Abstract
Many aspects of song learning in songbirds resemble characteristics of speech acquisition in humans. Genetic, anatomical and behavioural parallels have most recently been extended with demonstrated similarities in hemispheric dominance between humans and songbirds: the avian higher order auditory cortex is left-lateralized for processing song memories in juvenile zebra finches that already have formed a memory of their fathers’ song, just like Wernicke’s area in the left hemisphere of the human brain is dominant for speech perception. However, it is unclear if hemispheric specialization is due to pre-existing functional asymmetry or the result of learning itself. Here we show that in juvenile male and female zebra finches that had never heard an adult song before, neuronal activation after initial exposure to a conspecific song is bilateral. Thus, like in humans, hemispheric dominance develops with vocal proficiency. A left-lateralized functional system that develops through auditory-vocal learning may be an evolutionary adaptation that could increase the efficiency of transferring information within one hemisphere, benefiting the production and perception of learned communication signals.
The parallels between speech acquisition in humans and song learning in songbirds are numerous1,2,3,4,5. In both cases there is a sensitive period for auditory learning during which the infants or young birds are exposed to and memorize the sounds of adult conspecifics. In zebra finches (Taeniopygia guttata), a memory of the song of the tutor is usually formed between 25–35 days post hatching (dph)6, but the duration of the sensitive period is dependent on model availability; even after 65 days post hatching, birds will successfully copy song from an adult male introduced to their cage7,8. Under normal circumstances song becomes ‘crystallized’ and does not change anymore in adulthood (>120 dph), but birds with limited experience with song models can still modify their songs beyond the conventional sensitive period for song learning9,10,11. Similar to timing of model availability in birds described above, a second language can replace the first language in adopted children even when exposure to the second language did not happen until 3–8 years of age12. Thus, auditory and vocal learning phases overlap in humans and songbirds and the end of the sensitive phase for auditory learning depends on model availability. In contrast, learning to produce vocalizations progresses through distinct phases: from ‘cooing’ to ‘babbling’ in human infants and ‘subsong’ to ‘plastic song’ in songbirds13,14.
In humans, it is well known that regions within the superior temporal gyrus and sulcus, including Wernicke’s area, are involved in speech perception. The songbird analogue of the mammalian higher auditory cortex is localized in the auditory lobule of the avian forebrain (see Fig. 1). The auditory lobule consists of the caudo-medial Nidopallium (NCM) and Mesopallium (CMM), analogous to regions in Superior Temporal cortex in humans, including Wernicke’s Area, and the primary auditory area, Field L2. Results of several studies indicate that these regions are (part of) the neural substrate for auditory perception and recognition memory15,16,17. Converging evidence suggests that the NCM is also involved in acquisition, processing, or recognition of the memory of the tutor song specifically18,19,20,21,22, which is acquired early in life, with parallel or distributed loci for the representation of this memory existing within the song system23,24.
Figure 1. Parallels between avian and mammalian auditory systems.
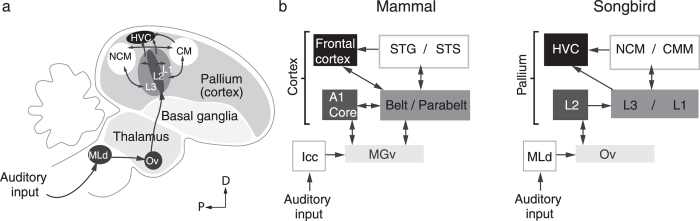
a) Simplified diagram (not to scale) of the avian auditory system. The ascending auditory pathway (dark grey arrows) includes a midbrain nucleus (MLd), a thalamic nucleus (Ov) and several cortical regions. The primary auditory cortex (Field L2) connects to the secondary auditory regions Field L3 and L1, which in turn are reciprocally connected to regions analogous to the mammalian auditory association cortex (NCM and medial and lateral CM). (b) Block diagram highlighting parallels between mammalian and songbird circuitry. Adapted from63,64,65,66,67.
Brain regions involved in human speech perception and production are functionally lateralized25,26. If such lateralization is related to successful auditory-vocal learning, Moorman and colleagues (2012) hypothesized that brain activation for auditory memory would be lateralized in juvenile songbirds that are in the middle of their song-learning period. When juvenile males were re-exposed to the song of their tutor, there was predominant activation in the left NCM, similar to that seen in humans27. Auditory memory was left lateralized (measured as a positive lateralization ratio) for males that already had imitated characteristics of their tutor’s song, but not for birds with less similarity to their tutors’ songs27. Activation in the pre-motor nucleus HVC (used as a proper noun), which forms part of a circuit equivalent to the ventral prefrontal cortex (including Broca’s area) in humans28, was dominant in the left hemisphere independent of the auditory stimulus to which the birds were exposed27. This suggests that lateralization of the auditory-vocal system may be necessary for successful vocal learning. During language learning in humans, left-lateralization becomes more significant with age and with increasing language proficiency29,30,31,32; when proficiency of a newly learned language is poor, there is a largely bilateral activation pattern33 and language impairments (developmental dysphasias) have been linked to a lack of lateralization in the core language areas34. If pronounced left-lateralization of the neural substrate for auditory processing is characteristic for successful development of acquired vocal skills, the question arises whether left-hemispheric dominance is absent at the onset of learning in other vocal learning species, such as songbirds. Establishing a parallel in lateralization of brain regions, or the absence thereof at the onset of the learning process, is key in relating findings from the songbird model to humans.
