Abstract
Background
The effectiveness of reintroducing oxaliplatin in patients with metastatic colorectal cancer refractory to standard chemotherapy has not been verified. We performed a single-arm, open-label, Phase II study to evaluate the safety and efficacy of reintroducing oxaliplatin.
Methods
Eligible patients had received prior chemotherapy including oxaliplatin and irinotecan that achieved a response or stable disease followed by confirmed disease progression ≥6 months previously during prior oxaliplatin-based therapy. The primary endpoint was the disease control rate (DCR) after 12 weeks of treatment starting. The DCR was defined as the sum of patients with complete response, partial response, and stable disease.
Results
Thirty-three patients were enrolled. The median age was 62 (range: 35–77) years and the male/female ratio was 19/14. Eastern Cooperative Oncology Group performance status was 0 in 84.8%. Fourteen primary tumors were in the colon and 19 were in the rectum. All patients received modified FOLFOX6 as the protocol treatment. After 12 weeks of treatment starting, the DCR was 39.4% (95% confidence interval 21.8–57.0) and the response rate (complete response and partial response) was 6.1%. The median number of chemotherapy cycles was five and the median total dose of oxaliplatin was 425 mg/m2. Median progression-free survival time was 98 days and median overall survival was 300 days. The incidence of grade ≥1 and grade ≥3 allergic reactions was 28.1% and 3.1%, respectively. The incidence of grade ≥1 and grade ≥3 peripheral sensory neuropathy was 53.1% and 0%, respectively. There were no other severe adverse events and no treatment-related deaths.
Conclusion
Reintroducing oxaliplatin can be both safe and effective. This may be a salvage option for patients with metastatic colorectal cancer who achieved a response or stable disease with prior oxaliplatin-based therapy followed by disease progression ≥6 months previously during prior oxaliplatin-based therapy.
Keywords: reintroduction, oxaliplatin, FOLFOX, advanced colorectal cancer, salvage-line
Introduction
Colorectal cancer is the leading cause of cancer death among women and third highest cause of cancer death among men in Japan.1 Oxaliplatin, irinotecan, fluorouracil (5-FU), and several molecular-targeting agents are key drugs in the treatment of metastatic colorectal cancer (mCRC).2–6 Most patients receive regimens that include a combination of these key drugs (such as 5-FU+leucovorin+oxaliplatin [FOLFOX], CapeOX, or 5-FU+leucovorin+irinotecan [FOLFIRI]) plus bevacizumab, and cetuximab or panitumumab is available if the tumor has wild-type Kirsten rat sarcoma (KRAS).7–14 Sequential use of the established regimens containing these key agents has been shown to prolong overall survival (OS) by up to 30 months, which is the current benchmark.15,16 Recently, new agents such as regorafenib17 and TAS-10218 have been approved as salvage therapy for mCRC in Japan. Because there was no valid standard treatment before the approval of these new agents, most patients with advanced cancer received best supportive care (BSC). On the other hand, many patients who only receive BSC if ineligible for clinical trials could still benefit from further intensive therapy.
In 2009, retrospective subgroup analysis of the OPTIMOX studies that investigated the effect of reintroducing oxaliplatin (“stop-and-go” concept) revealed promising results, and indicated that the primary response to oxaliplatin and the oxaliplatin-free interval influenced the effectiveness of reintroduction of oxaliplatin.19–22 However, no prospective clinical trials of this therapy have been performed.
A retrospective analysis of the efficacy of rechallenge therapy was reported before the OPTIMOX studies, in which the previous chemotherapy regimen with or without a biological agent was given to patients who had already shown resistance to treatment.23 However, these studies were not consistent with respect to the interval between primary and secondary treatment (rechallenge) or the clinical response to the initial therapy, meaning that the mechanism and role of rechallenge in clinical practice have remained unclear. In addition, repeating treatment to which the tumor has already acquired resistance is generally considered to be unacceptable. Thus, the rechallenge strategy is substantially different from the stop-and-go strategy used in the OPTIMOX trials that focused on decreasing the risk of cumulative toxicity.
To our knowledge, there have been no reports about prospective trials of rechallenge treatment for patients showing confirmed progression after induction therapy that have employed well-defined eligibility criteria. Also, at the time of planning our study, the clinical trials of regorafenib and TAS-102 had not started in Japan. Accordingly, we performed a single-arm, open-label Phase II study (RE-OPEN) to evaluate the safety and efficacy of reintroducing oxaliplatin from January 2011.
Patients and methods
Study design
RE-OPEN was a prospective, single-arm, open-label Phase II study performed at a single center in Japan. We evaluated the safety and efficacy of reintroducing oxaliplatin in mCRC patients whose tumors had not responded to prior chemotherapy with oxaliplatin and irinotecan. This study was performed in accordance with the Declaration of Helsinki and the ethical guidelines for clinical studies. The institutional review board at the Cancer Institute Hospital of the Japanese Foundation of Cancer Research approved the protocol and the study is registered with the University Hospital Medical Information Network Clinical Trials Registry (ID 000004884).
