Abstract
Purpose
To determine the incidence of chemotherapy-induced nausea/vomiting (CINV) and chemotherapy treatment delay and adherence among patients receiving palonosetron versus other 5-hydroxytryptamine receptor antagonist (5-HT3 RA) antiemetics.
Materials and methods
This retrospective claims analysis included adults with primary malignancies who initiated treatment consisting of single-day intravenous highly emetogenic chemotherapy (HEC) or moderately EC (MEC) regimens. Treatment delay was defined as a gap in treatment at least twice the National Comprehensive Cancer Network-specified cycle length, specific to each chemotherapy regimen. Treatment adherence was determined by the percentage of patients who received the regimen-specific recommended number of chemotherapy cycles within the recommended time frame.
Results
We identified 1,832 palonosetron and 2,387 other 5-HT3 RA (“other”) patients who initiated HEC therapy, and 1,350 palonosetron users and 1,379 patients on other antiemetics who initiated MEC therapy. Fewer patients receiving palonosetron experienced CINV versus other (HEC, 27.5% versus 32.2%, P=0.0011; MEC, 36.1% versus 41.7%, P=0.0026), and fewer treatment delays occurred among patients receiving palonosetron versus other (HEC, 3.2% versus 6.0%, P<0.0001; MEC, 17.0% versus 26.8%, P<0.0001). Compared with the other cohort, patients receiving palonosetron were significantly more adherent to the index chemotherapy regimen with respect to the recommended time frame (HEC, 74.7% versus 69.7%, P=0.0004; MEC, 43.1% versus 37.3%, P=0.0019) and dosage (HEC, 27.3% versus 25.8%, P=0.0004; MEC, 15.0% versus 12.6%, P=0.0019).
Conclusion
Palonosetron more effectively reduced occurrence of CINV in patients receiving HEC or MEC compared with other agents in this real-world setting. Additionally, patients receiving palonosetron had better adherence and fewer treatment delays than patients receiving other 5-HT3 RAs.
Keywords: palonosetron, adherence, CINV, delay of therapy, observational, health services research
Introduction
Nausea and vomiting are common chemotherapy-associated side effects ranked by patients as especially distressing.1–7 Chemotherapy-induced nausea and vomiting (CINV) can cause psychological distress, nutritional deficiencies, and reduced quality of life among patients receiving chemotherapy.5–8 Furthermore, its occurrence may potentially affect adherence to chemotherapy regimens, leading to treatment delays or receipt of fewer treatments or lower dosages than recommended.9,10 Such events may have an adverse effect on treatment efficacy, ultimately resulting in suboptimal clinical outcomes and potentially increased health care-related resource utilization and costs.3
Recognizing the importance of preventing and managing CINV, leading oncology societies have issued treatment guidelines11–13,29 recommending 5-hydroxytryptamine-receptor antagonists (5-HT3 RAs) as the preferred medication class to effectively prevent CINV in patients receiving highly emetogenic chemotherapy (HEC) or moderately EC (MEC).19,20 Compared to the older agents, palonosetron – a newer 5-HT3 RA – is pharmacologically distinct, with a longer half-life and greater receptor-binding affinity, allosteric binding to serotonin receptors with positive cooperativity, and cross talk with Neurokinin-1 (NK-1) receptors.21–23 While the early 5-HT3 RA compounds were considered equally efficacious,19 palonosetron demonstrated greater efficacy than active comparators in preventing CINV in patients receiving HEC or MEC in multiple clinical trials.20,24–26 Hatoum et al compared palonosetron with other 5-HT3 RAs in a real-world setting among patients with breast/lung cancer undergoing cisplatin/carboplatin treatments.19,27 They concluded that patients who received prophylaxis with palonosetron had a significantly lower risk of CINV events than those who had received other 5-HT3 RA agents. Furthermore, those breast/lung cancer patients receiving palonosetron experienced 49.5% and 29.1% fewer CINV days, respectively.27 Their study focused on serious CINV events resulting in hospital or emergency department admissions, and did not include CINV events occurring in an outpatient context. Craver et al found that prophylactic administration of palonosetron among patients with hematologic malignancies who were receiving HEC/MEC resulted in a 20.4% decrease in CINV event rate per cycle compared with patients receiving other 5-HT3 RAs.28 However, while 5-HT3 RA agents have been proven effective in preventing CINV, little is known regarding their impact on chemotherapy treatment adherence and delay. To address these questions, a real-world study was designed comparing patients who received palonosetron with those who received other 5-HT3 RAs on incidence of acute and delayed CINV and chemotherapy treatment delay and adherence. This study also contributes to the development of methods to assess medication adherence for intravenous (IV) agents.
Materials and methods
This was an observational nested case–control study using data from the HealthCore Integrated Research Database (HIRDSM). The HIRD is an integrated medical and pharmacy-claims and laboratory-result database of commercially insured patients from 14 major commercial health plans across the US representing approximately 45 million patient-lives dating as far back as January 1, 2001.
Cohort creation
The index date was defined as the earliest medical or pharmacy claim date for an IV HEC or MEC between January 1, 2002 and October 31, 2010. All patients included in the study were adults (≥18 years of age as of the index date) who had one or more medical claims with a diagnosis of primary malignant breast, lung, or colorectal neoplasm during the baseline period, which was defined as the 12 months before the index date. All patients had continuous medical and pharmacy health plan eligibility for at least 12 months pre- and 12 months postindex date. Patients were excluded if they 1) had a secondary malignant neoplasm or primary neoplasms at multiple sites, 2) had preindex HEC or MEC claims, 3) initiated multiday chemotherapy, 4) received oral chemotherapy alone or in combination with an IV formulation, 5) switched from a single-day-per-cycle chemotherapy regimen to multiday chemotherapy, or 6) had medical claim(s) for pregnancy, labor, or delivery in the 6 months postindex.
Lastly, in order to create clean comparison cohorts, patients receiving both palonosetron and any of the “other” 5-HT3 RAs any time during the course of one or more chemotherapy treatment cycles were excluded from the analysis. The remaining patients were stratified into either the palonosetron or other 5-HT3 RA treatment cohorts. Specifically, patients in the palonosetron group received only palonosetron and no other IV 5-HT3 RA agent (ie, dolasetron, granisetron, and/or ondansetron; see Table S1) as prophylactic or rescue therapy beginning 1 day before through 5 days after the start of any chemotherapy treatment cycle; those in the other 5-HT3 RA cohort were allowed to receive any prophylactic 5-HT3 RA agent other than palonosetron.
Assignment of chemotherapy regimens
Index HEC and MEC agents were defined as any chemotherapeutic agent classified as having a known high or moderate emetogenic potential (Table S2).29 Chemotherapy agents were identified using generic product identifier (GPI) and Healthcare Common Procedure Coding System (HCPCS) codes. Chemotherapy dose determined the HEC/MEC status of certain chemotherapy drugs (eg, cyclophosphamide and cisplatin) by calculating the index dose administered and then applying the National Comprehensive Cancer Network® (NCCN®)-recommended Guidelines available at the time of the study for classification (Table S3).29 Because only single-day administration regimens were included in the study, the average dose was equal to the average strength, as noted on medical or pharmacy claims. Body-surface area (BSA) was not available on claim forms, so published BSA estimates of cancer patients were used to determine the average dose per square meter.30 The standard estimates used were 1.91 m2 for men (95% confidence interval [CI] 1.90–1.92) and 1.71 m2 for women (95% CI 1.70–1.72).
For regimens involving a combination of chemotherapeutic agents, the agent with the highest emetic risk defined the risk of the combination (ie, one MEC agent and one HEC agent equaled an HEC regimen; one lowly EC [LEC] and one MEC equaled an MEC regimen).12,13 Two MEC agents were classified as HEC; however, two LEC agents remained a lowly emetogenic regimen (Table 1).31 Additional information on the step-by-step regimen identification can be found in the Supplementary materials.
Table 1.
Algorithm to identify HEC and MEC regimens
| Regimen makeup | HEC/MEC classification of regimen |
|---|---|
| Any HEC drug | HEC |
| Two or more MEC drugs | HEC |
| One MEC drug with or without low or minimal emetogenic chemotherapy (LEC) | MEC |
| Multiple LEC drugs | LEC |
Abbreviations: HEC, highly emetogenic chemotherapy; MEC, moderately EC; LEC, lowly EC; EC, emetogenic chemotherapy.
Claims for index chemotherapy agents dated 7 days or later after the beginning of the cycle were designated as the beginning of the subsequent cycle, and so on until the end of the 12-month observation period. The end of a chemotherapy cycle was determined using either the passing of the NCCN-recommended number of weeks between two cycles (Table 2), which was specific to each treatment regimen, or the start date of the subsequent treatment cycle, whichever occurred earlier.
Table 2.