Is hemispheric dominance underlying the neural substrate for vocal learning innate, or does it develop with skill acquisition? Pre-natal experience with language in human infants may shape lateralization of the neural substrates underlying speech perception35,36,37 and, therefore, the relationship between pre-existing functional asymmetry and lateralization resulting from learning is unclear. Similarly, the results of the study by Moorman et al. (2012) do not allow us to distinguish between the following hypotheses: 1) some birds were already left-dominant for neuronal activity in NCM when they first listened to the song of their tutor and therefore became better vocal imitators (pre-existing functional asymmetry), 2) in birds that sang accurate imitations while practicing their motor skills, the neural substrate for song memory became more left-lateralized through experience (lateralization resulting from proficiency), or 3) a small pre-existing bias in processing song with the left NCM became more pronounced in good learners (pre-existing functional asymmetry enhanced by proficiency). Here, we conducted similar experiments in previously isolated male and female zebra finches to distinguish between these hypotheses. Female zebra finches do not learn to produce a song, but develop a preference for the song of their father38,39. Comparing male and female zebra finches can thus reveal the neural mechanisms of auditory memory independent of song-production learning40,41,42. We hypothesized that if song acquisition parallels speech learning in humans, the neural response to song should initially be bilateral in the NCM of birds that have no prior experience with the vocalizations produced by adult conspecifics.
Results
To control the auditory environment and limit exposure to normal adult songs early in life, juvenile zebra finches were separated from their father when they were 7–10 days old (when hearing thresholds are still elevated compared to adults) and raised by their mother (‘isolates’)43. The juvenile males were put into individual sound-isolated boxes at ~37 days post hatching (dph), which is when male juveniles start to produce ‘subsong’. Experiments were performed between 45–98 dph, a period in development during which molecular responses are inducible in normally reared male and female zebra finches44,45. We exposed juvenile birds to one of three auditory stimuli: a 30-min playback of a song from an adult conspecific (Song), a rhythmic white-noise stimulus for which the RMS-amplitude and temporal envelope were exactly matched to the song stimuli in the experimental group (Noi; see Fig. 2 and Park & Clayton46) or to silence (Sil). Thus, the only difference between Song and Noise groups was the species-specific spectral and temporal characteristics present in the song-stimulus but not in the noise control stimulus (Fig. 2). The birds were sacrificed 30 min after stimulus exposure, which is optimal to induce protein expression in the auditory regions47, and their brains processed immunocytochemically for Zenk (the protein product of the immediate early gene ZENK, an acronym for zif-268, egr-1, ngf-Ia and krox-24), a marker for neuronal activation48(for review, see: Mello et al., 200449).
Figure 2. Auditory stimuli for playback experiments.
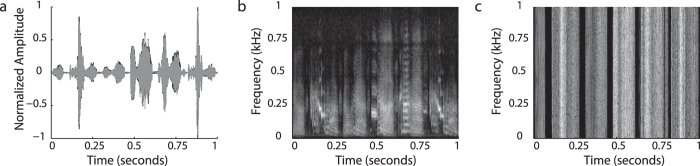
(a) An overlay of the amplitude plots of the tutor stimulus (black) and white noise control stimulus (grey), showing matching amplitude and temporal envelop of both stimuli. Spectrograms of the song (b) and control stimulus (c). Note the differences in spectro-temporal characteristics.
Differential modulation of the subdivisions of the avian auditory lobule
We quantified the number of Zenk-immunopositive neurons bilaterally in the NCM (Fig. 3) and CMM to investigate stimulus-specific lateralization. To examine the effects of auditory stimulus on the Zenk response in the different brain regions in both sexes, a repeated measures ANOVA with Hemisphere (left or right) as within-subjects factor and Stimulus (song, noise or silence) and Sex (male or female subjects) as between-subject factors was conducted for NCM as well as CMM. This analysis showed that there was a significant effect in NCM of Hemisphere (F1,25 = 11.22, p = 0.003) and a significant interaction between Hemisphere and Stimulus (F2,25 = 5.96, p = 0.008) but no interactions between Hemisphere and Sex (F1,25 = 0.001, n.s.) or Hemisphere, Stimulus and Sex (F2,25 = 2.83, n.s.). This effect was due to a stimulus-dependent response in the left NCM only (Song > Sil, p = 0.006, Song > Noi, p = 0.016 Bonferronni corrected post-hoc test, Fig. 3). In the right NCM, there were no stimulus-dependent responses; activation to Song or Noi was not significantly different than the background (or default) activation seen in the Sil group (p > 0.05 Bonferronni corrected post-hoc test). A similar analysis was performed in CMM, the results of which showed no significant effects of Hemisphere or Stimulus on neuronal activation (p > 0.05 for all factors and interactions between factors) but a significant effect of Sex (F1,21 = 5.30, p = 0.032; no interactions with other factors).
Figure 3. Zenk expression in the left and right NCM of juvenile zebra finches.
