, KH2PO4 0.6, Na2HPO4·2H2O 0.6, MgSO4·7H2O 1.2, NaHCO3 12, KHCO3 10, HEPES 10, Taurine 30, 2,3-butanedione monoxime (BDM) 10, glucose 5.5, and phenol red 0.032 (37°C, pH 7.4). Then, 7.5 mg/mL of liberase 1 (Roche Diagnostics, Mannheim, Germany) and trypsin 0.6% (Life Technologies, Carlsbad, CA, USA) as well as 0.125 mmol/L of CaCl2 were added to the perfusion solution. Once the tissue became flaccid, ventricular and atrial myocardium was separated by cutting off the atria above the atrioventricular valve level. The atrial myocardium was cut into small pieces and again dispersed in solution. Concentration of Ca2+ was stepwise increased every 7 min until desired concentration was reached. Cells were plated on laminin-coated recording chambers and left for 20 min to enable settling.
2.2.2. Human
Left atrial myocardium was rinsed, cut into small pieces and incubated at 37°C in a spinner flask filled with Ca2+-free solution containing 1.0 mg/mL of collagenase (Worthington type 1, 185 U/mg), 0.05 mg/mL of proteinase (Life Technologies) and (in mmol/L): NaCl 88, sucrose 88, KCl 5.4, NaHCO3 4, NaH2PO4 0.3, MgCl 1, HEPES 10, Taurine 20, glucose 10, and sodium pyruvate 5 (pH 7.4). After 45 min, the supernatant was discarded and fresh Ca2+-free solution containing only collagenase was added. The myocytes were incubated in the solution for 10–20 min and agitated using a Pasteur pipette. The supernatant containing dispersed cells was removed and centrifuged (59 g, 7 min). Fresh Ca2+-free solution with collagenase was added to the remaining tissue. This procedure was repeated 4–5 times. After every step, the centrifuged cells were resuspended in KB medium containing (mmol/L): Taurine 10, glutamic acid 70, KCl 25, KH2PO4 10, dextrose 22, EGTA 0.5, and bovine calve serum 10% (pH 7.4, KOH, room temperature). Only cell solutions containing elongated cardiomyocytes (CMs) with clear cross striations were selected for experiments, plated on laminin-coated recording chambers, and left to settle for 30 min.
2.3. Patch-clamp experiments
Ruptured-patch whole-cell voltage-clamp experiments were used to measure late INa using microelectrodes (2–3 MΩ). Pipettes were filled with (in mmol/L) 95 CsCl, 40 Cs-glutamate, 10 NaCl, 0.92 MgCl2, 5 Mg-ATP, 0.3 Li-GTP, 5 HEPES, 0.03 niflumic acid (to block Ca-activated chloride current), 0.02 nifedipine (to block Ca current), 0.004 strophanthidin (to block NCX current), 1 EGTA, and 0.36 CaCl2 (free [Ca2+]i,100 nmol/L; pH 7.2, CsOH). The bath solution contained (in mmol/L) 135 NaCl, 5 tetramethylammonium chloride, 4 CsCl, 2 MgCl2, 10 glucose, and 10 HEPES (pH 7.4, NaOH). To minimize contaminating Ca2+ currents during late INa measurements, Ca2+ was omitted from the bath solution. Anemonia sulcata toxin II (ATX-II; 0.5 nmol/L) vs. control was added to the bath solution. Access resistance was <7 MΩ. In all experiments, myocytes were mounted on the stage of a microscope (Nikon T 300). Fast capacitance was compensated in cell-attached configuration. Membrane capacitance and series resistance were compensated after patch rupture. Signals were filtered with 2.9 and 10 kHz Bessel filters and recorded with an EPC10 amplifier (HEKA Elektronik). Myocytes were held at −120 mV and late INa was elicited using a train of pulses to −35 mV (1000 ms duration, 10 pulses, and basic cycle length 2000 ms). Recordings were started 5 min after rupture. Experiments were conducted at room temperature. The measured current was integrated (between 50 and 100 ms) and normalized to the membrane capacitance.
For action potential recordings, ruptured-patch whole-cell voltage-clamp experiments were used to measure membrane potential (current-clamp configuration). For membrane potential measurements, microelectrodes were filled with (in mmol/L) 120 K-aspartate, 8 KCl, 7 NaCl, 1 MgCl2, 10 HEPES, and 5 Mg-ATP (pH 7.2, KOH). The bath solution contained (mmol/L) 135 NaCl, 5.4 KCl, 1 MgCl2, 1 CaCl2, 10 glucose, and 10 HEPES (pH 7.4, NaOH). Action potentials were continuously elicited by square current pulses of 1–2 nA amplitude and 1–5 ms duration at 3 Hz. Access resistance was typically ∼20 MΩ after patch rupture. When appropriate, ATX-II (0.5 nmol/L) or ranolazine (10 µmol/L) was added to the bath solution. Fast capacitance was compensated in cell-attached configuration. Membrane capacitance and series resistance were compensated after patch rupture. Signals were filtered with 2.9 and 10 kHz Bessel filters and recorded with an EPC10 amplifier (HEKA Elektronik). Recordings were started 5 min after rupture. All experiments were conducted at room temperature.
2.4. Intracellular Ca2+ imaging
2.4.1. Confocal microscopy (measurement of SR Ca2+ sparks)
2.4.1.1. Mouse
Isolated CMs were incubated at room temperature for 30 min with a Fluo-3 AM loading buffer (10 μmol/L, Molecular Probes), which in the treatment groups contained ATX-II (0.5 nmol/L) alone or in combination with Ranolazine (10 μmol/L, Gilead Sciences, Palo Alto, CA, USA), CaMKII inhibitor Autocamide-2-related inhibitory peptide (AIP, 1 μmol/L, Alexis Corp., Switzerland), Tetrodotoxin (TTX, 2 μmol/L), or KBR (0.1 μmol/L). AIP was used in its myristoylated form to ensure cell permeability. Experimental solution contained (in mmol/L): KCl 4, NaCl 140, MgCl2 1, HEPES 5, glucose 10, CaCl2 2 (pH 7.4, NaOH, room temperature), and the respective active agents. Cells were continuously superfused during experiments. To wash out the loading buffer and remove any extracellular dye as well as to allow enough time for complete de-esterification of Fluo-3 AM, cells were superfused with experimental solution for 5 min before experiments were started. Ca2+ spark measurements were performed with a laser scanning confocal microscope (LSM 5 Pascal, Zeiss) using a ×40 oil immersion objective. Fluo-3 was excited by an argon ion laser (488 nm) and emitted fluorescence was collected through a 505 nm long-pass emission filter. Fluorescence images were recorded in the line scan mode with 512 pixels per line (width of each scan line: 38.4 μm) and a pixel time of 0.64 μs. One image consists of 10 000 unidirectional line scans, equating to a measurement period of 7.68 s. Experiments were conducted at resting conditions after loading the SR with Ca2+ by repetitive field stimulation (at 3 Hz, 20 V). Typically shaped Ca2+ sparks were detected by eye and quantified by the program SparkMaster for ImageJ with the discrimination criterion 4.4 SD. The mean spark frequency of the respective cell (CaSpF) resulted from the number of sparks normalized to cell width and scan rate (100 μm−1 s−1). Spark size (CaSpS) was calculated as the product of spark amplitude (F/F0), duration, and width. From this, we inferred the average leak per cell by multiplication of CaSpS with CaSpF. For the illustration of detected Ca2+ sparks (Figures 2A, 3A, 4A, and 8A), representative confocal line scans from each group were converted into a surface plot using ImageJ (x-axis = time; y-axis = width; z-axis = brightness, respective range of grey values as annotated).