Chemotherapy index regimens
| Index HEC/MEC regimen (± LEC) | Emetogenicity | Number of recommended cyclesa | Allowed gap (weeks)b | Regimen duration (weeks) |
|---|---|---|---|---|
| Breast cancer | ||||
| Cyclophosphamide (600 mg/m2; with or without docetaxel) | If index dose >1,500 mg/m2 then HECc; if index dose ≤1,500 mg/m2 then MECc |
4 | 3 | 12 |
| Cyclophosphamide (600 mg/m2)/doxorubicin (60 mg/m2) | HECc | 4 | 3 | 12 |
| Cyclophosphamide (600 mg/m2)/doxorubicin (50 mg/m2) + docetaxel | HECc | 6 | 3 | 18 |
| Cyclophosphamide (600 mg/m2)/doxorubicin (60 mg/m2) + docetaxel | HECc | 4 | 3 | 12 |
| Cyclophosphamide (600 mg/m2)/doxorubicin (60 mg/m2) + paclitaxel (R1) | HECc | 4 | 2 | 8 |
| Cyclophosphamide (600 mg/m2)/doxorubicin (60 mg/m2) + paclitaxel (R2) | HECc | 4 | 3 | 12 |
| Cyclophosphamide (500 mg/m2)/doxorubicin (50 mg/m2) + 5-fluorouracil | HECc | 6 | 3 | 18 |
| Cyclophosphamide (100 mg/m2)/epirubicin (830 mg/m2) | HECc | 8 | 3 | 24 |
| Cyclophosphamide (500 mg/m2)/epirubicin (75 mg/m2) + 5-fluorouracil | HECc | 4 | 3 | 12 |
| Cyclophosphamide (500 mg/m2)/epirubicin (100 mg/m2) + docetaxel + 5-fluorouracil | HECc | 6 | 3 | 18 |
| Cyclophosphamide (600 mg/m2)/epirubicin (90 mg/m2) + paclitaxel + 5-fluorouracil | HECc | 4 | 3 | 12 |
| Carboplatin (500–900 mg/m2; with docetaxel or trastuzumab or both) | MECc | 6 | 3 | 18 |
| Carboplatin (150 mg) + paclitaxel | MECc | 3 | 1 | 3 |
| Carboplatin (500 mg) + paclitaxel | MECc | 3 | 3 | 9 |
| Carboplatin (150–900 mg/m2) + gemcitabine | MECc | 6 | 3 | 18 |
| Lung cancer | ||||
| Cisplatin (100 mg) + vinorelbine Cisplatin (75–80 mg) + vinorelbine Cisplatin (75 mg/m2) + gemcitabine Cisplatin (75 mg/m2) + docetaxel |
If index dose ≥50 mg/m2, then HECc; if index dose <50 mg/m2, then MECc |
4 4 4 4 |
4 3 3 3 |
16 12 12 12 |
| Cisplatin (75 mg/m2) + etoposide | 4 | 4 | 16 | |
| Cisplatin (75 mg/m2) + pemetrexed | 4 | 3 | 12 | |
| Carboplatin (150–900 mg/m2) + etoposide | MECc | 6 | 4 | 24 |
| Carboplatin (150–900 mg/m2) + gemcitabine | MECc | 6 | 3 | 18 |
| Carboplatin (500–900 mg/m2) + docetaxel | MECc | 6 | 3 | 18 |
| Carboplatin (500–900 mg) + paclitaxel | MECc | 3 | 3 | 9 |
| Carboplatin (150–900 mg) + paclitaxel | MECc | 3 | 1 | 3 |
| Colorectal cancer | ||||
| Oxaliplatin (85 mg/m2) + 5-fluorouracil | MECc | 12 | 2 | 24 |
| Oxaliplatin (85 mg/m2) + 5-fluorouracil + leucovorin | MECc | 12 | 2 | 24 |
| Oxaliplatin (130 mg/m2) + capecitabine | MECc | 8 | 3 | 24 |
Notes:
As per NCCN guidelines at the time of the study14–18 (the most recent NCCN guidelines indicate minor changes to the emetogenicity classification);
Hesketh rule in effect.31 Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V2.2011, Colon Cancer V3.2011, Rectal Cancer V4.2011, Small Cell Lung Cancer V2.2012, Non-Small Cell Lung Cancer V3.2011. © National Comprehensive Cancer Network, Inc 2015. All rights reserved. All accessed July 11, 2011. To view the most recent and complete version of the guidelines, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
Abbreviations: HEC, highly emetogenic chemotherapy; MEC, moderately EC; LEC, lowly EC; EC, emetogenic chemotherapy; NCCN, National Comprehensive Cancer Network.
Outcome measures
Acute CINV was identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes for nausea and vomiting, persistent vomiting, or volume depletion, or current procedural terminology codes for hydration, on the day of chemotherapy (Table S1). Delayed CINV was identified by the same ICD-9-CM and CPT codes for nausea and vomiting, volume depletion and hydration, as well as GPI/HCPCS codes for IV rescue medications (dexamethasone, fosaprepitant, diphenhydramine, promethazine, haloperidol, prochlorperazine, lorazepam, or metoclopramide) or 5-HT3 RAs (Table S1) between the day after chemotherapy and day 5 of the chemotherapy cycle of interest. CINV events were assessed on a patient- and cycle-level basis.
Each index chemotherapy regimen was assigned a total number of chemotherapy cycles and an allowed gap between chemotherapy cycles according to the recommendations of the 2011 NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) (Table 2).14–18 For example, a lung cancer patient on cisplatin (index dose of 100 mg/m2) and vinorelbine would be assumed to have initiated a therapy involving four treatment cycles with an allowed rest period of 4 weeks between each cycle.
Treatment delay was measured in two ways: 1) the proportion of patients who delayed their index chemotherapy based on the presence of a significant gap between two chemotherapy cycles, and 2) the mean and median time from the index date to the date of treatment delay. Delay of therapy was defined as a gap in treatment exceeding twice the NCCN-specified cycle length specific to each chemotherapy regimen (Table 2). The date of treatment delay was the date of the last chemotherapy cycle start date prior to delay plus one cycle length. For patients on combination regimens, delay of any one agent involved in the regimen constituted delay of the entire regimen. We also performed a sensitivity analysis around the permissible treatment gap, assigning a lower limit of 1.5 times the NCCN-recommended cycle length and an upper limit of three times the NCCN-recommended cycle length.
Treatment adherence was measured in four related ways: the percentage of patients who received the 1) recommended number of cycles for their specific chemotherapy regimen, as determined by NCCN guidelines, 2) recommended number of chemotherapy cycles for their regimen within the recommended time frame, 3) recommended chemotherapy dose within a 10% margin, and 4) recommended number of cycles within the specified time frame at the expected dose. We used measure 2 as our primary measure of adherence. Patients on multiagent regimes were required to be adherent with each component of the regimen to be considered adherent overall.
Statistical analysis
Descriptive statistics were used to characterize the incidence of acute and delayed CINV, as well as baseline patient characteristics, such as primary cancer site and chemotherapy regimen. Means/standard deviations were used for continuous data, and counts/relative frequencies were used for categorical data. Each baseline characteristic and study outcome was compared using unadjusted statistical tests between patients receiving palonosetron and those receiving all other 5-HT3 RAs. Continuous variables were compared using Student’s t-test or Wilcoxon rank-sum test, depending on the distributional characteristics. Categorical data were compared using χ2 tests.
Logistic regression models were used to estimate associations between antiemetic treatment (palonosetron versus other 5-HT3 RAs) and CINV (acute and/or delayed), delay of index chemotherapy regimen, and adherence to index chemotherapy regimen. Covariates in the multivariable regression analysis included age, sex, geographic region, health plan type, year of index date, cancer type, Deyo–Charlson Comorbidity Index (DCI) score,32 individual comorbidities, and baseline receipt of LEC, radiation, and antiemetics. All analyses were stratified by HEC and MEC regimens.
Results
Patient characteristics
We identified 1,832 HEC patients who received only palonosetron and no other 5-HT3 RA and 2,387 HEC patients who received other 5-HT3 RAs excluding palonosetron (Table 3). In the HEC group, the mean age was slightly higher among palonosetron users (52.0 versus 51.4 years for those receiving other 5-HT3 RAs, P=0.0345), and breast cancer was the most common malignancy (97.3% palonosetron and 96.0% other 5-HT3 RAs). The mean baseline DCI scores were 4.35 for patients receiving palonosetron and 4.56 for those receiving other 5-HT3 RAs (P=0.0211). Similarly, we identified 1,350 palonosetron users and 1,379 other 5-HT3 RA recipients who indexed on an MEC therapy. Within the MEC cohort, the mean age and DCI scores were slightly lower among palonosetron patients compared to those receiving other 5-HT3 RAs (56.8 versus 59.2 years, P<0.0001; 4.29 versus 4.55, P=0.0229; respectively). Breast (48.6%) and colon (29.3%) cancers were the most prevalent malignancies among palonosetron recipients, whereas lung (35.2%) and colon (34.0%) cancers were more common among those receiving other 5-HT3 RAs in the MEC group.