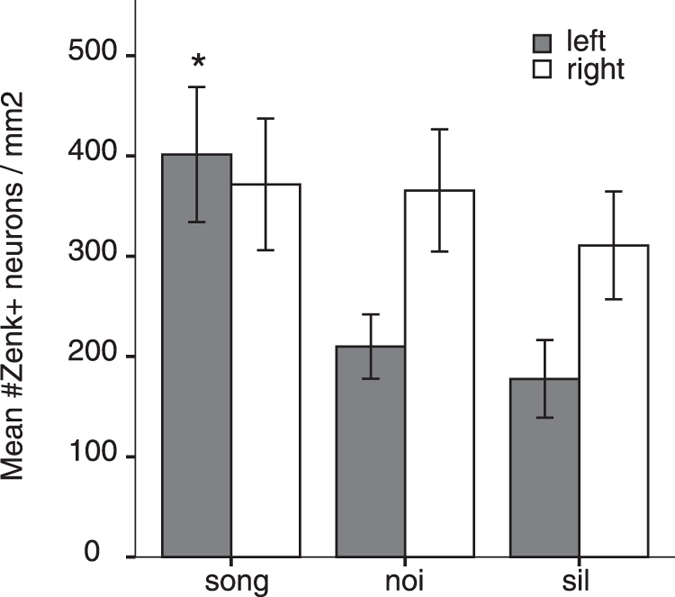
Mean number of Zenk-immunopositive neurons per square millimeter in the NCM is shown for the left (grey) and right (white) hemisphere. The mean activation level is higher in the NCM of zebra finches that were exposed to tutor song than to noise or silence, in the left hemisphere only (indicated with asterisk, see Results for statistics). Error bars represent the SEM.
Some males had produced songs in the hour before they were sacrificed. We measured the total duration (in seconds) of songs produced by these birds. There were no significant correlations between song production and neuronal activation in any of the regions investigated (Pearson’s correlation coefficient; p > 0.05 for NCM and CMM). We investigated whether the age at which the playback experiment was performed affected the levels of Zenk expression in each of the four regions and in each stimulus group independently; no significant correlations were found (Pearson’s correlation coefficient with Bonferroni adjusted α for testing in four regions: p = n.s. for all regions and all stimulus groups; before adjustments for multiple testing, right CMM in Song group: r = 0.65, N = 13, p = 0.016; left CMM in Noi group: r = 0.67, N = 9, p = 0.047; all other regions p > 0.05).
The first exposure to song: neuronal activation in the NCM is bilateral
Because of the stimulus-dependent hemispheric differences in neuronal activation in NCM described in the previous paragraph, we calculated the lateralization ratio ([L − R]/[L + R]) for each bird. This lateralization ratio normalizes individual lateralization levels in response to each stimulus and is not influenced by differences in absolute neuronal activation.
ANOVA confirmed a significant effect of Stimulus on lateralization ratios in the NCM (Fig. 4; F2,31 = 6.20, p = 0.007; Bonferroni corrected post-hoc tests: Song >Noi p = 0.008, Song >Sil p = 0.019). There were no significant effects of Sex (F1,31 = 0.00, p = n.s.) or between Stimulus*Sex (F2,31 = 2.78, p = n.s.). Thus, in birds that do not have previous experience with a song from an adult conspecific, there was a bilateral neuronal response in the NCM when first exposed to song (Fig. 4; mean lateralization ratio = 0.04; t = 0.738, p = n.s., n = 13). The lateralization ratios for the rhythmic noise and silence groups were significantly different from zero in the NCM, demonstrating right-hemispheric dominance when birds are not exposed to a model song (Noi: mean lateralization ratio = −0.24, t = −3.39, p = 0.007, n = 11; Sil: mean lateralization ratio = −0.25, t = −2.91, p = 0.027, n = 7). For comparison (see Fig. 4a in Moorman et al. 2012), lateralization ratios for birds that have already learned song and were re-exposed to their tutor song, ranged between −0.2 and 0.8 depending on learning proficiency27.
Figure 4. Lateralization ratios in the NCM and CMM after exposure to song, rhythmic white noise or silence.
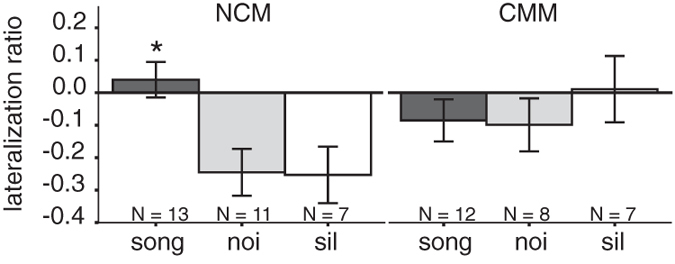
Lateralization ratios ([L − R]/[L + R]) were calculated for each subject from the number of Zenk-immunopositive cells per square millimeter. In the NCM, the data show equal bilateral activation in response to a song (mean lateralization ratio ± SEM; dark grey bar). In white-noise exposed birds (grey bar) and birds kept in silence (white bar), the lateralization ratio is negative, indicating stronger activation of the right hemisphere. Asterisk indicates a significant difference between the groups. For CMM, there was bilateral activation independent of stimulus condition.
Lateralization ratios for CMM are also shown in Fig. 4. In the CMM, lateralization ratios indicated bilateral activation independent of stimulus in both males and females (p > 0.05 for all factors and stimuli).