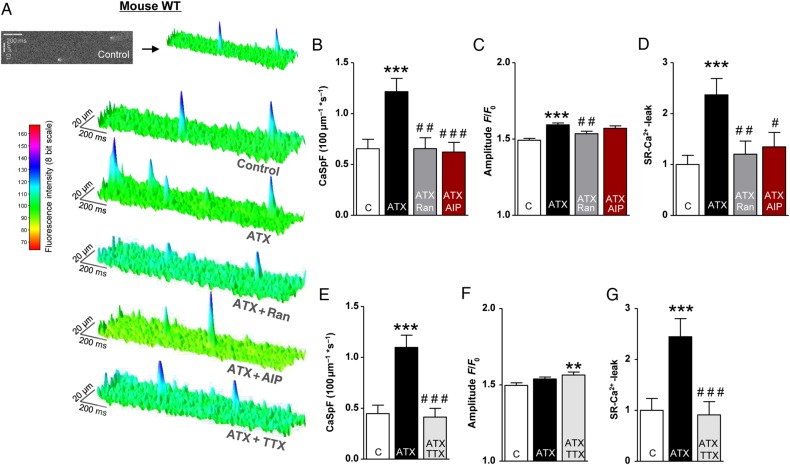
Figure 2.
Effects of late INa modulations and CaMKII inhibition on SR-Ca2+-leak in atrial CM of WT mice. (A) Surface plot of representative confocal line scans. (B–G) Mean values of CaSpF, Ca2+ spark amplitude, and the total calculated SR-Ca2+-leak in the presence of the respective drugs (B–D: n mice/cells = 6/122 vs. 6/123 vs. 6/108 vs. 6/119; E–G: n = 5/89 vs. 5/93 vs. 5/92). *significant vs. control; #significant vs. ATX-II. */#P < 0.05; **/##P < 0.01; ***/###P < 0.001.
Figure 3.
The impact of NCX inhibition and CaMKIIδc-KO on ATX-II-mediated induction of the SR-Ca2+-leak in murine atrial CMs. (A) Surface plot of representative confocal line scans. (B–D) Mean values of the diastolic CaSpF, spark amplitude, and the SR-Ca2+-leak (n mice/cells = 5/71 vs. 5/61 vs. 5/75). (E) Representative confocal line scans of atrial CM from CaMKIIδc-KO mice. (F) Mean values of the SR-Ca2+-leak (n = 5/148 vs. 5/140, P = 0.40). *significant vs. control; #significant vs. ATX-II. */#P < 0.05; **/##P < 0.01; ***/###P < 0.001.
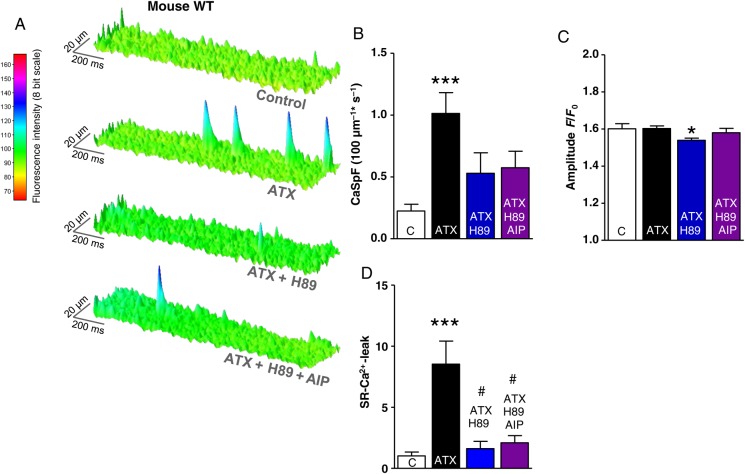
Figure 4.
Effects of late INa modifications and PKA inhibition on SR-Ca2+-leak in murine atrial CMs. (A) Surface plot of representative confocal line scans. (B–D) Mean values of the diastolic CaSpF, spark amplitude, and SR-Ca2+-leak (n mice/cells = 6/100 vs. 6/87 vs. 4/33 vs. 3/47). *significant vs. control; #significant vs. ATX-II; */#P< 0.05; **/##P<0.01; ***/###P< 0.001.
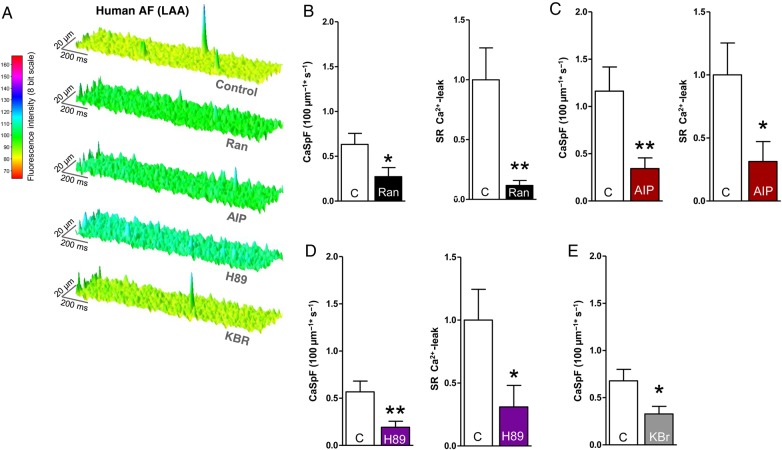
Figure 8.
Effects of late INa, CaMKII, and PKA inhibition on SR-Ca2+-leak in left atrial CMs of AF patients. (A) Surface plot of representative confocal line scans. Mean values of CaSpF and SR-Ca2+-leak after treatment with (B) Ranolazine (n patients/cells = 5/100 vs. 5/71), (C) AIP (n = 4/44 vs. 4/34), (D) H89 (n = 4/95 vs. 4/78), and (E) KBR (n = 5/124 vs. 5/120, P < 0.01). *significant vs. control; */#P< 0.05; **/##P< 0.01; ***/###P < 0.001.
2.4.1.2. Human
Isolated CMs were stained and measured as annotated above with the following differences: Fluo 3 AM loading buffer either contained Ranolazine (10 μmol/L), AIP (1 μmol/L), H89 (5 μmol/L), KBR (0.1 μmol/L), or no active agent. The experimental solution contained (in mmol/L): NaCl 136, KCl 4, NaH2PO4 0.33, NaHCO3 4, CaCl2 2, MgCl2 1.6, HEPES 10, glucose 10 (pH 7.4, NaOH, room temperature), and the respective active agents. Cells were stimulated at 1 Hz for 10 s (20 V) to ensure appropriate SR-Ca2+-load before measurements were started.
2.4.2. Epifluorescence microscopy (systolic Ca2+ transients and SR Ca2+ content)
2.4.2.1. Mouse
CMs were isolated and plated as described above and incubated with a Fluo-4 AM loading buffer (10 μmol/L, Molecular Probes) for 15 min. In the intervention groups, the loading buffer also contained the respective active agents as described above for mouse/human confocal microscopic measurements. After staining, the CMs were superfused with experimental solution (as described in the section ‘Confocal microscopy’) for 5 min before measurements were started to enable complete de-esterification of intracellular Fluo-4 and allow cellular rebalance of Ca2+ cycling properties. During measurements, CMs were continuously superfused with experimental solution. Measurements were performed with a Nikon Eclipse TE2000-U microscope provided with a fluorescence detection system (ION OPTIX Corp.). Cells were excited at 480 ± 15 nm, and the emitted fluorescence was collected at 530 ± 20 nm. Systolic Ca2+ transients were recorded at steady-state conditions under constant field stimulation (1, 2, and 4 Hz). To assess the SR Ca2+ content, the amplitude of caffeine-induced Ca2+ transients was measured. After stopping the stimulation during steady-state conditions at 1 Hz, caffeine (10 mmol/L, Sigma-Aldrich, St Louis, MO, USA) was applied directly onto the cell, leading to immediate and complete SR Ca2+ release. The recorded Ca2+ transients were analysed with the software IONWizardR (ION OPTIX Corp.). For the evaluation of the speed of Ca2+ reuptake into the SR, the decay constant k (reciprocal of Tau) of caffeine-induced Ca2+ transients was subtracted from the decay constant k of systolic Ca2+ transients at 1 Hz (ksys − kcaff).