Table 3.
Palonosetron versus other 5-HT3 RAs among patients initiating an HEC/MEC regimen
| Characteristics | HEC
|
MEC
|
||||
|---|---|---|---|---|---|---|
| Palonosetron groupa n=1,832 |
Other 5-HT3 RA groupb n=2,387 |
P-value | Palonosetron groupa n=1,350 |
Other 5-HT3 RA groupb n=1,379 |
P-value | |
| Female, n (%) | 1,805 (98.5) | 2,333 (97.7) | 0.0643 | 981 (72.7) | 857 (62.2) | <0.0001 |
| Age at index (years), mean ± SD | 52.04 (±9.52) | 51.41 (±9.6) | 0.0345 | 56.82 (±10.9) | 59.21 (±11.33) | <0.0001 |
| 18–44 | 394 (21.5) | 571 (23.9) | 0.1046 | 175 (13.0) | 144 (10.4) | <0.0001 |
| 45–64 | 1,276 (69.7) | 1,632 (68.4) | 858 (63.6) | 804 (58.3) | ||
| ≥65 | 162 (8.8) | 184 (7.7) | 317 (23.5) | 431 (31.3) | ||
| Geographic region, n (%) | ||||||
| Northeast | 288 (15.7) | 278 (11.7) | <0.0001 | 185 (13.7) | 173 (12.6) | <0.0001 |
| South | 585 (31.9) | 736 (30.8) | 445 (33.0) | 480 (34.8) | ||
| Midwest | 651 (35.5) | 488 (20.4) | 485 (35.9) | 406 (29.4) | ||
| West | 232 (12.7) | 773 (32.4) | 172 (12.7) | 276 (20.0) | ||
| Unknown | 76 (4.2) | 112 (4.7) | 63 (4.7) | 44 (3.2) | ||
| Health plan type, n (%) | ||||||
| HMO | 314 (17.1) | 487 (20.4) | 0.0047 | 238 (17.6) | 278 (20.2) | 0.0115 |
| POS | 99 (5.4) | 85 (3.6) | 49 (3.6) | 34 (2.5) | ||
| PPO | 1,286 (70.2) | 1,661 (69.6) | 898 (66.5) | 879 (63.7) | ||
| FFS | 12 (0.7) | 15 (0.6) | 7 (0.5) | 21 (1.5) | ||
| Other/unknown | 121 (6.6) | 139 (5.8) | 158 (11.7) | 167 (12.1) | ||
| Medicare planc | 134 (7.3) | 190 (8.0) | 0.4352 | 233 (17.3) | 353 (25.6) | <0.0001 |
| Index year, n (%) | ||||||
| 2002–2004 | 16 (0.9) | 736 (30.8) | <0.0001 | 8 (0.6) | 105 (7.6) | <0.0001 |
| 2005–2006 | 936 (51.1) | 924 (38.7) | 343 (25.4) | 515 (37.4) | ||
| 2007–2008 | 854 (46.6) | 670 (28.1) | 963 (71.3) | 685 (49.7) | ||
| 2009–2011 | 26 (1.4) | 57 (2.4) | 36 (2.7) | 74 (5.4) | ||
| Baseline medical conditions, n (%) | ||||||
| Breast cancer | 1,782 (97.3) | 2,291 (96.0) | 0.0228 | 656 (48.6) | 425 (30.8) | <0.0001 |
| Lung cancer | 50 (2.7) | 96 (4.0) | 0.0228 | 299 (22.2) | 485 (35.2) | <0.0001 |
| Colorectal cancer | 0 | 0 | NA | 395 (29.26) | 469 (34.0) | 0.0076 |
| Hypertension | 679 (37.1) | 871 (36.5) | 0.7015 | 700 (51.9) | 751 (54.5) | 0.1722 |
| Cerebrovascular disease | 33 (1.8) | 47 (2.0) | 0.6922 | 80 (5.9) | 101 (7.3) | 0.1422 |
| Heart failure | 26 (1.4) | 39 (1.6) | 0.5748 | 49 (3.6) | 76 (5.5) | 0.0187 |
| Renal diseased | 35 (1.9) | 46 (1.9) | 0.9689 | 60 (4.4) | 63 (4.6) | 0.8759 |
| Liver disease | 28 (1.5) | 34 (1.4) | 0.7808 | 40 (3.0) | 36 (2.6) | 0.5759 |
| Diabetes mellitus | 174 (9.5) | 194 (8.1) | 0.1179 | 216 (16) | 226 (16.4) | 0.7829 |
| Ischemic heart disease | 84 (4.6) | 107 (4.5) | 0.8738 | 165 (12.2) | 238 (17.3) | 0.0002 |
| Pulmonary diseasee | 211 (11.5) | 297 (12.4) | 0.3602 | 315 (23.3) | 460 (33.4) | <0.0001 |
| Osteoporosis | 197 (10.8) | 244 (10.2) | 0.5761 | 149 (11.0) | 149 (10.8) | 0.8459 |
| Mental health disorder | 441 (24.1) | 543 (22.8) | 0.3135 | 352 (26.1) | 375 (27.2) | 0.5083 |
| DCI score | ||||||
| Mean ± SD | 4.35 (±2.92) | 4.56 (±2.98) | 0.0211 | 4.29 (±2.88) | 4.55 (±2.95) | 0.0229 |
| Median (Q1–Q3) | 2 (2–8) | 3 (2–8) | 0.0264 | 3 (2–8) | 3 (2–8) | 0.0002 |
| Baseline therapies, n (%) | ||||||
| LEC | 93 (5.1) | 108 (4.5) | 0.4042 | 193 (14.3) | 205 (14.9) | 0.6734 |
| Radiation | 60 (3.3) | 113 (4.7) | 0.0179 | 237 (17.6) | 290 (21.0) | 0.0215 |
| 5-HT3 antiemetics | 675 (36.8) | 895 (37.5) | 0.6652 | 450 (33.3) | 374 (27.1) | 0.0004 |
| Non-5-HT3 antiemetics | 1,166 (63.7) | 1,359 (56.9) | <0.0001 | 828 (61.3) | 728 (52.8) | <0.0001 |
| Chemotherapeutic regimens, n (%) | ||||||
| Cyclophosphamide | 6 (0.3) | 7 (0.3) | 499 (37.0) | 287 (20.8) | ||
| Cyclophosphamide/doxorubicin | 1,656 (90.4) | 2,133 (89.4) | ||||
| Cyclophosphamide/epirubicin | 120 (6.6) | 151 (6.3) | ||||
| Cisplatin | 50 (2.7) | 96 (4.0) | 21 (1.6) | 13 (0.9) | ||
| Carboplatin | 435 (32.2) | 610 (44.2) | ||||
| Oxaliplatin | 395 (29.3) | 469 (34.0) | ||||
| Duration of study follow-up, days | ||||||
| Mean ± SD | 1,239.79 (±521.83) | 1,398.59 (±722.88) | <0.0001 | 1,036.1 (±414.11) | 1,089.16 (±517.92) | 0.0031 |
| Median (Q1–Q3) | 1,200 (835.5–1,648.5) | 1,295 (807–1,885) | <0.0001 | 994.5 (716–1,270) | 1,013 (648–1,407) | 0.1939 |
Notes:
Receipt of palonosetron and no other 5-HT3 RA during any cycle, measured from (HEC cycle-start date -1 day) until (HEC cycle-start date +5 days);
receipt of any 5-HT3 RA except palonosetron during any cycle, measured from (chemotherapy cycle-start date -1 day) until (chemotherapy cycle start date +5 days);
consisting of Medicare Advantage, supplemental, and Part D plans;
renal disease included kidney disease, nephrosis, nephritis, and renal function impairment, including dialysis;
pulmonary disease included asthma, bronchitis, pneumonia, emphysema, and COPD.
Abbreviations: HT, hydroxytryptamine; RAs, receptor antagonists; HEC, highly EC; MEC, moderately EC; LEC, lowly EC; NEC, non-EC; EC, emetogenic chemotherapy; SD, standard deviation; HMO, health maintenance organization; POS, point of service; PPO, preferred provider organization; FFS, fee for service; NA, not applicable; Q, quartile; COPD, chronic obstructive pulmonary disease; DCI, Deyo–Charlson Comorbidity Index.
Incidence of CINV
Within the HEC cohort, fewer palonosetron patients experienced CINV compared with those who received other 5-HT3 RAs (27.5% versus 32.2%, P=0.001; Table 4). Likewise, 19.1% and 14.8% of HEC patients receiving palonosetron experienced ≥1 acute and ≥1 delayed CINV event(s) respectively, compared to 20.5% and 20.2% of other 5-HT3 RA HEC patients. Furthermore, patients in the other 5-HT3 RA group experienced more CINV events per cycle than the palonosetron group (0.3 versus 0.2 events/cycle). In the MEC cohort, fewer palonosetron patients experienced CINV and CINV events per cycle compared with those who received other 5-HT3 RAs (36.1% versus 41.7%, P=0.003; 0.3 versus 0.4 events/cycle). MEC patients in the palonosetron cohort were significantly less likely to experience delayed CINV versus patients in the other 5-HT3 RA cohort (20.6% versus 29.5%, P<0.0001).