This study evaluated the efficacy of reintroducing oxaliplatin (in modified FOLFOX6 [mFOLFOX6] regimens) after patients with mCRC had failed prior chemotherapy containing oxaliplatin and irinotecan. The primary endpoint was the disease control rate (DCR) after 12 weeks of treatment starting, while the secondary endpoints were safety, overall response rate (ORR), progression-free survival (PFS), and OS. Simon’s two-stage design was used for statistical assumptions.24 The target sample size was 33 patients (18 in step 1 and 15 in step 2), assuming the expected DCR and threshold DCR after 12 weeks of treatment starting were 40% and 20%, respectively, with a one-sided α-level of 5% and a power of 80%. In step 1, it was planned to accrue 18 patients. If four or fewer of the 18 patients achieved disease control, the study would be stopped. Otherwise, 15 additional patients would be accrued for a total of 33. The null hypothesis would be rejected if eleven or more of the 33 patients achieved disease control.
Patients
Eligible patients had a histologically confirmed diagnosis of mCRC and had shown a response or stable disease before confirmed progression during previous oxaliplatin-based therapy at least 6 months before entering the study. They also met the following criteria: age 20 years or older; Eastern Cooperative Oncology Group (ECOG) performance status 0–2; history of previous irinotecan-based chemotherapy; measurable or evaluable disease; life expectancy ≥12 weeks; neutrophil count ≥1,500/mm3; platelet count ≥100,000/mm3; hemoglobin ≥9.0 g/dL; total bilirubin ≤1.5 times the upper limit of normal; aspartate aminotransferase and alanine aminotransferase ≤2.5 times the upper limit of normal (≤5.0 if liver metastases were present); serum creatinine ≤1.5 times the upper limit of normal; and signed informed consent.
Patients with any of the following conditions were excluded: severe peripheral sensory neuropathy (PSN); a history of serious hypersensitivity to drugs; active infection; interstitial lung disease, severe emphysema, or pulmonary fibrosis; paralytic or mechanical bowel obstruction; uncontrolled hypertension; uncontrolled diabetes; cirrhosis; clinically significant cardiovascular disease; history of myocardial infarction within the previous 3 months; uncontrolled angina pectoris or arrhythmia; multiple primary cancers within the past 5 years; pleural effusion requiring drainage ascites or pericardial effusion; uncontrolled diarrhea; brain tumor or brain metastases; clinically significant mental or psychological disease; and any other condition making a patient unsuitable for this study.
Chemotherapy
The mFOLFOX6 regimen was administered every 2 weeks. On day 1, oxaliplatin (85 mg/m2) was given as a 2-hour intravenous infusion and levoleucovorin (200 mg/m2) was given as a 2-hour intravenous infusion concurrently with oxaliplatin, immediately followed by intravenous bolus injection of 5-FU (400 mg/m2) and a 46-hour intravenous infusion of 5-FU (2,400 mg/m2).
Study treatment was delayed if any of the following criteria were applicable on the day when administration was scheduled or the previous day: a neutrophil count <1,500/mm3, a platelet count <75,000/mm3, active infection with fever ≥38.0°C, grade 2 or worse diarrhea, grade 3 or worse PSN, and other grade 2 or worse non-hematological toxicities. The oxaliplatin dose was reduced to 65 or 50 mg/m2 if grade 3–4 neutropenia or thrombocytopenia, grade 3–4 fatigue, persistent grade 2 or reversible grade 3 PSN, or any grade 3–4 non-hematological toxicities occurred. Bolus 5-FU was reduced to 300 mg/m2 if grade 3–4 neutropenia, thrombocytopenia, febrile neutropenia, nausea/vomiting, diarrhea, stomatitis, skin toxicity, or any other grade 3–4 non-hematological toxicities occurred. Infusional 5-FU in mFOLFOX6 therapy was also reduced to 2,000 or 1,600 mg/m2 according to the same criteria. The study was terminated if grade 3 toxicity persisted after a 21-day washout period or if grade 4 PSN or a grade 3–4 allergic reaction occurred. The study was also terminated if the patient required longer than 3 weeks to recover from an adverse event.
Evaluation of safety and efficacy
Data on the patients, including the results of imaging studies, were recorded in electronic clinical records. A multidisciplinary hospital CRC team confirmed patient eligibility. In all patients, adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.0 every 2 weeks or before each treatment cycle. Treatment was continued until any of the following occurred: disease progression, unmanageable toxicity, patient refusal, or transfer of the patient to another hospital. The baseline tumor response was assessed within 4 weeks before enrollment in the study, and the tumor response was then assessed prospectively every 6 weeks by computed tomography according to Response Evaluation Criteria for Solid Tumors version 1.1. The Independent Data and Safety Monitoring Committee of the RE-OPEN study group confirmed all safety and efficacy data obtained during this study.
Statistical analysis
The DCR was calculated from the number of patients who achieved a complete response (CR), partial response (PR), or stable disease with treatment, while the ORR was based on the number of patients who had CR or PR. PFS was defined as the interval between the date of starting treatment and the date of confirming disease progression or death. Data for patients without disease progression were censored on the date at which the patient was last confirmed to be alive. OS was calculated from the date of starting treatment until the date of death from any cause. In patients who were lost to follow-up, data were censored on the date when the patient was last confirmed to be alive. PFS and OS were estimated by the Kaplan–Meier method and were compared using the log-rank test, with predictive or prognostic factors being identified by univariate analysis. Multivariate analysis of the factors was conducted by using the Cox proportional hazards model to identify factors influencing PFS and OS. All analyses were carried out with Statistical Package for the Social Sciences software version 22.0 (IBM Corporation, Armonk, NY, USA) and P<0.05 was considered to indicate statistical significance.