2.5. Western blots
Mouse hearts were rapidly removed and immersed in oxygenated Krebs–Henseleit buffer (in mmol/L: NaCl 118, KCl 4.8, KH2PO4 1.2, CaCl2 2.5, MgSO4 1.2, pyruvic acid 2, glucose 5.5, Na2EDTA 0.5, and NaHCO3 25, pH 7.4). Mouse right atria were quickly excised and suspended in a chamber of the Danish Myo Technologies (DMT) myograph containing 8 mL of oxygenated Krebs–Henseleit buffer at 37°C. The spontaneously beating right atria were preincubated with late Na+ current, CaMKII, or PKA inhibitors for 30 min, and then incubated with 0.5 nmol/L of ATX-II for an additional 30 min. The atria were then snap-frozen in liquid nitrogen and stored at −80°C. Atria were lysed with Tissue Lyser (Qiagen, Valencia, CA, USA) in 0.2 mL of lysis buffer containing 20 mmol/L of Tris, pH 7.5, 2 mmol/L of EDTA, 10 mmol/L of EGTA, 0.25 mmol/L of sucrose, 1% Triton X-100, and 1 tablet of protease inhibitor/10 mL (Sigma, St Louis, MO, USA). Lysates were incubated on ice for 20 min and then centrifuged at 9408 g for 5 min at 4°C. The supernatant was saved. About 20 μg of protein was loaded with SDS sample buffer, run on a 10% SDS–PAGE, transferred, and probed for phospho-CaMKII at threonine 287 and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-phospholamban (PLN) at threonine 16, phospho-PLN at threonine 17, phospho-RyR2 at serine 2808, and phospho-RyR2 at serine 2814 (Badrilla, Leeds, UK). Secondary antibody was horseradish peroxidase-linked goat anti-rabbit (1 : 1000; PerkinElmer Life and Analytical Sciences, Waltham, MA, USA). The intensity of individual bands from western blots was quantified using ImageJ software, normalized to GAPDH, and then shown relative to the control group value.
2.6. FRET measurements and data analysis
Freshly isolated adult mouse atrial CMs from EPAC1-camps mice were plated onto laminin-coated coverslides and allowed to settle for 30 min. Subsequently, coverslides with isolated CMs were mounted in an imaging chamber (Attofluor cell chamber, Invitrogen) equipped with an electrode. After washing the cells once with Tyrode solution (in mmol/L: KCl 1, NaCl 149, MgCl2 1, HEPES 5, glucose 10, and CaCl2 1, pH 7.54), 400 µL of Tyrode solution was added to the chamber. The cells were preincubated for 7 min with Tyrode solution containing IBMX (Applichem, 0.1 mmol/L), NKY80 (Sigma-Aldrich, 0.01 mmol/L), KBR (0.1 μmol/L), or no inhibitor in the control group. Subsequently, myocytes were field-stimulated at 1 Hz and FRET measurements (Förster resonance energy transfer) were performed using an inverted fluorescent microscope (Nikon Ti) and the MicroManager software. The FRET donor cyan fluorescent protein (CFP) was excited at 440 nm using a CoolLED light source. Images were acquired every 5 s in CFP and YFP emission channels, with an exposure time of usually 10 ms as described. The compounds were diluted in Tyrode solution. ATX-II (0.5 nmol/L) was added to the imaging chamber 50 s after the start of the measurements, and 100 nmol/L of isoprenaline was added after the ATX-II reaction reached a stable plateau, usually after 5 min. FRET data analysis was performed using the Origin 8.5 software as previously described. To calculate the corrected FRET ratio, we subtracted the bleedthrough of the donor fluorescence (CFP) into the acceptor (YFP) channel using the equation: FRET ratio = (YFP − 0.63 × CFP)/CFP, where 0.63 is a bleedthrough coefficient measured in cells expressing CFP only and normalized the values to the initial FRET ratio recorded at the beginning of every experiment. These data are presented as YFP/CFP ratio (norm.) in the results.
2.7. Statistical analysis
All data are presented as mean ± SEM. Student's t-test was used except for Figures 1D and 7D (two-way ANOVA with the Bonferroni post hoc test). Values with P< 0.05 were considered as statistically significant.
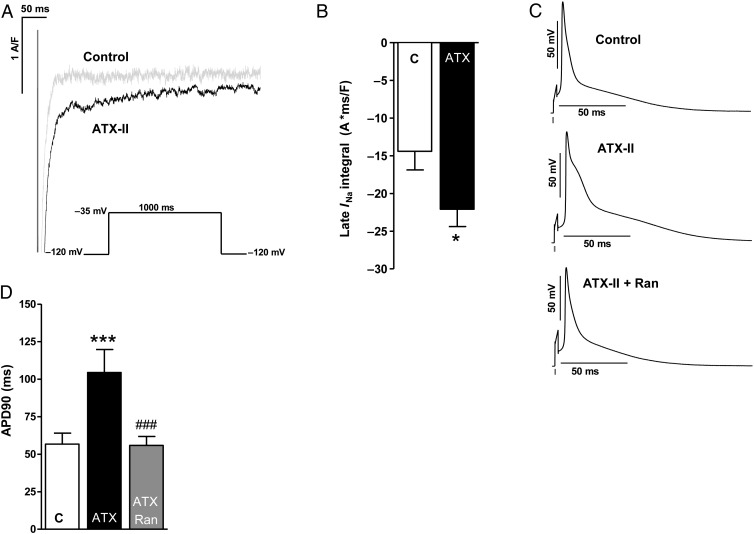
Figure 1.
Effects of ATX-II treatment (0.5 nmol/L) on late INa of murine atrial CMs. (A) Representative original patch-clamp recordings of late Na+ currents. (B) Mean values of late INa integral 50–100 ms (n mice/cells = 8/17 vs. 8/23). (C) Original recordings of action potentials (3 Hz) of untreated murine atrial CMs (above) as well as upon treatment with ATX-II (0.5 nmol/L, middle) and ATX-II + Ranolazine (10 µmol/L, below). (D) Quantification of action potential duration (APD90) after wash-in of ATX-II and Ranolazine (n mice/cells = 6/12 each). *significant vs. control; #significant vs. ATX-II; */#P < 0.05; **/##P < 0.01; ***/###P < 0.001.
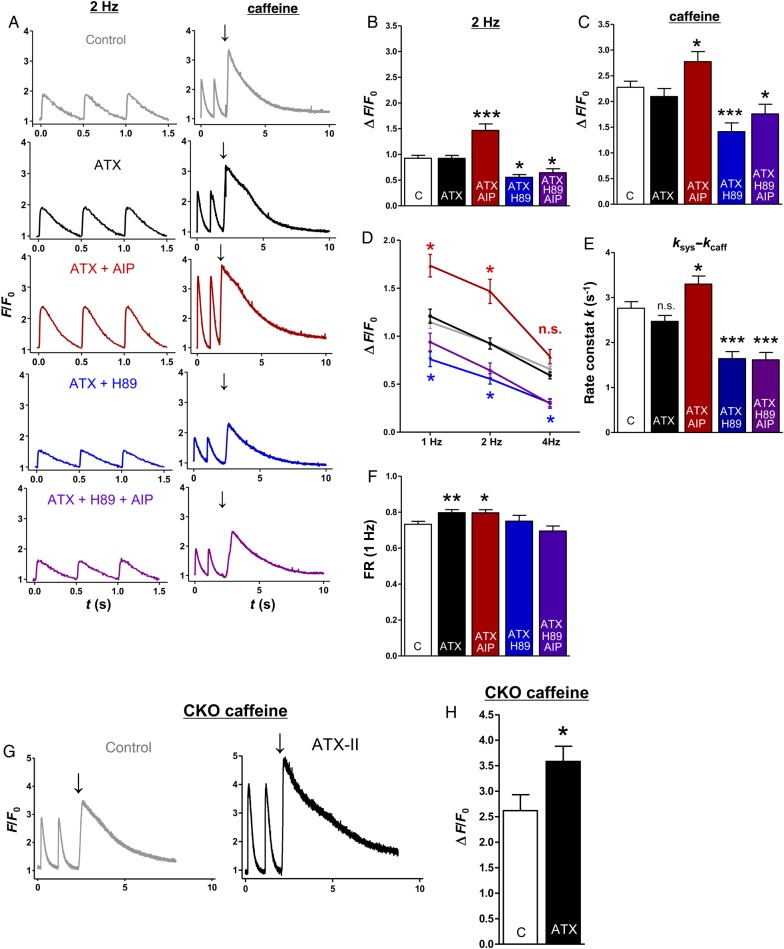
Figure 7.