Discussion
These findings establish that neuronal activation in isolated juvenile songbirds is bilateral in the NCM, a region functionally analogous to Wernicke’s area in the mammalian brain, when they are first exposed to a model song. In contrast, neuronal activation in the control groups, whether spontaneous (no stimulus) or evoked by a rhythmic white-noise stimulus, is right-dominant. This pattern of differential activation is due to modulation of the neuronal response in the left hemisphere only, with song inducing significantly stronger response than silence. The default processing state of the NCM in animals with no prior experience with song is thus one in which neuronal activation can be induced by song in the left hemisphere only, but not in the right hemisphere due to higher background activation. Thus, an innate bias to process song with the left hemisphere (this article) results in balanced neuronal activation between left and right hemispheres when birds are first exposed to song. Taken together with the results from Moorman et al. (2012) showing left-lateralization in juveniles that had already learned song27, the current results support the hypothesis that left-dominance of the neural substrate for song memory is not innate but develops with successful song imitation. Thus, these results confirm hypothesis 3 (outlined in the introduction): a pre-existing functional asymmetry in inducible neuronal activation becomes enhanced by proficiency. Whether hemispheric dominance arises due to an increase in activation in the left hemisphere, or a decrease in baseline activity levels in the right hemisphere remains to be determined.
Consistent with the current results, a previous study that measured absolute response magnitudes (ARMs) in the NCM of adult male and female zebra finches also showed a lack of lateralization in birds that had no experience with song50. However, ARMs to both songs and long calls were stronger in the right hemisphere in birds that had previous experience with either a tutor song or their own (isolate) song than in birds that had never heard song before50. In contrast, there is left-hemispheric dominance in IEG expression in birds exposed to their tutor’s song but not in birds exposed to a novel song27. Thus, the right-hemispheric dominance in experienced birds that was reported in the study by Phan and Vicario (2010) could be due to differences in auditory processing of the stimulus related to memory: an unfamiliar song50 vs. the tutor song27 or the method used (electrophysiology vs. immediate early gene expression). Alternatively, this could be due to the behavioural state of the subject: anesthetized50 vs. awake behaving27 birds.
It is relevant to note that ~40% of Zenk-positive neurons in the NCM are GABAergic51. Pharmacological application of GABA antagonists results in a more phasic and synchronized response pattern of auditory units in the NCM and abolishes sustained activity between the song syllables52. A role for inhibitory circuits in shaping auditory selectivity has been shown in the NCM of European starlings (Sturnus vulgaris) that had learned to associate certain song stimuli in a go/no go task53. We could interpret the left-hemispheric dominance for tutor song that develops with successful song imitation, as the result of stronger inhibition and thus higher selectivity for learned song. A pre-existing bias of the left NCM to process song would then allow the left NCM to become dominant for processing auditory memories, such as the memory of the tutor’s song or the bird’s own song, through strengthening and activation of inhibitory (and possibly disinhibitory) local circuits.
The presence of balanced bilateral activation in response to song in the current study could be explained by a hemispheric difference in modulation of the auditory response: in the left hemisphere neuronal activation was modulated differentially by the auditory stimuli, whereas in the right hemisphere there was no significant modulation (Fig. 3). This suggestion is also consistent with the behavioural preference for the bird’s own song, which can be abolished by blocking the production of estradiol in the left NCM only, but is not affected by the same treatment in the right NCM54. Lesions to the left ascending auditory pathway (nucleus Ovoidalis) have similar consequences on song discrimination in a go/no-go test, disrupting song discrimination when the BOS and a cage-mate’s song are used as stimuli whereas the right hemisphere seems to be involved in discriminating the subtle differences in harmonic profile of songs55. Thus, the right NCM seems to be dominant for perception of spectro-temporal characteristics of song and the left NCM for perception and recognition of familiar songs.
What is the neuronal mechanism through which the NCM becomes lateralized? In males that have no prior experience with a model song, the ERP, BOLD and IEG response lack a stimulus-specific response pattern41,42. Here, we show that the left-dominant IEG response typical for normally reared male juveniles27 is also absent in previously isolated birds. In juveniles that are raised with a song tutor, there is left-sided dominance for memorized song and bilateral processing of novel song27. Together, the results show that initial auditory processing is bilateral, and that the neural substrate for memorized song becomes left-dominant when vocal imitation becomes more accurate.
There is a parallel with human speech acquisition: functional specialization of the left-hemisphere for language becomes more pronounced with increasing complexity of language skills32. In addition, in bilingual toddlers, there is left lateralization for the dominant language and bilateral activation for the non-dominant language, indicating that left-lateralization develops with language proficiency similarly to song learning in songbirds30. Development of lateralization of the neural substrate for language may begin very early in development, possibly prenatally. In sleeping human neonates, there was a left-lateralized bias for processing forward versus backward speech56. Functional MRI experiments in 3-month old infants revealed left-hemispheric processing of auditory stimuli (forward and backward speech) in all infants (awake or asleep), whereas there was bilateral activation that was greater for backward speech versus forward speech in the posterior part of the superior temporal sulci only in awake infants57. Interestingly, language impairments such as developmental dysphasia, autism spectrum disorder, speech delay, stuttering and dyslexia, have all been linked to atypical or lack of lateralization (see34).
In conclusion, in juvenile songbirds with limited experience with song, there is balanced neuronal activation when first exposed to adult conspecific vocalizations; the left-dominance of brain regions that is characteristic of complex auditory-vocal skills is therefore likely a developmentally- and possibly evolutionarily-acquired trait.