Table 4.
Outcomes among palonosetron versus other 5-HT3 RAs in patients initiating an HEC/MEC regimen
| Outcomes of interest | HEC
|
MEC
|
||||
|---|---|---|---|---|---|---|
| Palonosetron group n=1,832 |
Other 5-HT3 RA group n=2,387 |
P-value | Palonosetron group n=1,350 |
Other 5-HT3 RA group n=1,379 |
P-value | |
| CINV | ||||||
| Patients experiencing ≥1 CINV event, n (%) | 504 (27.5) | 768 (32.2) | 0.0011 | 487 (36.1) | 575 (41.7) | 0.0026 |
| Patients experiencing ≥1 acute CINV event | 350 (19.1) | 490 (20.53) | 0.2513 | 361 (26.74) | 334 (24.22) | 0.1308 |
| Patients experiencing ≥1 delayed CINV event | 271 (14.79) | 482 (20.19) | <0.0001 | 278 (20.59) | 407 (29.51) | <0.0001 |
| Total number of cycles | 7,616 | 9,878 | 7,952 | 8,749 | ||
| Total number of events | 1,552 | 2,685 | 2,070 | 3,686 | ||
| Acute | 769 | 1,212 | 1,193 | 1,196 | ||
| Delayed | 783 | 1,473 | 877 | 2,490 | ||
| Events/cycle | 0.2038 | 0.2718 | 0.2603 | 0.4213 | ||
| Treatment delay | ||||||
| Treatment delay, n (%) | 59 (3.2) | 144 (6.0) | <0.0001 | 230 (17.0) | 369 (26.8) | <0.0001 |
| Treatment delay, lower limit, n (%) | 102 (5.6) | 199 (8.3) | 0.0005 | 363 (26.9) | 536 (38.9) | <0.0001 |
| Treatment delay, upper limit, n (%) | 19 (1.0) | 40 (1.7) | 0.08 | 101 (7.5) | 163 (11.8) | 0.0001 |
| Therapy length until delay (days) | ||||||
| Mean (± SD) | 76.28 (±22.65) | 76.32 (±22.62) | 0.9577 | 87.38 (±42.45) | 85.45 (±48.18) | 0.2659 |
| Median (Q1–Q3) | 67 (64–85) | 76 (64–85) | 0.0147 | 85 (62–111) | 85 (48–126) | 0.0147 |
| Treatment adherence, n(%) | ||||||
| Receipt of | ||||||
| 1. Recommended number of cycles | 1,607 (87.7) | 2,062 (86.4) | 0.2022 | 885 (65.6) | 824 (59.8) | 0.0017 |
| 2. Recommended number of cycles within the specified time frame | 1,368 (74.7) | 1,664 (69.7) | 0.0004 | 582 (43.1) | 514 (37.3) | 0.0019 |
| 3. Recommended number of cycles within the specified time frame at the expected dose | 500 (27.3) | 616 (25.8) | 0.0004 | 202 (15.0) | 173 (12.6) | 0.0019 |
| 4. Recommended dose | 616 (33.6) | 798 (33.4) | 0.8951 | 467 (34.6) | 512 (37.1) | 0.1673 |
Abbreviations: HT, hydroxytryptamine; RAs, receptor antagonists; HEC, highly EC; MEC, moderately EC; EC, emetogenic chemotherapy; CINV, chemotherapy-induced nausea/vomiting; SD, standard deviation; Q, quartile.
Chemotherapy treatment delay
Fewer chemotherapy treatment delays occurred among patients receiving palonosetron compared with other 5-HT3 RAs in both the HEC (3.2% versus 6.0%, P<0.0001) and MEC (17.0% versus 26.8%, P<0.0001) cohorts (Table 4). The results for delayed therapy remained consistent when using the upper and lower limits as defined earlier (see Table 4). Mean time to delay was similar across the palonosetron and other 5-HT3 RAs groups (approximately 76 days in the HEC cohort and 86 days in the MEC cohort).
Chemotherapy treatment adherence
In both the HEC and MEC cohorts, more patients receiving palonosetron were adherent to their chemotherapy regimen compared to those who received other 5-HT3 RAs for three of the four different adherence measures. In the HEC cohort, slightly more of those who received palonosetron completed the recommended number of chemotherapy cycles versus those who received other 5-HT3 RAs (87.7% versus 86.4%, respectively; P=0.2022). The difference was greater in the MEC cohort, with 65.6% of those receiving palonosetron and 59.8% of those receiving other 5-HT3 RAs completing the recommended number of chemotherapy cycles (P=0.0017). Compared with those who received other 5-HT3 RAs, significantly more patients receiving palonosetron completed the recommended number of chemotherapy cycles within the specified time frame (HEC, 74.7% versus 69.7%, respectively, P=0.0004; MEC, 43.1% versus 37.3%, respectively, P=0.0019) and at the expected doses (HEC, 27.3% versus 25.8%, respectively, P=0.0004; MEC, 15.0% versus 12.6%, respectively, P=0.0019) (Table 4). A similar proportion of patients in both the palonosetron and other 5-HT3 RA cohorts received the recommended chemotherapy doses for HEC (33.6% palonosetron and 33.4% other 5-HT3 RAs, P=0.8951) and MEC regimens (34.6% palonosetron and 37.1% other 5-HT3 RAs, P=0.1673).
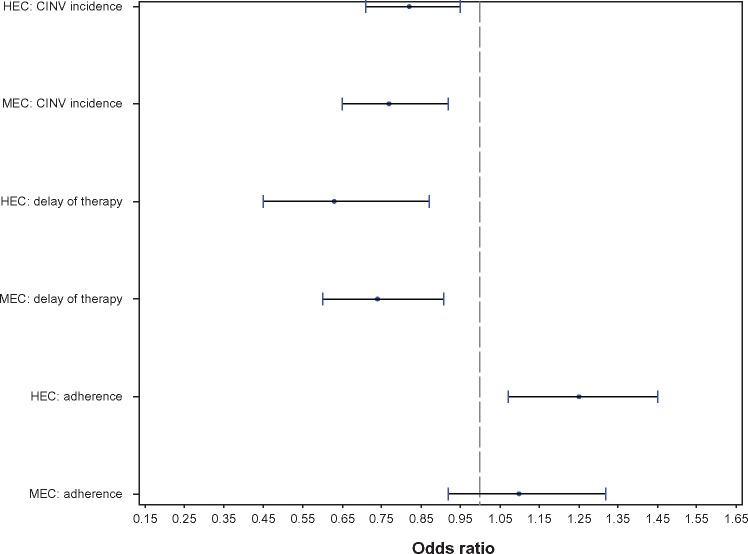
These findings were supported in a multivariable analysis (Figure 1). Treatment with palonosetron was associated with a reduced likelihood of CINV occurrence in the HEC (odds ratio [OR] 0.82, 95% CI 0.71–0.95) and MEC (OR 0.77, 95% CI 0.65–0.92) cohorts. Palonosetron treatment was also associated with fewer chemotherapy treatment delays in both cohorts (HEC, OR 0.63, 95% CI, 0.45–0.87; MEC, OR 0.74, 95% CI 0.60–0.91). Although palonosetron was associated with greater chemotherapy adherence in the HEC cohort (OR 1.25, 95% CI 1.07–1.45), no association was found in the MEC cohort (OR 1.1, 95% CI 0.92–1.32).
Figure 1.
Odds ratios and 95% confidence intervals for palonosetron versus other 5-HT3-RAs.
Notes: For CINV and delayed therapy, an odds ratio <1 is associated with improved outcomes; for adherence, an odds ratio <1 is associated with improved outcomes.
Abbreviations: HT, hydroxytryptamine; CINV, chemotherapy-induced nausea/vomiting; HEC, highly EC; MEC, moderately EC; EC, emetogenic chemotherapy.
Discussion
In this retrospective, observational, nested case–control study, patients who received prophylactic or rescue palonosetron had significantly fewer CINV events, fewer chemotherapy treatment delays, and higher adherence to their chemotherapy regimen compared with patients who received any other IV 5-HT3 RA medication. These findings were seen both among patients who were undergoing HEC treatment and those undergoing MEC treatment.