The impact of late INa modifications and CaMKII/PKA inhibition on stimulated and caffeine-induced Ca2+ transients of murine atrial CMs. (A) Representative original recordings (2 Hz, left) and caffeine-induced Ca2+ transients (right, arrow indicates caffeine application, WT). (B) Mean values of the amplitude (ΔF/F0) of stimulated Ca2+ transients (n mice/cells = 12/65 vs. 11/52 vs. 5/29 vs. 4/16 vs. 4/13 for B, D, E, and F). (C) Mean values of caffeine-induced Ca2+ transients (ΔF/F0; n = 10/42 vs. 11/37 vs. 5/27 vs. 4/15 vs. 4/13). (D) Mean values of Ca2+-transient amplitudes (ΔF/F0) at different stimulation frequencies. (E) Mean values of Ca2+-reuptake speed into the SR (ksys− kcaff). (F) Mean values of fractional Ca2+ release (FR, ΔF/F0 1 Hz/SR-Ca2+-load). (G) Representative original recordings of atrial CMs from CaMKIIδc-KO mice (arrow indicates caffeine application). (H) Mean values of caffeine-induced Ca2+ transients in CaMKIIδc-KO mice (n = 3/19 vs. 3/22; P < 0.05). *significant vs. control. */#P< 0.05; **/##P< 0.01; ***/###P< 0.001.
3. Results
3.1. Effects of late INa modulation and CaMKII inhibition on SR-Ca2+-leak
Atrial CMs isolated from hearts of wild-type (WT) mice were treated with Anemonia sulcata toxin II (ATX-II, 0.5 nmol/L), which led to an increase of the late INa integral by 53 ± 16% compared with untreated control (patch-clamp P < 0.05, Figure 1 A and B). The duration of the action potential (APD90) was prolonged by ∼1.8-fold by ATX-II (APD90 ATX-II vs. control = 104 ± 15 vs. 56 ± 6 ms, Figure 1C and D, P < 0.001). This effect could be antagonized by additional treatment with the late INa inhibitor Ranolazine (10 µmol/L; Figure 1C and D).
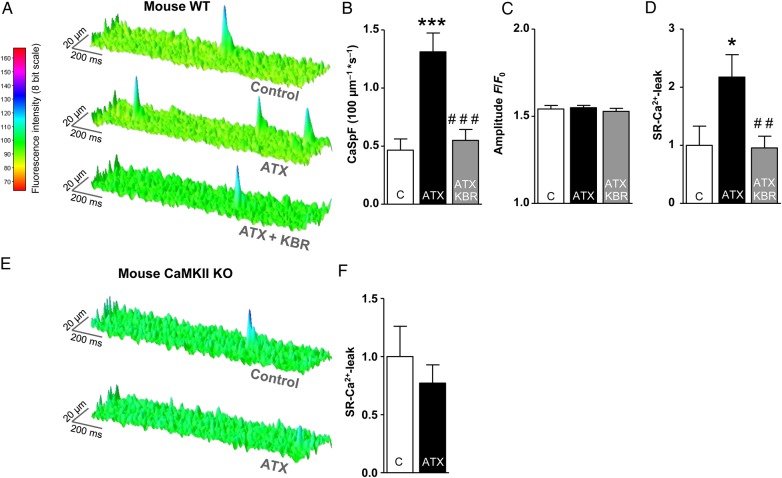
To evaluate the resulting effects on SR-Ca2+-leak, atrial CMs of WT mice were field-stimulated and scanned for diastolic Ca2+ sparks. The basal Ca2+ spark frequency (CaSpF, 0.65 ± 0.09 100 μm−1 s−1) was increased by ATX-II (0.5 nmol/L) to 1.22 ± 0.13 100 μm−1 s−1 (by 78 ± 44%, Figure 2A and B, P < 0.001). In addition, the amplitude of Ca2+ sparks was increased upon ATX-II treatment by 7 ± 1% (Figure 1C, P < 0.001) and the size of detected Ca2+ sparks was also increased (data not shown). Conclusively, the resulting calculated SR-Ca2+-leak (CaSpF × Ca2+ spark duration × width × amplitude) was increased 2.4-fold (Figure 2D, P < 0.001). Accordingly, inhibition of late INa with Ranolazine (10 µmol/L) prevented the ATX-II-induced increase in CaSpF (Figure 2B, ATX-II + Ran vs. ATX-II, P < 0.01) and SR-Ca2+-leak (Figure 2D, reduced by 49 ± 10% compared with ATX-II alone, P < 0.01). To exclude unspecific actions of Ranolazine, we additionally used the Na+ channel blocker TTX (2 μmol/L) in combination with ATX-II (Figure 2E–G), which also reversed the ATX-II effect on SR-Ca2+-leak (Figure 2G, P < 0.001) and increased spark amplitude (Figure 2F, P < 0.01). Interestingly, simultaneous CaMKII inhibition (AIP, 1 µmol/L, Figure 2A–D) as well as an inhibition of NCX (KBR, Figure 3A–D) also prevented the ATX-II-induced increase of CaSpF (P < 0.001 each) and SR-Ca2+-leak (P < 0.05 and <0.01, respectively), suggesting that CaMKII is activated via NCX-dependent Ca2+ overload. Importantly, ATX-II did not induce SR-Ca2+-leak in atrial CMs of CaMKIIδc-knockout mice (CKO) (Figure 3E and F, P = 0.46).
3.2. Effects of late INa modulation and PKA inhibition on SR-Ca2+-leak
Of note, an inhibition of PKA (H89 5 μmol/L) as well as an inhibition of both kinases (H89 5 μmol/L + AIP 1 μmol/L) also attenuated the ATX-II induced increase of CaSpF (Figure 4B, CaSpF control vs. ATX vs. ATX + H89 vs. ATX + H89 + AIP = 0.22 ± 0.05 vs. 1.01 ± 0.20 vs. 0.53 ± 0.17 vs. 0.57 ± 0.13 100 μm−1 s−1) and significantly reduced the calculated SR-Ca2+-leak compared with sole ATX-II treatment (Figure 4D, P < 0.05 each). PKA inhibition additionally reduced the amplitude of detected Ca2+ sparks (Figure 4C, P < 0.05).