Materials & Methods
Subjects
34 male and female juvenile zebra finches were reared in the animal facility of Wellesley College. Birds were maintained on a 16:8 light:dark cycle, lights on at 10:00 AM. All birds were kept in breeding cages with their parents and siblings until 7−11 days post-hatching (dph). At 9.7 dph (+/−0.25 SEM), the juveniles and their mother were separated from the father and transferred to a different room equipped with acoustically isolated cages each holding a single clutch. At 37 dph (+/−1 SEM; range 34–53 dph), juvenile males were transferred into individual, acoustically isolated holding cages. At 46 dph (+/−3 SEM; range 37–64 dph), juvenile females were also separated from their mother into individual, acoustically isolated holding cages. All experiments were performed during the equivalent of the plastic song stage in normally reared birds (~40–100 dph) (Immelmann 1969; Johnson et al. 2002). Mean age at the day of the experiment was 69 dph (+/−2.7 SEM; range 45–98 dph). Experimental procedures were in accordance with US law and approved by the Institutional Animal Care and Use Committee of Wellesley College (IACUC #1106).
Experimental procedures
One day prior to stimulus exposure, the birds were transferred to a sound-proof chamber equipped with a microphone and a speaker. On the day of the experiment, the lights were manually turned on for the duration of stimulus exposure. Stimulus presentation started between 10:00 AM and 11:00 AM and lasted 30 min. The birds were sacrificed 30 min after the end of the last stimulus presentation. The birds were kept in darkness during the 30 min post-stimulus period to stop them from vocalizing, and thereby to prevent their own vocalizations from evoking molecular neuronal activation. Subjects were exposed to one of the three treatments: a recording of the song of an adult zebra finch male (Song), a rhythmic white-noise stimulus (Noi) with the same temporal envelope as song and of equal amplitude and duration (Fig. 2) or silence (Sil). Thus, the only difference between the Song and Noi is that the experimental group heard a stimulus that contains species-specific spectrotemporal characteristics. Each 30 min stimulus consisted of one-minute loops in which 15 seconds of sound was followed by 45 seconds of silence. The stimulus songs were broadcast through a speaker and Windows Media Player controlled the sound pressure level at 65 dB mean SPL at 30 cm from the speaker. Sound recordings were made throughout the experiment to ensure that birds were awake during stimulus presentation, and to monitor vocal behavior during stimulus exposure.
Tissue collection
One hour after stimulus onset, the experimental subjects were anesthetized with 0.03 mL Natriumpentobarbital (intramuscular) (Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI) and subsequently perfused with phosphate buffer (PB, pH 7.4) containing 0.2% heparin, followed by fixation with 2% paraformaldehyde and 0.075% glutaraldehyde in PB. Whole brains were dissected out, separated by hemisphere and post-fixed at 4 °C in 2% paraformaldehyde and 0.075% glutaraldehyde in PB for 4 hours. Parasaggital sections (50 um) were made on a vibratome and stored in PB overnight at 4 °C or in cryoprotectant at –18 °C.
Immunocytochemistry
Sections were rinsed three times in PB (5 min. each rinse) and incubated in H2O2 (0.03%) for 8 minutes. Sections were rinsed three times with PBS and 0.01% BSA-c (acetylated albumin; BSA-c, Aurion, Wageningen, the Netherlands) for 10 minutes each and incubated with 5% normal goat serum (NGS) in 0.01% BSA-c for 30 minutes. Afterwards, sections were rinsed three times in PBS and 0.01% BSA-c for 5 minutes and incubated with primary polyclonal rabbit antiserum (Santa Cruz Biotechnology, Santa Cruz, CA; Cat. No. sc-189, 1:1,000) raised against the carboxy-terminus of mouse egr-1 (sequence STGLSDMTATFSPRTIEIC; see47) and 0.01% BSA-c overnight. Sections were rinsed again in PBS 10 times for 5 minutes, incubated with biotinylated goat anti-rabbit (IgG, dilution 1:500, Vector Laboratories, Burlingame, CA), for 1 hour (room temperature [RT]), and rinsed three times for 15 minutes in PBS. Afterwards, sections were incubated (RT) with ABC (avidin-biotinylated enzyme complex, Vector Elite Kit, Vector Laboratories) and rinsed in PBS twice for 5 minutes. Finally, sections were incubated in diaminobenzidine medium with 0.034% H2O2 for 4 minutes (RT). The reaction was stopped in distilled water. Sections were then rinsed in PBS, mounted on slides, dehydrated, and embedded in DPX (RT). Birds of different experimental groups were run in parallel with one another on the same well plate and controls were performed for which the primary or secondary antibodies were omitted. All procedures were performed cold (4 °C) unless otherwise specified.
Image analysis
Quantification of Zenk-immunopositive cells was performed for NCM and CMM on 422 × 562 um images of three sections at the lateral position (between 600 and 1000 mm from the midline18,21,58,59). For the NCM, a counting frame was placed at the extreme caudal pole of the nidopallium59. For the CMM, the frame was placed adjacent to the ventricle and the lamina mesopallialis. Distance from the midline was assessed by calculating the number of serial sections and this location was verified using the atlas of Vates et al. (1996)60, an unpublished atlas of the zebra finch brain by A. M. den Boer-Visser (which was also used in previous studies40,59) and a stereotaxic atlas that is available online61. Digital photographs were taken using a SPOT Insight 2 Mp camera (SPOT Imaging Solutions) and the QCapture 2.9 program (Quantitative Imaging Corporation) on a Nikon Eclipse 50i (Nikon Instruments) with 20x objective. Image analysis was carried out with a PC-based system equipped with Image J (NIH, Bethesda, MD)62. Counts of three sections per region per animal were averaged for further statistical analysis. Image analysis was performed ‘blind’ as to the experimental history of the subject.