Results from clinical trials have demonstrated the overall efficacy of palonosetron in preventing acute CINV in patients receiving HEC and in preventing acute or delayed CINV in patients receiving MEC.20,24–26 However, limited evidence is available regarding the effect of palonosetron on chemotherapy adherence and treatment delay in a real-world setting. To our knowledge, no previous study has evaluated this association. A previous administrative claims analysis evaluated the risk of serious CINV events associated with hospital or emergency department admissions among patients with breast or lung cancer undergoing MEC or HEC who received palonosetron compared with those who received any other 5-HT3 RA.19 Patients receiving palonosetron experienced a significantly reduced risk of serious CINV compared to those who received other 5-HT3 RAs, ranging from 31% to 45% among lung and breast cancer patients, respectively. Another recent study by Craver et al evaluated the risk of CINV among recipients of palonosetron versus other 5-HT3 RAs initiating HEC/MEC therapy in all medical settings,28 using a broader definition of CINV encompassing events occurring any time within 7 days of the chemotherapy cycle-start date. While both studies showed a reduction in CINV with palonosetron use as expected, an exploration of the effect of CINV risk reduction on chemotherapy adherence or delay was not conducted.
The real-world analysis in the current study demonstrated improved adherence to chemotherapy regimens among patients who received palonosetron compared with other 5-HT3 RA agents. The association between the use of antiemetics and adherence may have been underestimated: patients undergoing chemotherapy, particularly HEC, are more likely to have been prepared by their health care providers to expect nausea and vomiting; such preparedness has been shown to alleviate the reported incidence of nausea and vomiting in patients receiving chemotherapy.31 Additionally, some patients undergoing IV chemotherapy regimens will have advanced disease, and may therefore not have the option of delaying or discontinuing treatment because of nausea and vomiting.33
Future research exploring the association between reduced CINV and chemotherapy adherence would benefit from a cost analysis, which was not included in the current study. A therapy that improves chemotherapy adherence by reducing CINV events could potentially reduce costs, both direct (costs of antiemetic medications, physician visits, and hospitalizations) and indirect (lost workdays and intangibles, including lower quality of life and potential consequences of delayed or reduced chemotherapy treatment). Other chemotherapy-associated side effects, such as fatigue, insomnia, or dermatologic conditions, which cannot be easily identified through claims, may also affect treatment adherence.
The nature of the administrative claims database and the lack of granularity precluded us from identifying more than one CINV event per day or the severity of the CINV experienced. While our approach to identify CINV events from claims matches that used in clinical trials of antiemetics,19,28 others have used criteria that were either more strict (eg, nausea, vomiting, and dehydration associated with hospital admissions27) or that relied on patient diaries rather than diagnosis codes.24,25 The strategy used in the current study to define CINV did not capture patients using oral antiemetics or over-the-counter remedies, and the IV antiemetics may have been prescribed for reasons other than CINV (eg, for nausea and vomiting associated with migraine,34 surgery,35 or gastroparesis36). Nausea and vomiting occurring after day 5 of the chemotherapy cycle and before the subsequent cycle were not attributed to chemotherapy, and may have resulted in an underestimation of CINV events. Despite these limitations, the narrow time frame and broader medical setting used for identifying CINV in the current study design resulted in a conservative estimate of the impact of palonosetron and other 5-HT3 RAs on CINV in a real-world setting.
Administrative claims are designed for reimbursement rather than research, and may contain coding errors or omissions. Therefore, the claim-based algorithm developed to identify patients with early stage cancers may be susceptible to potential misidentification. Furthermore, standard definitions of adherence with IV chemotherapy regimens within an administrative claim database are lacking in the published literature. All patients were members of large commercial health plans in the US; the results may not be generalizable to patients outside the US or with other types of health insurance. While enrollment was limited to patients with single-day chemotherapy regimens, further research in patients receiving multiday regimens would be desirable. Because of concerns regarding patient selection and cohort size, the comparative analysis was limited to IV chemotherapy in general and IV 5-HT3 RAs as a class. Consistent with NCCN guideline recommendations, the analysis assumed that the non-5-HT3 RA components of the observed antiemetic regimens were similar across the palonosetron and other cohorts. NCCN guidelines were used to define chemotherapy regimens for this analysis, and did not allow for individualized treatment plans. BSA was needed to calculate the index dosage of cyclophosphamide and cisplatin; however, this information is unavailable in administrative claims. In the absence of US-based data, BSA estimates developed in a prior UK study30 were used to calculate index doses.
Conclusion
In this real-world retrospective analysis, patients receiving palonosetron were more adherent to their HEC/MEC regimens and experienced fewer treatment delays compared to patients receiving other 5-HT3 RAs. Palonosetron was also associated with a decrease in the occurrence of CINV events. These results highlight the importance of symptom control in the context of adherence to prescribed chemotherapy, which may ultimately influence treatment and disease outcomes, including costs. This study also presents an innovative approach to estimate adherence to IV chemotherapy using administrative claims data.
Supplementary materials
Assignment of index chemotherapy regimens
Identification of chemotherapeutic regimens involves a two-step process. Step one involves the identification of the highly emetogenic chemotherapy (HEC)/moderately EC (MEC) agents making up the regimen, which are identified within 6 days of the index date. For instance, for a breast cancer patient with a claim for cyclophosphamide on the index date (ie, the start date of the first chemotherapy cycle), medical and pharmacy claims are evaluated to determine the presence of another HEC/MEC drug (eg, doxorubicin, epirubicin, etc). If no other HEC/MEC drug is found, then the index dose is calculated by using the strength (as determined from the index medical or pharmacy claim) and the body surface-area estimate. A dose of >1,500 mg/m2 indicates receipt of HEC, while an index dose ≤1,500 mg/m2 indicates MEC. However, if doxorubicin is present, then the patient is classified as HEC as per the Hesketh algorithm.31 Step two of the chemotherapy-regimen identification involves claims for non-HEC/MEC chemotherapy agents also observed within 6 days of the index date (Table S3). In the aforementioned example, for a patient indexing on cyclophosphamide and doxorubicin, if a claim for another lowly EC or non-EC drug (eg, docetaxel, paclitaxel, etc) is found within ±6 days of the index date, then the patient’s adherence will be evaluated as per the NCCN Guidelines® recommendations for the appropriate cyclophosphamide/doxorubicin/docetaxel combination regimen. Where multiple regimen options were available for the drugs involved, additional analysis was performed to determine the specific regimen. This included determining the doses of the HEC/MEC components in order to pinpoint the specific regimen. For example, a doxorubicin dose of 50 and 60 mg/m2 would indicate a regimen involving six and four cycles, respectively (Table 2). Duration between the claims for the index HEC/MEC drugs was also used if the doses were insufficient in differentiating among the various regimen options. A combination involving cyclophosphamide/doxorubicin/paclitaxel could be assigned a regimen either 8 weeks or 12 weeks long if the gap between the first and second claim for cyclophosphamide/doxorubicin was found to be 14 or 21 days, respectively.
Table S1.
CINV codes and antiemetic therapies
| Generic name | GPI | HCPCS | ICD-9-CM diagnosis |
|---|---|---|---|
| 5-HT3 RAs | |||
| Dolasetron mesylate | 50250025x | J1260, Q0180 | |
| Granisetron | 50250035x | J1626, Q0166 | |
| Ondansetron | 50250065x | J2405, Q0179 | |
| Palonosetron | 50250070x | J2469 | |
| Other antiemetics | |||
| Dexamethasone | 22100020x | J1094, J1100, J7637, J7638, J7312, J8540 | |
| Fosaprepitant | 502800351021x | J1453 | |
| Promethazine | 41400020101210, 414000201020x, 414000201003x, 41400020101215, 414000201029x, 414000201052x | J2550, J2180, Q0169, Q0170 | |
| Prochlorperazine | 59200055x | J0780, Q0164, Q165, S0183 | |
| Metoclopramide | 523000201020x, 523000201003x, 523000201012x, 523000201013x, 523000201029x, 523000201072x, 5230002011x | J2765 | |
| Lorazepam | 571000600020x, 571000600003x, 571000600013x | J2060 | |
| Haloperidol | 5910001030x, 591000102020x, 5910001010x, 591000102013x | J1630, J1631 | |
| Diphenhydramine | 4120003010x, 412000303x, 60300020x, 6030990x | J1200, Q0163 | |
| Nausea | |||
| Nausea and vomiting | 22100020x | J1094, J1100, J7637, J7638, J7312, J8540 | 787.0x |
| Persistent vomiting | 5028002000x | J8501 | 536.2x |
| Volume depletion | 502800351021x | J1453 | 276.5x |
| Hydration (nontherapeutic administration) | 96360, 96361, 90760, 90761, 2018F, G0345 | ||
Abbreviations: CINV, chemotherapy-induced nausea/vomiting; HT, hydroxytryptamine; RAs, receptor antagonists; GPI, generic product identifier; HCPCS, Healthcare Common Procedure Coding System; ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification.
Table S2.