3.3. Effects of late INa augmentation on intracellular cAMP levels
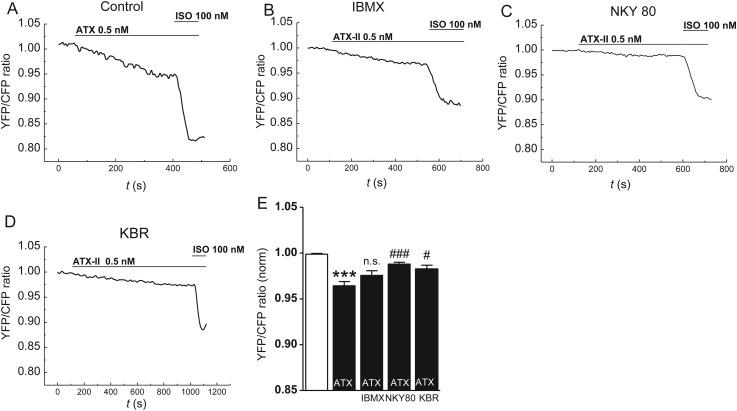
As PKA is activated by cAMP, we measured cytoplasmic cAMP levels upon ATX-II treatment in atrial CMs of EPAC-camps transgenic mice.14 Indeed, ATX-II (0.5 nmol/L) increased cAMP levels (FRET ratio ATX-II vs. control = 0.96 ± 0.0047 vs. 1.0 ± 0.007; P < 0.001, Figure 5A and E). This response made up for ∼28% of the maximal isoproterenol response (FRET ratio Iso 100 nmol/L vs. control = 0.88 ± 0.013 vs. 1.0 ± 0.0017; P < 0.001, Figure 5A). When we pre-incubated atrial CMs with the unselective phosphodiesterase (PDE) inhibitor IBMX (100 µmol/L, Figure 5B), the normalized YFP/CFP ratio upon ATX-II was still lowered compared to control (YFP/CFP ratio = 0.976 ± 0.005 vs. 1.0 ± 0.001, P < 0.001) and did not differ from untreated CMs exposed to ATX-II (YFP/CFP ratio = 0.964 ± 0.05). However, an inhibition of adenylyl cyclase (AC) isoforms AC5 and AC6 (NKY80, 10 µmol/L)15 attenuated the cAMP response to ATX-II (Figure 5C and E; YFP/CFP ratio NKY80 + ATX vs. ATX = 0.988 ± 0.02 vs. 0.964 ± 0.05, P < 0.001). Of note, the preincubation with the reverse mode NCX inhibitor KBR (0.1 μmol/L) also reduced ATX-II-induced cAMP production (Figure 5D and E; YFP/CFP ratio ATX-II + KBR vs. ATX-II = 0.982 ± 0.004 vs. 0.964 ± 0.005, P = 0.01).
Figure 5.
Effects of late INa induction on cellular cAMP levels in atrial CMs of mice. (A–D) YFP/CFP tracings in CMs of EPAC-camps transgenic mice upon ATX-II (0.5 nmol/L) and subsequent isoproterenol treatment (100 nmol/L). CMs were preincubated with a (B) PDE inhibitor IBMX (100 µmol/L), (C) an inhibitor of AC 5/6 (NKY80, 10 µmol/L), (D) an inhibitor of reverse-mode NCX KBR (0.1 µmol/L), or (A) no inhibitor. (E) Quantification of response of YFP/CFP ratio (n mice/cells = 10/39 vs. 3/8 vs. 3/10 vs. 3/11). *significant vs. steady-state before ATX-II; #significant vs. YFP/CFP ratio upon ATX-II in the control group. */ #P < 0.05; **/##P < 0.01; ***/###P< 0.001.
3.4. Effects of late INa modulation on phosphorylation of key Ca2+-handling proteins
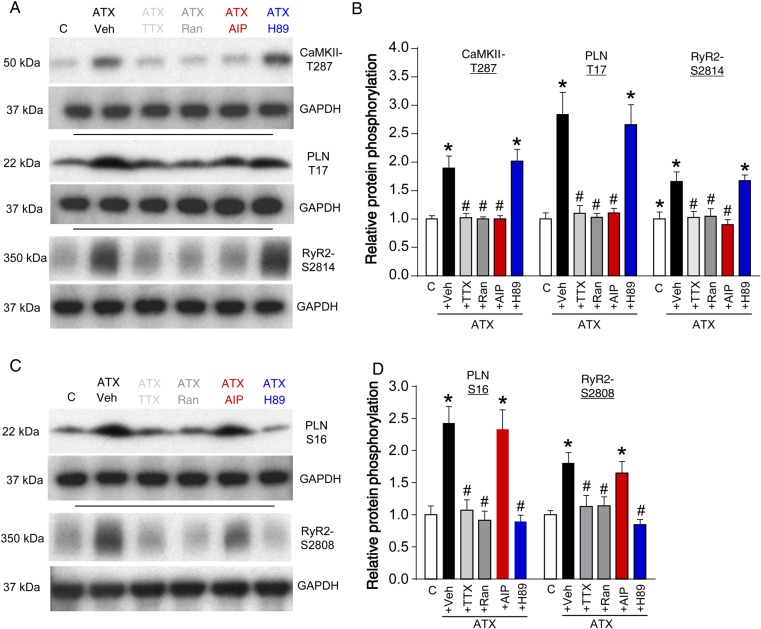
To further investigate the roles of CaMKII and PKA, we performed Western blots from extracted right murine atria that had been perfused with ATX-II and the respective inhibitors (Figure 6 and see Supplementary material online, Figure S1). An augmentation of late INa (ATX-II, 0.5 nmol/L) increased the phosphorylation of CaMKII target sites PLN-T17 and RyR2-S2814 (Figure 6A and B, P < 0.05 each). In addition, CaMKII autophosphorylation at T287 was increased (P < 0.05). The hyperphosphorylation of CaMKII targets could be prevented by Ran (10 µmol/L), TTX (2 µmol/L), and AIP (1 µmol/L), but not by H89 (5 µmol/L; Figure 6A and B, P < 0.05 each).
Figure 6.
Phosphorylation of CaMKII and PKA targets in murine atria upon modulations of late INa. Representative western blots of the phosphorylation status of (A) CaMKII and (C) PKA targets. (B and D) Western blot quantifications of the respective groups (n mice = 4 and P < 0.05 each). *significant vs. control; #significant vs. ATX-II + Veh (vehicle).
Similarly, PKA targets PLN-S16 and RyR2-S2808, were hyperphosphorylated in ATX-II-treated atrial myocardium vs. control (P < 0.05), which could be prevented by treatment with Ranolazine, TTX, or H89, but not with AIP (Figure 6A and B, P < 0.05 each).
3.5. Effects of late INa modulation and CaMKII/PKA inhibition on Ca2+ kinetics and SR-Ca2+-load
ATX-II treatment did not alter systolic Ca2+-transient amplitude (Figure 7A—left and B, P = 0.97) or SR-Ca2+ -load (Figure 7A—right and C; P = 0.35). Different stimulation frequencies showed similar results (Figure 7D). Simultaneous CaMKII inhibition, however, increased systolic Ca2+-transient amplitude at 2 Hz (Figure 7B, increased by 28 ± 7%, P < 0.001) and SR-Ca2+-load (increased by 22 ± 9% vs. control, Figure 7C, P < 0.05), suggesting that late INa activates SR refill independently of CaMKII, which maintains SR-Ca2+-load despite a markedly increased SR-Ca2+-leak (ATX-II vs. control, Figures 2D and 7C) and even leads to increased SR-Ca2+-load and Ca2+-transient amplitude when the CaMKII-dependent leak induction is prevented (ATX + AIP vs. control; Figures 2D and 7B and C). This hypothesis was confirmed in CaMKII-knockout mice. Here, the treatment with ATX-II also increased SR-Ca2+ load (by 37 ± 9%, Figure 7G and H, P < 0.05). In contrast, the inhibition of PKA (ATX + H89) decreased Ca2+-transient amplitude at 2 Hz (Figure 7B, P < 0.01) and SR-Ca2+-load (Figure 7C, P < 0.001). Furthermore, Ca2+ elimination kinetics were assessed. As a measure for the speed of Ca2+ uptake into the SR, the decay constant k of caffeine-induced Ca2+ transients (Ca2+ elimination predominantly via NCX) was subtracted from the decay constant of systolic transients (Ca2+ elimination via SERCA + NCX). Interestingly, ATX-II treatment did not significantly alter ksys− kcaff (Figure 7E, P = 0.15). Additional inhibition of CaMKII, however, yielded an acceleration of Ca2+ reuptake into the SR (ksys− kcaff ATX + AIP vs. control, increased by 34 ± 7%; P < 0.05). In contrast, inhibition of PKA decelerated SR reuptake (ATX + H89 vs. control, decreased by 33 ± 6%; P < 0.001). Moreover, ATX-II treatment increased the fractional Ca2+ release (Figure 7F, ratio F/F0/F/F0 caff, ATX-II vs. control = 0.80 ± 0.02 vs. 0.73 ± 0.02, P < 0.01), which was counteracted by PKA inhibition but not by CaMKII inhibition (Figure 7F).