Statistical analysis
Levels of Zenk expression were not normally distributed in three regions in the Song group, 2 regions in the Noise group and 2 regions in the Silence group (Kolmogorov-Smirnov test for normality, p < 0.05); data were natural-log transformed for further statistical analysis. A repeated measures ANOVA with Hemisphere (left or right) as within-subjects factor and Stimulus (song, noise or silence) and Sex (male or female subjects) as between-subject factors was conducted for NCM as well as CMM to examine the effects of playback stimulus on the levels of neuronal activation (measured as the number of Zenk+ neurons) in the different brain regions in both sexes (Bonferroni adjusted α = 0.025 for performing repeated measures ANOVA on both NCM and CMM). A lateralization ratio for NCM and for CMM was calculated by dividing the difference in Zenk expression levels between the two hemispheres by the total amount of Zenk expression of the two hemispheres: [L − R]/[L + R]. To test for lateralization effects in each stimulus group, we performed an ANOVA on the lateralization ratios followed by post hoc paired t-tests with Bonferroni corrections for multiple testing. One-sample t-tests were performed to investigate whether lateralization ratios were significantly different from zero (bilateral). Pearson’s correlation coefficient with Bonferroni correction for multiple testing was used to examine correlations between Zenk expression levels and the age at which the experiment was performed, and with the amount of songs produced by the birds. Data were analyzed using SPSS 21.0.0 (IBM Corporation).
Additional Information
How to cite this article: Chirathivat, N. et al. Hemispheric dominance underlying the neural substrate for learned vocalizations develops with experience. Sci. Rep. 5, 11359; doi: 10.1038/srep11359 (2015).
Acknowledgments
We thank Pat Carey and Val LePage for animal care, Mike Wiest and Barbara Beltz for thoughtful comments on the manuscript, and the following undergraduate research assistants for their contributions to stimulus preparation, behavioural experiments, perfusions, sectioning of brains or immunocytochemistry: Andrea Bae, Kaitlin Bohon, Savannah Hauge, Catherine Ho, Houda Khaled, Sima Lotfi, Shonda Luke, Milena Radoman and Madeleine Whitaker. Undergraduate research assistants received support from the Brachman Hoffman fund, the Patterson Summer Research Fund, Howard Hughes Medical Institutes summer research award, the Sophomore Early Research Program and the Wellesley College Neuroscience Program. Early Career funds from Wellesley College also supported this work.
Footnotes
Author Contributions S.M.H.G. conceived and designed, and N.C., S.C.R. and S.M.H.G. executed the study and analysed the data. S.M.H.G wrote the paper.
References
- Doupe A. J. & Kuhl P. K. Birdsong and human speech: Common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631 (1999). [DOI] [PubMed] [Google Scholar]
- Bolhuis J. J., Okanoya K. & Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nat. Rev. Neurosci. 11, 747–759, doi: 0.1038/nrn2931 (2010). [DOI] [PubMed] [Google Scholar]
- Bolhuis J. J. & Everaert M. Birdsong, Speech And Language: Exploring The Evolution Of Mind And Brain (The MIT Press, Cambridge, 2013). [Google Scholar]
- Mooney R. Neurobiology of song learning. Curr. Opin. Neurobiol. 19, 654–660, doi: 10.1016/j.conb.2009.10.004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard M. S. & Doupe A. J. Translating birdsong: songbirds as a model for basic and applied medical research. Annu. Rev. Neurosci. 36, 489–517, doi: 10.1146/annurev-neuro-060909-152826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper A. & Zann R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology 112, 458–470 (2006). [Google Scholar]
- Eales L. A. Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim. Behav. 33, 1293–1300 (1985). [Google Scholar]
- Yazaki-Sugiyama Y. & Mooney R. Sequential learning from multiple tutors and serial retuning of auditory neurons in a brain area important to birdsong learning. J. Neurophysiol. 92, 2771–2788, doi: 10.1152/jn.00467.2004 (2004). [DOI] [PubMed] [Google Scholar]
- Jones A. E., TenCate C. & Slater P. J. B. Early experience and plasticity of song in adult male zebra finches (Taeniopygia guttata). J. Comp. Psychology 110, 354–369 (1996). [Google Scholar]
- Eales L. A. Song learning in female-raised zebra finches: another look at the sensitive phase. Anim. Behav. 35, 1356–1365 (1987). [Google Scholar]
- Morrison R. G. & Nottebohm F. Role of a telencephalic nucleus in the delayed song learning of socially isolated zebra finches J. Neurobiol. 24, 1045–1064, doi: 10.1002/neu.480240805 (1993). [DOI] [PubMed] [Google Scholar]
- Pallier C. et al. Brain imaging of language plasticity in adopted adults: Can a second language replace the first? Cerebral Cortex 13, 155–161, doi: 10.1093/cercor/13.2.155 (2003). [DOI] [PubMed] [Google Scholar]
- Aronov D., Andalman A. S. & Fee M. S. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 320, 630–634 (2008). [DOI] [PubMed] [Google Scholar]