Chemotherapy codes and emetogenic potential
| Description | GPI code | HCPCS code | Emetogenicity | Formulation |
|---|---|---|---|---|
| Carmustine | 21102010x | J9050, C9437 | Other | IV |
| Cisplatin | 211000200020x | J9060, J9062, C9418 | Other | IV |
| Dacarbazine | 2170002000x | J9130, J9140, C9423 | HEC | IV |
| Mechlorethamine | 211010301021x | J9230 | HEC | IV |
| Streptozotocin | 21102030002105 | J9320 | HEC | IV |
| Alemtuzumab | 21353010x | J9010 | Minimal | IV |
| Arsenic trioxide | 21700008x | J9017 | MEC | IV |
| Azacitidine | 21300003x | J9025 | MEC | IV |
| Bendamustine | 21100009x | J9033 | MEC | IV |
| Carboplatin | 21100015x | J9045 | MEC | IV |
| Clofarabine | 21300008x | J9027, C9129 | MEC | IV |
| Dactinomycin | 212000200021x | J9120 | MEC | IV |
| Daunorubicin | 21200030x | J9150–J9151, C9424 | MEC | IV |
| Doxorubicin | 21200040x | J9000–J9001, C9415 | Other | IV |
| Epirubicin | 21200042x | J9178, J9180 | Other | IV |
| Idarubicin | 21200045x | J9211, C9429 | MEC | IV |
| Ifosfamide | 2110102500x, 219900024064x | J9208, C9427 | Other | IV |
| Irinotecan | 21550040x | J9206 | MEC | IV |
| Melphalan | 211010400021x, 211010401021x | J9245 | MEC | IV |
| Oxaliplatin | 21100028x | J9263, C9205 | MEC | IV |
| Temozolomide | 211040700021x | J9328, C9253 | MEC | IV |
| Aldesleukin | 21703020x | J9015 | Other | IV |
| Amifostine crystalline | 21758010x | J0207 | Other | IV |
| Cyclophosphamide | 21101020002x | J9070–J9097, C9420, C9421 | Other | IV |
| Cytarabine | 21300010x | J9098–J9110, C9422 | Other | IV |
| Interferon-α | 217000601x, 217000602x, 217000603x | J9212–J9215 | Other | IV |
| Altretamine | 21100005x | MEC/HEC | Oral | |
| Procarbazine | 21700050x | S0182 | MEC/HEC | Oral |
| Cyclophosphamide | 211010200003x | J8530 | Other | Oral |
| Imatinib | 21534035x | S0088 | Minimal/Low | Oral |
| Temozolomide | 211040700001x | J8700 | Other | Oral |
| Busulfan | 211000100003x | J0594, J8510 | Other | Oral |
| Estramustine phosphate sodium | 2140302010x | MEC/HEC | Oral | |
| Etoposide | 21500010x | J8560 | MEC/HEC | Oral |
| Lomustine | 211020200001x | S0178 | MEC/HEC | Oral |
Notes: Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Antiemesis, Version 1.2012. © National Comprehensive Cancer Network, Inc 2015. All rights reserved. Accessed August 11, 2011. To view the most recent and complete version of the guideline, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc. Other = NCCN emetogenicity rating depends on dosage.
Abbreviations: GPI, generic product identifier; HCPCS, Healthcare Common Procedure Coding System; HEC, highly EC; MEC, moderately EC; EC, emetogenic chemotherapy; IV, intravenous.
Table S3.
HEC/MEC regimens
| Agent | Regimen | Schedule | Regimen type | MEC/HEC |
|---|---|---|---|---|
| Cyclophosphamide | TC | • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles |
Single day | Depends on dosage |
| Cyclophosphamide/doxorubicin | TAC (with docetaxel) | • Doxorubicin 50 mg/m2 IV day 1 | Single day | HEC |
| • Cyclophosphamide 500 mg/m2 IV day 1 Cycled every 3 weeks for six cycles | ||||
| TAC (with docetaxel) | • Doxorubicin 60 mg/m2 on day 1 | Single day | HEC | |
| • Cyclophosphamide 500 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| AC | • Doxorubicin 60 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 2 weeks for four cycles | ||||
| • Doxorubicin 60 mg/m2 IV day 1 | Single day | HEC | ||
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| • Doxorubicin 60 mg/m2 IV day 1 | Single day | HEC | ||
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| • Doxorubicin 60 mg/m2 IV day 1 | Single day | HEC | ||
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| FAC/CAF (with 5-FU) | • Doxorubicin 50 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 500 mg/m2 IV day 1 Cycled every 3 weeks for six cycles | ||||
| Cyclophosphamide/epirubicin | FEC/CEF (with 5-FU) | • Epirubicin 75 mg/m2 IV day 1 | Single day | HEC |
| • Cyclophosphamide 500 mg/m2 day 1 Cycled every 3 weeks for four cycles | ||||
| EC | • Epirubicin 100 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 830 mg/m2 IV day 1 Cycled every 3 weeks for eight cycles | ||||
| FEC | • Epirubicin 100 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 500 mg/m2 day 1 Cycled every 3 weeks for three cycles | ||||
| FEC | • Epirubicin 90 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| Carboplatin | TCH | • Carboplatin AUC 6 IV day 1 Cycled every 3 weeks for six cycles |
Single day | MEC |
| CH (with trastuzumab) | • Carboplatin AUC 6 IV day 1 Cycled every 3 weeks for six cycles |
Single day | MEC | |
| CT (with docetaxel) | • Carboplatin AUC 6 IV day 1 Cycled every 3 weeks for six cycles |
Single day | MEC | |
| Cisplatin | CV (with vinorelbine) | • Cisplatin 100 mg/m2 day 1 Cycled every 4 weeks for four cycles |
Single day | HEC/depends on dosage |
| CV (with vinorelbine) | • Cisplatin 75–80 mg/m2 day 1 Cycled every 3 weeks for four cycles |
Single day | HEC/depends on dosage | |
| CG (with gemcitabine) | • Cisplatin 75 mg/m2 day 1 Cycled every 3 weeks for four to six cycles |
Single day | HEC/depends on dosage | |
| CD (with docetaxel) | • Cisplatin 75 mg/m2 day 1 Cycled every 3 weeks for four to six cycles |
Single day | HEC/depends on dosage | |
| CP (with pemetrexed) | • Cisplatin 75 mg/m2 day 1 Cycled every 3 weeks for four cycles |
Single day | HEC/depends on dosage | |
| Carboplatin | PC (with paclitaxel) | • Carboplatin AUC 6 day 1 Cycled every 3 weeks for four to six cycles |
Single day | MEC |
| PC (with paclitaxel) | • Carboplatin AUC 2 (initial) and 6 (last 2) weekly Total of three cycles |
Single day | MEC | |
| PG (with gemcitabine) | • Carboplatin AUC 2 day 1 Cycled every 3 weeks for six cycles |
Single day | MEC | |
| PE (with etoposide) | • Carboplatin AUC 2 day 1 Cycled every 4 weeks for six cycles |
Single day | MEC | |
| Oxaliplatin | FOLFOX | • Oxaliplatin 85 mg/m2 day 1 Cycled every 2 weeks for 6 months |
Single day | MEC |
| FOLFOX (modified) | • Oxaliplatin 85 mg/m2 day 1 Cycled every 2 weeks for 6 months |
Single day | MEC | |
| XELOX | • Oxaliplatin 130 mg/m2 day 1 Cycled every 3 weeks for eight cycles |
Single day | MEC |
Note: Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V2.2011, Colon Cancer V3.2011, Rectal Cancer V4.2011, Small Cell Lung Cancer V2.2012, Non-Small Cell Lung Cancer V3.2011 [All accessed July 11, 2011], Antiemesis V1.2012 [Accessed August 11, 2011]. © National Comprehensive Cancer Network, Inc 2015. All rights reserved. To view the most recent and complete version of the guidelines, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
Abbreviations: MEC, moderately emetogenic chemotherapy; HEC, highly EC; TC, taxotere/cyclophosphamide; IV, intravenous; TAC, Docetaxel/doxorubicin/cyclophosphomide; AC, Doxorubicin/cyclophosphomide; FAC/CAF, Flurouracil/doxorubicin/cyclophosphomide; 5-FU, 5-fluorouracil; FEC/CEF, Cyclophosphomide/Epirubicin/Flurouracil; TCH, Docetaxel/carboplatin/trastuzumab; CH, Docetaxel/carboplatin/trastuzumab; CT, Carboplatin/trastuzumab; CV, Cisplatin/vinorelbine; CG, Cisplatin/gemicitabine; CD, Cisplatin/docetaxel; CP, Cisplatin/pemetrexed; PC, Paclitaxel/Carboplatin; PG, Paclitaxel/Carboplatin/gemicitabine; PE, Paclitaxel/Carboplatin/etoposide; FOLFOX, Folinic acid/Fluorouracil/Oxaliplatin; XELOX, Capecitabine/Oxaliplatin.