3.6. Effects of late INa, CaMKII, and PKA inhibition in human AF
In human isolated left atrial CMs from patients with permanent AF (for patient characteristics see Table 1), inhibition of late INa (Ranolazine, 10 μmol/L) reduced CaSpF (Figure 8B, CaSpF Ran vs. control = 0.27 ± 0.10 vs. 0.63 ± 0.12 100 μm−1 s−1, P < 0.05) and reduced amplitude, width, and duration of Ca2+ sparks (data not shown, P < 0.001 each). This translated into a robust reduction of the SR-Ca2+-leak (Figure 8B, reduction by 88 ± 4%, P < 0.01). CaMKII inhibition (AIP 1 µmol/L) diminished CaSpF by 61 ± 9% (AIP vs. control = 0.34 ± 0.11 vs. 1.16 ± 0.26 100 μm−1 s−1, P < 0.01, Figure 8C) and reduced SR-Ca2+-leak by 69 ± 16% (Figure 8C, P < 0.05). Similar results were obtained upon PKA inhibition. The treatment of human AF CMs with H89 (5 µmol/L) significantly reduced the CaSpF by 66 ± 11% (Figure 8D, P < 0.01). This led to a reduction of calculated SR-Ca2+-leak (P < 0.05). Additionally, inhibition of reverse-mode NCX with KBR (0.1 µmol/L) also suppressed diastolic RyR2 leakiness in human AF (Figure 8E, CaSpF KBR vs. control = 0.33 ± 0.08 vs. 0.68 ± 0.12 100 μm−1 s−1, P < 0.05).
Table 1.
Characteristics of the patients from which human left atrial CMs were obtained
| n | Male (%) | Age (years) | AF (%) | LVEF (%) | ICM (%) | Diabet (%) | β-Block (%) | ACE-I (%) | Amiod (%) | Digit (%) | CCB (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 54.0 | 73.3 ± 1.8 | 100 | 50.6 ± 4.7 | 53.8 | 30.8 | 80.0 | 90.0 | 30.0 | 40.0 | 20.0 |
AF, atrial fibrillation; LVEF, left ventricular ejection fraction; ICM, ischaemic heart disease; Diabet, diabetes mellitus; β-block, β-blocker; ACE-I, ACE inhibitor; Amiod, Amiodarone; Digit, digitalis (Digoxin or Digitoxin); CCB, Ca2+ channel blockers.
4. Discussion
The present data show for the first time that an enhancement of late INa potently induces SR-Ca2+-leak in atrial CMs due to (i) an activation of CaMKII, which promotes diastolic RyR2 openings by hyperphosphorylation of RyR2 (Ser2814) and (ii) an activation of PKA (stimulation of AC 5/6 and increase of cAMP), which subsequently facilitates SR-Ca2+-accumulation by increased phosphorylation of PLN (Ser16). Importantly, both kinases are necessary for late INa-dependent SR-Ca2+-leak induction.
4.1. Late INa potently induces SR-Ca2+-leak
To our knowledge, no direct measurements of SR-Ca2+-leak as a function of late INa have been performed in atrial CMs. There is evidence in the literature that an enhanced late INa induces spontaneous diastolic depolarizations derived from both EADs and DADs. An interference of late INa with Ca2+ cycling properties has been suggested.16 However, the molecular basis of DADs downstream of an elevated Na+ influx is unknown. It is generally agreed that DADs arise from cytoplasmic Ca2+ overload leading to electrogenic extrusion of Ca2+ from the cell via NCX. Cytoplasmic Ca2+ overload might be explained by facilitation of Ca2+ channel reactivation due to late INa-induced APD prolongation,17 but increased SR-Ca2+-leak may also play a role. We used ATX-II to increase late INa18 and found the late INa integral to be increased by ∼65% upon ATX-II in our setting. We found that an enhancement of late INa increases SR-Ca2+-leak, but does not alter SR-Ca2+-load. The effects of ATX-II seem to be late INa-related as they can be blocked by Ranolazine and TTX. Importantly, ATX-II-treated murine atrial CMs showed similar CaSpF as human atrial CMs isolated from patients with permanent AF. In summary, our findings show the interdependence of Na+ current and Ca2+-cycling abnormalities in atrial CMs. This mechanism may significantly contribute to the increased SR-Ca2+-leak and cellular Ca2+ overload reported in human AF.11,12
4.2. Late INa activates CaMKII via a NCX-dependent mechanism
Hyperphosphorylation of RyR2 at S2814/2815 (mouse/human) has been shown to increase SR-Ca2+-leak in ventricular19 as well as in atrial myocardium.11,12 Our study extends molecular findings, suggesting that late INa activates CaMKII in rat ventricular CMs,20 to atrial myocardium. Of note, measurements of RyR2 function have not yet been performed in this context, either in ventricular or in atrial myocardium.20
Our data additionally show that CaMKII is essential for late INa-dependent SR-Ca2+-leak induction as it can be effectively prevented by the specific CaMKII inhibitor AIP and an elevated late INa failed to increase SR-Ca2+-leak in CaMKII-knockout mice. We further used KBR to inhibit the reverse mode of NCX function,21 which also suppressed SR-Ca2+-leak induction. It can thus be assumed that CaMKII is activated by Ca2+, which has entered the cell via NCX in the setting of Na+ overload. This mechanism may be pronounced in AF where NCX function is increased.12 Of note, it was shown that CaMKII, in turn, can phosphorylate Nav1.5, which enhances Na+ influx via late INa.22 Thus, cytoplasmic ion imbalance may establish a vicious circle in CMs, which maintains CaMKII activation and includes positive feedback on SR-Ca2+-leak and late INa.
4.3. Late INa increases cellular cAMP levels and activates PKA
PKA is also known to regulate RyR2 properties and was suggested to be involved in the disruption of diastolic RyR2 closure found in AF.13 When PKA was inhibited in the setting of an increased late INa, we could successfully prevent the induction of SR-Ca2+-leak. Thus, activation of PKA seems to be equally essential for the late INa-dependent induction of the SR-Ca2+-leak. Indeed, the western blot data demonstrate that the PKA-specific target sites at PLN (S16) and RyR2 (S2808) are hyperphosphorylated in ATX-II-treated atrial myocardium. The fact that PKA is activated by cytoplasmic Na+ influx via late INa has, to the best of our knowledge, not yet been described—either in atrial or in ventricular CMs—and further corroborates the link between Na+ currents and cellular Ca2+-cycling properties. Whereas the activation of CaMKII by Na+-induced cellular Ca2+ overload is rather expected, nothing is known about the mechanism of Na+-dependent PKA activation. PKA is an integral part of the β-adrenergic signalling pathway and is known to be activated by cAMP. We show that an enhancement of late INa by ATX-II induces a significant decrease of the measured YFP/CFP ratio in EPAC-camps transgenic mice,14 indicating an increase in cytoplasmic cAMP levels. The change in YFP/CFP ratio during ATX-II-treatment was pronounced as it made up for ∼28% of the maximal response upon β-adrenergic stimulation and can thus sufficiently explain PKA activation. We further used inhibitors of cardiac PDEs and ACs to elucidate if the late INa- related increase in cytoplasmic cAMP levels results from an inhibition of cardiac PDEs or an activation of ACs. Interestingly, preincubation with the unspecific PDE inhibitor IBMX did not block the cAMP response upon ATX-II treatment, showing that ATX-II does not exert its effect on cAMP levels by influencing PDE activity. In contrast, pre-inhibition of AC5 and 6 (the major cardiac AC isoforms) with NKY8015 clearly attenuated the cAMP response upon ATX-II. Thus, the enhanced late INa by ATX-II increases cytoplasmic cAMP levels by activating ACs. The fact that reverse-mode NCX inhibitor KBR also reduced ATX-II-dependent production of cAMP suggests a Ca2+-dependent activation of ACs.