- Lipkind D. et al. Stepwise acquisition of vocal combinatorial capacity in songbirds and human infants. Nature 498, 104–108, doi:10.1038/nature12173 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. V., Vicario D. S. & Clayton D. F. Song presentation induces gene-expression in the songbird forebrain. Proc. Natl. Acad. Sci. U. S. A. 89, 6818–6822 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. V., Nottebohm F. & Clayton D. F. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in zebra finch telencephalon. J. Neurosci. 15, 6919–6925 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew S. J., Vicario D. S. & Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc. Natl. Acad. Sci. U. S. A. 93, 1950–1955 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis J. J., Zijlstra G. G. O., den Boer-Visser A. M. & Van der Zee E. A. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc. Natl. Acad. Sci. U. S. A. 97, 2282–2285 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobes S. M. H. & Bolhuis J. J. Birdsong memory: a neural dissociation between song recognition and production. Curr. Biol. 17, 789–793, doi: 10.1016/j.cub.2007.03.059 (2007). [DOI] [PubMed] [Google Scholar]
- London S. E. & Clayton D. F. Functional identification of sensory mechanisms required for developmental song learning. Nat. Neurosci. 11, 579–586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobes S. M. H., Zandbergen M. A. & Bolhuis J. J. Memory in the making: localized brain activation related to song learning in young songbirds. Proc. R. Soc. B-Biol. Sci. 277, 3343–3351, doi: 10.1098/rspb.2010.0870 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan M. L., Pytte C. L. & Vicario D. S. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc. Natl. Acad. Sci. U. S. A. 103, 1088–1093 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. F., Gobes S. M. H., Murugan M., Olveczky B. P. & Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nat. Neurosci. 15, 1454–1459, doi: 10.1038/nn.3206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham M. E., Nordeen E. J. & Nordeen K. W. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata). Neurobiol. Learn. Mem. 66, 295–304 (1996). [DOI] [PubMed] [Google Scholar]
- Price C. J. et al. Hearing and saying - The functional neuro-anatomy of auditory word processing. Brain 119, 919–931, doi: 10.1093/brain/119.3.919 (1996). [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Hertz-Pannier L. & Dubois J. Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. Trends Neurosci. 29, 367–373 (2006). [DOI] [PubMed] [Google Scholar]
- Moorman S. et al. Human-like brain hemispheric dominance in birdsong learning. Proc. Natl. Acad. Sci. U. S. A. 109, 12782–12787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov C. I. & Jarvis E. Birds, Primates and Spoken Language Origins: Behavioral Phenotypes and Neurobiological Substrates. Frontiers in Evolutionary Neuroscience 4, doi: 10.3389/fnevo.2012.00012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S. K. et al. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage 14, 837–843, doi: 10.1006/nimg.2001.0875 (2001). [DOI] [PubMed] [Google Scholar]
- Conboy B. T. & Mills D. L. Two languages, one developing brain: event-related potentials to words in bilingual toddlers. Dev. Sci. 9, F1–F12, doi: 10.1111/j.1467-7687.2005.00453.x (2006). [DOI] [PubMed] [Google Scholar]
- Gaillard W. D. et al. Functional anatomy of cognitive development - fMRI of verbal fluency in children and adults. Neurology 54, 180–185 (2000). [DOI] [PubMed] [Google Scholar]
- Holland S. K. et al. Functional MRI of language lateralization during development in children. International Journal of Audiology 46, 533–551, doi: 10.1080/14992020701448994 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S. et al. Anatomical variability in the cortical representation of first and second language. Neuroreport 8, 3809–3815, doi: 10.1097/00001756-199712010-00030 (1997). [DOI] [PubMed] [Google Scholar]
- de Guibert C. et al. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia). Brain 134, 3044–3058, doi: 10.1093/brain/awr141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisilevsky B. S. et al. Effects of experience on fetal voice recognition. Psychol. Sci. 14, 220–224 (2003). [DOI] [PubMed] [Google Scholar]
- DeCasper A. J. & Prescott P. Lateralized processes constrain auditory reinforcement in human newborns. Hearing Research 255, 135–141, doi: 10.1016/j.heares.2009.06.012 (2009). [DOI] [PubMed] [Google Scholar]
- DeCasper A. J. & Fifer W. P. Of Human Bonding: Newborns Prefer their Mothers’ Voices. Science 208, 1174–1176 (1980). [DOI] [PubMed] [Google Scholar]
- Clayton N. S. Subspecies Recognition and Song Learning in Zebra Finches. Anim. Behav. 40, 1009–1017 (1990). [Google Scholar]
- Riebel K., Smallegange I. M., Terpstra N. J. & Bolhuis J. J. Sexual equality in zebra finch song preference: evidence for a dissociation between song recognition and production learning. Proc. Roy. Soc. B 269, 729–733 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra N. J., Bolhuis J. J., Riebel K., van der Burg J. M. M. & den Boer-Visser A. M. Localized brain activation specific to auditory memory in a female songbird. J. Comp. Neurol. 494, 784–791 (2006). [DOI] [PubMed] [Google Scholar]