Results
Outline of the study
Interim analysis was performed on the initial 18 patients enrolled in step 1. Disease control was observed in seven patients after 12 weeks of treatment starting with an acceptable safety profile. Since efficacy and safety of reintroducing oxaliplatin were suggested, an additional 15 patients were enrolled in step 2 according to the independent review committee’s judgment. Thus, final analysis was performed on a total of 33 patients.
Baseline patient characteristics
The 33 eligible patients were enrolled between February 2011 and August 2013 and their characteristics were as follows: median age 62 (range: 35–77) years; ECOG performance status 0 in 84.8%; adjuvant treatment in 39.4%, with two patients receiving oxaliplatin-based treatment; first-line chemotherapy with FOLFOX/XELOX in 61%/39%, first-line ORR of 60.6%, median oxaliplatin-free interval of 512 (range: 211–1,479) days, and a median oxaliplatin dose of 1,454 mg/m2. Most of the patients also received molecular-targeting agents. The details are shown in Table 1.
Table 1.
Baseline patient characteristics (n=33)
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| Male | 19 | 57.6 |
| Female | 14 | 42.4 |
| Age, years | ||
| Median | 62 | |
| Range | 35–77 | |
| ECOG performance status | ||
| 0 | 28 | 84.8 |
| 1 | 5 | 15.2 |
| Site of primary tumor | ||
| Colon | 14 | 42.4 |
| Rectum | 19 | 57.6 |
| Primary tumor | ||
| Resected | 30 | 90.9 |
| Unresected | 3 | 9.1 |
| Histology | ||
| Well | 7 | 21.2 |
| Moderately | 23 | 69.7 |
| Poorly | 1 | 3.0 |
| Unknown | 2 | 6.1 |
| Adjuvant treatment | ||
| Yes | 13* | 39.4 |
| No | 20 | 60.6 |
| KRAS status | ||
| Wild-type | 18 | 54.5 |
| Mutant | 15 | 45.5 |
| Sites of metastasis | ||
| Liver | 23 | 69.7 |
| Lung | 25 | 75.8 |
| Lymph nodes | 11 | 33.3 |
| Peritoneum | 4 | 12.1 |
| Other | 4 | 12.1 |
| Number of metastatic sites | ||
| 1 | 9 | 27.3 |
| ≥2 | 24 | 72.7 |
| Summary of prior oxaliplatin-based treatment | ||
| Treatment line | ||
| First | 26 | 78.8 |
| Second | 7 | 21.2 |
| Treatment regimen | ||
| FOLFOX | 20 | 60.6 |
| XELOX | 13 | 39.4 |
| Best overall tumor response | ||
| Complete response | 1 | 3.0 |
| Partial response | 19 | 57.6 |
| Stable disease | 13 | 39.4 |
| Total dose of oxaliplatin (mg/m2) | ||
| Median | 1,454 | |
| Range | 511–3,290 | |
| Oxaliplatin-free interval (days) | ||
| Median | 512 | |
| Range | 211–1,479 | |
| Exposure to molecular-targeting agents | ||
| None | 1 | 3.0 |
| Bevacizumab | 27 | 81.8 |
| Anti-EGFR (CET or PAN) | 19 | 57.6 |
Note:
Two patients received oxaliplatin-based treatment.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; FOLFOX, 5-fluorouracil, levoleucovorin and oxaliplatin; CET, cetuximab; PAN, panitumumab; KRAS, Kirsten rat sarcoma; XELOX, capectabine and oxaliplatin.
Treatment
The median number of treatment cycles was five (range: 1–14). The median cumulative dose was 425 (range: 85–1,190) mg/m2 for oxaliplatin, 1,850 (range: 400–5,600) mg/m2 for bolus 5-FU, and 11,400 (range: 2,400–33,600) mg/m2 for infusional 5-FU. The median relative dose intensity was 0.77 (range: 0.25–1.04) for oxaliplatin, 0.82 (range: 0.25–1.04) for bolus 5-FU, and 0.81 (range: 0.25–1.04) for infusional 5-FU.
Treatment was delayed in 22 patients (66.7%) for a median of one dose (range: 1–4) of each drug, with the delay being due to neutropenia in eleven patients (33.3%), thrombocytopenia in three, upper respiratory tract infection in two, allergic reaction in one, PSN in one, and personal reasons in two patients. Fourteen patients (45.4%) required dose reduction at least once, with the reason being neutropenia in eight (24.2%). Twenty-four patients (72.7%) continued treatment until disease progression, while the other reasons for discontinuing treatment were adverse events in two patients and refusal or personal reasons in seven patients. Of the 24 patients with confirmed disease progression, ten (30.3%) received subsequent chemotherapy (regorafenib in seven, another investigational drug in two, and mFOLFOX6 in one) and 14 patients (42.4%) received BSC.