4.4. Late INa-dependent activation of CaMKII and PKA exerts differential effects on Ca2+ cycling properties
The confocal microscopy and western blot data show that late INa activates CaMKII and PKA and that inhibition of either kinase can effectively prevent the late INa-dependent induction of SR-Ca2+-leak. SR-Ca2+-leak is, however, not only dependent on the properties of diastolic RyR2 closure, but also on SR-Ca2+-load (driving force). Surprisingly, late INa induction by ATX-II does not significantly influence systolic Ca2+-transient amplitude or SR-Ca2+-load compared to untreated control despite the markedly increased SR-Ca2+-leak, suggesting simultaneously increased Ca2+ reuptake into the SR. When CaMKII, however, was additionally inhibited (AIP) or knocked-out (CaMKIIδc-KO mice), an enhanced late INa increased Ca2+-transient amplitude and SR-Ca2+-load. These findings clearly suggest that another mechanism, independent of CaMKII, must be activated by late INa in atrial CMs, which maintains SR-Ca2+-load in the setting of an increased SR-Ca2+-leak and even augments SR-Ca2+-load when SR-Ca2+-leak is simultaneously suppressed via inhibition of CaMKII. We hypothesized that PKA is most likely responsible for this effect. Indeed, atrial CMs treated with H89 in addition to ATX-II showed both, decreased Ca2+-transient amplitude and decreased SR-Ca2+-load compared to untreated control or sole ATX-II treatment. Furthermore, we detected a deceleration of Ca2+ reuptake into the SR (ksys − kcaff) upon PKA inhibition. PKA thus enhances SR-Ca2+-uptake in atrial CMs—most likely by SERCA2a disinhibition through PLN phosphorylation,23 as demonstrated in the western blot experiments. In line with this interpretation, the rate of Ca2+ reuptake into the SR (ksys− kcaff) was found to be accelerated when late INa was increased and CaMKII was inhibited (ATX-II + AIP). The fact that late INa induction alone (ATX-II) did not accelerate Ca2+ reuptake into the SR may well be explained by the significantly higher SR-Ca2+-leak in this group counteracting cytosolic Ca2+ clearance by SERCA2a. The activation of SERCA2a by late INa can thus be unmasked by additional CaMKII inhibition that suppresses the increased SR-Ca2+-leak. Furthermore, fractional release is increased by ATX-II despite formally unchanged systolic Ca2+-transient amplitude and SR-Ca2+-load, which can effectively be prevented by H89 but not by AIP. It can thus be attributed to PKA-dependent phosphorylation of RyR2 (S2808).
The current data elucidate the specific function of PKA and CaMKII in the induction of SR-Ca2+-leak upon late INa activation and suggest that the mechanisms by which inhibition of CaMKII and PKA prevent SR-Ca2+-leak may be different. As inhibition of CaMKII successfully prevents diastolic Ca2+-leak-induction despite increased SR-Ca2+-load, it can be assumed that CaMKII primarily alters RyR2 open probability in the setting of an increased late INa and CaMKII inhibition effectively suppresses diastolic RyR2 leakiness. The effect of CaMKII-dependent PLN-T17 phosphorylation on SERCA2a activity seems to be rather minor in atrial CMs as Ca2+ elimination kinetics are even accelerated upon an inhibition of CaMKII in the setting of an elevated late INa. This can be explained by the suppression of the CaMKII-induced SR-Ca2+-leak that counteracts cytoplasmic Ca2+ clearance. In contrast, inhibition of PKA prevents the late INa-dependent diastolic leak induction in the setting of a decreased SR-Ca2+-load. Thus, the reduced SR-Ca2+-leak may only partly be explained by a lower PKA-dependent phosphorylation of RyR-S2808, but more likely by the decreased driving force for leak due to reduced SR-Ca2+-reuptake. Importantly, the activity of both kinases is essential for the late INa-dependent induction of SR-Ca2+-leak to occur. PKA thus facilitates CaMKII-dependent SR-Ca2+-leak induction by maintaining SR-Ca2+-load.
4.5. Inhibition of CaMKII, PKA, and late INa reduces SR-Ca2+-leak in human AF
Slowed inactivation of INa has been reported in a canine model of chronic AF,24 and we have recently shown that late INa is also enhanced in atrial CMs from patients with AF.6 Moreover, we and others have demonstrated that SR-Ca2+-leak is elevated in human AF and can be reduced by CaMKII inhibition.11,12 We sought to find out whether the newly established mechanism of a cellular interdependence between late INa and SR-Ca2+-leak can be applied to human AF. Therefore, we evaluated the effects of late INa, CaMKII, and PKA inhibition on SR-Ca2+-leak in CMs from patients with permanent AF. Indeed, inhibition of CaMKII by AIP as well as inhibition of PKA with H89 reduced diastolic RyR2 leakiness. This confirms the involvement of both kinases and the translatability of the mechanisms elucidated in murine atrial CMs to human AF. The fact that inhibition of reverse-mode NCX (KBR) also reduced CaSpF in human AF suggests a Ca2+-dependent activation of PKA (via ACs) and CaMKII. Importantly, a reduction of SR-Ca2+-leak can also be achieved by inhibition of late INa with Ranolazine confirming the importance of pathologic Na+ influx as an upstream activator of protein kinases and subsequent SR-Ca2+-leak induction. Thus, late INa inhibition could potentially be used as a treatment strategy to improve Ca2+ homeostasis in AF.
4.6. Limitations of the study
(i) The mouse dataset relies on an induction of late INa by ATX-II. This toxin has often been used for this purpose, mostly in ventricular CMs, and has been shown to delay Na+ channel inactivation and consequently induce Ca2+ overload.25,26 In our current study, ATX-II concentration was titrated to yield an increase of late INa current in a range that is comparable to the magnitude found in cardiac disease and thus to approximate a previously healthy murine atrial CM to the compromised Na+ gating found in human AF.6 Although the translatability of our results is shown by the fact that inhibition of the respective kinases as well as of late INa successfully suppresses SR-Ca2+-leak in human AF, unspecific actions of ATX-II cannot be completely excluded. Ca2+ overload may play an important role regarding the effects of ATX-II. This, however, may equally apply to an endogenously increased late INa. (ii) The calculation of spark size was performed by multiplying spark width by spark duration and amplitude. This calculation has repeatedly been used before.27 It incorporates the main features of spontaneous Ca2+ release and enables a valid comparison of SR-Ca2+-leak between groups relative to each other. However, a distinct absolute amount of Ca2+ loss cannot be inferred from this calculation. (iii) The observation of unchanged SR-Ca2+-load upon late INa induction is based on CMs that were prestimulated at 1 Hz. Changes in SR loading at higher frequencies cannot be completely excluded. (iv) There is evidence in the literature about the existence of a non-spark SR-Ca2+-leak.28,29 Although we did not directly measure the non-spark leak in our present study, this would most likely not change its significance as currently available data suggest that it coexists with Ca2+-spark-mediated SR-Ca2+-leak, and that both forms of diastolic Ca2+-loss are apparently not altered independently in certain cell types and diseases.29 Further studies investigating the non-spark-related leak in relation to late INa current and CaMKII/PKA activity may nevertheless be useful. However, Ca2+ sparks and waves may be of particular relevance as they constitute potent arrhythmogenic triggers. (v) BDM was used during murine cell isolation to increase cell quality and cell yield due to a reduction in actin–myosin interaction and ATP consumption. There are, however, concerns about BDM acting as a chemical phosphatase. As these side effects were shown to be dose-dependent,30 we used a very low concentration of BDM (10 mmol/L). At this concentration, no significant effects of BDM on protein phosphorylation could be detected.19 (vi) The use of caffeine in combination with Fluo dyes is an established method but might lead to some quenching of the indicator. This effect should be equally present in all groups and is known to be Ca2+-dependent. Therefore, the detected differences in SR-Ca2+-load would, if anything, rather be underestimated in this study.