Acknowledgments
The authors acknowledge Russell Knoth of Eisai Pharmaceuticals, Inc., for his input on the study design and manuscript. The authors also acknowledge Cheryl Jones for her editorial assistance in preparing the manuscript. Funding for this study was provided by Eisai, Inc., which distributes palonosetron.
Footnotes
Disclosure
SRP was an employee of HealthCore at the time of the study, and MG and RAQ are current employees of HealthCore, an independent research organization that received funding from Eisai Pharmaceuticals for the conduct of this study. HSR is a consultant to Eisai Pharmaceuticals. SRP is now an employee of CTI Clinical Trial and Consulting Services, Cincinnati, OH, USA.
References
- 1.de Boer-Dennert M, de Wit R, Schmitz PI, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer. 1997;76:1055–1061. doi: 10.1038/bjc.1997.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickok JT, Roscoe JA, Morrow GR, King DK, Atkins JN, Fitch TR. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: a University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97:2880–2886. doi: 10.1002/cncr.11408. [DOI] [PubMed] [Google Scholar]
- 3.Ihbe-Heffinger A, Ehlken B, Bernard R, et al. The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol. 2004;15:526–536. doi: 10.1093/annonc/mdh110. [DOI] [PubMed] [Google Scholar]
- 4.Shih YC, Xu Y, Elting LS. Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer. 2007;110:678–685. doi: 10.1002/cncr.22823. [DOI] [PubMed] [Google Scholar]
- 5.Farrell C, Brearley SG, Pilling M, Molassiotis A. The impact of chemotherapy-related nausea on patients’ nutritional status, psychological distress and quality of life. Support Care Cancer. 2013;21:59–66. doi: 10.1007/s00520-012-1493-9. [DOI] [PubMed] [Google Scholar]
- 6.Roscoe JA, Morrow GR, Colagiuri B, et al. Insight in the prediction of chemotherapy-induced nausea. Support Care Cancer. 2010;18:869–876. doi: 10.1007/s00520-009-0723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer. 2005;13:219–227. doi: 10.1007/s00520-004-0710-6. [DOI] [PubMed] [Google Scholar]
- 8.Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–4478. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 9.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox PM, Fetting JH, Nettesheim KM, Abeloff MD. Anticipatory vomiting in women receiving cyclophosphamide, methotrexate, and 5-FU (CMF) adjuvant chemotherapy for breast cancer. Cancer Treat Rep. 1982;66:1601–1604. [PubMed] [Google Scholar]
- 11.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 12.Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist. 2007;12:1143–1150. doi: 10.1634/theoncologist.12-9-1143. [DOI] [PubMed] [Google Scholar]
- 13.Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24:2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- 14.Carlson R, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer, Version 2. 2011. [Accessed: July 11, 2011]. © 2015 National Comprehensive Cancer Network, Inc. Available at NCCN.org.
- 15.Engstrom P, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Colon Cancer, Version 3. 2011. [Accessed: July 11, 2011]. © 2015 National Comprehensive Cancer Network, Inc. Available at NCCN.org.
- 16.Engstrom P, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Rectal Cancer, Version 4. 2011. [Accessed: July 11, 2011]. © 2015 National Comprehensive Cancer Network, Inc. Available at NCCN.org.
- 17.Kalemkerian G, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Small Cell Lung Cancer, Version 2. 2012. [Accessed: July 11, 2011]. © 2015 National Comprehensive Cancer Network, Inc. Available at NCCN.org.
- 18.Ettinger D, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer, Version 3. 2011. [Accessed: July 11, 2011]. © 2015 National Comprehensive Cancer Network, Inc. Available at NCCN.org.
- 19.Hatoum HT, Lin SJ, Buchner D, Cox D. Comparative clinical effectiveness of various 5-HT3 RA antiemetic regimens on chemotherapy-induced nausea and vomiting associated with hospital and emergency department visits in real world practice. Support Care Cancer. 2012;20:941–949. doi: 10.1007/s00520-011-1165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartzberg L, Jackson J, Jain G, Balu S, Buchner D. Impact of 5-HT3 RA selection within triple antiemetic regimens on uncontrolled highly emetogenic chemotherapy-induced nausea/vomiting. Expert Rev Pharmacoecon Outcomes Res. 2011;11:481–488. doi: 10.1586/erp.11.47. [DOI] [PubMed] [Google Scholar]
- 21.Stoltz R, Cyong JC, Shah A, Parisi S. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in US and Japanese healthy subjects. J Clin Pharmacol. 2004;44:520–531. doi: 10.1177/0091270004264641. [DOI] [PubMed] [Google Scholar]
- 22.Rojas C, Stathis M, Thomas AG, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008;207:469–478. doi: 10.1213/ane.0b013e318172fa74. [DOI] [PubMed] [Google Scholar]
- 23.Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT3 and tachykinin NK1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol. 2012;684:1–7. doi: 10.1016/j.ejphar.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 24.Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441–1449. doi: 10.1093/annonc/mdl137. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98:473–482. doi: 10.1002/cncr.11817. [DOI] [PubMed] [Google Scholar]
- 26.Gralla R, Lichinitser M, Van der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–1577. doi: 10.1093/annonc/mdg417. [DOI] [PubMed] [Google Scholar]
- 27.Lin SJ, Hatoum HT, Buchner D, Cox D, Balu S. Impact of 5-HT3 receptor antagonists on chemotherapy-induced nausea and vomiting: a retrospective cohort study. BMC Health Serv Res. 2012;12:215. doi: 10.1186/1472-6963-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craver C, Gayle J, Balu S, Buchner D. Palonosetron versus other 5-HT3 receptor antagonists for prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies treated with emetogenic chemotherapy in a hospital outpatient setting in the United States. J Med Econ. 2011;14:341–349. doi: 10.3111/13696998.2011.582908. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger D, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Antiemesis, Version 1. 2012. [Accessed: August 11, 2011]. © 2015 National Comprehensive Cancer Network, Inc. Available at NCCN.org.
- 30.Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS One. 2010;5:e8933. doi: 10.1371/journal.pone.0008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 33.Taylor SE, Lichtman RR, Wood JV. Compliance with chemotherapy among breast cancer patients. Health Psychol. 1984;3:553–562. doi: 10.1037//0278-6133.3.6.553. [DOI] [PubMed] [Google Scholar]
- 34.Silberstein S. Practice parameter: evidence-based guidelines for migraine headaches (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754–762. doi: 10.1212/wnl.55.6.754. [DOI] [PubMed] [Google Scholar]
- 35.Carlisle JB. A meta-analysis of prevention of postoperative nausea and vomiting: randomised controlled trials by Fujii et al compared with other authors. Anaesthesia. 2012;67:1076–1090. doi: 10.1111/j.1365-2044.2012.07232.x. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
CINV codes and antiemetic therapies
| Generic name | GPI | HCPCS | ICD-9-CM diagnosis |
|---|---|---|---|
| 5-HT3 RAs | |||
| Dolasetron mesylate | 50250025x | J1260, Q0180 | |
| Granisetron | 50250035x | J1626, Q0166 | |
| Ondansetron | 50250065x | J2405, Q0179 | |
| Palonosetron | 50250070x | J2469 | |
| Other antiemetics | |||
| Dexamethasone | 22100020x | J1094, J1100, J7637, J7638, J7312, J8540 | |
| Fosaprepitant | 502800351021x | J1453 | |
| Promethazine | 41400020101210, 414000201020x, 414000201003x, 41400020101215, 414000201029x, 414000201052x | J2550, J2180, Q0169, Q0170 | |
| Prochlorperazine | 59200055x | J0780, Q0164, Q165, S0183 | |
| Metoclopramide | 523000201020x, 523000201003x, 523000201012x, 523000201013x, 523000201029x, 523000201072x, 5230002011x | J2765 | |
| Lorazepam | 571000600020x, 571000600003x, 571000600013x | J2060 | |
| Haloperidol | 5910001030x, 591000102020x, 5910001010x, 591000102013x | J1630, J1631 | |
| Diphenhydramine | 4120003010x, 412000303x, 60300020x, 6030990x | J1200, Q0163 | |
| Nausea | |||
| Nausea and vomiting | 22100020x | J1094, J1100, J7637, J7638, J7312, J8540 | 787.0x |
| Persistent vomiting | 5028002000x | J8501 | 536.2x |
| Volume depletion | 502800351021x | J1453 | 276.5x |
| Hydration (nontherapeutic administration) | 96360, 96361, 90760, 90761, 2018F, G0345 | ||
Abbreviations: CINV, chemotherapy-induced nausea/vomiting; HT, hydroxytryptamine; RAs, receptor antagonists; GPI, generic product identifier; HCPCS, Healthcare Common Procedure Coding System; ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification.
Table S2.