Efficacy
The tumor response was assessed in 31 of the 33 patients (Table 2). It was not evaluable in two patients due to refusal to cooperate after treatment. The ORR was 6.1% (95% confidence interval [CI] −2.5–14.7), and disease control was achieved in 39.4% (95% CI 21.8–57.0) of the patients after 12 weeks of treatment starting. Overall disease control rate was 66.7% (95% CI 49.7–83.6). A waterfall plot of the best tumor response in 29 patients is shown in Figure 1. Two patients were excluded from this analysis because their target lesions became unmeasurable at the first evaluation, but were subsequently classified as having progressive disease after the appearance of new metastatic lesions. Tumor shrinkage was observed in 45.5% of the enrolled patients. The median follow-up period was 271.0 (range: 43–983) days. Median PFS was 98.0 days (95% CI 55.7–140.3; Figure 2), while median OS was 300.0 days (95% CI 229.3–370.7). In addition, median OS from the initiation of first-line treatment was 1,471.0 days (95% CI 1,128.2–1,813.8; Figure 3). According to the results of univariate analysis, PFS was significantly longer in patients with DCR after 12 weeks of treatment starting (hazard ratio [HR] 0.20, P=0.001) and patients with a history of adjuvant therapy (HR 0.35, P=0.026), while there was no significant improvement of PFS in patients with a performance status of 0 (P=0.085), a single metastasis (P=0.055), or a primary tumor located in the colon (P=0.113). OS was significantly longer in men (HR 0.36, P=0.012) and in patients with well or moderately differentiated adenocarcinoma (HR 0.07, P=0.033), while there was no significant improvement of OS in patients with disease control after 12 weeks of treatment starting (P=0.090) and those with a single metastasis (P=0.062). However, wild-type KRAS positivity (P=0.305), use of anti-epidermal growth factor receptor (EGFR) agents in third-line therapy (P=0.227), the interval from the last dose of oxaliplatin (P=0.825), and the total dose of oxaliplatin (P=0.293) were not prognostic factors for OS. In addition, PFS (P=0.922) and OS (P=0.660) were not significantly influenced by whether the first-line regimen was XELOX or FOLFOX (Table 3).
Table 2.
Tumor response after 12 weeks of treatment starting (n=33)
| Status | n | % |
|---|---|---|
| Complete response | 0 | 0 |
| Partial response | 2 | 6.1 |
| Stable disease | 11 | 33.3 |
| Progressive disease | 18 | 54.5 |
| Not evaluable | 2* | 6.1 |
| Disease control rate after 12 weeks of treatment starting, % | 39.4 (95% CI 21.8–57.0) | |
| Overall disease control rate, % | 66.7** (95% CI 49.7–83.6) | |
| Overall response rate, % | 6.1 (95% CI −2.5–14.7) | |
Note:
Tumor response was not evaluable in two patients who refused to cooperate after treatment initiation.
Overall disease control was achieved in 22 patients.
Abbreviation: CI, confidence interval.
Figure 1.
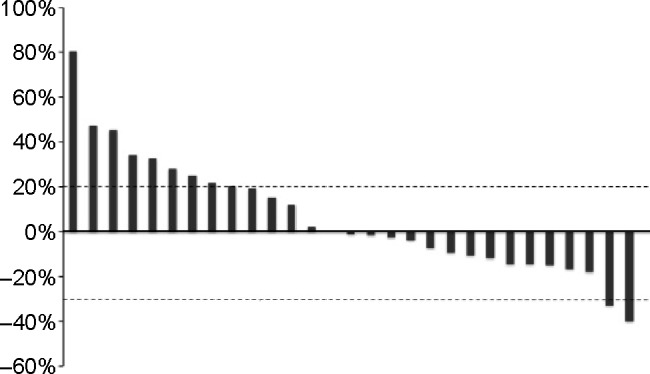
Waterfall plot of the best tumor response in patients with metastatic colorectal cancer receiving reintroduction of oxaliplatin-based therapy.
Note: Tumor shrinkage was observed in 15/33 patients (45.5%).
Figure 2.
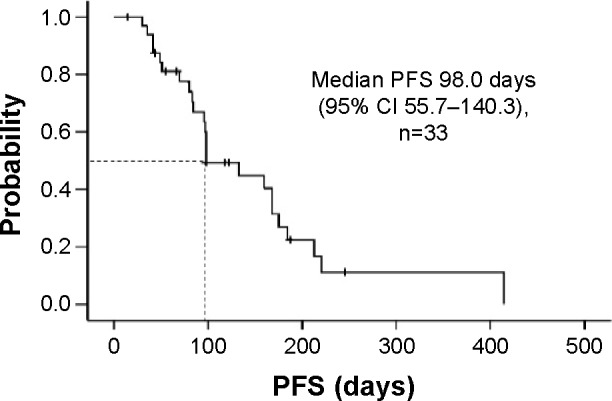
Progression-free survival in patients with metastatic colorectal cancer receiving reintroduction of oxaliplatin-based therapy.
Abbreviations: CI, confidence interval; PFS, progression-free survival.
Figure 3.