- Maul K. K. et al. The Development of Stimulus-Specific Auditory Responses Requires Song Exposure in Male But Not Female Zebra Finches. Dev. Neurobiol. 70, 28–40, doi: 10.1002/dneu.20751 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszycki M. L., Sluzas E. M., Sundberg K. A., Newman S. W. & DeVoogd T. J. Immediate early gene (ZENK) responses to song in juvenile female and male zebra finches: Effects of rearing environment. J. Neurobiol. 66, 1175–1182 (2006). [DOI] [PubMed] [Google Scholar]
- Amin N., Doupe A. & Theunissen F. E. Development of selectivity for natural sounds in the songbird auditory forebrain. J. Neurophysiol. 97, 3517–3531 (2007). [DOI] [PubMed] [Google Scholar]
- Jin H. & Clayton D. F. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron 19, 1049–1059 (1997). [DOI] [PubMed] [Google Scholar]
- Bailey D. J. & Wade J. FOS and ZENK responses in 45-day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav. Brain Res. 162, 108–115 (2005). [DOI] [PubMed] [Google Scholar]
- Park K. H. & Clayton D. F. Influence of restraint and acute isolation on the selectivity of the adult zebra finch zenk gene response to acoustic stimuli. Behav. Brain Res. 136, 185–191 (2002). [DOI] [PubMed] [Google Scholar]
- Mello C. V. & Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J. Comp. Neurol. 393, 426–438 (1998). [DOI] [PubMed] [Google Scholar]
- Mello C. V. & Jarvis E. D. in Neuroscience Of Birdsong (eds Zeigler H. P. & Marler P. ) 381–397 (Cambridge University Press, 2008). [Google Scholar]
- Mello C. V., Velho T. A. F. & Pinaud R. in Behavioral Neurobiology Of Birdsong Vol. 1016 Annals of the New York Academy of Sciences 263–281 (New York Academy of Sciences, 2004). [DOI] [PubMed] [Google Scholar]
- Phan M. L. & Vicario D. S. Hemispheric differences in processing of vocalizations depend on early experience. Proc. Natl. Acad. Sci. U. S. A. 107, 2301–2306, doi: 10.1073/pnas.0900091107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R. et al. GABAergic neurons participate in the brain’s response to birdsong auditory stimulation. Eur. J. Neurosci. 20, 1318–1330 (2004). [DOI] [PubMed] [Google Scholar]
- Pinaud R. et al. Inhibitory network interactions shape the auditory processing of natural communication signals in the songbird auditory forebrain. J. Neurophysiol. 100, 441–455, doi: 10.1152/jn.01239.2007 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. V., Jeanne J. M. & Gentner T. Q. Local inhibition modulates learning-dependent song encoding in the songbird auditory cortex. J. Neurophysiol. 109, 721–733, doi: 10.1152/jn.00262.2012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L., Coleman M. J., Oyama R. K. & Schlinger B. A. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc. Natl. Acad. Sci. U. S. A. 107, 3852–3857, doi: 10.1073/pnas.0906572107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J., Williams H. & Nottebohm F. Hemispheric-differences in avian song discrimination Proc. Natl. Acad. Sci. U. S. A. 89, 1372–1375, doi: 10.1073/pnas.89.4.1372 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena M. et al. Sounds and silence: An optical topography study of language recognition at birth. Proc. Natl. Acad. Sci. U. S. A. 100, 11702–11705 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Dehaene S. & Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science 298, 2013–2015 (2002). [DOI] [PubMed] [Google Scholar]
- Bolhuis J. J., Hetebrij E., Den Boer-Visser A. M., De Groot J. H. & Zijlstra G. G. O. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur. J. Neurosci. 13, 2165–2170 (2001). [DOI] [PubMed] [Google Scholar]
- Terpstra N. J., Bolhuis J. J. & den Boer-Visser A. M. An analysis of the neural representation of birdsong memory. J. Neurosci. 24, 4971–4977 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates G. E., Broome B. M., Mello C. V. & Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata). J. Comp. Neurol. 366, 613–642 (1996). [DOI] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler B. E. & Bischof H.-J. A stereotaxic atlas of the brain of the zebra finch, Taeniopygia Guttata - with special emphasis on telencephalic visual and song system nuclei in transverse and sagittal sections (Bethesda (MD): National Library of Medicine (US), NCBI; c2007, 2007). [Google Scholar]
- Gobes S. M. H. et al. Differential responsiveness in brain and behavior to sexually dimorphic long calls in male and female zebra finches. J. Comp. Neurol. 516, 312–320 (2009). [DOI] [PubMed] [Google Scholar]
- Woolley S. M. N. Early experience shapes vocal neural coding and perception in songbirds. Developmental Psychobiology 54, 612–631, doi: 10.1002/dev.21014 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P. & Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc. Natl. Acad. Sci. U. S. A. 97, 11800–11806 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J. H. & Hackett T. A. Subdivisions of auditory cortex and processing streams in primates. Proc. Natl. Acad. Sci. U. S. A. 97, 11793–11799 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J. H., Hackett T. A. & Tramo M. J. Auditory processing in primate cerebral cortex. Curr. Opin. Neurobiol. 9, 164–170 (1999). [DOI] [PubMed] [Google Scholar]
- Bolhuis J. J., Gobes S. M. H., Terpstra N. J., den Boer-Visser A. M. & Zandbergen M. A. Learning-Related Neuronal Activation in the Zebra Finch Song System Nucleus HVC in Response to the Bird’s Own Song. PLoS ONE 7, e41556–e41556 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