4.7. Clinical relevance
Our data support the potentially high antiarrhythmic potency of late INa inhibition as this approach reduces SR-Ca2+-leak in murine and human atrial CMs by interfering with the activity of CaMKII and PKA, both of which are known to exert deleterious effects in human cardiac disease. Inhibition of late INa appears to be a feasible way to control CaMKII activity in atrial myocardium and may overcome the current lack of cardiac-specific and clinically applicable CaMKII inhibitors. As CaMKII is crucially involved in neuronal processes, a systemic CaMKII inhibition could produce severe neurological side effects. Furthermore, our data show that inhibition of late INa as a therapeutic approach not only reduces CaMKII activation, but also interferes with the cAMP–PKA system and thus includes effects usually attributed to β-blockers. Of note, Ranolazine is currently being evaluated in clinical trials as a new treatment option for patients with AF (RAFFAELO Trial, EudraCT no.: 2011-002789-18, HARMONY Trial, EudraCT no.: 2011-001134-42) and new, even more selective and potent late INa inhibitors are currently under both experimental and clinical investigation (e.g. GS-6615; NCT01847391).
5. Summary
The dataset presented in this study shows that enhancement of late INa in atrial CM exerts significant effects on Ca2+ homeostasis. It leads to an NCX-dependent activation of CaMKII and an AC-mediated increase in cytoplasmic cAMP levels with subsequent activation of PKA. This results in hyperphosphorylation of the respective target sites at RyR2 and PLN and triggers a significant increase in SR-Ca2+-leak. Importantly, activation of both CaMKII and PKA is necessary for increased SR-Ca2+-leak to occur in the atria. Owing to the fact that SR-Ca2+-reuptake is simultaneously facilitated, increased SR-Ca2+-leak does not alter SR-Ca2+-load. Increased SR-Ca2+-turnover, however, is potentially highly arrhythmogenic. The increased diastolic SR-Ca2+-leak in human AF can effectively be reduced by CaMKII inhibition as well as by inhibition of late INa.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
L.S.M., S.S., and G.H. are funded by the Deutsche Forschungsgemeinschaft (DFG) through the SFB 1002. S.S. is supported by the German Heart Foundation/German Foundation of Heart Research through a research grant. Funding to pay the Open Access publication charges for this article was provided by the Deutsche Forschungsgemeinschaft (DFG) through the SFB 1002.
Acknowledgements
We gratefully acknowledge the technical assistance of T. Schulte, K. Hansing, and T. Sowa.
Conflict of interest: L.S.M. receives research grants from Gilead and is involved in clinical trials with Gilead and Menarini. S.S. and L.S.M. receive speaker's honoraria from Berlin-Chemie. L.B., P.F., and L.Y. are employees of Gilead Sciences, Inc.
References
- 1.Coraboeuf E, Deroubaix E, Coulombe A. Effect of tetrodotoxin on action potentials of the conducting system in the dog heart. Am J Physiol 1979;236:H561–H567. [DOI] [PubMed] [Google Scholar]
- 2.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 2008;45:32–43. [DOI] [PubMed] [Google Scholar]
- 3.Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol 1996;497(Pt 2):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol 1997;500(Pt 3):631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail 2007;9:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, Belardinelli L, Maier LS. Altered Na+ currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol 2010;55:2330–2342. [DOI] [PubMed] [Google Scholar]
- 7.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 2006;92(Suppl 4):iv6–iv14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiyosue T, Arita M. Late sodium current and its contribution to action potential configuration in guinea pig ventricular myocytes. Circ Res 1989;64:389–397. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Shryock JC, Belardinelli L. A slowly inactivating sodium current contributes to spontaneous diastolic depolarization of atrial myocytes. Am J Physiol Heart Circ Physiol 2009;297:H1254–H1262. [DOI] [PubMed] [Google Scholar]
- 10.Wit AL, Boyden PA. Triggered activity and atrial fibrillation. Heart Rhythm 2007;4:S17–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res 2010;106:1134–1144. [DOI] [PubMed] [Google Scholar]
- 12.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 2005;111:2025–2032. [DOI] [PubMed] [Google Scholar]
- 14.Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol 2009;7:e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand CS, Hocker HJ, Gorfe AA, Cavasotto CN, Dessauer CW. Isoform selectivity of adenylyl cyclase inhibitors: characterization of known and novel compounds. J Pharmacol Exp Ther 2013;347:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasserstrom JA, Sharma R, O'Toole MJ, Zheng J, Kelly JE, Shryock J, Belardinelli L, Aistrup GL. Ranolazine antagonizes the effects of increased late sodium current on intracellular calcium cycling in rat isolated intact heart. J Pharmacol Exp Ther 2009;331:382–391. [DOI] [PubMed] [Google Scholar]
- 17.Sipido KR, Callewaert G, Carmeliet E. Inhibition and rapid recovery of Ca2+ current during Ca2+ release from sarcoplasmic reticulum in guinea pig ventricular myocytes. Circ Res 1995;76:102–109. [DOI] [PubMed] [Google Scholar]
- 18.Isenberg G, Ravens U. The effects of the Anemonia sulcata toxin (ATX II) on membrane currents of isolated mammalian myocytes. J Physiol 1984;357:127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer TH, Herting J, Tirilomis T, Renner A, Neef S, Toischer K, Ellenberger D, Forster A, Schmitto JD, Gummert J, Schondube FA, Hasenfuss G, Maier LS, Sossalla S. Ca2+/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology. Circulation 2013;128:970–981. [DOI] [PubMed] [Google Scholar]
- 20.Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, Hirakawa R, Budas GR, Rajamani S, Shryock JC, Belardinelli L. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol 2011;301:C577–C586. [DOI] [PubMed] [Google Scholar]
- 21.Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem 1996;271:22391–22397. [DOI] [PubMed] [Google Scholar]
- 22.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 2006;116:3127–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii J, Maruyama K, Tada M, MacLennan DH. Expression and site-specific mutagenesis of phospholamban. Studies of residues involved in phosphorylation and pentamer formation. J Biol Chem 1989;264:12950–12955. [PubMed] [Google Scholar]
- 24.Yagi T, Pu J, Chandra P, Hara M, Danilo P, Jr, Rosen MR, Boyden PA. Density and function of inward currents in right atrial cells from chronically fibrillating canine atria. Cardiovasc Res 2002;54:405–415. [DOI] [PubMed] [Google Scholar]
- 25.Hoey A, Harrison SM, Boyett MR, Ravens U. Effects of the Anemonia sulcata toxin (ATX II) on intracellular sodium and contractility in rat and guinea-pig myocardium. Pharmacol Toxicol 1994;75:356–365. [DOI] [PubMed] [Google Scholar]
- 26.Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol 2006;41:1031–1038. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH. Phospholamban ablation rescues sarcoplasmic reticulum Ca2+ handling but exacerbates cardiac dysfunction in CaMKIIdeltaC transgenic mice. Circ Res 2010;106:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brochet DX, Xie W, Yang D, Cheng H, Lederer WJ. Quarky calcium release in the heart. Circ Res 2011;108:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bovo E, Mazurek SR, Blatter LA, Zima AV. Regulation of sarcoplasmic reticulum Ca2+ leak by cytosolic Ca2+ in rabbit ventricular myocytes. J Physiol 2011;589:6039–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stapleton MT, Fuchsbauer CM, Allshire AP. BDM drives protein dephosphorylation and inhibits adenine nucleotide exchange in cardiomyocytes. Am J Physiol 1998;275:H1260–H1266. [DOI] [PubMed]