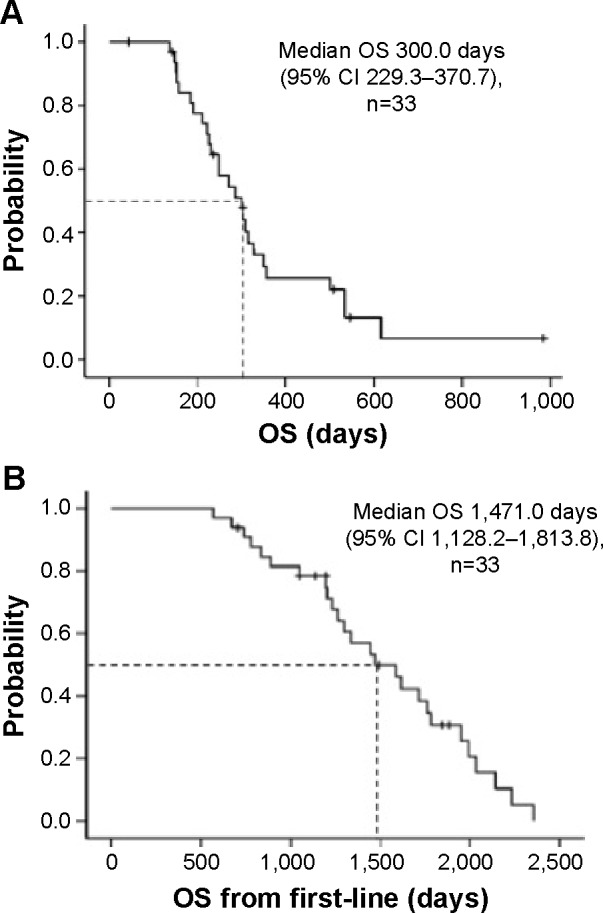
Overall survival (A) and overall survival from the initiation of first-line treatment (B) in patients with metastatic colorectal cancer receiving reintroduction of oxaliplatin-based therapy.
Abbreviations: CI, confidence interval; OS, overall survival.
Table 3.
Univariate analysis of factors influencing survival (n=33)
| Subgroup | HR | 95% CI | P-value |
|---|---|---|---|
| Progression-free survival | |||
| ECOG performance status | |||
| 0 | 0.37 | 0.12–1.15 | |
| 1 | 1 | 0.085 | |
| Site of primary tumor | |||
| Colon | 0.48 | 0.20–1.19 | |
| Rectum | 1 | 0.113 | |
| Adjuvant treatment | |||
| No | 1 | ||
| Yes | 0.35 | 0.14–0.88 | 0.026 |
| Number of metastatic sites | |||
| 1 | 0.30 | 0.09–1.03 | |
| ≥2 | 1 | 0.055 | |
| DC after 12 weeks of treatment starting | |||
| No | 1 | ||
| Yes | 0.20 | 0.08–0.51 | 0.001 |
| Overall survival | |||
| Sex | |||
| Male | 0.36 | 0.16–0.80 | |
| Female | 1 | 0.012 | |
| Histology | |||
| Well or moderately | 0.07 | 0.01–0.81 | |
| Poor | 1 | 0.033 | |
| Number of metastatic sites | |||
| 1 | 0.35 | 0.12–1.05 | |
| ≥2 | 1 | 0.062 | |
| DC after 12 weeks of treatment starting | |||
| No | 1 | ||
| Yes | 0.49 | 0.22–1.12 | 0.090 |
Abbreviations: CI, confidence interval; DC, disease control; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
Multivariate analysis of the 33 patients showed that a better performance status (HR 0.06; 95% CI 0.01–0.34, P=0.001), a single metastasis (HR 0.03; 95% CI 0.01–0.20, P=0.001), and disease control after 12 weeks of treatment starting (HR 0.05; 95% CI 0.01–0.27, P<0.001) were independent determinants of longer PFS. In the case of OS, male sex (HR 0.25; 95% CI 0.10–0.67, P=0.005) and a single metastasis (HR 0.27; 95% CI 0.08–0.89, P=0.031) were revealed to be positive prognostic indicators (Table 4).
Table 4.
Multivariate analysis of factors influencing survival (n=33)
| Subgroup | HR | 95% CI | P-value |
|---|---|---|---|
| Progression-free survival | |||
| ECOG performance status | |||
| 0 | 0.06 | 0.01–0.34 | |
| 1 | 1 | 0.001 | |
| Number of metastatic sites | |||
| 1 | 0.03 | 0.01–0.20 | |
| ≥2 | 1 | <0.001 | |
| DC after 12 weeks of treatment starting | |||
| No | 1 | ||
| Yes | 0.05 | 0.01–0.27 | <0.001 |
| Overall survival | |||
| Sex | |||
| Male | 0.25 | 0.10–0.67 | |
| Female | 1 | 0.005 | |
| Number of metastatic sites | |||
| 1 | 0.27 | 0.08–0.89 | |
| ≥2 | 1 | 0.031 |
Abbreviations: CI, confidence interval; DC, disease control; ECOG, Eastern Co operative Oncology Group; HR, hazard ratio.
Safety
Adverse events for 32 patients are summarized in Table 5. One patient refused to continue the protocol treatment during the initial cycle. The most common grade 3 or 4 adverse events were neutropenia (28.1%) and diarrhea (6.3%). Allergic reactions occurred in 28.1% of the patients, with one (3.1%) grade 3 episode. Allergic reactions were more frequent in patients with a high total dose of oxaliplatin (mean 2,483 mg/m2) than in those with a low total dose of oxaliplatin (mean 1,954 mg/m2; t-test, P=0.074). There were no other severe treatment-related adverse events and no deaths during treatment.
Table 5.