Chemotherapy codes and emetogenic potential
| Description | GPI code | HCPCS code | Emetogenicity | Formulation |
|---|---|---|---|---|
| Carmustine | 21102010x | J9050, C9437 | Other | IV |
| Cisplatin | 211000200020x | J9060, J9062, C9418 | Other | IV |
| Dacarbazine | 2170002000x | J9130, J9140, C9423 | HEC | IV |
| Mechlorethamine | 211010301021x | J9230 | HEC | IV |
| Streptozotocin | 21102030002105 | J9320 | HEC | IV |
| Alemtuzumab | 21353010x | J9010 | Minimal | IV |
| Arsenic trioxide | 21700008x | J9017 | MEC | IV |
| Azacitidine | 21300003x | J9025 | MEC | IV |
| Bendamustine | 21100009x | J9033 | MEC | IV |
| Carboplatin | 21100015x | J9045 | MEC | IV |
| Clofarabine | 21300008x | J9027, C9129 | MEC | IV |
| Dactinomycin | 212000200021x | J9120 | MEC | IV |
| Daunorubicin | 21200030x | J9150–J9151, C9424 | MEC | IV |
| Doxorubicin | 21200040x | J9000–J9001, C9415 | Other | IV |
| Epirubicin | 21200042x | J9178, J9180 | Other | IV |
| Idarubicin | 21200045x | J9211, C9429 | MEC | IV |
| Ifosfamide | 2110102500x, 219900024064x | J9208, C9427 | Other | IV |
| Irinotecan | 21550040x | J9206 | MEC | IV |
| Melphalan | 211010400021x, 211010401021x | J9245 | MEC | IV |
| Oxaliplatin | 21100028x | J9263, C9205 | MEC | IV |
| Temozolomide | 211040700021x | J9328, C9253 | MEC | IV |
| Aldesleukin | 21703020x | J9015 | Other | IV |
| Amifostine crystalline | 21758010x | J0207 | Other | IV |
| Cyclophosphamide | 21101020002x | J9070–J9097, C9420, C9421 | Other | IV |
| Cytarabine | 21300010x | J9098–J9110, C9422 | Other | IV |
| Interferon-α | 217000601x, 217000602x, 217000603x | J9212–J9215 | Other | IV |
| Altretamine | 21100005x | MEC/HEC | Oral | |
| Procarbazine | 21700050x | S0182 | MEC/HEC | Oral |
| Cyclophosphamide | 211010200003x | J8530 | Other | Oral |
| Imatinib | 21534035x | S0088 | Minimal/Low | Oral |
| Temozolomide | 211040700001x | J8700 | Other | Oral |
| Busulfan | 211000100003x | J0594, J8510 | Other | Oral |
| Estramustine phosphate sodium | 2140302010x | MEC/HEC | Oral | |
| Etoposide | 21500010x | J8560 | MEC/HEC | Oral |
| Lomustine | 211020200001x | S0178 | MEC/HEC | Oral |
Notes: Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Antiemesis, Version 1.2012. © National Comprehensive Cancer Network, Inc 2015. All rights reserved. Accessed August 11, 2011. To view the most recent and complete version of the guideline, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc. Other = NCCN emetogenicity rating depends on dosage.
Abbreviations: GPI, generic product identifier; HCPCS, Healthcare Common Procedure Coding System; HEC, highly EC; MEC, moderately EC; EC, emetogenic chemotherapy; IV, intravenous.
Table S3.
HEC/MEC regimens
| Agent | Regimen | Schedule | Regimen type | MEC/HEC |
|---|---|---|---|---|
| Cyclophosphamide | TC | • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles |
Single day | Depends on dosage |
| Cyclophosphamide/doxorubicin | TAC (with docetaxel) | • Doxorubicin 50 mg/m2 IV day 1 | Single day | HEC |
| • Cyclophosphamide 500 mg/m2 IV day 1 Cycled every 3 weeks for six cycles | ||||
| TAC (with docetaxel) | • Doxorubicin 60 mg/m2 on day 1 | Single day | HEC | |
| • Cyclophosphamide 500 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| AC | • Doxorubicin 60 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 2 weeks for four cycles | ||||
| • Doxorubicin 60 mg/m2 IV day 1 | Single day | HEC | ||
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| • Doxorubicin 60 mg/m2 IV day 1 | Single day | HEC | ||
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| • Doxorubicin 60 mg/m2 IV day 1 | Single day | HEC | ||
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| FAC/CAF (with 5-FU) | • Doxorubicin 50 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 500 mg/m2 IV day 1 Cycled every 3 weeks for six cycles | ||||
| Cyclophosphamide/epirubicin | FEC/CEF (with 5-FU) | • Epirubicin 75 mg/m2 IV day 1 | Single day | HEC |
| • Cyclophosphamide 500 mg/m2 day 1 Cycled every 3 weeks for four cycles | ||||
| EC | • Epirubicin 100 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 830 mg/m2 IV day 1 Cycled every 3 weeks for eight cycles | ||||
| FEC | • Epirubicin 100 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 500 mg/m2 day 1 Cycled every 3 weeks for three cycles | ||||
| FEC | • Epirubicin 90 mg/m2 IV day 1 | Single day | HEC | |
| • Cyclophosphamide 600 mg/m2 IV day 1 Cycled every 3 weeks for four cycles | ||||
| Carboplatin | TCH | • Carboplatin AUC 6 IV day 1 Cycled every 3 weeks for six cycles |
Single day | MEC |
| CH (with trastuzumab) | • Carboplatin AUC 6 IV day 1 Cycled every 3 weeks for six cycles |
Single day | MEC | |
| CT (with docetaxel) | • Carboplatin AUC 6 IV day 1 Cycled every 3 weeks for six cycles |
Single day | MEC | |
| Cisplatin | CV (with vinorelbine) | • Cisplatin 100 mg/m2 day 1 Cycled every 4 weeks for four cycles |
Single day | HEC/depends on dosage |
| CV (with vinorelbine) | • Cisplatin 75–80 mg/m2 day 1 Cycled every 3 weeks for four cycles |
Single day | HEC/depends on dosage | |
| CG (with gemcitabine) | • Cisplatin 75 mg/m2 day 1 Cycled every 3 weeks for four to six cycles |
Single day | HEC/depends on dosage | |
| CD (with docetaxel) | • Cisplatin 75 mg/m2 day 1 Cycled every 3 weeks for four to six cycles |
Single day | HEC/depends on dosage | |
| CP (with pemetrexed) | • Cisplatin 75 mg/m2 day 1 Cycled every 3 weeks for four cycles |
Single day | HEC/depends on dosage | |
| Carboplatin | PC (with paclitaxel) | • Carboplatin AUC 6 day 1 Cycled every 3 weeks for four to six cycles |
Single day | MEC |
| PC (with paclitaxel) | • Carboplatin AUC 2 (initial) and 6 (last 2) weekly Total of three cycles |
Single day | MEC | |
| PG (with gemcitabine) | • Carboplatin AUC 2 day 1 Cycled every 3 weeks for six cycles |
Single day | MEC | |
| PE (with etoposide) | • Carboplatin AUC 2 day 1 Cycled every 4 weeks for six cycles |
Single day | MEC | |
| Oxaliplatin | FOLFOX | • Oxaliplatin 85 mg/m2 day 1 Cycled every 2 weeks for 6 months |
Single day | MEC |
| FOLFOX (modified) | • Oxaliplatin 85 mg/m2 day 1 Cycled every 2 weeks for 6 months |
Single day | MEC | |
| XELOX | • Oxaliplatin 130 mg/m2 day 1 Cycled every 3 weeks for eight cycles |
Single day | MEC |
Note: Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V2.2011, Colon Cancer V3.2011, Rectal Cancer V4.2011, Small Cell Lung Cancer V2.2012, Non-Small Cell Lung Cancer V3.2011 [All accessed July 11, 2011], Antiemesis V1.2012 [Accessed August 11, 2011]. © National Comprehensive Cancer Network, Inc 2015. All rights reserved. To view the most recent and complete version of the guidelines, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
Abbreviations: MEC, moderately emetogenic chemotherapy; HEC, highly EC; TC, taxotere/cyclophosphamide; IV, intravenous; TAC, Docetaxel/doxorubicin/cyclophosphomide; AC, Doxorubicin/cyclophosphomide; FAC/CAF, Flurouracil/doxorubicin/cyclophosphomide; 5-FU, 5-fluorouracil; FEC/CEF, Cyclophosphomide/Epirubicin/Flurouracil; TCH, Docetaxel/carboplatin/trastuzumab; CH, Docetaxel/carboplatin/trastuzumab; CT, Carboplatin/trastuzumab; CV, Cisplatin/vinorelbine; CG, Cisplatin/gemicitabine; CD, Cisplatin/docetaxel; CP, Cisplatin/pemetrexed; PC, Paclitaxel/Carboplatin; PG, Paclitaxel/Carboplatin/gemicitabine; PE, Paclitaxel/Carboplatin/etoposide; FOLFOX, Folinic acid/Fluorouracil/Oxaliplatin; XELOX, Capecitabine/Oxaliplatin.