Adverse events according to Common Terminology Criteria for Adverse Events grade (n=32*)
| Adverse events | All grade
|
Grade ≥ 3
|
||
|---|---|---|---|---|
| n | % | n | % | |
| Hematological | ||||
| Leukopenia | 19 | 59.4 | 2 | 6.3 |
| Neutropenia | 17 | 53.1 | 9 | 28.1 |
| Anemia | 8 | 25.0 | 0 | 0 |
| Thrombocytopenia | 18 | 56.3 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 |
| Non-hematological | ||||
| Anorexia | 14 | 43.8 | 1 | 3.1 |
| Nausea | 16 | 50.0 | 1 | 3.1 |
| Vomiting | 4 | 12.5 | 0 | 0 |
| Diarrhea | 16 | 50.0 | 2 | 6.3 |
| Stomatitis | 14 | 43.8 | 0 | 0 |
| Total bilirubin increased | 3 | 9.4 | 0 | 0 |
| AST increased | 16 | 50.0 | 0 | 0 |
| ALT increased | 12 | 37.5 | 0 | 0 |
| Creatinine increased | 4 | 12.5 | 0 | 0 |
| Allergic reaction | 9 | 28.1 | 1 | 3.1 |
| Sensory neuropathy | 17 | 53.1 | 0 | 0 |
Note:
One patient refused to continue treatment during the first cycle.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Discussion
Oxaliplatin is one of the key drugs for mCRC, and it is usually combined with 5-FU plus leucovorin or with oral fluoropyrimidine drugs in regimens such as FOLFOX, XELOX, or SOX.7,8,25 Oxaliplatin is also considered appropriate for use with biological drugs such as anti-vascular endothelial growth factor (VEGF) or anti-EGFR agents according to the results of previous trials.8,9,11,15,16 However, PSN is an unavoidable side effect as the total dose of oxaliplatin increases, which requires discontinuation of treatment followed by resumption of oxaliplatin after recovery from neuropathy.19,20 The RE-OPEN study was performed to investigate the efficacy of reintroducing oxaliplatin in patients who had previously been treated with oxaliplatin-based regimens until disease progression. It is important to note that reintroduction of oxaliplatin, as performed in RE-OPEN, is different from resumption of treatment after an oxaliplatin-free interval due to PSN. We will consider the indications for reintroducing oxaliplatin, the safety of reintroduction, and its role in salvage treatment.
In 2004, the results of a retrospective analysis of reintroduction of FOLFOX in 29 patients after a break in treatment or following progression on another regimen were reported.23 Initial FOLFOX achieved PR in 83% and stable disease in 14%, while 3% had progressive disease and the reason was considered to be the low dose intensity of treatment. During initial treatment, 31% of the patients developed grade 3 PSN, but the actual reasons for discontinuation of treatment were not clear in this report. Between the end of initial administration and reintroduction of FOLFOX, 55% of the patients received 5-FU/leucovorin-based or irinotecan-based treatment and 45% were observed without chemotherapy. The median interval until resuming FOLFOX was 12 weeks in patients under observation, 48 weeks in patients receiving other chemotherapy, and 30 weeks in the entire study population. Thus, the interval showed a difference of 36 weeks between the two groups. After reintroduction, a median of six cycles of FOLFOX was given, with a median oxaliplatin dose intensity of 43/mg/m2/week and a cumulative dose of 492 mg/m2. The objective response rate after reintroduction was 20.7% for all 29 patients, being 31% for patients under observation during the interval (n=13) and 12% for those receiving other chemotherapy (n=16). The DCR was 73%, being 93% for patients under observation and 56% for those given other chemotherapy. As for survival, the median PFS and OS were 18 weeks and 42 weeks, respectively. PFS was 27 weeks and OS was 58 weeks for patients under observation in the interval, while the corresponding times were 11 weeks and 36 weeks for patients receiving additional therapy. Reintroduction was not recommended in oxaliplatin-refractory patients, except those with an extremely low oxaliplatin dose intensity during initial treatment, because patients who achieved a good response to initial treatment tended to show higher response after reintroduction. However, the reasons for administering a low dose intensity that led to a poor initial response were not described. Also, various strategies were employed in the interval between oxaliplatin-based treatments, and the reasons for discontinuation of oxaliplatin or other agents were unclear. Therefore, the true usefulness of reintroduction for patients who had acquired resistance to oxaliplatin was not revealed by this study.
Following this report, the OPTIMOX-1 and OPTIMOX-2 studies were performed to prospectively investigate the effect of discontinuing and reintroducing oxaliplatin according to the stop-and-go strategy with an oxaliplatin-free interval to reduce the incidence of severe PSN.19–21 During the oxaliplatin-free interval after induction therapy, maintenance with a simplified leucovorin/5-FU (sLV5FU2) regimen was done in OPTIMOX-1 and patients stayed off chemotherapy in OPTIMOX-2. According to the results, stop-and-go oxaliplatin therapy combined with sLV5FU2 as maintenance therapy achieved the improvement of PFS and OS, suggesting that a chemotherapy-free interval was undesirable except for possible improvement in quality of life.
A pooled analysis of data from the OPTIMOX-1 and OPTIMOX-2 studies was performed to elucidate the influence of initial FOLFOX treatment on the response rate, PFS, and OS after reintroduction of oxaliplatin.26 The study population was divided into two groups with an oxaliplatin-free interval of <6 and ≥6 months. In the <6 months group, the response rate, DCR, PFS, and OS were 14%, 45%, 3.0 months, and 8.8 months, respectively. In the ≥6 months group, the response rate, DCR, PFS, and OS were 22%, 63%, 5.5 months, and 16.8 months, respectively. The median PFS and OS were significantly better in the group with an oxaliplatin-free interval ≥6 months. Thus, an oxaliplatin-free interval of at least 6 months seems to be appropriate for improving sensitivity to oxaliplatin after reintroduction by the stop-and-go approach. Furthermore, the authors concluded that the oxaliplatin-free interval and the initial sensitivity to oxaliplatin should be taken into consideration when deciding treatment for mCRC.
According to previous reports,23,26 a longer oxaliplatin-free interval and initial sensitivity to oxaliplatin with disease control are valid criteria for reintroduction. However, there was no information provided about the details of the biological agents administered during/after reintroduction of oxaliplatin or alternative treatments during the chemotherapy-free interval or after oxaliplatin retreatment. Moreover, these studies were not designed to investigate the efficacy of reintroducing oxaliplatin in patients whose tumors had acquired resistance to initial oxaliplatin-based chemotherapy.
To our knowledge, the present study is the first prospective trial with well-defined eligibility criteria to investigate reintroduction of oxaliplatin for patients with confirmed disease progression on initial oxaliplatin-based treatment. The strengths of RE-OPEN were as follows: the oxaliplatin-free interval was ≥6 months, the initial response to oxaliplatin-based therapy (CR, stable disease, or PR) was included in the eligibility criteria, and initial treatment with oxaliplatin was continued until confirmed disease progression. Review of prior oxaliplatin-based treatment revealed that adequate oxaliplatin doses were administered, with the median total dose being 1,454 mg/m2, indicating that most of the tumors were actually refractory to oxaliplatin. Furthermore, other cytotoxic agents and biological agents (including bevacizumab and anti-EGFR agents) were given to all patients with wild-type KRAS in this study. Hence, our reintroduction study targeted patients who were refractory not only to oxaliplatin but also to other cytotoxic and biological agents, ie, their tumors were resistant to all standard chemotherapy regimens except for new agents such as regorafenib and TAS-102.17,18 It was considered that our strategy should improve the outcome of patients indicated for BSC if the study showed positive results. According to multivariate analysis, a better performance status, solitary metastasis, and disease control after 12 weeks of treatment starting were factors with an independent influence on PFS. These factors should therefore be taken into account when making decisions about salvage treatment as well as use of newer agents (regorafenib and TAS-102). Finally, OS from the initiation of first-line treatment was a median of 48 months in RE-OPEN, which exceeds the world benchmark of 30 months.15,16
Maintenance therapy with 5-FU/leucovorin or oral fluoropyrimidines is the worldwide standard when an oxaliplatin-free interval is required in order to reduce severe PSN and delay disease progression in mCRC patients.19–21,27 Although our reintroduction method differs from the stop-and-go strategy, both strategies employ an oxaliplatin-free interval that could be useful for diminishing PSN in clinical practice. There has been little information about the toxicity of reintroduction of oxaliplatin-based therapy. In the present study, the safety profile of reintroduction was acceptable. All patients started mFOLFOX6 treatment with full doses of all agents and hematological toxicity was not more severe than expected. The relative dose intensity of oxaliplatin was >0.7 and that of 5-FU was >0.8, which seem to be sufficient for salvage therapy in patients with previous heavy treatment. The reason for the lack of grade 3 PSN may be the short duration compared with prior treatment, but acute progression of PSN was not observed either. On the other hand, as we previously reported, allergic reactions caused by oxaliplatin are critical with respect to reintroduction therapy.28 Allergic reactions are thought to be treatment cycle-dependent and there is a low incidence of severe events. Nevertheless, careful monitoring is required during infusion of oxaliplatin.
In the present study, maintenance therapy was not permitted in the oxaliplatin-free interval, and all agents were ceased. Therefore, the actual efficacy and safety of reintroduction of oxaliplatin could be evaluated by our study. Recently approved agents such as regorafenib and TAS-102 are now standard key drugs that have shown superiority to BSC in Phase III trials.17,18
The present study had several limitations, including a small number of patients and no control arm as BSC. However, the efficacy of the RE-OPEN strategy in terms of PFS, OS, and tumor response was comparable with the results of Phase III trials, and the rate of post-treatment chemotherapy was also consistent across these three trials at approximately 30% of enrolled patients.17,18 This means that the sequential treatment pattern for mCRC patients using this strategy could be reintroduction of oxaliplatin, regorafenib monotherapy, and TAS-102 monotherapy. In RE-OPEN, all patients received mFOLFOX6 as widely used in clinical practice. Although the schedules of XELOX and FOLFOX differ from each other, the dose intensities of oxaliplatin are almost the same. Furthermore, XELOX has also been shown to be a substitute for FOLFOX by a Phase III trial in terms of efficacy and safety.8 In RE-OPEN, we focused on the efficacy of reintroduction of oxaliplatin regardless of prior oxaliplatin-based regimens, and no statistically significant difference in multivariate analysis was observed for efficacy between the prior XELOX and FOLFOX groups. We thereby consider the results of reintroduction could be applied to the two different regimens. Concerning toxicity, reintroduction of oxaliplatin seemed to be favorable because patients were familiar with management of oxaliplatin-induced toxicity (except allergic reactions) and considered it to be easier compared with other treatment.
Thus, reintroduction could be one more treatment option added to the new agents, and sequential use of all these strategies until disease progression could be the new approach to improve the survival of mCRC patients. However, translational studies to select tailored sequential therapy for different patients will be needed for each strategy. According to previous research, EGFR, VEGF, and insulin have been shown to activate the phosphatidyl inositol 3-kinase/Akt pathway to reduce the treatment efficacy of oxaliplatin.29–33 This mechanism supports that EGFR or VEGF inhibitors might increase the antitumor effect of oxaliplatin in not only front-line treatment but also salvage-line treatment for mCRC. We are currently planning a new study of reintroduction therapy combining oxaliplatin with a biological agent or new agent, and we hope that the median OS of mCRC patients will reach over 5 years in the near future.
Conclusion
Reintroducing oxaliplatin was demonstrated to be a reasonable option for selected patients with mCRC who have previously shown resistance to oxaliplatin, have an oxaliplatin-free interval ≥6 months, and achieved disease control during prior oxaliplatin-based therapy. Sequential use of reintroduction therapy and new agents could become a new approach to treatment of mCRC.
Acknowledgments
We thank the participating patients, their family members, and all researchers involved in this study. We are grateful to Drs Hiroyuki Uetake and Atsushi Sato for their support as the independent review committee, and to Yuki Horiike and Kaori Kobayashi for collecting data. This study was presented as a poster at the 2015 Gastrointestinal Cancers Symposium (http://meetinglibrary.asco.org/content/139456-158), and this paper is the first to publish the overall results as an original full length article.
Footnotes
Disclosure
The authors report no conflicts of interest with this paper.
References
- 1.Center for Cancer Control and Information Services, National Cancer Center Cancer Statistics in Japan. 2013. [Accessed May 21, 2015]. Available from: http://ganjoho.jp/data/professional/statistics/backnumber/2013/cancer_statistics_2013.pdf.
- 2.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 6.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 8.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2007;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 9.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2006;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 11.Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 12.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 13.Karapetis C, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 14.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 15.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 16.Venook AP, Niedzwiecki D, Lenz HJ, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) J Clin Oncol. 2014;32(5 Suppl):Abstr LBA3. [Google Scholar]
- 17.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 18.Yoshino T, Mizunuma N, Yamazaki K, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13:993–1001. doi: 10.1016/S1470-2045(12)70345-5. [DOI] [PubMed] [Google Scholar]
- 19.Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer – a GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 20.Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27:5727–5733. doi: 10.1200/JCO.2009.23.4344. [DOI] [PubMed] [Google Scholar]
- 21.de Gramont A, Buyse M, Abrahantes JC, et al. Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol. 2007;25:3224–3229. doi: 10.1200/JCO.2006.10.4380. [DOI] [PubMed] [Google Scholar]
- 22.de Gramont A, Chibaudel B, Bourges O, et al. Definition of oxaliplatin sensitivity in patients with advanced colorectal cancer previously treated with oxaliplatin-based therapy. J Clin Oncol. 2009;27:15 Suppl. Abstr 4024. [Google Scholar]
- 23.Maindrault-Goebel F, Tournigand C, André T, et al. Oxaliplatin reintroduction in patients previously treated with leucovorin, fluorouracil and oxaliplatin for metastatic colorectal cancer. Ann Oncol. 2004;15:1210–1214. doi: 10.1093/annonc/mdh305. [DOI] [PubMed] [Google Scholar]
- 24.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Takahari D, Matsumoto H, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278–1286. doi: 10.1016/S1470-2045(13)70490-X. [DOI] [PubMed] [Google Scholar]
- 26.Chibaudel B, Tournigand C, Bonnetain F, et al. Platinum-sensitivity in metastatic colorectal cancer: towards a definition. Eur J Cancer. 2013;49:3813–3820. doi: 10.1016/j.ejca.2013.07.150. [DOI] [PubMed] [Google Scholar]
- 27.Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12:642–653. doi: 10.1016/S1470-2045(11)70102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suenaga M, Mizunuma N, Shinozaki E, et al. Management of allergic reactions to oxaliplatin in colorectal cancer patients. J Support Oncol. 2008;6:373–378. [PubMed] [Google Scholar]
- 29.Chen J, Huang XF, Qiao L, Katsifis A. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol. 2011;2:27–33. doi: 10.3978/j.issn.2078-6891.2010.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baricevic I, Roberts DL, Renehan AG. Chronic insulin exposure does not cause insulin resistance but is associated with chemo-resistance in colon cancer cells. Horm Metab Res. 2014;46:85–93. doi: 10.1055/s-0033-1354414. [DOI] [PubMed] [Google Scholar]
- 31.Gaur S, Chen L, Ann V, et al. Dovitinib synergizes with oxaliplatin in suppressing cell proliferation and inducing apoptosis in colorectal cancer cells regardless of RAS-RAF mutation status. Mol Cancer. 2014;13:21. doi: 10.1186/1476-4598-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonetti A, Giuliani J, Muggia F. Targeted agents and oxaliplatin-containing regimens for the treatment of colon cancer. Anticancer Res. 2014;34:423–434. [PubMed] [Google Scholar]
- 33.Papadimitriou K, Rolfo C, Dewaele E, et al. Incorporating anti-VEGF pathway therapy as a continuum of care in metastatic colorectal cancer. Curr Treat Options Oncol. 2015;16:333. doi: 10.1007/s11864-015-0333-9. [DOI] [PubMed] [Google Scholar]


